Abstract
Context
The neurobiologic basis of late life depressive symptoms is not well understood.
Objective
To test the hypothesis that neurodegeneration and neuronal density in brainstem aminergic nuclei are related to late life depressive symptoms.
Design
Longitudinal clinical-pathologic cohort study.
Setting
Residences of participants in the Chicago metropolitan area.
Participants
A total of 124 older persons without dementia in the Rush Memory and Aging Project who had annual evaluations for a mean of 5.7 years (SD = 2.8), died, and underwent a neuropathologic examination that provided estimates of the densities of Lewy bodies, neurofibrillary tangles, and aminergic neurons in the locus coeruleus, dorsal raphe nucleus, substantia nigra, and ventral tegmental area.
Main Outcome Measure
Number of depressive symptoms on the Center for Epidemiological Studies Depression scale averaged across annual evaluations (mean = 1.61, SD = 1.48, range: 0–6, skewness = 0.94).
Results
Brainstem Lewy bodies were associated with depressive symptoms and the association was attenuated in those on antidepressant medication. Brainstem tangles were associated with more depressive symptoms in those without cognitive impairment but fewer symptoms in those with mild cognitive impairment. Lower density of tyrosine-hydroxylase-immunoreactive neurons in the ventral tegmental area was robustly associated with higher level of depressive symptoms (estimate = −0.014, SE = 0.003, p<0.001, increase in adjusted R2 = 16.3%). The association was not modified by medications or cognitive impairment. Neither tyrosine-hydroxlyase-immunoreactive neurons in the locus coeruleus nor tryptophan-hydroxlyase-immunoreactive neurons in the dorsal raphe nucleus were related to depressive symptoms.
Conclusions
The results suggest that the mesolimbic dopamine system, especially the ventral tegemental area, plays an important role in late life depressive symptoms.
INTRODUCTION
Late life depressive symptoms are common1 and associated with increased risk of disability,2 dementia,3 and death.4 Because brainstem aminergic nuclei play an important role in the pathophysiology of depression5 and are early sites of age-related neurodegeneration,6,7 it has long been hypothesized that late life depressive symptoms are partly due to neurodegenerative changes in these nuclei. 8–11 Support for the hypothesis has been inconsistent, likely due to several factors. Few studies have had more than 50 participants 8,10,12 or used continuous measures of depressive symptoms,9,10 limiting statistical power. Also, much prior research has been conducted on individuals with dementia.6,8,9,10,13–15 However, dementia introduces substantial error into the measurement of depressive symptoms16,17 in addition to extensive pathology elsewhere in the brain, and the neurodegenerative changes associated with dementia are robustly related to cognitive,18 sensory,19 and motor20 symptoms years before dementia onset, suggesting that associations with depressive symptoms might be easier to identify during this period.11 Finally, despite evidence that brainstem dopamine neurons play an important role in regulating depression related behavior,21–23 previous studies have primarily focused on the locus coeruleus and dorsal raphe nucleus.
The present study examines the relationship of neurodegenerative lesions and aminergic neurons in 4 brainstem nuclei to level of depressive symptoms in late life. At annual intervals for a mean of 5.7 years, older persons without dementia had structured evaluations that included a standard self report measure of depressive symptoms. At death, there was a uniform neuropathological examination that yielded estimates of the densities of neurofibrillary tangles, Lewy bodies, and aminergic neurons in the locus coeruleus, dorsal raphe nucleus, substantia nigra, and ventral tegmental area. We tested the hypothesis that higher pathologic burden and lower neuronal density are associated with higher level of depressive symptoms.
METHODS
Participants
The Rush Memory and Aging Project is an ongoing clinical-pathologic study that began in 1997 and includes annual clinical evaluations and brain autopsy at death.24 Persons were recruited from social service agencies, subsidized housing facilities, retirement communities, and churches in the Chicago metropolitan region. After a presentation on the project, interested individuals met for further discussions with staff who obtained written informed consent. The project was approved by the institutional review board of Rush University Medical Center.
There had been 550 deaths (in a total of 1,533 participants) at the time of these analyses. A brain autopsy had been performed in 439 (79.8%) and a uniform neuropathologic examination had been completed in the first consecutive 417 individuals. From these, 170 individuals were selected to provide a wide range of cognitive, motor, and affective functioning proximate to death for a clinical-pathologic substudy of brainstem aminergic nuclei, as described elsewhere.25,26 We excluded 45 individuals with a diagnosis of dementia (see Clinical Evaluation section), and 1 person was missing data on depressive symptoms. Analyses are based on the remaining 124 individuals. They had a mean age at death of 87.7 (SD = 5.7). They had completed a mean of 14.3 years of education (SD = 2.5); 69.4 % were women and 43.5% had mild cognitive impairment (see Clinical Evaluation section).
