Abstract
Background
After U.S. licensure, parenterally administered medications are identified using non-specific drug codes. Accurately identifying these medications is critical to safety and effectiveness research. Methods to identify medications prior to assignment of specific drug codes have not been well described.
Objectives
To describe a generalized approach using non-specific drug codes to identify parenteral therapies in Medicare claims and to assess the ability of that approach to identify tocilizumab (TCZ), a new biologic agent approved in 2010.
Methods
We used 2008–2010 Medicare data for a cohort of rheumatoid arthritis patients for algorithm development. Our algorithm classified non-specific drug codes based upon: (1) ICD9 codes; (2) unit values (i.e. dose); (3) codes for infusion/injection procedures; (4) expected versus observed total reimbursement amount and reimbursement per unit. We assessed algorithm performance by linking to an arthritis registry to examine external validity.
Results
Of 472,803 claims with non-specific drug codes, 9,762 claims satisfied the TCZ algorithm. 74.3% of 9,762 claims were classified as TCZ by exact unit price or allowed amount, 4.4% by unique doses, 21.3% by diagnosis code and small deviation from unit price or allowed amount. The algorithm demonstrated good performance characteristics: sensitivity 94% (95% CI 80–99), specificity 100% (99–100) and PPV 97% (84–100).
Conclusion
Claims-based algorithms in Medicare or similar data systems can accurately identify newly approved biologics administered parenterally prior to the assignment of specific drug codes.
Keywords: rheumatoid arthritis, Medicare, Part D, biologics, tocilizumab, denosumab, certolizumab, linkage, registry
Introduction
Accurate identification of medication exposures is essential to validly answer questions related to comparative effectiveness and to conduct pharmacovigilance for new medications. Administrative claims data are commonly used for these purposes because the large sample sizes provide the necessary power to assess both uncommon exposures and rare adverse events. However, these databases suffer from potential misclassification of drug exposures, especially for newly licensed parenteral medications such as biologic therapies when administered by a healthcare provider rather than self-administered by patients. In these circumstances, Medicare providers, for example, obtain reimbursement for medications using Healthcare Common Procedure Coding System (HCPCS) codes. Claims for newly licensed medications use a non-specific HCPCS code (e.g. J3490, J3590) until, and often for some time after, a unique HCPCS code specific to each drug is assigned. These unique, permanent HCPCS codes typically are assigned one to two years after a drug comes to market. This scenario can lead to misclassification of the date of first use, cumulative dose, and duration of exposure if investigators fail to capture use of the new medication because they search only for the drug-specific HCPCS code (1). To further compound the problem, it is relatively common for multiple medications to be reimbursed under the same nonspecific HCPCS codes during the same calendar time.
In light of these issues, we present an example of a general framework for identifying and classifying parenteral medications reimbursed under non-specifics HCPCS codes using claims data from the U.S. Medicare program. The particular medication of interest chosen for this example was tocilizumab (TCZ), an interleukin 6 receptor antagonist indicated for the treatment of rheumatoid arthritis (RA). The validity of the approach was examined using an external validation procedure facilitated by a linkage between the Medicare data and a large arthritis registry that served as a reference standard for medication exposure.
Methods
Data Source and Key Variables
We used claims data obtained from the Chronic Conditions Warehouse (CCW) from 2008–2010 for a cohort of Medicare beneficiaries with RA and other types of inflammatory conditions that often have rheumatologic manifestations (e.g. spondyloarthritis, inflammatory bowel disease). Key variables examined included: ICD9 diagnosis codes for the expected disease indication; the observed Medicare-allowed reimbursement amount, the expected Medicare-allowed reimbursement amount, the number of units administered (a proxy for dose in milligrams), the reimbursed unit price (a derived variable computed as total reimbursed amount divided by units administered), and claims for infusion or injection procedures linked to the same claim ID (occurring on the same day). We searched both the Medicare carrier files and the outpatient revenue center files for these data.
Medication used for this example
The example chosen for this analysis was TCZ, an intravenously administered biologic medication used for the treatment of RA. TCZ was approved for use in RA by the Food and Drug Administration in January 2010 and is dosed by weight at either 4mg/kg or 8mg/kg. TCZ is generally prescribed only by rheumatology specialists. Identifying TCZ was complicated by the existence of another RA medication, certolizumab pegol (CZP), approved for RA in May of 2009 and assigned a permanent HCPCS code on January 1st, 2010. Like TCZ, CZP is administered once monthly, but CZP is administered by subcutaneous injection at a fixed dose (400mg). It can be administered by a healthcare provider or self-administered. Although CZP was assigned a permanent HCPCS code before the licensure date of TCZ, based upon past work, we expected to observe continued albeit declining use of the non-specific HCPCS code for CZP extending into 2010. Additionally, a third biologic, denosumab (DMAB), an injectable biologic used for osteoporosis that is administered every 6 months at a fixed dose (60mg), was approved for use in May of 2010. DMAB it is not indicated for RA nor does it have any known effect on the signs and symptoms of arthritis (2). However, it was commonly expected to be administered by rheumatologists. Relevant information about these three biologics and their associated allowable reimbursement were derived from both clinical knowledge and public information available from CMS (3).
