Abstract
Objective
We hypothesized that metachronous colorectal liver metastases (CLM) have different biology after failure of oxaliplatin (FOLFOX) compared to 5-fluorouracil (5-FU) or no chemotherapy for adjuvant treatment of colorectal cancer (CRC).
Background
It is unclear whether patients treated with liver resection for metachronous CLM after adjuvant FOLFOX for CRC have worse outcomes than those who received 5-FU or no chemotherapy.
Methods
We identified 341 patients who underwent hepatectomy for metachronous CLM (disease-free interval ≥12 months, 1993–2010). Mass-spectroscopy genotyping for somatic gene mutations in CLM was performed in a subset of 129 patients.
Results
Adjuvant treatment for primary CRC was FOLFOX in 77 patients, 5-FU in 169 patients, and no chemotherapy in 95 patients. Node-positive primary was comparable between FOLFOX and 5-FU but lower in the no-chemotherapy group (P < 0.0001). Median metastasis size was smaller in the FOLFOX group (2.5 cm) than in the 5-FU (3.0 cm) or no-chemotherapy (3.5 cm) groups, (P = 0.008) although prehepatectomy chemotherapy utilization, metastases number, and carcinoembryonic antigen levels were similar. Disease-free survival (DFS) and overall survival (OS) rates after hepatectomy were worse in patients treated with adjuvant FOLFOX [DFS at 3 years: 14% vs 38% (5-FU) vs 45% (no-chemo), OS at 3 years: 58% vs 70% (5-FU) vs 84% (no-chemo)]. On multivariate analysis, adjuvant FOLFOX was associated with worse DFS (P < 0.0001) and OS (P < 0.0001). Mutation analysis revealed ≥1 mutations in 57% of patients (27/47) after FOLFOX, 29% (12/41) after 5-FU, and 32% (13/41) after no chemotherapy (P = 0.011).
Conclusions
Adjuvant FOLFOX for primary CRC is associated with a high rate of somatic mutations in liver metastases and inferior outcomes after hepatectomy for metachronous CLM.
Keywords: colorectal cancer, metachronous colorectal liver metastases, somatic gene mutations
Colorectal cancer (CRC) is one of the most common malignancies worldwide and the second most common cause of cancer death in Western countries.1 CRC resection with regional lymphadenectomy is the primary treatment of choice. The postoperative survival in these patients is significantly associated with tumor stage on the basis of the TNM classification system, which takes into consideration the depth of tumor penetration in the bowel wall and the extent of lymph node involvement.2 Patients with positive lymph node metastases have a higher risk of local recurrence and metastasis, especially in the liver. Therefore, systemic therapy after resection of node-positive CRC has routinely been used to reduce the incidence of relapse.3
Adjuvant chemotherapy with fluorouracil (5-FU) and leucovorin (FL) was established in the 1990s for stage III CRC to reduce recurrence and prolong survival.4–6 Since 2004, oxaliplatin, in combination with 5-FU and FL (FOLFOX), has been used to treat stage II or stage III CRC after surgery. The MOSAIC randomized trial demonstrated that patients treated with this modern chemotherapy regimen have higher disease-free (DFS) and overall survival (OS) rates than those treated with 5-FU and FL alone.7,8
Despite adjuvant chemotherapy, approximately 20% of patients develop metachronous colorectal liver metastases (CLM) within 3 years.9 Modern chemotherapy with FOLFOX has increased the recurrence-free survival rate after resection of the primary CRC; however, metastatic liver disease has not been completely eliminated. One fourth of patients with CLM are candidates for liver resection; curative hepatectomy, combined with perioperative systemic therapy, leads to 5-year OS rates as high as 58%.10 However, in this era of modern chemotherapy, it is unclear whether patients treated with liver resection for metachronous CLM after adjuvant FOLFOX therapy for the primary CRC have poorer outcomes than those who received 5-FU or no chemotherapy.
In this retrospective study, we hypothesized that metachronous CLM have different biologic characteristics after failure of oxaliplatin compared with after 5-FU or no chemotherapy for adjuvant treatment of CRC. To investigate this hypothesis, we performed a survival analysis on 3 groups of patients who had undergone liver resection for metachronous CLM (diagnosis ≥ 1 year after resection of the CRC) and had received FOLFOX, 5-FU, or no chemotherapy for the primary tumor. The biologic characteristics of the CLM were evaluated on the basis of the presence of somatic gene mutations that are known to be associated with unfavorable outcome in metastatic CRC.
PATIENTS AND METHODS
Study Inclusion Criteria
We queried the prospectively maintained hepatobiliary surgical database at The University of Texas MD Anderson Cancer Center (Houston, TX) to identify consecutive patients who had undergone surgery for CLM between January 1993 and December 2010. Clinicopathologic data (described in detail in the Statistical Analysis section) were extracted from the patients’ medical records. Patients who had been treated with radiofrequency ablation (RFA) only or concomitant hepatectomy and RFA were not included in this analysis. All patients with disease-free interval between resection of the primary CRC and diagnosis of the CLM less than 12 months were considered to have synchronous CLM and were excluded. Patients who had undergone hepatectomy for metachronous CLM and had received adjuvant chemotherapy for the primary CRC other than 5-FU or FOLFOX did not fulfill the inclusion criteria. Institutional review board approval (Protocol PA11–0607) was obtained before data retrieval and analysis.
