Abstract
Organochalcogens, particularly ebselen, have been used in experimental and clinical trials with borderline efficacy. (PhSe)2 and (PhTe)2 are the simplest of the diaryl dichalcogenides and share with ebselen pharmacological properties. In view of the concerns with the use of mammals in studies and the great number of new organochalcogens with potential pharmacological properties that have been synthesized, it becomes important to develop screening protocols to select compounds that are worth to be tested in vivo. This study investigated the possible use of isolated human white cells as a preliminary model to test organochalcogen toxicity. Human leucocytes were exposed to 5–50 μM of ebselen, (PhSe)2, or (PhTe)2. All compounds were cytotoxic (Trypan's Blue exclusion) at the highest concentration tested, and Ebselen was the most toxic. Ebselen and (PhSe)2 were genotoxic (Comet Assay) only at 50 μM, and (PhTe)2 at 5–50 μM. Here, the acute cytotoxicity did not correspond with in vivo toxicity of the compounds. But the genotoxicity was in the same order of the in vivo toxicity to mice. These results indicate that in vitro genotoxicity in white blood cells should be considered as an early step in the investigation of potential toxicity of organochalcogens.
1. Introduction
Selenium (Se) is an essential microelement for human and animal nutrition [1]. It is important for selenoprotein synthesis, where it is present as the aminoacid selenocysteine [2]. Several selenoenzymes, such as Glutathione Peroxidase (GPx) and Thioredoxin Reductase (TrxR), are important for the cell defense against oxidative stress [3, 4]. Taking this role of Se in living beings, many therapeutic trials explored the use of inorganic forms of Se as pharmacological agents [5]. However, inorganic forms of Se, such as selenite and selenate, are poorly absorbed and present many toxic effects at high concentrations [6]. Consequently, the interest in organic forms of selenium, that can be less toxic and better absorbed than Se (IV) and Se (VI), has increased.
Tellurium (Te) is chemically related to Se and can be occasionally found in some proteins in bacteria, yeast, and fungi, but no functional telluroproteins have been found in animal cells [7]. In contrast to Se, Te does not have biological function [8]. However, the literature has demonstrated immunomodulatory, antioxidant, and anticancer properties of various organotellurides [9, 10]. Organotellurium compounds can also mimic Glutathione Peroxidase activity [11], and, consequently, these compounds can be potential antioxidants, effective against some cell damaging agents [12–14].
Ebselen and Diphenyl Diselenide ((PhSe)2) are two organoselenium compounds that are recognized as promising pharmacological agents presenting antioxidant, anti-inflammatory, neuroprotective, and other beneficial properties [9]. These compounds can exert their pharmacological effects by mimicking the native Glutathione Peroxidase enzyme (GPx-like activity) or by being a substrate of TrxR. The selenol intermediate formed after their reduction can reduce the levels of reactive oxygen species (ROS) in the cell and prevent oxidative damage to lipids, proteins, and DNA [15–18]. Diphenyl Ditelluride ((PhTe)2) is an organotellurium compound that also showed antioxidant and other in vitro pharmacological properties [9]. Therefore, the experimental use of organoselenium and -tellurium compounds in different models of human diseases has increased [19–23].
On the other hand, ebselen, (PhSe)2, and (PhTe)2 can be toxic when administered at high doses. This toxicity is thought to be associated with inhibition of thiol- and/or selenol-containing enzymes, which can increase ROS formation, lipid peroxidation, and DNA damage [24–27].
However, the quantity of new organoselenium and -tellurium compounds with pharmacological potential that have been synthesized is increasing rapidly. Consequently, information about the toxicity of new organochalcogens is needed. However, we do not have a simple preliminary test to determine the potential toxicity of a great number of new compounds. This point is critical both in view of the time required to perform assays with vertebrates and the need of ethical adherence to the 3R principal in the use of experimental animals. Here we compare the toxicity of ebselen (which has been used in different clinical trials), (PhSe)2 (which is a very simple and pharmacologically active diselenide), and (PhTe)2 (a simple and pharmacologically active ditelluride which is also very toxic in vivo to rodents) in human white blood cells to determine whether these cells could be used to do a preliminary screening of potentially toxic new organochalcogens.
In short, the aim of this study was to define the cytotoxic concentrations of ebselen, (PhSe)2, and (PhTe)2 in freshly isolated white human blood cells. Therefore, human leucocytes were exposed to compounds, and their potencial cytotoxic and genotoxic effects were measured using Trypan's Blue Exclusion and Comet Assay Tests.