Approach to developing and validating claims algorithm for assigning TCZ
For each claim that used a non-specific HCPCS code (J3490, J3590, J9999, C9399, Q4082) in 2010, we extracted all variables described above. The observed reimbursement amount for the claim was divided by the units supplied (i.e. dose) and was compared to the expected allowed unit price from the published CMS reimbursement schedule (Table 1). This procedure was repeated for the observed total reimbursed amount of the claim and also compared against the expected reimbursement amounts for all biologically-plausible typical TCZ doses (Table 1). The maximum dose recommended in the TCZ prescribing information is 800mg (4). If the amounts matched exactly (i.e. within $0.0001 for difference in observed versus expected single unit reimbursement, or $0.08 for the difference in observed versus expected total claim reimbursement, calculated as the product of $0.0001/unit × 800 units), the claim was considered TCZ. If the reimbursement amounts did not match exactly, we then examined the units dispensed for unique unit values that would identify TCZ as distinct from CZP and DMAB.
Table 1.
Description of Key Variables Used in Claims-Based Algorithm to Identify Tocilizumab and Other Biologics using Non-Specific HCPCS Codes
| Tocilizumab | Certolizumab Pegol | Denosumab (for osteoporosis) |
|
|---|---|---|---|
| Non Specific J code | J3490, J3590, J9999, C9399, Q4082 | ||
| Specific J code (date assigned) | J3262 (January 2011) C9264 (July 2010, institutional use only) |
J0718 (January 2010) C9249 (April 2009, institutional use only) |
J0897 (January 2012) C9272 (October 2010, institutional use only) |
| Associated diagnosis code sought on claim* | 714 (rheumatoid arthritis) | 714 (rheumatoid arthritis), 555 (Crohn’s disease), 556 (ulcerative colitis), 6960 (psoriatic arthritis), 6961 (psoriasis), 7200 (ankylosing spondylitis) | 733 (disorders of bone and cartilage, including osteoporosis) |
| Unit price** and effective date | 3.519/Jan 1, 2010 | 3.417/Jan 1, 2009 | 14.575/Oct 1, 2010 |
| 3.477/Oct 1, 2010 | 3.515/Apr 1, 2009 | ||
| 3.584/Jul 1, 2009 | |||
| 3.800/Oct 1, 2009 | |||
| Unit Count (i.e. dose) | |||
| Typical | 200,400,600,800 | 200,400 | 60 |
| Unique from other drugs | Combination of 200s and 80s(not multiple of 100s) | N/A | 60 |
| Possible Unit Count | 1, 2, 3, 4… | 1,2 | 1 |
| Infusion code*** | 96413, 96415 | none | none |
| Injection code*** | 96372, 96374, 96375 | 96372, 96374, 96375, 96401 | |
| Expected dosing frequency | Every 4 weeks | Every 4 weeks | Every 6 months |
N/A = not applicable
not necessarily limited to the disease indication for the drug, to allow the potential for ‘off-label’ use for related conditions
obtained from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html)
linked to the same claim, occurring on the same calendar day. These codes include the most common infusion and injection procedures used. A full list is available from the authors.
If the claim was not classifiable following the above procedures, we then allowed for some small mismatch in the observed versus expected unit reimbursement or total allowed reimbursement. The optimal cutoff for the difference between the observed unit and total reimbursement for a TCZ claim minus the expected reimbursement was unknown. However, since TCZ was approved and available in the U.S. in January 2010, if the algorithm was perfectly accurate, it would be expected to identify TCZ only in that month and beyond and not at all before that date. We therefore examined the ratio of the mean monthly number of claims for non-specific HCPCS code that the algorithm classified as TCZ after its licensure date (numerator) divided by mean monthly number of claims for non-specific HCPCS codes that the algorithm classified as TCZ in the 3 months before plus the 12 months after its licensure date (denominator). A ratio very close to 1.0 would provide evidence that the algorithm had correctly classified a non-specific HCPCS code claim as TCZ, since any claims the algorithm identified as TCZ prior to its approval date had to be misclassified. As the degree of misclassification increases, the ratio is expected to move closer to 0. In a loop that started with a maximal difference in the (observed – expected) unit reimbursement amount of $0.001/unit, the ratio of claims identified as TCZ after versus (before + after) its licensure date was plotted as a function of this difference. The process was repeated, sequentially increasing the (observed-expected) unit reimbursement amount by $0.001/unit each cycle of the loop. These plotted ratios were visually inspected for inflection points to select cutoffs to maximize specificity vs. sensitivity. This maximal difference in the unit price then was multiplied by 800 to provide a maximal difference in the total reimbursement amount of the largest TCZ recommended dose, 800mg (4).