Preoperative CLM Assessment
Preoperative assessment included a medical history, physical examination, laboratory evaluation, and imaging studies. Helical computed tomography of chest, abdomen, and pelvis with liver protocol was used to define the extent and location of CLM. Fluorodeoxyglucose positron emission tomography was used in selected patients to rule out extensive extrahepatic disease and confirm the metastatic nature of atypical lesions.11 Treatment plans were based on the location and extent of CLM, the presence of extrahepatic disease, and radiographic response to preoperative chemotherapy. The decision to administer preoperative chemotherapy was made by the treating physicians. Hepatectomy was considered in patients in whom computed tomographic volumetry data indicated that all CLM could be safely resected with preservation of a sufficient future liver remnant. In patients with an anticipated insufficient future liver remnant, preoperative portal vein embolization was used to induce hypertrophy.12
Surgical Procedure
During laparotomy, the peritoneal cavity was inspected to identify previously unrecognized extrahepatic disease. Intraoperative sonography of the liver was performed to confirm and to better define the location of CLM and their relation to portal pedicles and hepatic veins. Parenchymal transection was performed under total or selective hepatic inflow vascular exclusion using the Cavitron ultrasonic surgical aspirator (CUSA, Valleylab, Boulder, CO), and hemostasis was achieved using saline-linked cautery (dissecting sealer DS 3.0, Tissuelink Medical, Inc, Dover, NH).13
Postoperative Evaluation
Postoperative mortality was defined as any death within 90 days after liver resection, and postoperative morbidity was defined as any complication within the same time period. Postoperative complications were graded according to a standard classification.14 Major complications were classified as complications requiring surgical, endoscopic, or radiologic intervention (grade III); life-threatening complications requiring intensive care management (grade IV); and death (grade V). Postoperative liver insufficiency was defined as a postoperative peak serum bilirubin level higher than 7 mg/dL.15
All specimens were subjected to histologic evaluation to confirm the diagnosis of metastatic CRC, the degree of pathologic response of CLM to preoperative chemotherapy,16 and the width of the tumor-free surgical margin.17
Somatic Gene Mutation Profiling
To assess the tumor biologic characteristics in patients who received adjuvant FOLFOX, 5-FU, or no chemotherapy for the primary CRC, mass-spectroscopy genotyping for somatic gene mutations was performed. DNA extracted from formalin-fixed paraffin-embedded resected CLM was analyzed with Sequenom MassArray technology (Sequenom, Inc, San Diego, CA) using the protocol developed in one of our institutional core facilities.18 A total of 159 point mutations in 33 genes commonly involved in solid tumors including KRAS, BRAF, NRAS, PIK3CA, FBWX7, and CTNNB1 were tested. Sequenom’s MassARRAY system utilizes polymerase chain reaction amplification and single-base primer extension for mutation detection.19–21 The analytical sensitivity of the assay [limit of detection (LOD) 5%–10% of mutant DNA in total DNA] is higher than conventional Sanger sequencing (LOD: 10%–20%) and similar to pyrosequencing (LOD: 5%–10%).22,23 The advantages offered by the MassARRAY system include high-throughput screening for many hot-spot mutations in parallel, use of minimal DNA (10–50 ng) isolated from formalin-fixed paraffin-embedded tissues, ability to detect coexisting multiple mutations, and cost and time effectiveness.
Statistical Analysis
Quantitative and qualitative variables were expressed as medians (range) and frequencies. Comparisons between groups were analyzed with the chi-square or Fisher exact tests for proportions and the Mann-Whitney U test or Kruskal-Wallis H test for continuous variables, as appropriate. Patients were stratified by type of adjuvant chemotherapy for the CRC and the clinicopathologic characteristics of patients who received adjuvant FOLFOX were compared with those of patients who received 5-FU or no adjuvant chemotherapy. Somatic gene mutation rates were also compared between the 3 patient groups. OS and DFS rates were calculated from the date of liver resection to the date of last follow-up or recurrence, respectively, using the Kaplan-Meier method and were compared using log-rank tests.
To identify factors associated with OS and DFS in the entire study cohort (N = 341), we evaluated the following clinicopathologic variables in a univariate analysis: sex (male vs female), age (> 65 vs ≤ 65 years), primary tumor location (rectum vs colon), regional lymph nodes status of the primary tumor (positive vs negative), number of CLM (multiple vs solitary), adjuvant chemotherapy for CRC (FOLFOX vs 5-FU vs no chemotherapy), diameter of the largest CLM (>3 vs ≤3 cm), preoperative carcinoembryonic antigen (CEA) level (>5 ng/mL vs ≤5 ng/mL), preoperative chemotherapy for CLM (administered vs not), portal vein embolization (performed vs not), blood transfusion required (yes vs no), liver resection margins status on microscopic analysis (R1 vs R0), pathologic response to preoperative chemotherapy (major vs minor), postoperative chemotherapy for CLM (administered vs not), and postoperative complications (yes vs no).
All variables associated with OS or DFS with P < 0.05 in the univariate proportional hazards models were entered into a Cox multivariate regression model with backward elimination. P < 0.05 were considered statistically significant. Statistical analyses were performed using the software IBM SPSS Statistics, version 19 (IBM, Armonk, NY).