Assessment of Depressive Symptoms
A 10-item version27 of the Center for Epidemiological Studies Depression scale28 was used to assess depressive symptoms at each annual clinical evaluation. For each item, the interviewer read a brief statement (e.g., “I felt depressed”) and the participant indicated whether or not he or she had felt that way much of the past week. The score is the number of symptoms reported (range: 0–10). This 10-item scale is less burdensome than the original 20-item scale but has similar psychometric properties27 and has been associated with adverse health outcomes including dementia29 and death.4 Supported by prior factor analyses,27,30 we created subscores of negative affect (3 items: I felt depressed, I felt lonely, I felt sad), positive affect (2 items: I was happy, I enjoyed life), somatic symptoms (3 items: I felt like everything I did was an effort, My sleep was restless, I could not get going), and interpersonal problems (2 items: People were unfriendly, I felt that people disliked me), as previously described.31
Clinical Evaluation
The annual evaluations included a structured medical history, neuropsychological testing, and neurological examination.24 Prescription and over-the-counter medications were inspected, identified, and coded using the Medi-Span Drug Data Base system.32 The neurological examination included a modified version33 of the motor portion of the Unified Parkinson’s Disease Rating Scale,34 as previously described.35 After each evaluation, an experienced clinician diagnosed dementia and mild cognitive impairment. Classification of dementia was based on the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association which require a history of cognitive decline and impairment in at least 2 cognitive domains.36 The diagnosis of mild cognitive impairment required impairment in 1 or more cognitive domains in the absence of dementia.37 Upon death, all clinical data were reviewed by a board-certified neurologist blinded to all pathologic data and final diagnoses of mild cognitive impairment and dementia were made.
Neuropathologic Examination
The brain was removed a median of 6.0 hours (interquartile range = 3.1) after death which occurred a median of 9.3 months (interquartile range = 7.4) after the last complete clinical evaluation. Examiners blinded to all clinical data followed a standard protocol for tissue preservation, tissue sectioning, and quantification of pathologic findings.20
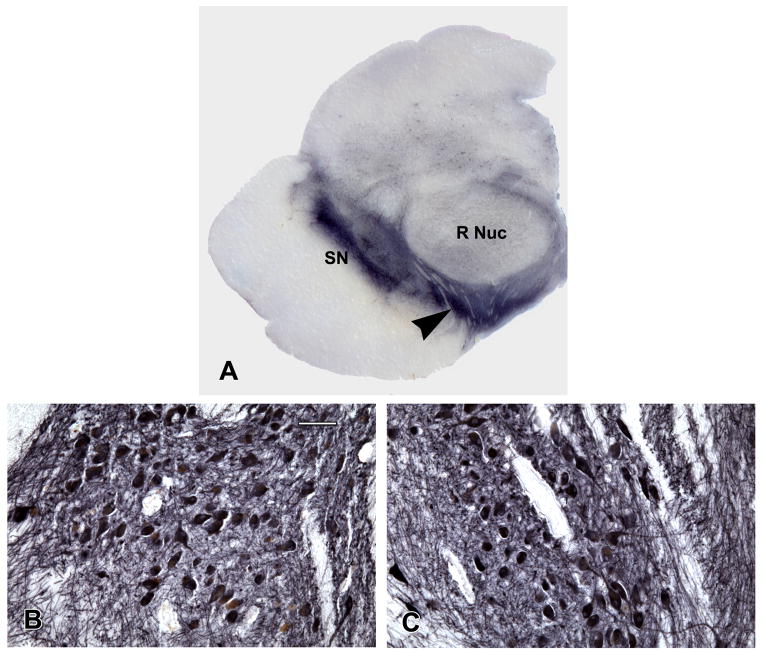
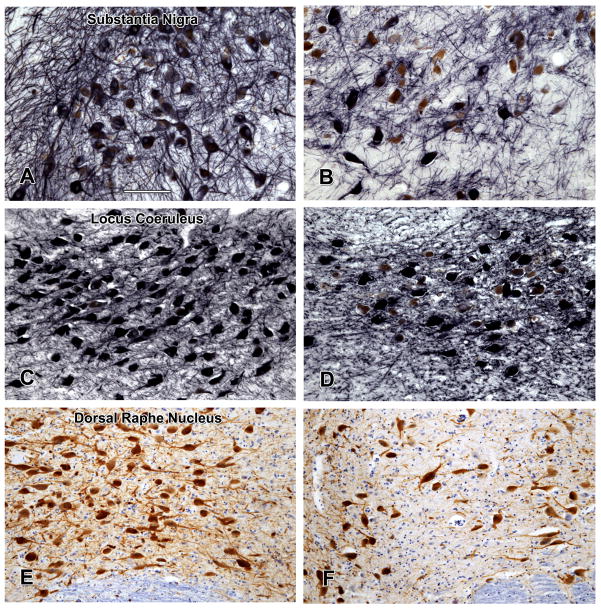
To examine the nuclei of interest, consecutive transverse blocks of fixed tissue were taken at four brainstem levels as previously described.25,26 The first block included the substantia nigra and paranigral nucleus of the ventral tegmental area (Figure 1, part A); the second block included the rostral level of the dorsal raphe nucleus, the trochlear nucleus, and the decussation of the superior cerebellar peduncles; the third block included the caudal level of the dorsal raphe nucleus, rostral levels of the locus coeruleus nuclei, and the mesencephalic nucleus of the trigeminal nerve; and the fourth block included the main body of the locus coeruleus. Following routine processing, blocks were embedded in paraffin and 6 μm sections stained with hematoxylin-eosin were used to survey the regions of interest. Blocks without anatomically matching levels of the regions of interest were excluded. Immunohistochemistry was done using an indirect immunoenzyme horseradish peroxidase method with 3, 3′diaminobenzidine as the chromogen in a Leica Bond Max autostainer (Leica Microsystems, Buffalo Grove, IL) using the Bond™ Polymer Refine Detection Kit (Leica Microsystems). Tyrosine-hydroxylase positive and negative neurons in the substantia nigra, ventral tegmental area, and locus coeruleus were identified using a monoclonal anti-tyrosine-hydroxylase antibody (1:750; Immunostar Hudson, WI). Nickel chloride (5%) added to the 3, 3′diaminobenzidine substrate (Vector Laboratories, Burlingame, CA) enhanced the signal of the tyrosine-hydroxylase positive neurons and their processes to a brownish black color. The count of ventral tegmental area neurons was based on the paranigral nucleus (Figure 1, part A). The figures show examples of high and low density of tyrosine-hydroxylase positive neurons in the paranigral nucleus of the ventral tegmental area (Figure 1, parts B and C), substantia nigra (Figure 2, parts A and B), and locus coeruleus (Figure 2, parts C and D). Serotonergic neurons in the dorsal raphe nucleus were identified using an anti-tryptophan-hydroxylase monoclonal antibody (1:1000; Sigma Chemical Co., St. Louis, MO). Examples of high (part E) and low (part F) density of tryptophan-hydroxylase positive neurons are shown in Figure 2.