After determining this optimal cutoff based upon visual inspection, the number of non-specific HCPCS claims in the CMS data was therefore plotted as a function of calendar time both before and after application of the TCZ algorithm. After this step that confirmed that the performance of the algorithm had removed the majority of non-specific HCPCS claims prior to January 2010, the final criterion of the algorithm added a restriction of all claims identified as TCZ to require them to occur on or after January 2010. This final algorithm was then subjected to external validation.
External validation
In order to validate the TCZ algorithm against an external reference standard, we previously linked the CMS data to the Consortium of Rheumatology Researchers of North America (CORRONA) arthritis registry that has enrolled patients with RA and other types of inflammatory arthritis (5, 6). Details of the linkage have been presented previously (7), and the accuracy of the linkage is >= 95%. Patients eligible for this analysis were those who were enrolled in the CORRONA registry during the entire year and also observable in Medicare in 2010. ‘Observable’ was defined as having part A, part B and part D Medicare coverage and not being enrolled in Medicare Advantage for all twelve months of 2010. We required Part D coverage because patients can obtain parenteral drugs, including TCZ, as part of their pharmacy benefit rather than their medical benefit and bring them to their healthcare provider for administration. This prevents the provider from having to be financially responsible for reimbursement of the medication. Information regarding TCZ exposure from the arthritis registry was cross-classified against the Medicare data to determine the performance characteristics of the TCZ identification algorithm. This cross-classification was done at a person level rather than at a claim level given that the arthritis registry does not explicitly capture every administration of biologic therapies but rather classifies individuals as exposed or unexposed at each visit. Registry visits occur at approximately 4 month intervals.
Statistical analysis
Descriptive statistics were used to summarize all data and provide 95% confidence intervals for sensitivity, specificity, and positive predictive values using the binomial distribution. All analyses were conducted in SAS 9.3 (Cary, NC). The university institutional review board approved the study protocol. Use of the CMS data was governed by a Data Use Agreement, which prohibited showing cell sizes < 11.
Results
Using CMS data of the RA cohort from 2008–2010, we identified 472,803 claims with non-specific HCPCS codes. In total, and after applying the procedures described above, we identified 9,280 claims (1,807 unique patients) as TCZ, 243 claims (89 unique patients) as CZP and 1,588 (1,536 unique patients) as DMAB. Among the TCZ claims, 883(9.5%) were identified as TCZ based upon an exact match with the observed minus expected unit price reimbursed, 6,010 (64.8%) based upon an exact match in the observed to expected total reimbursement allowed amount for the claim, and, 407(4.4%) due to unique units dispensed (i.e. dose) [Table 2].
Table 2.
Criteria used to Identify Tocilizumab in Final Algorithm
| Hierarchy* | Criterion (applied sequentially) | Additional Criterion |
Frequency of claims meeting these criteria |
|---|---|---|---|
| 1 | |Observed – expected| unit price <=0.0001 | None | 9.5% |
| 2 | |Observed – expected| total allowed amount <= 0.08** | None | 64.8% |
| 3 | Unit values (dose) are a linear combination of 80 and 200, excluding 200, 400, 600, 800*** | None | 4.4% |
| 4 | |Observed – expected| unit price allowed amount <= $0.033**** | Diagnosis for RA (714.X) | 2.1% |
| 5 | |Observed – Expected | total allowed amount <= $26.40** | Diagnosis for RA (714.X) | 19.3% |
The hierarchy describes the order in which the criteria were applied. Claims were classified as TCZ if they met the criteria in ANY of these rows.
