Abstract
Based on accumulating evidence that the 3D topography and the chemical features of a growth surface influence neuronal differentiation, we combined these two features by evaluating the cytotoxicity, proliferation, and differentiation of the rat PC12 line and human neural stem cells (hNSCs) on chitosan (CS), cellulose acetate (CA), and polyethersulfone (PES)-derived electrospun nanofibers that had similar diameters, centered in the 200 to 500 nm range. None of the nanofibrous materials were cytotoxic compared to 2D (e.g., flat surface) controls; however, proliferation generally was inhibited on the nanofibrous scaffolds although to a lesser extent on the polysaccharide-derived materials compared to PES. In an exception to the trend towards slower growth on the 3D substrates, hNSCs differentiated on the CS nanofibers proliferated faster than the 2D controls and both cell types showed enhanced indication of neuronal differentiation on the CS scaffolds. Together, these results demonstrate beneficial attributes of CS for neural tissue engineering when this polysaccharide is used in the context of the defined 3D topography found in electrospun nanofibers.
Keywords: Electrospun nanofibers, polysaccharides, tissue engineering scaffolds, human neural stem cells, rat PC12 cells
1. Introduction
Several features position polysaccharides as attractive biomaterials for use in tissue engineering, stem cell research, and regenerative medicine. Three factors in particular have contributed to the growing potential of sugar-based materials for these applications. The first is the long-standing recognition that the oligosaccharide components of glycoproteins and glycolipids ubiquitously displayed on the surfaces of mammalian cells are essential for the function of these molecules; for example they play many roles at the nexus of cell adhesion and signaling (Hakomori, 2002). The second factor has been the realization that extracellar polysaccharides, which are major structural components of the extracellular matrix (ECM), also play critical roles in modulating signaling (Du & Yarema, 2010). For example the ECM can spatially sequester signaling molecules, which can enhance their ability to engage signaling pathways to promote and guide nerve regeneration (Krick, Tammia, Martin, Höke & Mao, 2011) as well as direct asymmetric cell division (Hayes, Tudor, Nowell, Caterson & Hughes, 2008). The third factor is the ongoing need for new materials with specific and controllable biological activity, a high degree of safety, and biodegradability for use in tissue engineering and regenerative medicine (Tirtsa Ehrenfreund-Kleinman & Domb, 2006); polysaccharides meet all of these criteria.
Chitosan, an abundant natural polysaccharide, has many attractive qualities for tissue engineering because of its biocompatibility, biodegradability, non-toxicity, and its pH dependent solubility facilitating its processing into micro- and nano-scaffolds (Kim et al., 2008). The chemical structure of chitosan is similar to glycosaminoglycans such as hyaluronic acid present in mammalian ECM insofar as both contain amino-sugar residues, and its hydrophilicity enhances its interaction with growth factors, cellular receptors, and adhesion proteins (Kumar, 2000). Previous studies showed that chitosan is biocompatible with neural cells (Freier, Koh, Kazazian & Shoichet, 2005) with its surface amines and its low equilibrium water content contributing to the viability, survival, and integration of neural precursor cells during prolonged periods of cell culture (Scanga, Goraltchouk, Nussaiba, Shoichet & Morshead, 2010). Chitosan scaffolds can also enhance cell proliferation (Prabhakaran et al., 2008) and accelerate repair in the peripheral nervous system (Pfister, Papaloïzos, Merkle & Gander, 2007). Similarly, Ao et al. demonstrated the excellent affinity of multimicrotubule chitosan-containing conduits for nerve cells (Ao et al., 2006), and most recently, chitosan-based nerve grafts have successfully repaired the 50 mm nerve defects in rhesus monkeys after 12 months of implantation (Hu et al., 2013).
In addition to the chemical composition of a biomaterial, surface properties such as topography, surface charge, and protein adsorption influence cell behavior by providing appropriate signals for cell growth, differentiation and subsequent tissue formation (Lim & Mao, 2009). In this regard, electrospun nanofibers have gained considerable interest because their architecture is similar to the naturally-occurring protein fibrils in the extracellular environment and each individual nano-scale fiber has a high surface to volume and aspect ratio allowing for a high level of surface area contact of the scaffold with the cell. Because scaffolds consisting of electrospun fibers mimic the extracellular matrix (ECM), they have been used in recent years to enhance nerve regeneration both in vitro and in vivo. For example, electrospun nanofibers fabricated from poly-l-lactic acid (PLLA) and poly-ε-caprolactone (PCL) have been used successfully as scaffolding materials for nerve tissue engineering (Ghasemi-Mobarakeh, Prabhakaran, Morshed, Nasr-Esfahani & Ramakrishna, 2010; Koh, Yong, Chan & Ramakrishna, 2008). Furthermore, tuning the fiber dimension and pattern of PLLA fibers regulates the viability, proliferation, and neurite outgrowth of neonatal mouse cerebellum C17.2 cells (He et al., 2010). Hurtado et al. investigated for repairing a completely transected spinal cord with a 3-mm defect in a rat model (Hurtado et al., 2011) by filling the spinal cord gap four weeks after surgery with cells enclosed in PLLA electrospun fiber conduits. Kim et al. carried out both in vitro and in vivo studies on the impacts of aligned electrospun poly(acrylonitrile-co-methylacrylate) fibers on neuron outgrowth and Schwann cell migration and repairing a 17-mm nerve gap in a rat peripheral nerve injury model (Kim, Haftel, Kumar & Bellamkond, 2008).