2. Materials and Methods
2.1. Chemicals
Ebselen, (PhSe)2, (PhTe)2, Trypan's Blue, dextran, and tungstosilicic acid were obtained from Sigma-Aldrich (St. Louis, MO). All the other reagents were obtained from standard chemical suppliers.
2.2. Sample Preparation
Leucocytes were isolated from heparinized venous blood obtained from healthy volunteers. The protocol of study was reviewed and approved by the appropriate institutional review board from Guidelines of the Committee of UFSM (0089.0.243.000-07).
2 mL of dextran 5% (dissolved in Phosphate Buffer Saline 1%) was added to 8 mL of blood. The tube was gently mixed and left to stand at room temperature for 45 min. Afterwards, the supernatant was centrifuged (480 ×g, 10 min) and plasma was discarded. The pellet was washed with erythrocyte lysis solution (NH4Cl 150 mM; NaHCO3 10 mM; EDTA 1 mM) and centrifuged (480 ×g, 2 min). The supernatant was discarded and the pellet was washed twice with 1 mL erythrocyte lysis solution. After the second centrifugation, the pellet was suspended in 2 mL Hank's buffer solution (KCl 5.4 mM; Na2HPO4 0.3 mM; KH2PO4 0.4 mM; NaHCO3 4.2 mM; MgCl2 0.5 mM; NaCl 122.6 mM; D-glicose 10 mM, Tris-HCl 10 mM; CaCl2 1.3 mM; pH 7.4). The concentration of leucocytes was adjusted to 2000 cells/μL.
2.3. Leucocytes Exposure to Organochalcogens
Leucocytes were exposed to ebselen, (PhSe)2, and (PhTe)2 at 5, 10, and 50 μM or an equal volume of DMSO (final concentration of 0.5%) during 3 hours at 37°C. Positive control group was treated with hydrogen peroxide (H2O2) 2 mM and sodium azide 1 mM.
2.4. Trypan's Blue Exclusion Test
Trypan's Blue exclusion test was performed according to Mischel and Shiingi [28]. After 3 hours of incubation, 50 μL of Trypan's Blue 0.4% was mixed with 50 μL of leucocytes and left to stand at room temperature for 5 minutes. Cell viability was determined microscopically (400x magnification) and expressed as number of viable cells divided by the total number of cells multiplied by 100.
2.5. Comet Assay
Comet Assay was performed according to Collins [29] with some modifications. After three hours of incubation, 15 μL of the sample was mixed with 90 μL of low-melting point agarose 0.75% and placed in a slide precoated with agarose 1%. A coverslip was added and the samples were left to solidify at 4°C. The coverslips were removed and the slides were placed on a lysis solution (NaCl 2.5 M; EDTA 100 mM; Tris-HCl 8 mM; Triton X-100 1%; pH 10–10.5) during 24 hours at 4°C. Afterwards, the slides were incubated in an electrophoresis solution (NaOH 300 mM; EDTA 1 mM; pH 13.5) for 20 minutes at 4°C and the electrophoresis was performed (25 V; 300 mA; 7 W) for 20 minutes. All the steps were performed in the dark until this moment. After electrophoresis, the slides were washed in a neutralizing solution (Tris-HCl 400 mM; pH 7.5) three times, washed with distilled water, and left to dry. The slides were rehydrated and fixed (Trichloroacetic acid 15%; ZnSO4 5%; glycerol 5%), washed three with distilled water, and left to dry. Afterwards, the rehydrated slides were stained (Na2CO3 5%; NH4NO3 0.1%; AgNO3 0.1%; H4[W12SiO40] 0.25%; formaldehyde 0.15%). The slides were immersed in acetic acid 1%, washed, and left to dry.
One hundred cells randomly selected were analyzed in each sample according to tail size and intensity in five classes. The damage score for each cell can range between 0 (no damage) and 4 (maximum damage), according to Figure 1. Damage index (DI) was defined as follows: DI = 1n1 + 2n2 + 3n3 + 4n4, where n1 represents the number of cells with damage level 1, n2 represents the number of cells with damage level 2, n3 represents the number of cells with damage level 3, and n4 represents the number of cells with damage level 4. At least two different individuals analyzed the slides under blind conditions.