Figure 1.
(A) Low power photomicrograph of a hemisection of the midbrain at the level of the red nucleus (R Nuc) immunostained with tyrosine hydroxylase antibody. The substantia nigra (SN) is present laterally and the paranigral nucleus of the ventral tegmental area (arrowhead) is present medially and is traversed by the exiting third nerve fibres. The paranigral nucleus having high (B) and low (C) density of neurons is shown. Scale bar = 125 μm.
Figure 2.
Tyrosine-hydroxylase immunostaining demonstrates high (A,C) and low (B,D) neuronal densities in one quadrant of the substantia nigra and in the locus coeruleus while tryptophan-hydroxylase immunostaining demonstrates high (E) and low (F) neuronal densities in the dorsal raphe nucleus. The medial longitudinal fasciculus is present in the lower border of the photomicrographs. Scale bar = 125 μm.
Neuronal density, neurofibrillary tangles, and Lewy bodies were quantitated at one level of the substantia nigra and the ventral tegmental area, two levels of the dorsal raphe nucleus, and two levels of the right and left locus coeruleus. The immunohistochemical preparations were used to outline the regions of interest with the Stereo Investigator Program software (MBF Biosciences, Williston, VT) attached to an Olympus BX60 microscope with a motorized stage. These outlines were superimposed on slides used to determine the neuron, neurofibrillary tangle, and Lewy body counts in the locus coeruleus, dorsal raphe nucleus, and ventral tegmental area. The counting frame and motorized stage facilitated counting in contiguous fields in the outlined regions. In these fields, unstained and immunostained neurons with nuclei were manually selected at a magnification of 400x. Density of neurons, tangles, and Lewy bodies in the four quandrants of the substantia nigra were determined as described previously.25
Analyses were based on the density/mm2 of immunostained neurons. The inter-rater reliability of these neuronal density measures, estimated in a subset of 12 participants, was high (locus coeruleus r = 0.97, dorsal raphe nucleus r = 0.96, substantia nigra r = 0.97, ventral tegmental area r = 0.99, all p<0.001). Their discriminant validity is supported by previous research linking neuronal density in the nigra to subclinical parkinsonism25 and neuronal density in the locus coeruleus to cognitive decline.26
Neurofibrillary tangles were identified using a monoclonal anti-paired helical filament-tau phosphorylated antibody, clone AT8 (1:2000; Thermo Scientific, Rockford, IL, USA) and Lewy bodies were identified using a monoclonal phosphorylated antibody to alpha-synuclein (1:20,000; Wako Chemical USA Inc, Richmond, VA).20 The total number of neurofibrillary tangles and Lewy bodies in each brainstem nucleus within the outlined area referred to above were counted manually in the regions of interest and results were expressed as density/mm2.
To assess pH, 250–300 mg of frozen cerebellum was homogenized (Polytron Kinematica PT10-35GT) in 5 volumes of nuclease free water adjusted to pH 7.2. The pH of each homogenate was measured twice at room temperature (VWR Symphony SB70pH meter). Analyses are based on the mean of the 2 readings, which were highly correlated (r = 0.998, p<0.001).
Statistical Analysis
All analyses included terms to adjust for the effects of age at death, sex, and education. A mixed-effects model was used to determine whether depressive symptoms changed over time. The mean depressive symptoms score was regressed on postmortem variables in a series of linear regression models. The initial model had a term for the composite measure of brainstem Lewy bodies. The model was repeated controlling for pH, postmortem interval, and time from last clinical evaluation to death; controlling for parkinsonism; separately analyzing Lewy bodies within each nucleus; with depression subscores as outcomes; with an indicator for antidepressant use and its interaction with Lewy bodies; and with an indicator for mild cognitive impairment and its interaction with Lewy bodies. Similar analyses were conducted with the measures of brainstem tangle density. The 4 neuronal density scores were modeled separately, ventral tegmental area neurons and nigral neurons were modeled together, and ventral tegmental area neurons were modeled controlling for pH, postmortem interval, and time from last clinical evaluation to death. We also assessed whether ventral tegmental area neurons interacted with antidepressant medication use or mild cognitive impairment, accounted for the association of Lewy bodies with depressive symptoms, or were related to depression subscores.
RESULTS
At the initial evaluation, depressive symptom scores ranged from 0 to 5 (mean = 1.48, SD = 1.66, skewness = 1.04). During a mean of 5.7 years of annual follow-up, depressive symptoms did not appear to change (eFigure 1). In a mixed-effects model adjusted for age, sex, and education, there was no evidence that depressive symptoms changed over time (estimate for time = 0.005, SE = 0.029, p = 0.85) or that residual variation not captured by the model was related to the brainstem postmortem variables. Therefore, we averaged scores across evaluations to create a composite index of late life depressive symptoms (mean = 1.61, SD = 1.48, range: 0–6, skewness = 0.94) as done in previous clinical-pathologic research.38,39 The composite score was not related to age at death (r = −0.00, p = 0.97), sex (t [122] = 1.42, p = 0.16), education (r = −0.09, p = 0.30), pH (r = −0.12, p = 0.19), postmortem interval ( r = 0.11, p = 0.22), or time from last clinical evaluation to death (r=0.17, p=0.052).