As described above, chitosan as a material and electro-spun nanofibers as a scaffold design strategy hold promise for neural tissue engineering. However, to date these two approaches have not been systematically combined by investigating electrospun chitosan nanofibers as scaffolds for nerve cell growth and differentiation. To fill this void, the present study explores the electrospinning of two polysaccharides, chitosan (CS) and cellulose acetate (CA), to obtain the nanofibrous scaffolds with similar structures. We predict that unique features of CS, specifically that it is the only natural cationic polysaccharide with a more hydrophilic and positively charged surface than CA, will provide enhanced benefits for biocompatibility, viability, and differentiation of nerve cells. The results obtained from nanofibrous scaffolds constructed from these two sugar-based materials will be compared with structurally-similar polyethersulfone electrospun (PES) fibers as well as with cells grown under standard, widely used 2D conditions (e.g., on collagen- and laminin-coated tissue culture plastic for rat PC12 and human neural stem cells, hNSCs, respectively). The resulting findings verified that a combination of the chemical properties of CS together with nanofiber topography constituted highly compatible scaffolds for nerve tissue regeneration.
2. Materials and Methods
2.1 Materials
All chemicals were purchased from Sigma-Aldrich unless otherwise stated. Polyethersulfone granules (Mw = 55,000) was purchased from Goodfellow Cambridge Limited. Cellulose acetate (CA, Mv = 30 kDa and DS = 2.45) and chitosan (CS, Mv = 405 kDa and DDA = 82.5%) were obtained from Sigma-Aldrich. Rat pheochromocytoma (PC12) cells and human neural stem cells (hNSC) were purchased from the American Tissue Culture Collection and EMD Millipore, respectively.
2.2 Electrospinning of polyethersulfone (PES), cellulose acetate (CA), and chitosan (CS) fibers
By adopting and optimizing methodology from the literature (Du & Hsieh, 2009; Lin, Chua, Christopherson, Lim & Mao, 2007; Supaphol & Sangsanoh, 2006), homogeneous solutions of 17% PES with 0.5% OTAB (trimethyloctadecylammonium bromide), 15% CA, and 3.5% CS were selected to dissolve in DMSO (dimethyl sulfoxide), 2/1 acetone/DMAc, and 7/3 TFA/DCM (trifluoroacetic acid/dichloromethane) separately under constant stirring in order to make the similar diameter electrospun nanofibers. All solution concentrations reported were weight percentages except CS, which was weight to volume. Each solution was electrospun at ambient temperature through a 27G needle at a rate of 1.0 mL h−1 controlled by a syringe pump (KD Scientific, model 200) and under 10–20 kV DC voltage (Gamma High Voltage Supply, ES 30-0.1 P). The distance between the syringe tip and the grounded collector was kept at 15–20 cm. Nanofibers were collected directly onto grounded 15 mm diameter glass coverslips. The PES and CA nanofiber samples were washed thoroughly in distilled water and subsequently in ethanol to remove any residual solvents, dried thoroughly under vacuum, and stored in a desiccator. The CS nanofibers were first neutralized in Na2CO3 aqueous solution (Sangsanoh & Supapho, 2006), and then treated as mentioned above. To quantify the fiber diameters, samples were processed and imaged on a field-emission SEM (JEOL 6700F), and analyzed by NIH Image J software. A minimum of 100 fibers were measured for each sample group.
Fibrous matrices were prepared for cell culture studies as follows: fiber meshes were first secured to the coverslips by applying a small amount of surgical glue (B-401 secure adhesive, Factor II, Inc.) to the edge of the coverslip. Then the matrices were washed in PBS (phosphate-buffered saline) and sterilized with 70% ethanol, followed by exposure to UV in the biosafety cabinet for 30 min. Fibers were coated with collagen for experiments using PC12 cells and with poly-d-lysine / laminin for experiments using hNSCs.