Figure 1.
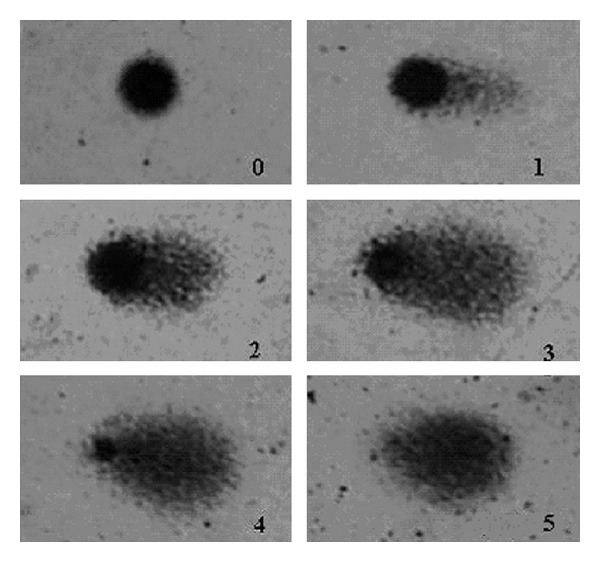
Damage levels considered for analysis in Comet Assay. Level 5 was excluded from our evaluation.
2.6. Statistical Analysis
Statistical analyses were performed using analysis of variance (ANOVA) followed by Newman-Keuls multiple test when appropriate. The results are expressed as mean ± SEM for four independent replicates. The difference was considered significant when P < 0.05.
3. Results and Discussion
Organoselenium compounds, such as ebselen and (PhSe)2, are known as pharmacologically active compounds, exhibiting antioxidant, anti-inflammatory, neuroprotective, and antimutagenic properties [9, 20, 22, 30, 31]. At low concentrations, these compounds protect cells against the insults generated by ROS production, depleting H2O2 via their GPx-mimic activity [32]. In fact, ebselen was used in clinical trials with borderline efficacy [19]. Therefore, the interest in the use of organochalcogens as therapeutic agents has increased in the last years.
Despite their pharmacological properties, organochalcogens can be hepato-, reno-, and neurotoxic to mammals when administered at high doses [33–36]. Accordingly, (PhSe)2 administration caused genotoxicity and prooxidant effects in mice [37, 38]. These toxic effects of ebselen, (PhSe)2, and (PhTe)2 can be secondary to thiol oxidation of critical target proteins, for instance, lactate dehydrogenase [39], Na+/K+ ATPase [9, 40], and δ-aminolevulinic acid dehydratase (δ-ALAD) [24, 41, 42]. Recently, we have demonstrated that (PhTe)2 can also inhibit important antioxidant selenoenzymes [27].
The data available in the literature about organochalcogens toxicity are scarce, mainly in human cells. So, this study examined comparatively the potential cytotoxic and genotoxic effects of ebselen, (PhSe)2, and (PhTe)2 in human leucocytes.
DMSO did not modify cell viability. At 50 μM, ebselen, (PhSe)2, and (PhTe)2 caused a significant decrease in cell viability when compared to the control groups. However, the effect of ebselen (a decrease of about 60%) was higher than that of (PhSe)2 (a decrease of about 20%) and that of (PhTe)2 (a decrease of about 25% in leucocyte viability, Figure 2). At lower concentrations, ebselen, (PhSe)2, and (PhTe)2 did not cause significant decrease in cell viability (Figure 2).
Figure 2.
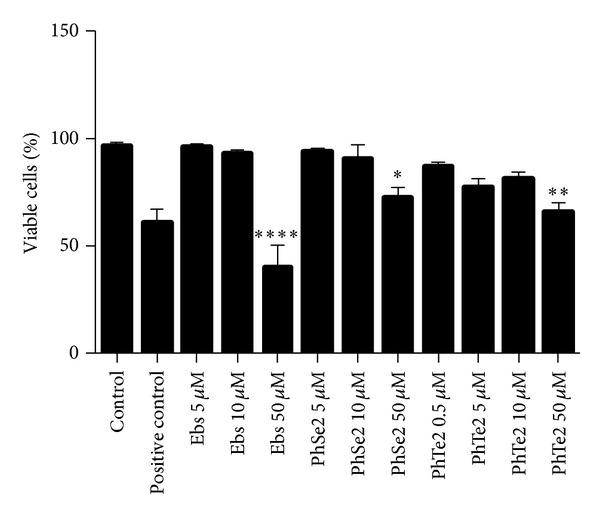
Cellular viability of human leucocytes exposed to organochalcogens for 3 hours. The results are expressed as mean ± SEM from four replicates. One-way ANOVA followed by Newman-Keuls (*P < 0.05, **P < 0.01, and ****P < 0.0001).