RESULTS
Patients and Treatment
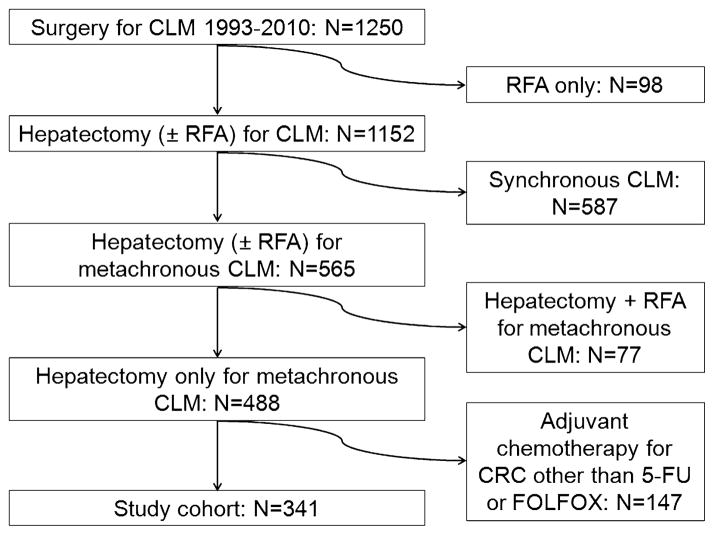
Among 1250 consecutive patients with CLM treated at MD Anderson during the study period, 98 patients had been treated with RFA only and were excluded from the study. Patients with synchronous CLM (N = 587) (disease-free interval < 12 months) were also excluded. Concomitant hepatectomy and RFA had been performed in 77 of the remaining 565 patients with metachronous CLM; these patients were excluded from the analysis. Of the remaining 488 patients treated with curative hepatectomy for metachronous CLM, 147 had undergone adjuvant chemotherapy for the primary tumor with agents other than 5-FU or FOLFOX and were thus not included in the analysis. The final study cohort consisted of 341 patients who underwent curative liver resection for metachronous CLM and had received FOLFOX (N = 77), 5-FU (N = 169), or no adjuvant systemic therapy (N = 95) after the resection of the primary CRC (Fig. 1).
FIGURE 1.
Study inclusion criteria.
Patient Characteristics by Adjuvant Chemotherapy Type for CRC
Patients’ characteristics, listed by adjuvant chemotherapy type for CRC, are summarized in Table 1. Patients who received no adjuvant chemotherapy for CRC have been treated in our center from 1993 to the end of the study period (2010). Patients who received adjuvant 5-FU have been treated in the same time period (1993–2010). FOLFOX has been used for the adjuvant therapy of CRC since 2005. Patients’ median age in the FOLFOX group was significantly lower than that in the 5-FU and no chemotherapy groups (P = 0.035). The number of node-positive primary tumors was similar between FOLFOX and 5-FU but lower in the no-chemotherapy group (P < 0.0001). The median metastasis size was smaller in the adjuvant FOL-FOX group (2.5 cm) than in the 5-FU (3.0 cm) and no-chemotherapy (3.5 cm) groups (P = 0.008). Postoperative complications were more common in the FOLFOX group (P = 0.047), but there was no difference in the major complication rates among the 3 groups (P = 0.204). The remaining patient characteristics in the 3 groups were similar, including prehepatectomy chemotherapy utilization (P = 0.110), CLM number (P = 0.579), and preoperative CEA serum level (P = 0.239).
TABLE 1.
Characteristics of Patients who Underwent Hepatectomy for Metachronous CLM by Adjuvant Chemotherapy Type for CRC
| Variable | No Adjuvant Chemotherapy (N = 95) | 5-FU (N = 169) | FOLFOX (N = 77) | P* | P† |
|---|---|---|---|---|---|
| Male sex, % | 68 | 57 | 58 | 0.166 | 0.810 |
| Median age, (range), y | 60 (37–84) | 62 (23–82) | 57 (32–87) | 0.035 | 0.015 |
| Age > 65 y, % | 40 | 38 | 25 | 0.074 | 0.042 |
| Median DFS after CRC resection (range), mo | 20 (12–142) | 20 (12–204) | 19 (12–48) | 0.187 | 0.177 |
| Rectal primary tumor (%) | 31 | 29 | 30 | 0.952 | 0.857 |
| Positive nodes for primary tumor, % | 8 | 68 | 79 | <0.0001 | 0.059 |
| Median number of CLM (range) | 1 (1–10) | 1 (1–7) | 1 (1–9) | 0.579 | 0.420 |
| Multiple CLM (%) | 43 | 37 | 38 | 0.619 | 0.954 |
| Median size of CLM, (range), cm | 3.5 (3–15) | 3 (0.5–17) | 2.5 (0.4–8.5) | 0.008 | 0.036 |
| Size > 3 cm, % | 52 | 50 | 36 | 0.092 | 0.052 |
| Median CEA (range), ng/mL | 4 (1–2477) | 7 (1–387) | 5 (1–210) | 0.239 | 0.092 |
| CEA > 5 ng/mL | 47 | 56 | 46 | 0.275 | 0.168 |
| Preoperative chemotherapy for CLM, % | 61 | 50 | 61 | 0.110 | 0.098 |
| Portal vein embolization, % | 8 | 4 | 13 | 0.09 | 0.027 |
| Median estimated blood loss, (range), mL | 250 (0–2000) | 300 (0–3000) | 250 (0–2000) | 0.770 | 0.507 |
| Transfusions, % | 13 | 9 | 9 | 0.604 | 0.965 |
| Postoperative complications, % | 31 | 21 | 35 | 0.047 | 0.023 |
| Major postoperative complications, % | 15 | 10 | 17 | 0.204 | 0.094 |
| Positive surgical margins, % | 13 | 11 | 10 | 0.884 | 0.831 |
| Resection for recurrence, % | 17 | 15 | 10 | 0.449 | 0.298 |
| Major pathologic response to preoperative chemotherapy for CLM, % | 60 | 38 | 43 | 0.06 | 0.545 |
| Postoperative chemotherapy for CLM, % | 66 | 67 | 54 | 0.137 | 0.054 |
Comparison of patients with FOLFOX vs 5-FU vs no chemotherapy for adjuvant treatment of CRC.
Comparison of patients with FOLFOX vs 5-FU for adjuvant treatment of CRC.