Because the measures of tangles and Lewy bodies in the nuclei clustered together in a factor analysis (Table 1), we used composite measures of tangle density (n = 124, mean = 1.66, SD = 1.51, range: 0–5.44, skewness = 1.80) and Lewy body density (n = 124, mean = 0.09, SD = 0.29 range: 0–0.88, skewness = 3.52) based on all 4 nuclei in primary analyses. The composite measures were not correlated (r = 0.13, p = 0.16). Higher density of tangles (r = 0.24, p =0.006) but not Lewy bodies (r= 0.03, p = 0.75) was related to older age at death; neither was related to sex, education, pH, postmortem interval, or time from last clinical evaluation to death.
Table 1.
Information on density of tangles and Lewy bodies in brainstem aminergic nuclei
| Rotated Factor Loadings | ||||||
|---|---|---|---|---|---|---|
| Pathologic Measure | N | Mean | SD | Skewness | Factor 1 | Factor 2 |
| Locus coeruleus tangles | 118 | 1.82 | 2.01 | 2.50 | 0.82 | 0.02 |
| Dorsal raphe nucleus tangles | 117 | 3.41 | 3.19 | 1.81 | 0.71 | 0.15 |
| Substantia nigra tangles | 106 | 0.30 | 0.45 | 2.36 | 0.84 | −0.10 |
| Ventral tegmental area tangles | 109 | 0.91 | 1.67 | 2.46 | 0.75 | −0.09 |
| Locus coeruleus Lewy bodies | 117 | 0.22 | 0.79 | 4.25 | −0.09 | 0.87 |
| Dorsal raphe nucleus Lewy bodies | 117 | 0.01 | 0.07 | 5.92 | −0.03 | 0.62 |
| Substantia nigra Lewy bodies | 111 | 0.05 | 0.23 | 5.72 | 0.00 | 0.82 |
| Ventral tegmental area Lewy bodies | 109 | 0.08 | 0.40 | 6.93 | 0.07 | 0.52 |
Lewy Bodies and Depressive Symptoms
In a linear regression model adjusted for age at death, sex, and education, higher density of brainstem Lewy bodies was associated with higher level of depressive symptoms (estimate = 1.133, SE = 0.443, p = 0.012, increase in adjusted R2 = 4.0%). Results were similar after controlling for pH, postmortem interval, and time from last evaluation to death (estimate = 1.159, SE = 0.437, p = 0.009) or parkinsonism proximate to death (estimate = 1.168, SE = 0.450, p = 0.011). The association was observed in 3 of the 4 nuclei (estimate for locus coeruleus = 0.363, SE = 0.175, p = 0.040; estimate for dorsal raphe nucleus = 5.302, SE = 2.000, p = 0.009; estimate for substantia nigra = 1.301, SE = 0.609, p = 0.035; estimate for ventral tegmental area = 0.420, SE = 0.368, p = 0.26). In analyses of subscores, higher Lewy body density was marginally related to negative affect (estimate = 0.495, SE = 0.237, p = 0.039), robustly related to somatic symptoms (estimate = 0.634, SE = 0.204, p = 0.002), and not related to positive affect (estimate = 0.007, SE = 0.107, p = 0.95) or interpersonal problems (estimate = −0.005, SE = 0.033, p = 0.88).
We conducted additional analyses to determine whether antidepressant medication use (32.3% during at least part of the observation period; eTable 1) or mild cognitive impairment (43.5%) was obscuring the association of Lewy bodies with depressive symptoms. There was no interaction with mild cognitive impairment (estimate = 0.859, SE = 0.991, p = 0.39) but there was with medication use (estimate = −2.476, SE = 0.913, p = 0.008): the association of Lewy bodies with depressive symptoms was attenuated in those on antidepressant medication compared to those not on antidepressant medication.
Tangles and Depressive Symptoms
The composite measure of brainstem tangle density was not related to depressive symptoms (estimate = −0.038, SE = 0.090, p = 0.68); adjustment for pH, postmortem interval, and time from last evaluation to death yielded comparable results (estimate = 0.087, SE = 0.093, p = 0.35); and a subsequent analysis with a quadratic term for tangles (estimate = −0.061, SE = 0.039, p = 0.12) did not suggest a nonlinear association. No association with tangles was present in the individual nuclei (estimate for locus coeruleus = 0.031, SE = 0.071, p = 0.66; estimate for dorsal raphe nucleus = −0.007, SE = 0.045, p = 0.88; estimate for substantia nigra = 0.482, SE = 0.332, p = 0.15; estimate for ventral tegmental area = −0.105, SE = 0.087, p = 0.23). There was no interaction with antidepressant medication use (estimate = 0.027, SE = 0.217, p = 0.90) but there was with mild cognitive impairment (estimate = −0.491, SE = 0.190, p = 0.011): higher tangle density was associated with higher level of depressive symptoms in those without cognitive impairment but with lower level of symptoms in those with mild cognitive impairment (eFigure 2).