2.3 Cell culture
PC12 cells were cultured in complete medium consisting of high glucose DMEM supplemented with 10% heat inactivated horse serum, 5% calf serum, and 1% of a 100× stock solutions of penicillin and streptomycin (pen/strep) at 37 °C and 5% CO2. Collagen coated fiber matrices in 24-well plates were incubated in medium 2 h prior to seeding cells. PC12 cells were seeded at a density of 10000 cells/well in complete medium for cell viability assays. For differentiation studies, cells were seeded at a density of 5,000 cells/well in DMEM with 1% horse serum and pen/strep with 50 ng/ml nerve growth factor (NGF).
hNSCs were expanded in neurobasal medium supplemented with 2mM L-glutamine, 1xB27, 10 ng/ml leukemia inhibitory factor (LIF; all above from Invitrogen), and 20 ng/ml human recombinant basic fibroblast growth factor (bFGF; Sigma). ENStem-A™ Neuronal Differentiation Medium from EMD Millipore was used for neural differentiation studies. Cells were seeded at a density of 100,000 cells/well into 24-well culture dishes in triplicate.
2.4 Determination of cell toxicity, viability, and proliferation
A CytoTox 96 Nonradioactive assay (Promega) was used to assess the cytotoxicity of the electrospun nanofibers constructed from each of the materials. This colorimetric assay measures lactose dehydrogenase (LDH) in the supernatant, which is released upon cell lysis. Fiber samples were cultured with PC12 cells up to three days and similar numbers of cell grown on 2D tissue culture plastic were used as control. The media was sampled and assayed at day 1, day 2, and day 3 after seeding following the manufacturer's protocol and the absorbance was measured at 490 nm. Each experiment was done in triplicate. The spectrophotometer was calibrated to zero absorbance using culture medium without cells.
The viability and proliferation of cells on the fiber scaffolds were determined using a WST-1 assay (Biovision) after day 1, 3, and 5 of culture in a complete medium. At pre-determined intervals, culture medium was removed by aspiration and the cells/scaffolds were washed with PBS. Serum-free medium (450 µl) and WST solution (50 µl) were added to each well. The samples were covered in foil and incubated at 37°C for 2.5 h. An aliquot (100 µl) from each sample was removed, transferred to a 96-well plate, and analyzed with a spectrophotometric plate reader (µQuant, Bio-Tek). Controls of each sample type without cells were also analyzed to calculate background absorbance values for correction. The absorbance at 450 nm was recorded for each well for n = 5 replicates for each sample and time point. All values were corrected for background absorbance and normalized to cells grown on tissue culture polystyrene.
2.5 Analysis of PC12 neurite outgrowth
After day 7 of seeding PC12 cells in differentiation medium supplemented with NGF, neurite outgrowth was characterized by staining actin filaments with phalloidin (Invitrogen) and analyzing with confocal laser scanning microscopy (CLSM, Zeiss LSM 710) by preparing the samples as previously reported (Du, Che, Wang, Aich & Yarema, 2011). Briefly, the cells on the fiber scaffolds were rinsed thoroughly with PBS, fixed with 4% paraformaldehyde in PBS for 20 min, and then permeabilized with 0.1% Triton X-100 for 15 min. Nonspecific labeling was minimized by incubating samples in a blocking buffer composed of 2.5% bovine serum albumin (BSA) in PBS for 30 min. Samples were then incubated with phalloidin (1:500; Invitrogen) in blocking buffer for 1.0 h, washed with PBS, and kept in the dark for analysis. A minimum of n = 5 random areas for each of three replicate samples were imaged.
Morphometric analysis (Wang, Che, Du, Ha & Yarema, 2010) was performed on digitized images of live cells taken under phase contrast microscope. Images of two fields per well were taken with an average of 10 cells per field. The neurite growth, neurites per differentiated cells, and percentage of differentiated cells were characterized for the differentiation of PC-12 cells following published precedent (Cooke et al., 2010; Das, Freudenrich & Mundy, 2004; Leach, Brown, Jacot, A & Wong, 2007). Briefly, neurite growth was determined by manually tracing the length of the longest neurite per cell for all cells in a field that had an identifiable neurite and for which the entire neurite arbor could be visualized. The neurite length was defined as the straight-line distance from the neurite tip to the junction between the cell body and neurite base. The number of differentiated cells was determined by visual examination of the field and counting cells that had at least one neurite with a length equal to the cell body diameter, and expressed as a percentage of the total cells in the field. Data from two fields in each well and a total five wells were pooled.