Postoperative Mortality and Morbidity
The postoperative 90-day mortality rate was 2% (6 patients died). Three patients died as a result of postoperative liver insufficiency after an extended hepatectomy following prolonged preoperative chemotherapy. Two deaths were related to pulmonary infection, and 1 patient died of thromboembolic complications (pulmonary embolism). The postoperative 90-day morbidity rate was 27% (93 of 341 patients). Thirteen percent of patients experienced a major complication that necessitated operative, endoscopic, or radiologic intervention.
Long-Term Survival
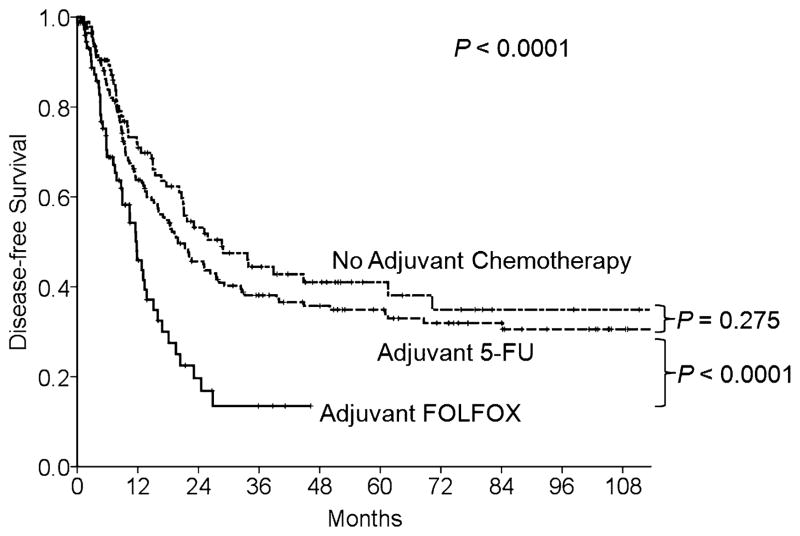
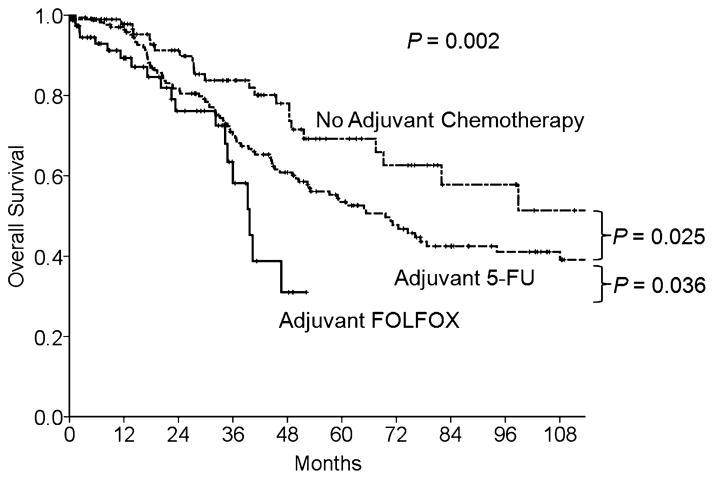
At a median follow-up duration of 53 months (1–196 months), the 3- and 5-year DFS rates of the entire cohort were 36% and 33%, respectively. The 3- and 5-year OS rates were 72% and 55%, respectively. The DFS rates after resection of CLM were significantly lower in patients treated with adjuvant FOLFOX than in patients treated with 5-FU or no chemotherapy after resection of the primary CRC (DFS at 3 years: 14% vs 38% vs 45%, respectively, P < 0.0001) (Fig. 2). Likewise, OS rates were lower in FOLFOX patients than in 5-FU and no chemotherapy patients (OS at 3-years: 58% vs 70% vs 84%, respectively, P = 0.002) (Fig. 3).
FIGURE 2.
DFS by adjuvant chemotherapy type for primary CRC in 341 patients who underwent hepatectomy for metachronous CLM.
FIGURE 3.
OS by adjuvant chemotherapy type for primary CRC in 341 patients who underwent hepatectomy for metachronous CLM.
Predictors of Outcome
The results of univariate and multivariate analyses of factors associated with DFS are summarized in Table 2. On univariate analysis, positive lymph node metastases for the primary tumor (P = 0.023), adjuvant FOLFOX therapy for the primary CRC (P < 0.0001), pre-operative chemotherapy for CLM (P = 0.028), and positive surgical margins at CLM resection (P = 0.012) were associated with poor DFS. On multivariate analysis, only the adjuvant FOLFOX therapy for the primary CRC [hazard ratio (HR) = 1.52, 95% confidence interval (CI): 1.23–1.89, P < 0.0001] was independently associated with worse DFS.
TABLE 2.