This constant was obtained from the previous row, multiplied by 800 (the maximum recommended TCZ dose)
certolizumab pegol is typically administered as a 400mg (200mg × 2) injection; exact multiples of 200mg are therefore not specific for TCZ
derived from Figure 1
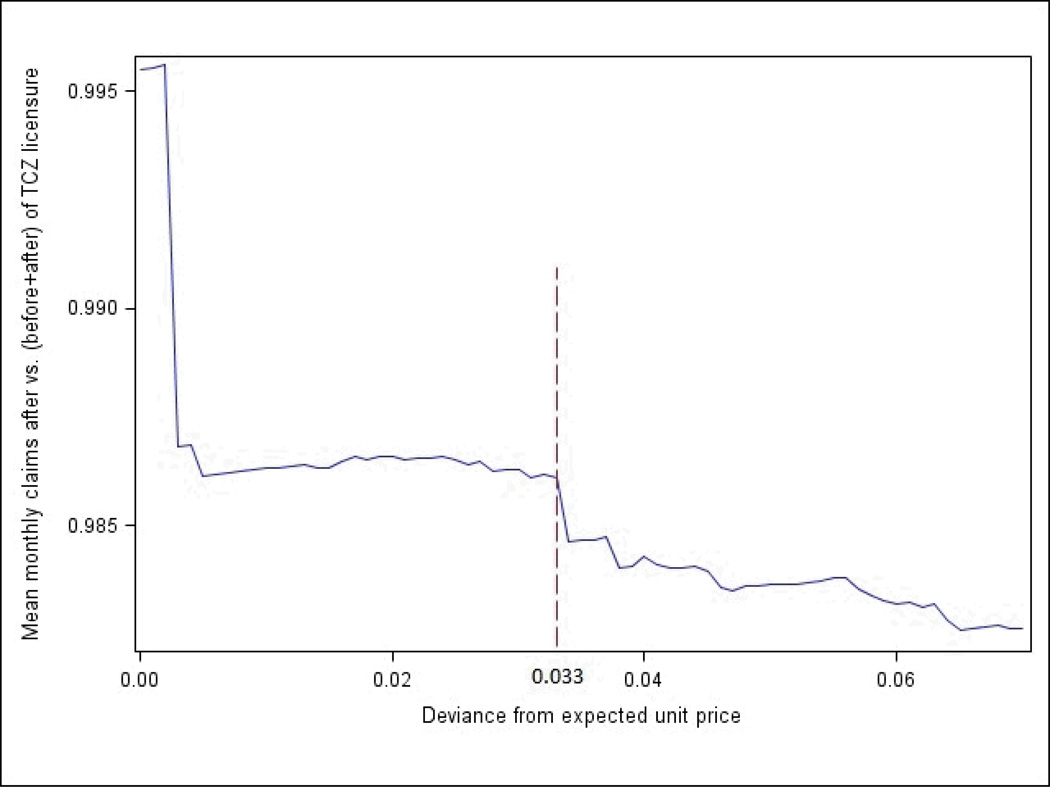
To increase sensitivity, steps 4 and 5 of the algorithm allowed for slight mismatch in the difference between the observed and expected unit price or total reimbursement. Plotting the differences in unit costs according to the ratio of mean monthly claims after TCZ licensure date vs. before and after, a major inflection point was observed at $0.033 (Figure 1); therefore, this value was used in the TCZ algorithm in step 4 (Table 2). This difference in the unit price was then multiplied by 800 (the highest recommended TCZ dose) to yield a maximal difference between the observed and expected total reimbursement amount of $26.40.
Figure 1.
Ratio of Non-Specific HCPCS Code Claims After Versus (Before + After) The Date of Tocilizumab Licensure according to the Difference between the Observed and Expected Unit Reimbursement
The cutoff selected for use in the TCZ algorithm (Table 2, step 4) was determined based upon visual inspection of the figure.
Based on this approach, an additional 191 (2.1%) claims were identified as TCZ based upon the presence of an RA diagnosis on the claim as well as an observed minus expected difference in the unit reimbursed amount of less or equal to $0.033 (Table 2). An additional 1,789 (19.3%) claims were identified as TCZ based upon the presence of an RA diagnosis on the claim as well as an observed minus expected difference in the total claim allowed reimbursement amount of <= $26.40.