2.6 Immunocytochemistry and imaging of hNSCs on fiber matrices
hNSCs cultures were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 2.5% BSA buffer as described above. Primary antibody against β-tubulin III (1:500; neuronal marker; rabbit polyclonal anti-TUJ1; Millipore/Chemicon) was applied in blocking buffer overnight at 4 °C. Cells were then washed in PBS and treated with secondary antibodies coupled to Cy3 (1:400 dilution; Jackson ImmunoResearch) for 60 min. After two washes in PBS, cells were stained with 4′, 6′-diamidino-2-phenylindole (DAPI) and then mounted using ProLong Gold anti-fade reagent (Invitrogen). In control sections, primary antibodies were omitted from the staining procedure. Microscopy was performed using a Nikon TE2000 fluorescence microscope. Three samples from each type of matrix were examined under the microscope.
2.7 Reverse transcriptase polymerase chain reaction analysis of hNSCs neural differentiation
RNA was isolated from ten separate fibrous scaffolds using Trizol (Invitrogen) following standard protocols. RNA concentrations were obtained using a Nanodrop 2000 spectrophotometer. One micorgram of RNA was reverse-transcribed to cDNA using the High Capacity RNA-to-cDNA Kit (Applied Biosystems). Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed on the cDNA using a Step One Plus system (Applied Biosystems) and TaqMan primers and reagents (Applied Biosystems). Relative expression levels of each gene compared to GAPDH were determined using the 2−ΔΔCt method. The reference condition chosen was 2D cell culture plate; all data were normalized to this condition.
2.8 Statistical analysis
All data are represented as the mean ± the standard deviation. Statistical significance was determined using a two-tailed, paired t test (Excel), where values of p < 0.05 were considered statistically significant.
3. Results and Discussion
3.1 Characterization of fiber diameters
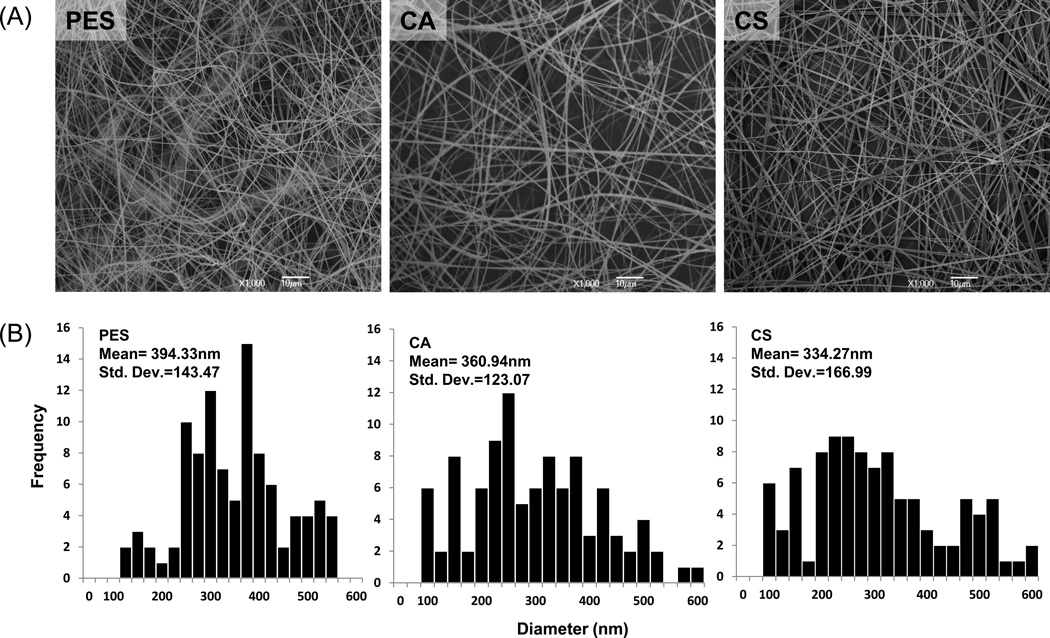
We constructed electrospun PES, CA, and CS nanofibrous scaffold based on results from the Mao group, which previously optimized fiber diameter to influence neural cell differentiation and proliferation (Christopherson, Song & Mao, 2009). As shown from the SEM micrographs presented in Figure 1, bead-free and continuous PES, CA, and CS electrospun fiber substrates with similar range diameters of 394±143 nm, 360±123 nm, and 334±166 nm were prepared using the optimized protocols described in the Method section of this report.
Figure 1. Characterization of electrospun nanofibers.