Univariate and Multivariate Analysis of Clinicopathologic Variables Associated With DFS in 341 Patients Who Underwent Hepatectomy for Metachronous CLM
| Variable | Univariate Analysis
|
Multivariate Analysis*
|
|||||
|---|---|---|---|---|---|---|---|
| N = 341 (%) | Median DFS, mo | P | HR | 95% CI | P | ||
| Sex | Male | 60 | 19 | 0.340 | |||
| Female | 40 | 20 | |||||
| Age, y | >65 | 35 | 19 | 0.268 | |||
| ≤65 | 65 | 20 | |||||
| Primary tumor | Rectal | 29 | 20 | 0.820 | |||
| Colon | 71 | 18 | |||||
| Lymph nodes for primary | Positive | 54 | 16 | 0.023 | NS | ||
| Negative | 46 | 25 | |||||
| Number CLM | Multiple | 61 | 15 | 0.065 | |||
| Solitary | 39 | 22 | |||||
| Adjuvant therapy for CRC | None | 28 | 29 | < 0.0001 | 1.52 | 1.23–1.89 | < 0.0001 |
| 5-FU | 49 | 20 | |||||
| FOLFOX | 23 | 12 | |||||
| Size, cm | > 3 | 47 | 20 | 0.434 | |||
| ≤3 | 53 | 18 | |||||
| CEA, ng/mL | > 5 | 51 | 17 | 0.924 | |||
| ≤5 | 49 | 16 | |||||
| Preoperative chemotherapy for CLM | Yes | 55 | 15 | 0.028 | NS | ||
| No | 44 | 25 | |||||
| Portal vein embolization | Yes | 7 | 11 | 0.174 | |||
| No | 93 | 20 | |||||
| Transfusions | Yes | 10 | 16 | 0.602 | |||
| No | 90 | 20 | |||||
| Positive surgical margins at CLM resection | Yes | 12 | 13 | 0.012 | NS | ||
| No | 88 | 20 | |||||
| Pathologic response to preoperative chemotherapy for CLM (n = 189) | Major | 48 | 16 | 0.089 | |||
| Minor | 52 | 12 | |||||
| Postoperative chemotherapy for CLM | Yes | 64 | 18 | 0.113 | |||
| No | 36 | 23 | |||||
| Complications | Yes | 27 | 19 | 0.687 | |||
| No | 73 | 20 | |||||
Cox regression multivariate analysis included all variables with P < 0.05 in univariate analysis.
NS indicates not significant.
The results of the analysis of OS predictors are shown in Table 3. On univariate analysis, positive lymph node metastases for the primary tumor (P = 0.022), multiple CLM (P = 0.009), adjuvant FOLFOX therapy for the primary CRC (P = 0.002), largest CLM larger than 3 cm (P = 0.002), and positive surgical margins at the CLM resection (P = 0.003) were predictive of poor OS. On multivariate analysis, multiple CLM (HR = 1.52, 95% CI: 1.07–2.17, P = 0.021), adjuvant FOLFOX therapy for the primary CRC (HR = 1.86, 95% CI: 1.36–2.53, P < 0.0001), largest CLM larger than 3 cm (HR = 1.89, 95% CI: 1.31–2.73, P = 0.001), and positive surgical margins at CLM resection (HR = 1.82, 95% CI: 1.13–2.93, P = 0.014) remained significant predictors of OS.
TABLE 3.
Univariate and Multivariate Analysis of Clinicopathologic Variables Associated With OS in 341 Patients Who Underwent Hepatectomy for Metachronous CLM
| Variable | Univariate Analysis
|
Multivariate Analysis*
|
|||||
|---|---|---|---|---|---|---|---|
| N = 341 (%) | Median OS, mo | P | HR | 95% CI | P | ||
| Sex | Male | 60 | 61 | 0.087 | |||
| Female | 40 | 94 | |||||
| Age, y | > 65 | 35 | 59 | 0.103 | |||
| ≤65 | 65 | 94 | |||||
| Primary | Rectal | 29 | 94 | 0.265 | |||
| Colon | 71 | 65 | |||||
| Lymph nodes for primary | Positive | 54 | 59 | 0.022 | NS | ||
| Negative | 46 | 94 | |||||
| Number CLM | Multiple | 61 | 53 | 0.009 | 1.52 | 1.07–2.17 | 0.021 |
| Solitary | 39 | 108 | |||||
| Adjuvant therapy for CRC | None | 28 | NR | 0.002 | 1.86 | 1.36–2.53 | < 0.0001 |
| 5-FU | 49 | 70 | |||||
| FOLFOX | 23 | 40 | |||||
| Size, cm | >3 | 47 | 50 | 0.002 | 1.89 | 1.31–2.73 | 0.001 |
| ≤3 | 53 | NR | |||||
| CEA, ng/mL | >5 | 51 | 57 | 0.209 | |||
| ≤5 | 49 | 71 | |||||
| Preoperative chemotherapy for CLM | Yes | 55 | 76 | 0.978 | |||
| No | 44 | 72 | |||||
| Portal vein embolization | Yes | 7 | 45 | 0.534 | |||
| No | 93 | 71 | |||||
| Transfusions | Yes | 10 | 41 | 0.227 | |||
| No | 90 | 71 | |||||
| Positive surgical margins at CLM resection | Yes | 12 | 40 | 0.003 | 1.82 | 1.13–2.93 | 0.014 |
| No | 88 | 77 | |||||
| Pathologic response to preoperative chemotherapy for CLM (n = 189) | Major | 48 | NR | 0.264 | |||
| Minor | 52 | 114 | |||||
| Postoperative chemotherapy for CLM | Yes | 64 | 67 | 0.417 | |||
| No | 36 | 82 | |||||
| Complications | Yes | 27 | 57 | 0.129 | |||
| No | 43 | 75 | |||||
Cox regression multivariate analysis included all variables with P < 0.05 in univariate analysis.
NR indicates not reached; NS, not significant.
Somatic Gene Mutation Profiling
Among 341 patients in this series, a total of 210 patients operated in the most recent years (FOLFOX = 70, 5-FU = 70, and no chemotherapy = 70) were selected for specimen analysis. Paraffin blocks and sufficient tissue for somatic gene mutation analysis using Sequenom MassArray technology were available in 129 patients (FOLFOX = 47, 5-FU = 41, no chemotherapy = 41). The tumor biologic characteristics of patients treated with adjuvant FOLFOX, 5-FU, or no chemotherapy for the primary CRC were assessed according to the proportions of somatic gene mutations found in each group. One or more mutations were found in 57% of patients (27/47) after FOLFOX, 29% of patients (12/41) after 5-FU, and 32% of patients (13/41) after no chemotherapy (P = 0.011). The mutations included the genes KRAS, BRAF, NRAS, CTNNB1, FBWX7, and PIK3CA. The differences in mutation rates among the groups were related to the proportions of KRAS mutations in each group (P = 0.008). Other mutations were similarly distributed among the 3 groups (Table 4).