Aminergic Neuronal Density and Depressive Symptoms
There were 6 to 13 participants per nucleus with missing neuronal data due to missing or insufficient tissue. The distributions of aminergic neuronal density in the locus coeruleus (n = 118, mean = 44.1, SD = 17.8, skewness = 0.39), dorsal raphe nucleus (n = 117, mean = 105.4, SD = 28.3, skewness = 0.54), substantia nigra (n = 113, mean = 31.1, SD = 11.9, skewness = 0.01), and ventral tegmental area (n=111, mean = 82.8, SD = 47.9, skewness = 0.33) are shown in eFigure 3. None of the density measures was related to age at death, sex, or education; their crude correlations with time from last clinical evaluation to death, postmortem interval, pH, and brainstem pathology are shown in eTable 2.
We constructed a series of linear regression models adjusted for age at death, sex, and education to assess the hypothesized association between brainstem aminergic neurons and depressive symptoms. Higher density of tyrosine-hydroxylase-immunoreactive neurons in the ventral tegmental area (Figure 3; estimate = −0.014, SE = 0.003, p<0.001, increase in adjusted R2 = 16.3%) and substantia nigra (estimate = −0.025, SE = 0.012, p = 0.033, increase in adjusted R2 = 1.5%), but not the locus coeruleus (estimate = −0.011, SE = 0.008, p = 0.18), was related to lower level of depressive symptoms, with no association for tryptophan-hydroxylase-immunoreactive neurons in the dorsal raphe nucleus (estimate = 0.000, SE = 0.005, p = 0.999). When the ventral tegmental area and substantia nigra were included in the same model, only neurons in the ventral tegmental area were related to depressive symptoms (estimate = −0.012, SE = 0.003, p <0.001). Adjustment for pH, postmortem interval, and time from last clinical evaluation to death did not affect the relation of ventral tegmental area neurons to depressive symptoms (estimate = −0.013, SE = 0.003, p<0.001). Use of antidepressant medication was not related to density of ventral tegmental area neurons (t[109] = −1.21, p = 0.23). Neither antidepressants (estimate = 0.006, SE = 0.005, p = 0.26) nor mild cognitive impairment (estimate = 0.001, SE = 0.006, p = 0.88) modified the association of ventral tegmental area neurons with depressive symptoms.
Figure 3.
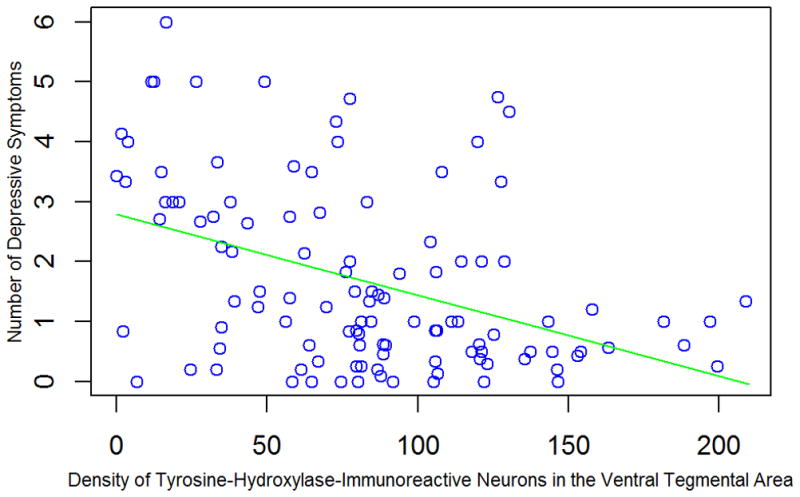
Relation of density of tyrosine-hydroxylase-immunoreactive neurons in the ventral tegmental area to depressive symptoms, adjusted for age at death, sex, and education.
Because lower density of ventral tegmental area neurons was associated with higher density of brainstem Lewy bodies (r = −0.19 p = 0.046), we modeled them together. In this analysis, the association of Lewy bodies with depressive symptoms was attenuated (estimate = 0.615, SE = 0.434, p=0.16) but the association of neurons with symptoms persisted (estimate = −0.013, SE = 0.003, p<0.001), suggesting that the association of brainstem Lewy bodies with depressive symptoms was due in part to the density of ventral tegmental area neurons.
To further investigate how ventral tegmental area neurons were related to depressive symptoms, we analyzed different symptom domains. Lower density of tyrosine-hydroxylase- immunoreactive neurons in the ventral tegmental area was associated with negative affect (estimate = −0.007, SE = 0.002, p <0.001), lack of positive affect (estimate = −0.002, SE = 0.001, p = 0.003), and somatic symptoms (estimate = −0.004, SE = 0.001, p = 0.007) but not with interpersonal problems (estimate = −0.0003, SE = 0.0001, p = 0.17).
COMMENT
During a mean of approximately 5 years of observation, depressive symptoms were assessed annually in nearly 125 older persons who subsequently died and had a neuropathologic examination. Higher density of tyrosine-hydroxylase-immunoreactive neurons in the ventral tegmental area was associated with lower level of depressive symptoms, with 16% of the variability in symptoms explained by individual differences in density. The results suggest that the mesolimbic dopamine system plays an important role in late life depressive symptoms.
Animal models21–23 and human neuroimaging studies40,41 have highlighted the potential importance of mesolimbic dopaminergic mechanisms in depression, particularly for aspects of apathy and anhedonia. However, we are not aware of previous clinical-pathologic studies of ventral tegmental area neuronal density and depressive symptoms. Higher neuronal ratings in the substantia nigra were associated with lower likelihood of depressive symptoms in a study of older persons without dementia.14 The association in the present study between density of nigral dopaminergic neurons and depressive symptoms is consistent with this finding and extends it by more precisely quantifying nigral neurons and by showing that the association is largely confounded by density of dopaminergic neurons in the ventral tegmental area. We did not find density of noradrenergic neurons in the locus coeruleus or serotonergic neurons in the dorsal raphe nucleus to be related to depressive symptoms. These null results are in agreement with most 42,43 but not all44 studies of the locus coeruleus and with the weight of the evidence from studies of dorsal raphe nucleus neurons in depression, which have reported levels to be lower11,45 no different,12,46 and higher47 than controls.