(A) Scanning electron microscopy (SEM) characterization of electrospun fiber matrices prepared from 17% PES w/0.5% OTAB in DMSO, 15% CA in 2/1 acetone/DMAc, and 3.5% CS in 7/3 TFA/DCM are shown; the images were acquired at high magnification (1,000 ×). Scale bars are 10 µm. (B) Histograms of fiber diameters, obtained from a minimum of 100 measurements, are shown for fibers prepared from each material.
3.2 Characterization of PC12 cell cytotoxity and proliferation on nanofiber scaffolds
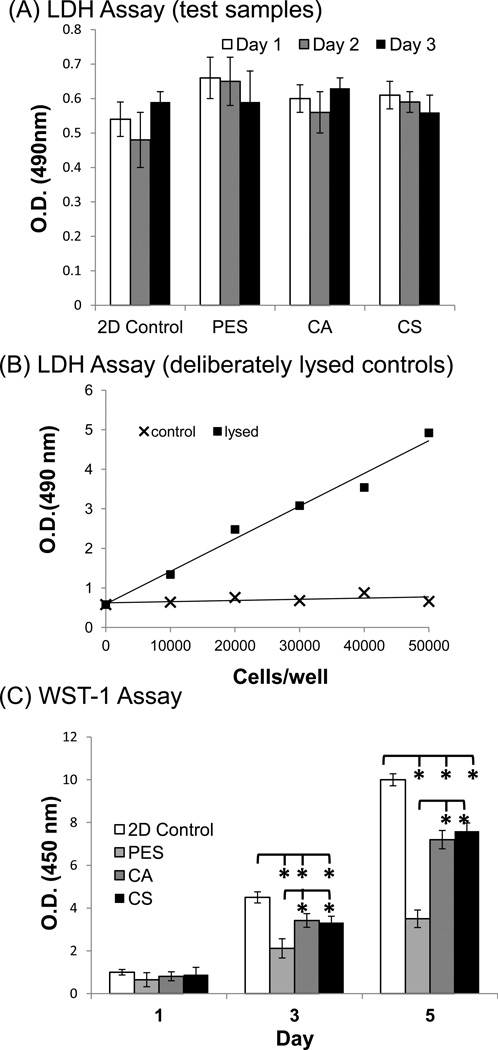
The potential cytotoxicity of the electrospun PES, CA, and CS scaffolds was investigated by measuring the presence of lactose dehydrogenase (LDH), a cytoplasmic enzyme released from injured cells in culture media. As shown in Fig. 2A, LDH levels from PC12 cells cultured on 2D culture plate and 3D nanofibrous matrices were comparable and were maintained at similar levels throughout the three-day culture period. Although the number of cells present at various time points in this experiment was not carefully characterized (10,000 were seeded in each case), a comparison with fully lysed cells over a range of increasing cell numbers (Fig. 2B) showed that the assay was linear up to 50,000 cells (with OD values ranging from ~0.5 to 6) and the signal did not measurably increase for non-lysed cells (remaining at an OD value of ~0.5). Therefore, because this assay did not result in a measurable increase in OD values over the background level for PC12 cells grown on the nanofibrous scaffolds under any of the conditions, we concluded that neither the materials nor topographic features of the nanofibers were cytotoxic.
Figure 2. Cellular compatibility assessment of PC12 cells on 2D tissue culture plates and PES, CA, and CS nanofibrous matrices in proliferation media.
(A) Cytotoxicity was measured using the lactose dehydrogenase (LDH) assay over a three day period. (B) Quantification of deliberately lysed cells with the LDH assay verified that the results shown in panel A were both in the linear range of the assay and that none of the conditions resulted in measurable toxicity higher than that experienced by untreated controls. (C) The proliferation of PC12 cells was estimated using the WST-1 assay after 1, 3, or 5 days of growth on each substrate. Asterisks denote statistical significance (p < 0.05) by comparing (i) each type of nanofiber with collagen-coated 2D tissue culture plastic and (ii) the two polysaccharide-based nanofibers (i.e., CA and CS) with the PES scaffolds. Bars represent mean ±standard deviation (n =5).
Furthermore, metabolic activity measured by the WST-1 assay (Fig. 2C) indicated that cell numbers on all three nanofiber mats increased more than 2.5 times the initial seeded cell population throughout the cell culture period; this result is consistent with the lack of cytotoxicity discussed above. However, the situation was more complex insofar as all of the samples grown on nanofibers exhibited reduced proliferation compared to collagen-coated 2D tissue culture plastic-grown samples. A further nuance was that the two samples grown on polysaccharide nanofibers (i.e., the CA and CS samples) experienced statistically significantly increased proliferation compared to PES (as also shown in Fig. 2C). These results indicated that, as predicted in the Introduction, both the chemical composition of the material and the topography of the surface influence the biological fate of neural cells.