DMSO did not modify damage index (DI) of DNA in human blood leucocytes. Ebselen and (PhSe)2 at 50 μM and (PhTe)2 at 5, 10 and 50 μM caused a significant increase in DI when compared to the control group (Figure 3). Statistical analysis indicated that the effect of 50 μM ebselen and (PhTe)2 on DI was higher than that caused by (PhSe)2 (Figure 3). At 5 and 10 μM, (PhTe)2 increased DI, whereas ebselen and (PhSe)2 did not cause DNA damage at these concentrations.
Figure 3.
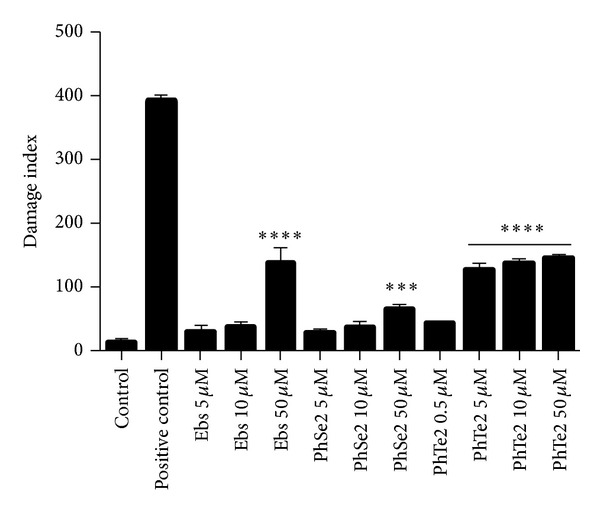
DI of human leucocytes exposed to organochalcogen for 3 hours. Data are expressed as mean ± SEM of four independent experiments done in duplicate. One-way ANOVA followed by Newman-Keuls (***P < 0.001 and ****P < 0.0001).
Thus, regardless of their structural differences, the toxicity of these compounds can have a common molecular mechanism, that is, oxidation of thiol groups in critical proteins [22, 42, 43]. However, here we observed that ebselen exhibited higher cytotoxicity in human leucocytes than (PhSe)2 and (PhTe)2. The higher toxicity of ebselen may be related to its capacity to induce thiol oxidation on lactate dehydrogenase [39] and mitochondrial complexes I and II [44] more than (PhSe)2 and (PhTe)2, which can cause the impairment of cell respiration and, consequently, cell death. Additionally, we observed that ebselen was more genotoxic than (PhSe)2, and (PhTe)2 was the most genotoxic of the three compounds. A report in the literature shows that (PhTe)2 induces cell death via oncosis [45], which is a different type of cell death than that induced by ebselen [9, 46] and (PhSe)2 [47]. The different genotoxicity potential may be related to differences in the interaction of these compounds with the reparing DNA machinery, in addition to differences in the reactivity with critical thiol-containing proteins.
4. Conclusion
In summary, this study shows that ebselen, (PhSe)2, and (PhTe)2 can cause cytotoxicity and genotoxicity in human leucocytes, that was expressed, respectively, by a decrease in cell viability in Trypan's Blue exclusion test and an increase of DI in Comet Assay, where the cytotoxic effect of ebselen was more pronounced, while (PhTe)2 presented the highest genotoxic effect in freshly isolated human leucocytes. Here, the acute cytotoxicity did not correspond with in vivo toxicity of the compounds [9], probably because (PhTe)2 induces cell death by a different way than that induced by ebselen and (PhSe)2, or otherwise they can have some common steps (for instance, oxidation of thiol proteins, but with different potency and perhaps with some different targets). However, the genotoxicity was in the same order of the in vivo toxicity to mice (i.e., (PhTe)2 > ebselen > (PhSe)2) [9], confirming that the use of Comet Assay in human leucocytes is a good strategy for a preliminary study of genotoxicity. These results indicate that in vitro genotoxicity in white blood cells should be considered as an early step in the investigation of potential toxicity of organochalcogens before performing in vivo studies with vertebrates. However, more studies are needed to elucidate the toxic effects of ebselen, (PhSe)2 and (PhTe)2, and their mechanisms of action in different cell types.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This paper is under the financial support of CNPq, CAPES, and FAPERGS.