TABLE 4.
Somatic Gene Mutation Rates in 129 Patients who Underwent Hepatectomy for Metachronous CLM by Adjuvant Chemotherapy Type for the CRC
| Mutation | No Adjuvant Chemotherapy (N = 41) | 5-FU (N = 41) | FOLFOX (N = 47) | P* | P† |
|---|---|---|---|---|---|
| Patients with somatic gene mutations | 13 (32%) | 12 (29%) | 27 (57%) | 0.011 | 0.008 |
| KRAS | 8 (20%) | 9 (22%) | 22 (47%) | 0.008 | 0.015 |
| BRAF | 1 (2%) | 2 (5%) | 0 | 0.317 | 0.126 |
| NRAS | 1 (2%) | 0 | 3 (6%) | 0.217 | 0.100 |
| CTNNB1 | 0 | 1 (2%) | 0 | 0.339 | 0.282 |
| FBWX7 | 2 (5%) | 1 (2%) | 0 | 0.317 | 0.282 |
| PIK3CA | 4 (10%) | 5 (12%) | 3 (6%) | 0.640 | 0.344 |
Comparison of patients with FOLFOX vs 5-FU vs no chemotherapy for adjuvant treatment of CRC.
Comparison of patients with FOLFOX vs 5-FU for adjuvant treatment of CRC.
DISCUSSION
Patients with primary CRC and lymph node metastases and those at high risk for metachronous CLM (stage II/III) have been treated with adjuvant chemotherapy, including oxaliplatin in recent years.3,7,8 Nevertheless, some of these patients will develop CLM, which can be successfully treated if they can be completely resected with histologically negative margins.24,25 In this study, we analyzed DFS and OS rates after resection of metachronous CLM according to adjuvant chemotherapy type for the primary tumor. After controlling for primary and metastatic disease stage, the primary risk factor associated with poor outcome was treatment with adjuvant FOLFOX after resection of CRC. These findings suggest that the type of adjuvant therapy given after colon resection impacts the tumor biology of the subsequent metastases. To validate this hypothesis, we analyzed somatic gene mutations in CLM. We found a higher rate of mutations in FOLFOX-treated patients, with KRAS mutational status being entirely responsible for this difference.
Prior to the oxaliplatin-era, published series reporting on patients who developed metachronous CLM indicated 46% to 62% 3-year DFS and 64% to 75% 3-year OS rates.9,26,27 Our retrospective analysis of prospectively collected CLM patient data demonstrated that the natural history of the subset of metachronous CLM from stage III CRC may have changed after the introduction of oxaliplatin-based chemotherapy. Patients with metachronous CLM treated with 5-FU experienced longer survivals (3-year DFS 38% and OS 70%) than those treated with FOLFOX (3-year DFS 18% and OS 58%). Clinical trials indicate that the use of FOLFOX after primary resection prevents or delays CLM in a larger number of patients than does 5-FU alone,7,8 but it may at the same time contribute to the selection of patients with a more aggressive form of metastatic disease—that is, resistant to oxaliplatin and responsible for a poorer OS and DFS after resection of metachronous CLM.
Two prospective randomized studies on adjuvant chemotherapy in stage II and III colon cancer patients have demonstrated that patients who recur after adjuvant FOLFOX have shorter OS than patients who recur after randomization to adjuvant 5-FU.8,28 In the MOSAIC trial, the median time from relapse to death was 21 months for the FOLFOX group and 24 months for the 5-FU group.8 This has been previously attributed to the lower efficacy of oxaliplatin regimens upon retreatment of previously FOLFOX-treated patients. According to this hypothesis, these patients have fewer effective chemotherapy regimens and a resulting lower OS. However, our data supports the alternate suggestion that FOLFOX-resistant colon cancer has a different biology than 5-FU–resistant tumors. Preclinical studies suggest that oxaliplatin resistant cell lines develop epithelial-to-mesenchymal transition, characterized by a migratory and proinva-sive phenotype.29,30 As DFS after hepatectomy is dictated by unrecognized microscopic disease outside of the visible metastases, such migratory behavior may contribute to the higher recurrence rates after FOLFOX.
KRAS mutation in primary tumors represents a modest prognostic marker for metastatic CRC patients in some, but not all, clinical series. However, it is clearly associated with resistance to epidermal growth factor receptor inhibitors.31–34 KRAS mutation in CLM has also been shown to be associated with lower survival and accelerated disease progression in patients with resected CLM in an era predating FOLFOX chemotherapy.35 The same study reported a low rate of KRAS mutations (16%), similar to our study, after resection of metachronous CLM. KRAS mutation analysis was additionally used in 2 previous studies to assess the minimum surgical margins in resected CLM.36,37 KRAS mutation has recently been associated with higher rates of lung metastases, a common location of recurrence for patients with resected CLM.32 The current study is the first to characterize KRAS mutation in patients undergoing curative liver resection for metachronous CLM in the era of adjuvant FOLFOX chemotherapy for CRC; the higher rate of KRAS mutation in patients treated with FOLFOX underscores the association between modern chemotherapy and the long-term selection of worse tumor biology.