3.3 PC12 cell differentiation on nanofiber scaffolds
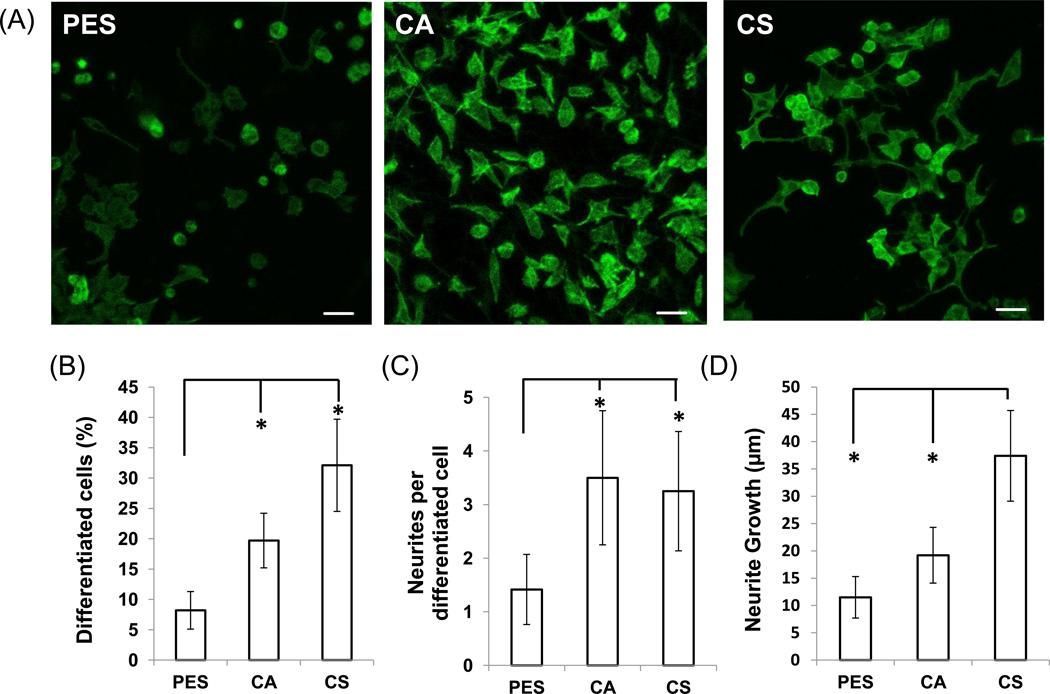
PC12 cells, a clonal rat pheochromocytoma line (Greene & Tischler, 1976) that responds to nerve growth factor (NGF) by extending long nerve-like processes and expressing markers of neural differentiation, have been a useful model system for studying the differentiation of nerve cells. In this study, PC12 differentiation on fibrous matrices was evaluated 5 days after seeding by staining the cells with phalloidin to observe actin filaments (Fig. 3A). On the PES substrate, most cells maintained a round shape while only a few exhibited a bipolar shape and formed neurite extensions. In contrast, on the CA substrate, more cells presented a bipolar shape and projected short neurites and on the CS substrates the greatest proportion of PC12 cells exhibited a neuronlike shape with long neurites.
Figure 3. Differentiation of PC-12 cells after 5 days of incubation on PES, CA, or CS fibers.
(A) Images of actin-stained PC-12 cells differentiated for 5 days on PES, CA, and CS fibers (scale bar, 20 µm). Endpoints relevant to differentiation were then evaluated; these included determining: (B) the number of differentiated cells (cells with at least one neurite with a length equal to or greater than the diameter of the cell body were counted and expressed as a percentage of the total number of cells in a field), (C) the number of neurites per differentiated cell, and (D) neurite growth (the length of the longest neurite was determined for cells with at least one identified neurite). Asterisks denote statistical significance (p < 0.05) by comparison with other fiber substrates as indicated. Bars represent mean ± standard deviation (n=5).
PC12 cell differentiation was quantitatively evaluated by first measuring the number of cells with at least one neurite outgrowth equal in length or longer than the cell body diameter (Fig. 3B); such cells were defined to be “differentiated.” Both polysaccharide substrates supported significantly higher differentiation than PES (20% for CA and 32% for CS vs. 8% for PES). Next, an analysis of the number of neurites per differentiated cells again showed that both polysaccharide-derived fibers induced more neurites per cell (CA at 3.5 ± 1.3 and CS at 3.3±1.1, which were statistically identical) compared to the PES fibers (1.4± 0.6; *p < 0.05) (Fig. 3C).