References
- 1.Combs GF, Jr., Combs SB. The nutritional biochemistry of selenium. Annual Review of Nutrition. 1984;4:257–280. doi: 10.1146/annurev.nu.04.070184.001353. [DOI] [PubMed] [Google Scholar]
- 2.Holben DH, Smith AM. The diverse role of selenium within selenoproteins: a review. Journal of the American Dietetic Association. 1999;99(7):836–843. doi: 10.1016/S0002-8223(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 3.Papp LV, Lu J, Holmgren A, Khanna KK. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxidants and Redox Signaling. 2007;9(7):775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 4.Zhong L, Arnér ESJ, Holmgren A. Structure and mechanism of mammalian thioredoxin reductase: the active site is a redox-active selenolthiol/selenenylsulfide formed from the conserved cysteine-selenocysteine sequence. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(11):5854–5859. doi: 10.1073/pnas.100114897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutation Research. 2001;475(1-2):123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 6.Alfthan G, Aro A, Arvilommi H, Huttunen JK. Selenium metabolism and platelet glutathione peroxidase activity in healthy Finnish men: effects of selenium yeast, selenite, and selenate. American Journal of Clinical Nutrition. 1991;53(1):120–125. doi: 10.1093/ajcn/53.1.120. [DOI] [PubMed] [Google Scholar]
- 7.Bienert GP, Schüssler MD, Jahn TP. Metalloids: essential, beneficial or toxic? Major intrinsic proteins sort it out. Trends in Biochemical Sciences. 2008;33(1):20–26. doi: 10.1016/j.tibs.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Taylor A. Biochemistry of tellurium. Biological Trace Element Research. 1996;55(3):231–239. doi: 10.1007/BF02785282. [DOI] [PubMed] [Google Scholar]
- 9.Nogueira CW, Zeni G, Rocha JBT. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chemical Reviews. 2004;104(12):6255–6285. doi: 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]
- 10.Avila DS, Benedetto A, Au C, et al. Organotellurium and organoselenium compounds attenuate Mn-induced toxicity in Caenorhabditis elegans by preventing oxidative stress. Free Radical Biology and Medicine. 2012;52(9):1903–1910. doi: 10.1016/j.freeradbiomed.2012.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braga AL, Alberto EE, Soares LC, Rocha JBT, Sudati JH, Roos DH. Synthesis of telluroamino acid derivatives with remarkable GPx like activity. Organic and Biomolecular Chemistry. 2009;7(1):43–45. doi: 10.1039/b814990a. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C-M, Brattsand R, Hallberg A, et al. Diaryl tellurides as inhibitors of lipid peroxidation in biological and chemical systems. Free Radical Research. 1994;20(6):401–410. doi: 10.3109/10715769409145639. [DOI] [PubMed] [Google Scholar]
- 13.Kanski J, Drake J, Aksenova M, Engman L, Butterfield DA. Antioxidant activity of the organotellurium compound 3-[4-(N,N-dimethylamino)benzenetellurenyl]propanesulfonic acid against oxidative stress in synaptosomal membrane systems and neuronal cultures. Brain Research. 2001;911(1):12–21. doi: 10.1016/s0006-8993(01)02541-0. [DOI] [PubMed] [Google Scholar]
- 14.Jacob C, Arteel GE, Kanda T, Engman L, Sies H. Water-soluble organotellurium compounds: catalytic protection against peroxynitrite and release of zinc from metallothionein. Chemical Research in Toxicology. 2000;13(1):3–9. doi: 10.1021/tx990156g. [DOI] [PubMed] [Google Scholar]
- 15.De Freitas AS, Rocha JBT. Diphenyl diselenide and analogs are substrates of cerebral rat thioredoxin reductase: a pathway for their neuroprotective effects. Neuroscience Letters. 2011;503(1):1–5. doi: 10.1016/j.neulet.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Mugesh G, Panda A, Singh HB, Punekar NS, Butcher RJ. Glutathione peroxidase-like antioxidant activity of diaryl diselenides: a mechanistic study. Journal of the American Chemical Society. 2001;123(5):839–850. doi: 10.1021/ja994467p. [DOI] [PubMed] [Google Scholar]