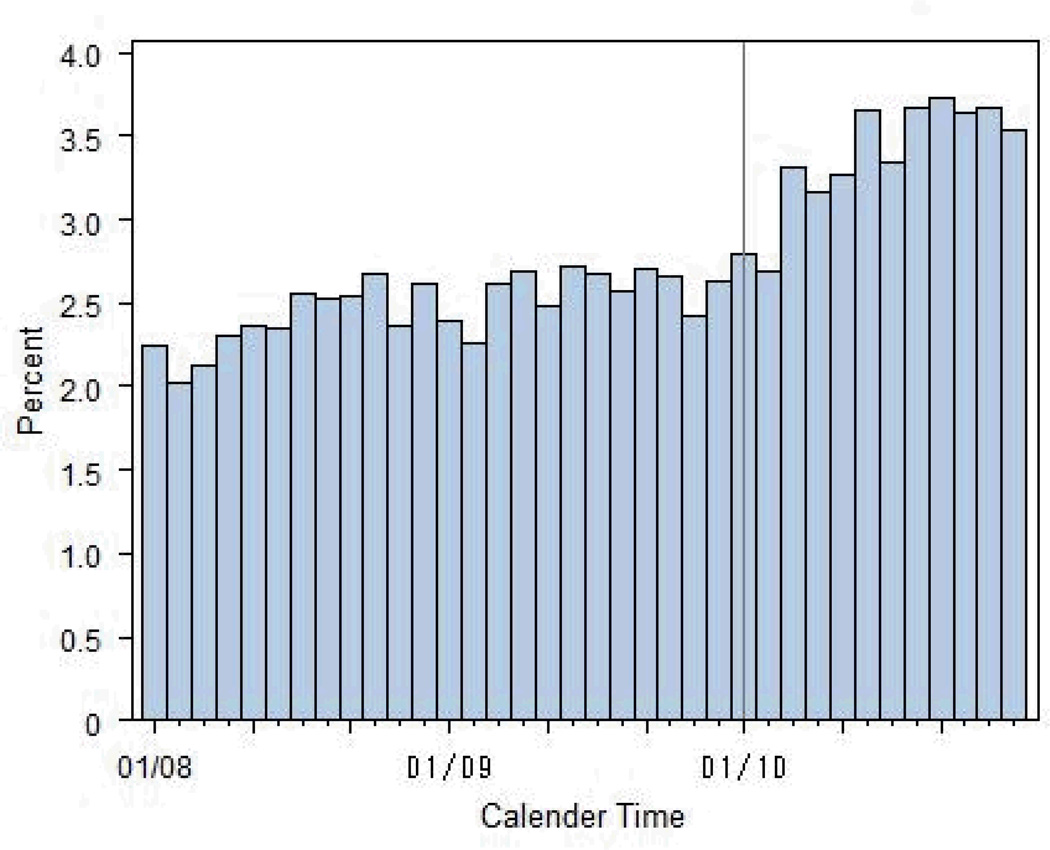
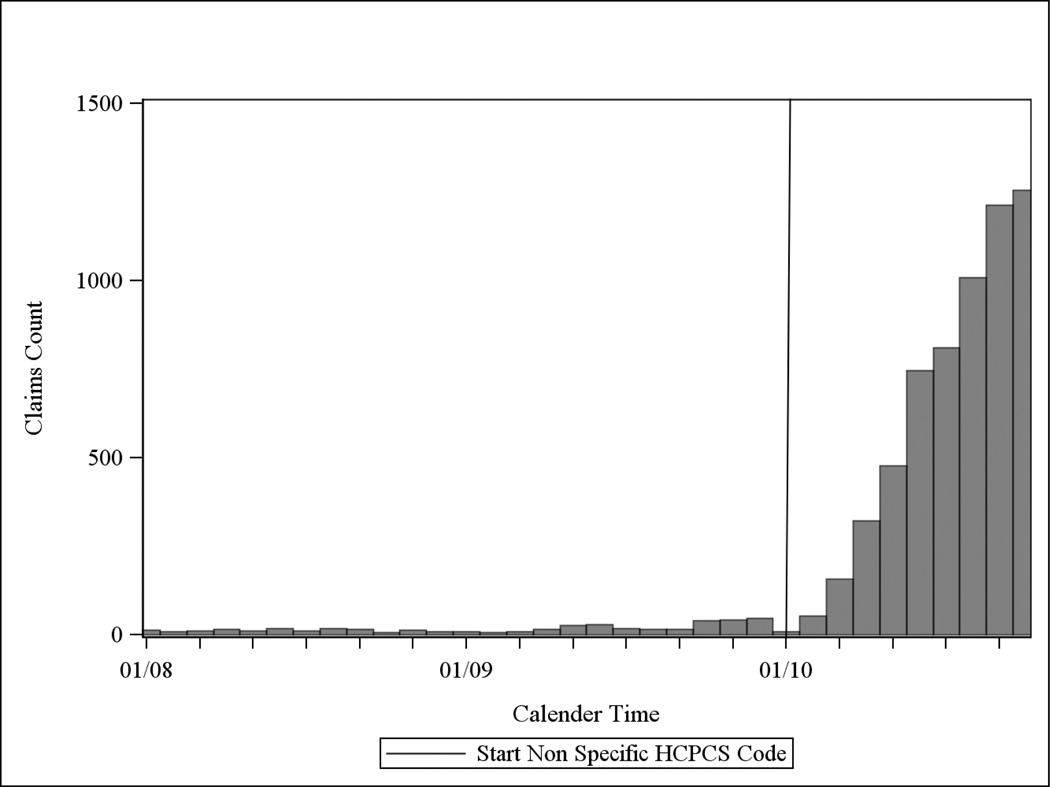
Figure 2a describes the distribution of claims for non-specific HCPCS by calendar time before applying the TCZ algorithm. Figure 2b show the same distribution after applying the TCZ algorithm described in Table 2, but before any restriction by calendar time was implemented. As shown, the vast majority (95%) of non-specific HCPCS claims prior to the TCZ licensure date of January 2010 were removed by the algorithm. The final TCZ algorithm then applied a restriction of all preliminarily-identified TCZ claims to those occurring in January 2010 or later. An additional 335 filled TCZ claims for 72 people were reimbursed by Medicare Part D.