Contrary to the results presented so far that show an enhanced effect for both polysaccharide scaffolds compared to PES, the results shown in Fig. 3D demonstrate an added benefit for CS over CA scaffolds in promoting neurite outgrowth. Specifically, the cells cultured on PES, CA, and CS fiber matrices had neurites with longest mean lengths of 11.5 ± 3.8 µm, 19.2 ± 5.1 µm, and 37.4 ± 8.3 µm, respectively. The cells grown on the CS fibers had significantly longer neurites than cells grown on either the PES or CA fibers (*p < 0.05) while there was no significant difference in the mean neurite length between the cells grown on PES and CA fibers (although the polysaccharide scaffold showed a trend towards longer projections). These results demonstrated that not only were polysaccharides beneficial as materials used for scaffold formation, but that CS nanofibers provided an added benefit in the form of enhanced neurite outgrowth over CA nanofibers.
3.4 Effect of fibrous scaffolds on hNSC proliferation
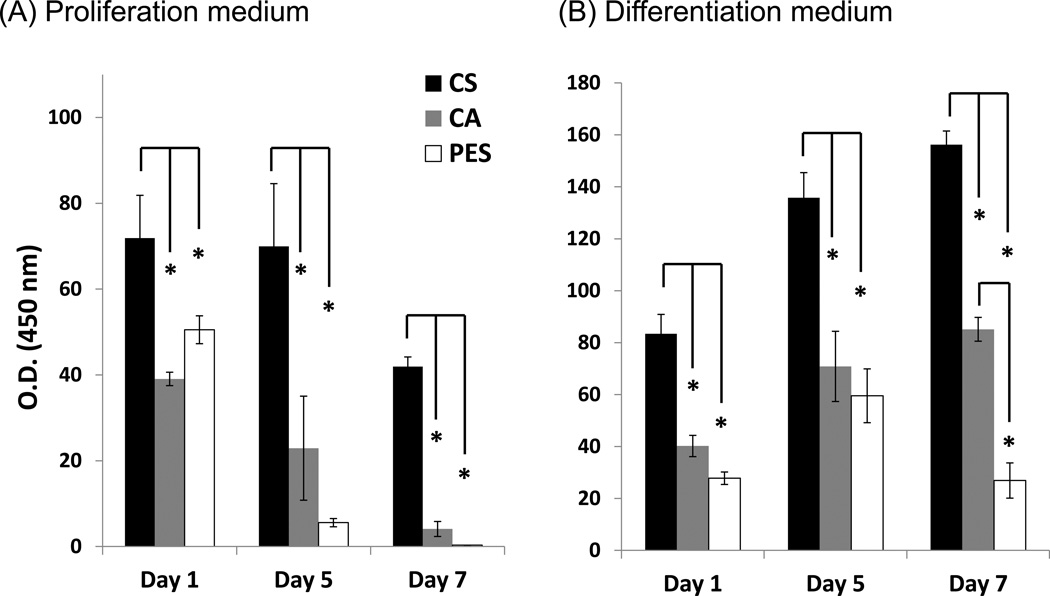
The WST-1 assay was used to evaluate hNSC cell viability in proliferation (Fig. 4A) and then separately in differentiation (Fig. 4B) media. Both assays showed that the CS nanofibrous scaffolds supported cell growth than their CA or PES counterparts (2D cultures were arbitrarily defined as the “100%” metric in these assays). The hNSC cells revealed lower proliferation on all of the investigated fibrous scaffolds compared to 2D controls in proliferation media up to 7 days with the relative number of cells compared to the control continually dropping over time. After 7 days, the only nanofiber-grown cells with substantial proliferation were those incubated on CS scaffolds and even these had fewer than 50% of cells found in the 2D controls; this number, however, was much higher than the cells remaining on the CA (4.1±0.7%) and PES fibers (0.3%). Remarkably, in ENStem-A™ Neuronal Differentiation Medium, higher cell numbers of hNSCs were evident in cultures grown on CS nanofibers by days 5 and 7, which were 135.8±7.5% and 156.2±5.3% higher compared to 2D controls; growth on CS was even more impressive when compared with PES and CA nanofibers, both of which lagged behind the 2D controls. In more detail, there was no significant difference between PES and CA scaffolds at day 5 although by day 7 more cells were present on the CA compared to PES scaffolds (85.1±4.6% for CA vs. 26.9±6.8% for PES). Overall, these results mirror the PC12 findings in that both sets of polysaccharide-derived scaffolds showed benefits over PES nanofibers and 2D growth surfaces in certain assays while in other assays, the CS-derived materials provided added benefits over the CA-based scaffolds.