- 17.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radical Biology and Medicine. 1993;14(3):313–323. doi: 10.1016/0891-5849(93)90028-s. [DOI] [PubMed] [Google Scholar]
- 18.Zhao R, Holmgren A. A novel antioxidant mechanism of ebselen involving ebselen diselenide, a substrate of mammalian thioredoxin and thioredoxin reductase. The Journal of Biological Chemistry. 2002;277(42):39456–39462. doi: 10.1074/jbc.M206452200. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Sano K, Takakura K, et al. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke. 1998;29(1):12–17. doi: 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- 20.Rossato JI, Ketzer LA, Centurião FB, et al. Antioxidant properties of new chalcogenides against lipid peroxidation in rat brain. Neurochemical Research. 2002;27(4):297–303. doi: 10.1023/a:1014907228580. [DOI] [PubMed] [Google Scholar]
- 21.Sanmartín C, Plano D, Palop JA. Selenium compounds and apoptotic modulation: a new perspective in cancer therapy. Mini-Reviews in Medicinal Chemistry. 2008;8(10):1020–1031. doi: 10.2174/138955708785740625. [DOI] [PubMed] [Google Scholar]
- 22.Nogueira CW, Rocha JBT. Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds. Archives of Toxicology. 2011;85(11):1313–1359. doi: 10.1007/s00204-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 23.Ip C, Thompson HJ, Zhu Z, Ganther HE. In vitro and in vivo studies of methylseleninic acid: evidence that a monomethylated selenium metabolite is critical for cancer chemoprevention. Cancer Research. 2000;60(11):2882–2886. [PubMed] [Google Scholar]
- 24.Barbosa NBV, Rocha JBT, Zeni G, Emanuelli T, Beque MC, Braga AL. Effect of organic forms of selenium on δ-aminolevulinate dehydratase from liver, kidney, and brain of adult rats. Toxicology and Applied Pharmacology. 1998;149(2):243–253. doi: 10.1006/taap.1998.8373. [DOI] [PubMed] [Google Scholar]
- 25.Rosa RM, Moura DJ, Romano e Silva AC, Saffi J, Pêgas Henriques JA. Antioxidant activity of diphenyl diselenide prevents the genotoxicity of several mutagens in Chinese hamster V79 cells. Mutation Research. 2007;631(1):44–54. doi: 10.1016/j.mrgentox.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Santofimia-Castaño P, Salido GM, Gonzáles A. Ebselen alters mitochondrial physiology and reduces viability of rat hippocampal astrocytes. DNA and Cell Biology. 2013;32(4):147–155. doi: 10.1089/dna.2012.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Comparsi B, Meinerz DF, Franco JL, et al. Diphenyl ditelluride targets brain selenoproteins in vivo: inhibition of cerebral thioredoxin reductase and glutathione peroxidase in mice after acute exposure. Molecular and Cellular Biochemistry. 2012;370:173–182. doi: 10.1007/s11010-012-1408-6. [DOI] [PubMed] [Google Scholar]
- 28.Mischell BB, Shiingi SM. Selected Methods in Cellular Immunology. W.H. Freeman; 1980. [Google Scholar]
- 29.Collins AR. The comet assay for DNA damage and repair: principles, applications, and limitations. Molecular Biotechnology . 2004;26(3):249–261. doi: 10.1385/MB:26:3:249. [DOI] [PubMed] [Google Scholar]
- 30.Farina M, Frizzo MES, Soares FAA, et al. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicology Letters. 2003;144(3):351–357. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- 31.de Freitas AS, Funck VR, Rotta MDS, et al. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Research Bulletin. 2009;79(1):77–84. doi: 10.1016/j.brainresbull.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Mugesh G, Singh HB. Synthetic organoselenium compounds as antioxidants: glutathione peroxidase activity. Chemical Society Reviews. 2000;29(5):347–357. [Google Scholar]