The factors responsible for the observed individual differences in density of dopaminergic neurons in the ventral tegmental area are not known, and because the present study did not include persons who died prior to old age, it is uncertain whether the association between dopaminergic neurons in the ventral tegmental area and depressive symptoms is specific to late life. That the association was observed after excluding individuals with dementia and controlling for postmortem markers linked to dementia suggests that some of the variability in dopamine neuron density was longstanding. It is possible, therefore, that a less populated ventral tegmental area due to neurodevelopmental or other causes occurring earlier in adulthood somehow increases vulnerability to depressive symptoms. However, a substantial subgroup had few dopamine neurons in the ventral tegmental area (eFigure 3) and older age had a nearly significant correlation with lower neuronal density (eTable 2), suggesting that age related pathologic processes are also involved. Because Parkinson’s disease also involves a loss of brain stem dopaminergic neurons for uncertain reasons, it provides a useful point of comparision. Depressive symptoms are common in the disease48 and are reduced by pramipexole, 49,50 a dopamine agonist, suggesting that late life depressive symptoms in some persons without Parkinson’s disease might also be alleviated by pharmacologic enhancement of dopamine transmission with agonists or with reuptake inhibitors such as bupropion51 or nomifensine.52
Neurodegenerative lesions in brainstem aminergic nuclei were related to depressive symptoms, but the associations were weak and conditional on other factors. Thus, the presence of Lewy bodies in brainstem aminergic nuclei was related to depressive symptoms, consistent with a previous clinical-pathologic study12 and the prominent place of depressive symptoms in synucleinopathies such as Parkinson’s disease and Lewy body disease. However, the association was only seen in those not on antidepressants and controlling for ventral tegmental area neuronal density eliminated it.
There was no overall association between brainstem tangles and depressive symptoms, consistent with previous research on persons without dementia12,44 and with most9,44 but not all13 studies of persons with dementia. In contrast to previous research, the present study included clinical classification of mild cognitive impairment, and higher brainstem tangle density was associated with higher level of depressive symptoms in persons without cognitive impairment but with lower level of symptoms in those with mild cognitive impairment. This suggests that tangles make a very modest contribution to depressive symptoms prior to the onset of cognitive impairment, but that as cognitive problems develop, depressive symptoms may be less consistently reported, possibly because they are harder to remember or acknowledge.16,17 In addition, incipient cognitive decline may disrupt the continuity of mood states and their ability to regulate behavior. These observations are in line with longitudinal data that suggest little increase in depressive symptoms during the development of mild cognitive impairment and dementia.31,53,54
Study strengths and limitations should be noted. There was a high rate of participation in the annual clinical evaluations and brain autopsy, minimizing bias due to selective attrition. Uniform annual evaluations and the application of widely used diagnostic criteria allowed us to reliably classify mild cognitive impairment and dementia. The measure of depressive symptoms was based on a standard scale administered at regular intervals for several years, which probably enhanced assessment of the enduring the tendency to be depressed while reducing measurement error and the impact of episodic fluctuations in mood. An important limitation is that the cohort was selected and so replication of these findings will be important. In addition, the findings for the ventral tegmental area are based on the paranigral nucleus and it is uncertain whether they will generalize to other subpopulations of ventral tegmental area dopaminergic neurons.55
Supplementary Material
Acknowledgments
The authors thank the many Illinois residents for participating in the Rush Memory and Aging Project; Traci Colvin, MPH, and Karen Skish, MS, for study coordination; John Gibbons, MS, and Greg Klein, MS, for data management; Wenqing Fan, MS, for statistical programming; and the Neuropathologic Laboratory. This research was supported by the National Institute on Aging (R01AG17917, R01AG15819, R01AG24871, R01AG34374, R01AG33678) and the Illinois Department of Public Health.
Footnotes
Statistical analyses were conducted by Dr. Yu
The authors have no conflicts of interest to disclose.
Dr. Wilson had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The funding organizations had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of this manuscript.