Figure 2.
a: Distribution of the Number of Claims for Non-Specific HCPCS Codes Prior to Application of Tocilizumab Algorithm in Medicare Data 2008–2010
Note: TCZ was approved January 2010, as indicated by the solid vertical line
b: Distribution of the Number of Claims for Non-Specific HCPCS Codes Following Application of Tocilizumab Algorithm* in Medicare Data 2008–2010
* but prior to any calendar time restriction. Note: TCZ was approved January 2010, as indicated by the solid vertical line
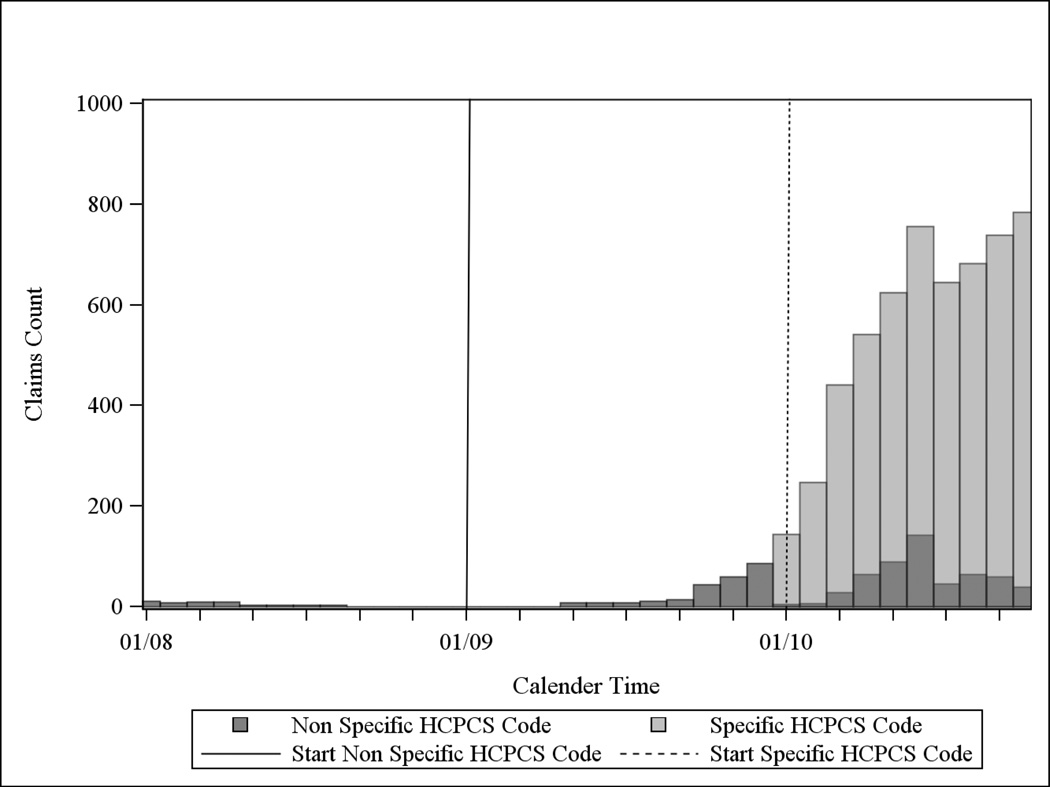
c: Distribution of the Number of Claims for Non-Specific HCPCS Codes Initially Identified as Certolizumab in Medicare Data, 2008–2010*
* but prior to any calendar time restriction. Note that CZP was reimbursed under a non-specific HCPCS code prior to 2010 and acquired a specific code in January of 2010 (dotted vertical line).
d: Distribution of the Number of Claims for Non-Specific HCPCS Codes Initially Identified as Denosumab in Medicare Data, 2008–2010*
* but prior to any calendar time restriction.
A similar set of procedures was followed for both CZP and DMAB (figures 2c and 2d). The group of non-specific HCPCS code claims in 2010 (dark grey bars) in Figure 2c could have been misclassified as TCZ given their similarities in reimbursed amounts, although there were many more 2010 CZP claims using the specific HCPCS code (light grey bars). Additionally, many more CZP claims for RA were reimbursed under Medicare Part D (data not shown).