- 33.Maciel EN, Flores EMM, Rocha JBT, Folmer V. Comparative deposition of diphenyl diselenide in liver, kidney, and brain of mice. Bulletin of Environmental Contamination and Toxicology. 2003;70(3):470–476. doi: 10.1007/s00128-003-0010-8. [DOI] [PubMed] [Google Scholar]
- 34.Meotti FC, Borges VC, Zeni G, Rocha JBT, Nogueira CW. Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and Ebselen for rats and mice. Toxicology Letters. 2003;143(1):9–16. doi: 10.1016/s0378-4274(03)00090-0. [DOI] [PubMed] [Google Scholar]
- 35.Farina M, Soares FAA, Zeni G, Souza DO, Rocha JBT. Additive pro-oxidative effects of methylmercury and ebselen in liver from suckling rat pups. Toxicology Letters. 2004;146(3):227–235. doi: 10.1016/j.toxlet.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Straliotto MR, Mancini G, De Oliveira J, et al. Acute exposure of rabbits to diphenyl diselenide: a toxicological evaluation. Journal of Applied Toxicology. 2010;30(8):761–768. doi: 10.1002/jat.1560. [DOI] [PubMed] [Google Scholar]
- 37.Rosa RM, Hoch NC, Furtado GV, Saffi J, Henriques JAP. DNA damage in tissues and organs of mice treated with diphenyl diselenide. Mutation Research. 2007;633(1):35–45. doi: 10.1016/j.mrgentox.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Degrandi TH, De Oliveira IM, D’Almeida GS, et al. Evaluation of the cytotoxicity, genotoxicity and mutagenicity of diphenyl ditelluride in several biological models. Mutagenesis. 2010;25(3):257–269. doi: 10.1093/mutage/geq002. [DOI] [PubMed] [Google Scholar]
- 39.Lugokenski TH, Mller LG, Taube PS, Rocha JBT, Pereira ME. Inhibitory effect of ebselen on lactate dehydrogenase activity from mammals: a comparative study with diphenyl diselenide and diphenyl ditelluride. Drug and Chemical Toxicology. 2011;34(1):66–76. doi: 10.3109/01480541003782294. [DOI] [PubMed] [Google Scholar]
- 40.Borges VC, Rocha JBT, Nogueira CW. Effect of diphenyl diselenide, diphenyl ditelluride and ebselen on cerebral Na+, K+-ATPase activity in rats. Toxicology. 2005;215(3):191–197. doi: 10.1016/j.tox.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Saraiva RA, Bueno DC, Nogara PA, Rocha JBT. Molecular docking studies of disubstituted diaryl diselenides as mammalian δ-aminolevulinic acid dehydratase enzyme inhibitors. Journal of Toxicology and Environmental Health A. 2012;75(16-17):1012–1022. doi: 10.1080/15287394.2012.697810. [DOI] [PubMed] [Google Scholar]
- 42.Rocha JBT, Saraiva RA, Garcia SC, Gravina FS, Nogueira CW. Aminolevulinate dehydratase (δ-ALA-D) as marker protein of intoxication with metals and other pro-oxidant situations. Toxicology Research. 2012;1(2):85–102. [Google Scholar]
- 43.Yang CF, Shen HM, Ong CN. Intracellular thiol depletion causes mitochondrial permeability transition in ebselen-induced apoptosis. Archives of Biochemistry and Biophysics. 2000;380(2):319–330. doi: 10.1006/abbi.2000.1939. [DOI] [PubMed] [Google Scholar]
- 44.Puntel RL, Roos DH, Seeger RL, Rocha JBT. Mitochondrial transfer chain complexes inhibition by different organochalcogens. Toxicology in Vitro. 2013;27:59–70. doi: 10.1016/j.tiv.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Roy S, Hardej D. Tellurium tetrachloride and diphenyl ditelluride cause cytotoxicity in rat hippocampal astrocytes. Food and Chemical Toxicology. 2011;49(10):2564–2574. doi: 10.1016/j.fct.2011.06.072. [DOI] [PubMed] [Google Scholar]
- 46.Yang C-F, Shen H-M, Ong C-N. Ebselen induces apoptosis in HepG2 cells through rapid depletion of intracellular thiols. Archives of Biochemistry and Biophysics. 2000;374(2):142–152. doi: 10.1006/abbi.1999.1574. [DOI] [PubMed] [Google Scholar]
- 47.Posser T, De Paula MT, Franco JL, Leal RB, Da Rocha JBT. Diphenyl diselenide induces apoptotic cell death and modulates ERK1/2 phosphorylation in human neuroblastoma SH-SY5Y cells. Archives of Toxicology. 2011;85(6):645–651. doi: 10.1007/s00204-010-0602-0. [DOI] [PubMed] [Google Scholar]