References
- 1.Beekman ATF, Copeland JRM, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307–311. doi: 10.1192/bjp.174.4.307. [DOI] [PubMed] [Google Scholar]
- 2.Bruce ML, Seeman TE, Merrill SS, Blazer DG. The impact of depressive symptoms on physical disability: MacArthur Studies of Successful Aging. Am J Public Health. 1994;84:1796–1799. doi: 10.2105/ajph.84.11.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Sano MP, Tang MX, Taylor S, Gurland BJ, Wilder D, Stern Y, Mayeux R. Depressed mood and the incidence of Alzheimer’s disease in the elderly living in the community. Arch Gen Psychiatry. 1996;53:175–182. doi: 10.1001/archpsyc.1996.01830020093011. [DOI] [PubMed] [Google Scholar]
- 4.Wilson RS, Bienias JL, Mendes de Leon CF, Evans DA, Bennett DA. Negative affect and mortality in older persons. Am J Epidemiol. 2003;158:827–835. doi: 10.1093/aje/kwg224. [DOI] [PubMed] [Google Scholar]
- 5.Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- 6.Zweig RM, Ross CA, Hedreen JC, Steele C, Cardillo JE, Whitehouse PJ, Folstein MF, Price DL. The neuropathology of aminergic nuclei in Alzheimer’s disease. Ann Neurol. 1988;24:233–242. doi: 10.1002/ana.410240210. [DOI] [PubMed] [Google Scholar]
- 7.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 8.Förstl H, Burns A, Luthert P, Cairns N, Lantos P, Levy R. Clinical and neuropathological correlates of depression in Alzheimer’s disease. Psychol Med. 1992;22:877–884. doi: 10.1017/s0033291700038459. [DOI] [PubMed] [Google Scholar]
- 9.Chen CPLH, Eastwood SL, Hope T, McDonald B, Francis PT, Esiri MM. Immunocytochemical study of the dorsal and median raphe nuclei in patients with Alzheimer’s disease prospectively assessed for behavioral changes. Neuropath Appl Neurobiol. 2000;26:347–355. doi: 10.1046/j.1365-2990.2000.00254.x. [DOI] [PubMed] [Google Scholar]
- 10.Matthews KL, Chen CPLH, Esiri MM, Keene J, Minger SL, Francis PT. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol Psychiatry. 2002;51:407–416. doi: 10.1016/s0006-3223(01)01235-5. [DOI] [PubMed] [Google Scholar]
- 11.Syed A, Chatfield M, Matthews F, Harrison P, Brayne C, Esiri MM. Depression in the elderly: pathological study of raphe and locus coeruleus. Neuropath Appl Neurobiol. 2005;31:405–413. doi: 10.1111/j.1365-2990.2005.00662.x. [DOI] [PubMed] [Google Scholar]
- 12.Tsopelas C, Stewart R, Savva GM, Brayne C, Ince P, Thomas A, Matthews FE Medical Research Council Cognitive Function and Ageing Study. Neuropathological correlates of late-lfe depression in older people. Brit J Psychiatry. 2011;198:109–114. doi: 10.1192/bjp.bp.110.078816. [DOI] [PubMed] [Google Scholar]
- 13.Zubenko GS, Mossy J. Major depression in primary dementia. Clinical and neuropathologic correlates. Arch Neurol. 1988;45:1182–1186. doi: 10.1001/archneur.1988.00520350020008. [DOI] [PubMed] [Google Scholar]
- 14.Chan-Palay V, Asan E. Alterations in catecholamine neurons of the locus coeruleus in senile dementia of the Alzheimer type and in Parkinson’s disease with and without dementia and depression. J Comp Neurol. 1989;287:373–392. doi: 10.1002/cne.902870308. [DOI] [PubMed] [Google Scholar]
- 15.Hoogendijk WJ, Sommer IE, Pool CW, Kamphorst W, Hofman MA, Eikelenboom P, Swabb DF. Lack of association between depression and losss of neurons in the locus coeruleus in Alzheimer disease. Arch Gen Psychiatry. 1999;56:45–51. doi: 10.1001/archpsyc.56.1.45. [DOI] [PubMed] [Google Scholar]
- 16.Gilley DW, Wilson RS. Criterion-related validity of the Geriatric Depression Scale in Alzheimer’s disease. J Clin Exp Neuropsychol. 1997;19:489–499. doi: 10.1080/01688639708403739. [DOI] [PubMed] [Google Scholar]
- 17.Gilley DW, Wilson RS, Fleischman DA, Harrison DW, Goetz CG, Tanner CM. Impact of Alzheimer’s-type dementia and information source on the assessment of depression. Psychol Assess. 1995;7:42–48. [Google Scholar]
- 18.Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age-related cognitive decline. Neurology. 2010;75:1070–1078. doi: 10.1212/WNL.0b013e3181f39adc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson RS, Arnold SE, Schneider JA, Tang Y, Bennett DA. The relationship between cerebral Alzheimer’s disease pathology and odour identification in old age. J Neurol Neurosurg Psychiatry. 2007;78:30–35. doi: 10.1136/jnnp.2006.099721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider JA, Li J-L, Li Y, Wilson RS, Kordower JH, Bennett DA. Neurofibrillary tangles in the substantia nigra are related to gait impairment in older persons. Ann Neurol. 2006;59:166–173. doi: 10.1002/ana.20723. [DOI] [PubMed] [Google Scholar]
- 21.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaundhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchman AS, Nag S, Shulman JM, Lim AS, VanderHorst VG, Leurgans SE, Schneider JA, Bennett DA. Locus coeruleus neuron density and parkinsonism in older adults without Parkinson’s disease. Mov Disord. 2012;27:1625–1631. doi: 10.1002/mds.25142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, Schneider JA, Bennett DA. Neural reserve, neuronal density in the locus coeruleus, and cognitive decline. Neurology. doi: 10.1212/WNL.0b013e3182897103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohout FJ, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993;5:79–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Wilson RS, Barnes LL, Mendes de Leon CF, Aggarwal NT, Schneider JA, Bach J, Pilat J, Beckett LA, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology. 2002;59:364–370. doi: 10.1212/wnl.59.3.364. [DOI] [PubMed] [Google Scholar]