Additional factors examined in the medical claims data were not essential elements of the final algorithm but are reported descriptively. Almost all (98.4%) claims identified as TCZ had an ICD9 diagnosis code for RA, and 74% had HCPCS codes for intravenous infusion on the same claim ID. For HCPCS claims identified as CZP, 72% had claims for a subcutaneously injected medication on the same claim ID. Corresponding results for DMAB were identical to CZP; 72% had claims for an injection. The dosing interval for claims identified as TCZ was also examined descriptively. Both the mode and median interval between consecutive TCZ claims was 28 days. The inter-quartile range of the dosing interval was 28 and 32 days, consistent with the expected infusion frequency of once monthly TCZ.
Using the CORRONA arthritis registry, there were 2,161 registry participants who were fully observable in the CMS data for the entirety of 2010. Of these, 34 individuals used TCZ in 2010 (median [IQR] number of TCZ claims = 5 [3, 8]); the algorithm correctly identified 32 of them, yielding a sensitivity of 94% (95% CI 80, 99), specificity of 100% (99, 100), and positive predictive value of 97% (84, 100). For the two people the algorithm failed to identify as TCZ users, one of them had a non-specific HCPCS code for a biologic but had an (observed – expected) difference in total reimbursement that was greater than $26.40. The second individual had no claim for any non-specific HCPCS code in the CMS data. Neither of these two patients filled TCZ under their Medicare part D benefit. The algorithm also identified one person in the registry as a TCZ user that was not reported as such to the registry. Upon further query of the site, the individual was confirmed to be a TCZ user in 2010 and the registry data was acknowledged to have been mis-entered.
Discussion
Using administrative data from the U.S. Medicare program in a population of patients with inflammatory diseases, we described a generalized approach to classify parenteral medications (e.g. biologics) that are initially reimbursed and identified in claims data under the Part B medical benefit using non-specific HCPCS procedure codes. The approach was applied to classify TCZ, a biologic used for the treatment of RA. The algorithm was externally validated and at least in this small sample, shown to have good performance characteristics, yielding high sensitivity, specificity, and PPV. These results suggest that this approach can be used to validly identify other parenteral medications shortly after their initial licensure and before they are assigned a permanent and unique HCPCS code, which typically takes approximately 1–2 years. The methodologicalmethodologic approach described should have high generalizability to other therapeutics in as much as none of these methods explicitly required any feature unique to the treatment of arthritis.
The implications of our study are important for researchers interested in studying the safety and effectiveness of newly licensed medications that are reimbursed using non-specific HCPCS codes. Failure to identify these medications will result in not identifying exposed patients and reduce the statistical power available for early pharmacoepidemiologic research. It will also misclassify the date of first exposure for those who remain on the medication once it obtains its specific HCPCS code, which may have ‘downstream’ effects. Assessment of adverse events that more commonly occur shortly after starting therapy will be biased be depletion of susceptible patients. In calculating adherence to a new medication in an analysis that ignore the non-specific HCPCS codes, only those patients who remain on the therapy long enough for it to receive its permanent HCPCS code will be identified as exposed. In general, this will have the effect of biasing adherence upwards because only prevalent users are included in such an analysis. For example, a study conducted by our group showed that mean proportion of days covered (PDC) for new users of a parenterally administered bisphosphonate (intravenous ibandronate) would be 6% higher than it actually was when the non-specific HCPCS codes were used (8). Moreover, 15% of the dates of first exposure to intravenous ibandronate would have been misclassified had not the non-specific HCPCS codes been used.
A few additional comments merit consideration when applying this approach in future studies. First, reimbursement rates will change over time. Typically, the wholesale acquisition cost (WAC) is the initial metric by which allowable reimbursement amounts are assigned. After approximately 6 months following the date of licensure, the price then will fluctuate quarterly based upon the Average Sales Price (ASP); of note, insurance carriers other than Medicare (e.g. commercial health plans) may reimburse other amounts. Codes for IV infusions or subcutaneous injections on the same calendar day as administration of the drug may therefore be more important in these circumstances. In this analysis, the IV infusion or injection procedure codes likely would have been helpful to discriminate between TCZ and CZP given their commonalities in their RA disease indication with associated diagnosis codes, and somewhat similar unit reimbursement amounts. This was not necessary in this example given that CZP was assigned a permanent HCPCS code prior to the licensure of TCZ, and the majority of CZP claims were reimbursed under part D, not part B. Additionally, some healthcare providers will submit claims with a unit value of 1 on all non-specific HCPCS code claims and could submit multiple claims on the same day, making identification of unique unit values more difficult. A potential solution may be to aggregate these claims into a single claim and summing their unit values and reimbursement amounts together. As another consideration, it is possible that local Medicare carriers have specific coding rules in their Local Coverage Determinations (LCDs) for reimbursement, and these should be examined to evaluate for particular restrictions or coding rules across geographic areas. Finally, claims may be denied if they do not meet coverage restrictions and therefore the allowed reimbursement on such claims is zero, which will make an assessment of unit or total reimbursement unhelpful. In general, all of these challenges are surmountable but do merit careful examination of the data as well as the regional reimbursement rules and allowable reimbursement amounts within the healthcare setting in which the non-specific HCPCS claims are being identified.