This study is limited by its retrospective nature. The selection of adjuvant chemotherapy regimens in routine patient care is based on many clinical and pathologic factors not fully captured by multivariate analysis; however, the degree of magnitude of the observed effect and the inclusion of multiple prognostic variables argues against this. A prospective study to confirm our findings may not be feasible because chemotherapy with FOLFOX for lymph-node positive CRC is currently the standard of care on the basis of randomized trials.3,7,8 Efforts to replicate this finding from completed randomized adjuvant studies are ongoing. Our study is also limited by the fact that somatic gene mutation profiling using Sequenom MassArray technology could only be performed in a subset of 129 patients. However, this high-throughput technology enabled the testing of 159 different mutations on 33 different genes, allowing evaluation of genes and pathway interactions that could not be evaluated with a single gene study. Another possible limitation of this study is the absence of KRAS status evaluation of the primary tumor. Thus, it was not possible to determine whether a discordance in mutation rates existed between patients who did and did not receive oxaliplatin after resection of the primary. However, numerous studies of KRAS mutational status in primary and metastatic disease sites have shown high concordance rates, ranging from 84% to 100%38–44, whereas only one study, in 21 patients, reported a low concordance rate of 52%.45
In conclusion, this study suggests that oxaliplatin-based adjuvant therapy may provide a selection pressure favoring a chemotherapy-resistant subset enriched for KRAS mutations while on balance preventing liver recurrences in patients with KRAS wild-type tumors. This change may be responsible for the early recurrence and lower OS observed after resection of metachronous CLM. The selection of patients with chemotherapy-resistant CLM and predestined worse prognosis represents a new challenge for hepatobiliary surgeons in an era that is characterized by multimodal therapy of CLM and the increasing use of perioperative chemotherapy with molecular profiling.16,46,47
Acknowledgments
The authors thank Ann Sutton and Ruth J. Haynes for their assistance with manuscript preparation.
Footnotes
Disclosure: This research was supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CA016672). The authors declare no conflicts of interest.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.American Joint Committee on Cancer. Colon and rectum. In: Edge SB, Byrd DR, Compton CC, et al., editors. AJCC Cancer Staging Manual. 7. New York, NY: Springer; 2010. pp. 143–159. [Google Scholar]
- 3.Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol. 2011;29:3768–3774. doi: 10.1200/JCO.2011.36.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N Engl J Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 5.O’Connell MJ, Laurie JA, Kahn M, et al. Prospectively randomized trial of postoperative adjuvant chemotherapy in patients with high-risk colon cancer. J Clin Oncol. 1998;16:295–300. doi: 10.1200/JCO.1998.16.1.295. [DOI] [PubMed] [Google Scholar]
- 6.Wolmark N, Rockette H, Mamounas E, et al. Clinical trial to assess the relative efficacy of fluorouracil and leucovorin, fluorouracil and levamisole, and fluorouracil, leucovorin, and levamisole in patients with Dukes’ B and C carcinoma of the colon: results from National Surgical Adjuvant Breast and Bowel Project C-04. J Clin Oncol. 1999;17:3553–3559. doi: 10.1200/JCO.1999.17.11.3553. [DOI] [PubMed] [Google Scholar]
- 7.Andre T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 8.Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. doi: 10.1200/JCO.2008.20.6771. [DOI] [PubMed] [Google Scholar]
- 9.Scheele J, Stang R, Altendorf-Hofmann A, et al. Resection of colorectal liver metastases. World J Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 10.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg. 2004;239:818–825. doi: 10.1097/01.sla.0000128305.90650.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truant S, Huglo D, Hebbar M, et al. Prospective evaluation of the impact of [18F]fluoro-2-deoxy-D-glucose positron emission tomography of resectable colorectal liver metastases. Br J Surg. 2005;92:362–369. doi: 10.1002/bjs.4843. [DOI] [PubMed] [Google Scholar]
- 12.Ribero D, Abdalla EK, Madoff DC, et al. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386–1394. doi: 10.1002/bjs.5836. [DOI] [PubMed] [Google Scholar]
- 13.Aloia TA, Zorzi D, Abdalla EK, et al. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mullen JT, Ribero D, Reddy SK, et al. Hepatic insufficiency and mortality in 1,059 noncirrhotic patients undergoing major hepatectomy. J Am Coll Surg. 2007;204:854–862. doi: 10.1016/j.jamcollsurg.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 16.Blazer DG, III, Kishi Y, Maru DM, et al. Pathologic response to preoperative chemotherapy: a new outcome end point after resection of hepatic colorectal metastases. J Clin Oncol. 2008;26:5344–5351. doi: 10.1200/JCO.2008.17.5299. [DOI] [PubMed] [Google Scholar]
- 17.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715–724. doi: 10.1097/01.sla.0000160703.75808.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabriel S, Ziaugra L, Tabbaa D. SNP genotyping using the Sequenom MassARRAY iPLEX platform. Curr Protoc Hum Genet. 2009;Chapter 2(Unit 2.12) doi: 10.1002/0471142905.hg0212s60. [DOI] [PubMed] [Google Scholar]
- 20.Pearce M, Ehrich M. Somatic Mutation Analysis in Tumor Samples using the Sequenom. MassARRAY® System. Nat Methods. 2010 http://www.nature.com/app_notes/nmeth/2010/101304/full/an7545.html.