- 30.Shafer AB. Meta-analysis of the factor structures of 4 depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- 31.Wilson RS, Hoganson GM, Rajan KB, Barnes LL, Mendes de Leon CF, Evans DA. The temporal course of depressive symptoms during the development of Alzheimer’s disease. Neurology. 2010;75:21–26. doi: 10.1212/WNL.0b013e3181e620c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Medi-span, Inc. Master drug database documentation manual. Indianapolis, IN: Medi-span, Inc; 1995. [Google Scholar]
- 33.Bennett DA, Shannon KM, Beckett LA, Goetz CG, Wilson RS. Metric properties of nurses’ ratings of parkinsonian signs with a modified Unified Parkinson’s Disease Rating Scale. Neurology. 1997;49:1580–1586. doi: 10.1212/wnl.49.6.1580. [DOI] [PubMed] [Google Scholar]
- 34.Fahn S, Elton R. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden C, Goldstein M, Caine D, editors. Recent Developments in Parkinson’s Disease. Vol. 12. Folorham Park, NJ: MacMillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- 35.Wilson RS, Schneider JA, Bienias JL, Evans DA, Bennett DA. Parkinsonianlike signs and risk of incident Alzheimer disease in older persons. Arch Neurol. 2003;60:539–544. doi: 10.1001/archneur.60.4.539. [DOI] [PubMed] [Google Scholar]
- 36.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS/ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 37.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 38.Wilson RS, Schneider JA, Bienias JL, Arnold SE, Evans DA, Bennett DA. Depressive symptoms, clinical AD, and cortical plaques and tangles in older persons. Neurology. 2003;61:1102–1107. doi: 10.1212/01.wnl.0000092914.04345.97. [DOI] [PubMed] [Google Scholar]
- 39.Bennett DA, Wilson RS, Schneider JA, Bienias JL, Arnold SE. Cerebral infarctions and the relationship of depressive symptoms to level of cognitive functioning in older persons. Am J Geriatr Psychiatry. 2004;12:211–219. [PubMed] [Google Scholar]
- 40.Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinz HJ, Zilles K, Düzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- 42.Zhu MY, Klimek V, Dilley GE, Haycock JW, Stockmeier C, Overholser JC, Meltzer HY, Ordway GA. Elevated levels of tyrosine hydroxylase in the locus coeruleus in major depression. Biol Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 43.Gos T, Krell D, Bielau H, Brisch R, Trübner K, Steiner J, Bernstein HG, Jankowski Z, Bogerts B. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is eleveated in violent suicidal depressive patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:513–520. doi: 10.1007/s00406-008-0825-8. [DOI] [PubMed] [Google Scholar]
- 44.Baumann B, Danos P, Diekmann S, Krell D, Bielau H, Geretsegger C, Wurthmann C, Bernstein HG, Bogerts B. Tyrosine hydroxylase immunoreactivity in the locus coeruleus is reduced in depressed non-suicidal patients but normal in depressed suicide patients. Eur Arch Psychiatry Clin Neurosci. 1999;249:212–219. doi: 10.1007/s004060050089. [DOI] [PubMed] [Google Scholar]
- 45.Hendricksen M, Thomas AJ, Ferrier IN, Ince P, O’Brien JT. Neuropathological study of the dorsal raphe nuclei in late-life depression and Alzheimer’s disease with and without depression. Am J Psychiatry. 2004;161:1092–1102. doi: 10.1176/appi.ajp.161.6.1096. [DOI] [PubMed] [Google Scholar]
- 46.Baumann B, Bielau H, Krell D, Agelink MW, Diekmann S, Wurthmann C, Trübner K, Bernstein HG, Danos P, Bogerts B. Circumscribed numerical deficit of dorsal raphe neurons in mood disorders. Psychol Med. 2002;32:93–103. doi: 10.1017/s0033291701004822. [DOI] [PubMed] [Google Scholar]
- 47.Underwood MD, Khaibulina AA, Ellis SP, Moran A, Rice PM, Mann JJ, Arango V. Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims. Biol Psychiatry. 1999;46:472–483. doi: 10.1016/s0006-3223(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 48.Aarsland D, Påhlhagen S, Ballard CG, Ehrt U, Svenningsson P. Depression in parkinson’s disease – epidemiology, mechanisms and management. Nat Rev Neurol. 2012;8:35–47. doi: 10.1038/nrneurol.2011.189. [DOI] [PubMed] [Google Scholar]
- 49.Barone P, Poewe W, Albrecht S, Debieuvre C, Massey D, Rascol O, Tolosa E, Weintraub D. Pramipexole for the treatment of depressive symptoms in patients with Parkinson’s disease: a randomized, double-blind, placebo-controlled trial. Lacent Neurology. 2010;91:573–580. doi: 10.1016/S1474-4422(10)70106-X. [DOI] [PubMed] [Google Scholar]
- 50.Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, Hametner EM, Poewe W, Rascol O, Goetz CG, Sampaio C. The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, Ritz L, Biggs MM, Warden D, Luther JF, Shores-Wilson K, Niederehe G, Fava M STAR*D Study Team. Bupropin-SR, Sertraline, or Venlafaxine-XR after failure of SSRIs for depression. N Engl J Med. 2006;354:1231–1242. doi: 10.1056/NEJMoa052963. [DOI] [PubMed] [Google Scholar]
- 52.Feighner JP, Merideth CH, Claghorn JL. Multicenter placebo-controlled evaluation of nomifensine treatment in depressed outpatients. J Clin Psychiatry. 1984;45:47–51. [PubMed] [Google Scholar]
- 53.Amieva H, Goff ML, Millett X, Orgogozo JM, Peres K, Barberger-Gateau P, Jacqmin-Gadda H, Dartigues JF. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol. 2008;64:492–498. doi: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 54.Wilson RS, Arnold SE, Beck TL, Bienias JL, Bennett DA. Change in depressive symptoms during the prodromal phase of Alzheimer disease. Arch Gen Psychiatry. 2008;65:439–446. doi: 10.1001/archpsyc.65.4.439. [DOI] [PubMed] [Google Scholar]
- 55.Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.