The strengths of our study include assessment of this approach and algorithm in a large population of patients with autoimmune diseases. Given that the uptake of new expensive medications such as biologics may be slow, the size of the dataset permitted examination of this topic with a reasonable sample size. The presence of two additional biologics that were reimbursed using the non-specific HCPCS codes and used for RA or administered by healthcare providers who treat RA increased the complexity of the problem. Despite this, the algorithm still showed good performance characteristics. As a further and notable strength, the external validity of the procedure was assessed through a novel linkage with a large outpatient arthritis registry.
Despite these strengths, our results must be interpreted in light of the study design. Even with the linked registry data of more than 2100 people, there were only 34 TCZ users that met eligibility criteria for this analysis. Moreover, the external validation procedure was at a person level not a claim level, a necessary requirement given the precision of the medication exposure data available in the registry. Finally, we recognize that a registry is unlikely to be a perfect gold standard by which to assess medication capture in administrative data. Even though we required that participants in the linked registry to have Medicare, patients may have other options by which they could obtain medications (e.g. clinical trials, drug assistance programs). These would not be assessable in claims data. Moreover, and as we found, it is possible that data errors in the registry may not completely capture all medication use accurately. When we queried the registry site about the one patient we classified as a TCZ user based upon the claims data who could not be confirmed in the registry (i.e. a ‘false positive’), the site acknowledged this omission as a data entry error and subsequently corrected it. However, we considered this individual to be misclassified in our results in order to be conservative given that we did not systematically subject all data to this level of scrutiny; larger datasets may have a higher ‘false positive’ rate. This finding illustrates another potential opportunity for linkages between administrative claims and registry data, in as much as each data source can provide complementary information to the other to maximize the validity and completeness of capture of exposure and outcome data.
In conclusion, it is possible to develop an algorithm to identify newly approved medications using non-specific HCPCS code with acceptable sensitivity and PPV. The most useful information from the data is the number of units (i.e. dose), unit price, allowed amount by the insurer/plan, ICD9 diagnosis code, calendar time, and perhaps the presence of infusion or injection HCPCS codes. This approach is likely to be useful to support research using administrative claims data on the comparative effectiveness and safety of newly approved medications shortly after licensure.
Acknowledgement
This work was supported by (R01 HS018517). Dr. Curtis receives support from the NIH (AR 053351).
Disclosures: JRC received consulting fees and research grants from Amgen, Abbott, BMS, Pfizer, Eli Lilly, Janssen, UCB, Roche/Genentech, and CORRONA. ED, MLK, HY, NCW receive research support from Amgen. Dr. Lewis has served as a consultant for Amgen, Millennium Pharmaceuticals, Prometheus, Lilly, Shire, Nestle, and Abbott. He has served on a Data and Safety Monitoring Board for clinical trials sponsored by Pfizer. He has received research support from Shire, Centocor, and Takeda. ED receives research support from Amgen.
References
- 1.DiMartino LDCL, Williams RL, et al. Using Medicare Administrative Data to Conduct Postmarketing Surveillance of Follow-on Biologics: Issues and Opportunities. Food and Drug Law Journal. 2008;63(4):891–900. [PubMed] [Google Scholar]
- 2.Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58(5):1299–1309. doi: 10.1002/art.23417. [DOI] [PubMed] [Google Scholar]
- 3. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/index.html.
- 4.Tocilizumab package insert [Google Scholar]
- 5.Curtis JR, Jain A, Askling J, Bridges SL, Jr, Carmona L, Dixon W, et al. A comparison of patient characteristics and outcomes in selected European and U.S. rheumatoid arthritis registries. Semin Arthritis Rheum. 2010;40(1):2–14. doi: 10.1016/j.semarthrit.2010.03.003. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kremer JM. The CORRONA database. Autoimmun Rev. 2006;5(1):46–54. doi: 10.1016/j.autrev.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Chen L, Beukelman T, Bharat A, Xie F, Saag KG, et al. Methods To Link a National Arthritis Cohort with Medicare Administrative Claims Data International Society of Pharmacoepidemiology. Barcelona, Spain: 2012. [Google Scholar]
- 8.Curtis JR, Yun H, Matthews R, Saag KG, Delzell E. Adherence with intravenous zoledronate and intravenous ibandronate in the United States Medicare population. Arthritis Care Res (Hoboken) 2012;64(7):1054–1060. doi: 10.1002/acr.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]