- 21.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 22.Fumagalli D, Gavin PG, Taniyama Y, et al. A rapid, sensitive, reproducible and cost-effective method for mutation profiling of coln cancer and metastatic lymph nodes. BMC Cancer. 2010;10:101. doi: 10.1186/1471-2407-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Athanasios C, Tsiatis AC, Norris-Kirby A, et al. Comparison of Sanger sequencing, pyrosequencing, and melting curve analysis for the detection of KRAS mutations: diagnostic and clinical implications. J Mol Diagn. 2010;12:425–432. doi: 10.2353/jmoldx.2010.090188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1271–1280. doi: 10.1245/s10434-006-9045-5. [DOI] [PubMed] [Google Scholar]
- 25.Charnsangavej C, Clary B, Fong Y, et al. Selection of patients for resection of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol. 2006;13:1261–1268. doi: 10.1245/s10434-006-9023-y. [DOI] [PubMed] [Google Scholar]
- 26.Tsai MS, Su YH, Ho MC, et al. Clinicopathological features and prognosis in resectable synchronous and metachronous colorectal liver metastasis. Ann Surg Oncol. 2007;14:786–794. doi: 10.1245/s10434-006-9215-5. [DOI] [PubMed] [Google Scholar]
- 27.Bockhorn M, Frilling A, Fruhauf NR, et al. Survival of patients with synchronous and metachronous colorectal liver metastases—is there a difference? J Gastrointest Surg. 2008;12:1399–1405. doi: 10.1007/s11605-008-0508-9. [DOI] [PubMed] [Google Scholar]
- 28.Yothers GA, O’Connel MJ, Colangelo L, et al. 5-FU and leucovorin (Lv) with or without oxaliplatin (Ox) for adjuvant treatment of stage II and III colon cancer: long-term follow-up of NSABP C-07 with survival analysis. Gastrointest Cancers Symp. 2010:Abstract 401. [Google Scholar]
- 29.Yang AD, Fan F, Camp ER, et al. Chronic oxaliplatin resistance induces epithelial-to-mesenchymal transition in colorectal cancer cell lines. Clin Cancer Res. 2006;12:4147–4153. doi: 10.1158/1078-0432.CCR-06-0038. [DOI] [PubMed] [Google Scholar]
- 30.Kopetz S, Lesslie DP, Dallas NA, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res. 2009;69:3842–3849. doi: 10.1158/0008-5472.CAN-08-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 32.Tie J, Lipton L, Desai J, et al. KRAS mutation is associated with lung metastasis in patients with curatively resected colorectal cancer. Clin Cancer Res. 2011;17:1122–1130. doi: 10.1158/1078-0432.CCR-10-1720. [DOI] [PubMed] [Google Scholar]
- 33.De Roock W, Claes B, Bernasconi D, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 2010;11:753–762. doi: 10.1016/S1470-2045(10)70130-3. [DOI] [PubMed] [Google Scholar]
- 34.Molinari F, Felicioni L, Buscarino M, et al. Increased detection sensitivity for KRAS mutations enhances the prediction of anti-EGFR monoclonal antibody resistance in metastatic colorectal cancer. Clin Cancer Res. 2011;17:4901–4914. doi: 10.1158/1078-0432.CCR-10-3137. [DOI] [PubMed] [Google Scholar]
- 35.Nash GM, Gimbel M, Shia J, et al. KRAS mutation correlates with accelerated metastatic progression in patients with colorectal liver metastases. Ann Surg Oncol. 2010;17:572–578. doi: 10.1245/s10434-009-0605-3. [DOI] [PubMed] [Google Scholar]
- 36.Kokudo N, Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137:833–840. doi: 10.1001/archsurg.137.7.833. [DOI] [PubMed] [Google Scholar]
- 37.Holdhoff M, Schmidt K, Diehl F, et al. Detection of tumor DNA at the margins of colorectal cancer liver metastasis. Clin Cancer Res. 2011;17:3551–3557. doi: 10.1158/1078-0432.CCR-10-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oudejans JJ, Slebos RJ, Zoetmulder FA, et al. Differential activation of ras genes by point mutation in human colon cancer with metastases to either lung or liver. Int J Cancer. 1991;49:875–879. doi: 10.1002/ijc.2910490613. [DOI] [PubMed] [Google Scholar]
- 39.Etienne-Grimaldi MC, Formento JL, Francoual M, et al. K-Ras mutations and treatment outcome in colorectal cancer patients receiving exclusive fluoropy-rimidine therapy. Clin Cancer Res. 2008;14:4830–4835. doi: 10.1158/1078-0432.CCR-07-4906. [DOI] [PubMed] [Google Scholar]
- 40.Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2008;26:4217–4219. doi: 10.1200/JCO.2008.18.7286. [DOI] [PubMed] [Google Scholar]
- 41.Santini D, Loupakis F, Vincenzi B, et al. High concordance of KRAS status between primary colorectal tumors and related metastatic sites: implications for clinical practice. Oncologist. 2008;13:1270–1275. doi: 10.1634/theoncologist.2008-0181. [DOI] [PubMed] [Google Scholar]
- 42.Italiano A, Hostein I, Soubeyran I, et al. KRAS and BRAF mutational status in primary colorectal tumors and related metastatic sites: biological and clinical implications. Ann Surg Oncol. 2010;17:1429–1434. doi: 10.1245/s10434-009-0864-z. [DOI] [PubMed] [Google Scholar]
- 43.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watanabe T, Kobunai T, Yamamoto Y, et al. Heterogeneity of KRAS status may explain the subset of discordant KRAS status between primary and metastatic colorectal cancer. Dis Colon Rectum. 2011;54:1170–1178. doi: 10.1097/DCR.0b013e31821d37a3. [DOI] [PubMed] [Google Scholar]
- 45.Vermaat JS, Nijman IJ, Koudijs MJ, et al. Primary colorectal cancers and their subsequent hepatic metastases are genetically different: implications for selection of patients for targeted treatment. Clin Cancer Res. 2012;18:688–699. doi: 10.1158/1078-0432.CCR-11-1965. [DOI] [PubMed] [Google Scholar]
- 46.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–1016. doi: 10.1016/S0140-6736(08)60455-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chun YS, Vauthey JN, Boonsirikamchai P, et al. Association of computed tomography morphologic criteria with pathologic response and survival in patients treated with bevacizumab for colorectal liver metastases. JAMA. 2009;302:2338–2344. doi: 10.1001/jama.2009.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]