Figure 4. Proliferation of hNSCs cultured on CS, CA, and PES fibrous scaffolds.
The cells were grown in (A) proliferation medium and (B) ENStem-A™ Neuronal Differentiation Medium and analyzed after 1, 5, and 7 days. Optical density (O.D.) readings from WST-1 assays were used to estimate cell numbers; in all experiments the signal obtained from cells grown on each type of nanofibrous scaffold were normalized to the signal from cells grown on 2D controls (which were laminin-coated tissue culture plastic in all cases and that arbitrarily were set at a value of 100) for the same number of days. Asterisks denote statistical significance (p < 0.05) for the comparisons made between various nanofibrous scaffolds, as indicated. Error bars represent mean ± standard deviation (n =3).
3.5 Fibrous CS scaffolds enhance hNSC differentiation
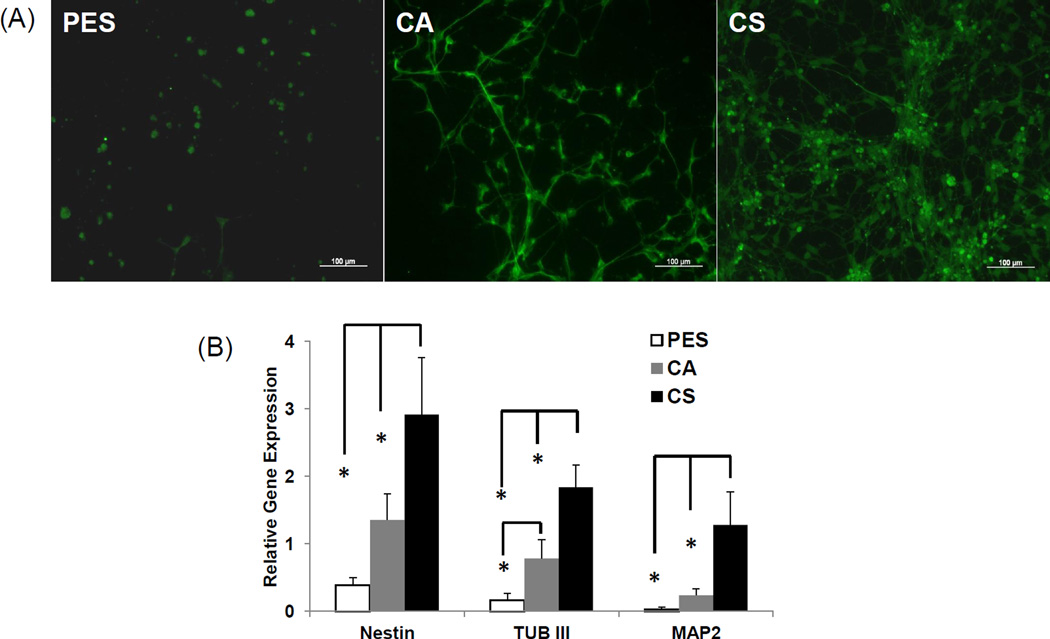
Immunocytochemical analysis (Fig. 5A) indicated that the differentiation of hNSCs cultured on polysaccharide-based nanofiber scaffolds were higher than for cells on PES substrate, where more cells remained in the undifferentiated state and fewer neurons were observed. The morphology of hNSCs grown on the three types of nanofibers suggested that the CS scaffolds preferentially promoted differentiation of the cells into mature neurons with long axons. However, hNSC differentiation is typically based on patterns of gene expression, not on morphology (Cooke et al., 2010), leading us to evaluate mRNA levels by qRT-PCR as summarized in Fig. 5B for nestin (a neural stem cell marker), β-tubulin (early neurons marker), and MAP2 (a mature neuronal marker). In all cases, the level of expression for these genes was substantially higher for cells incubated on the CS nanofibers than for the other conditions, again illustrating the superiority of this material for neural cell differentiation; for β-tubulin the cell grown on CA also showed a statistically significant up-regulation compared to the PES nanofibers.
Figure 5. Neuronal differentiation of hNSCs cultured on CS, CA, and PES scaffolds.
(A) Immunofluorescent images of cells cultured on PES, CA, CS for 7 days in differentiation media. The phenotypic differentiation of hNSCs was assessed by immunocytochemistry for TUJ1, a class III β-tubulin protein that marks early neurons ((Campbell, Sampathkumar, Weier & Yarema, 2007)green). (B) RT-PCR for neural differentiation markers, nestin (a neural stem cell marker), β-tubulin (early neuron marker), and MAP2 (a mature neuronal marker) by day 7. The reference condition was widely used laminin coated, 2D tissue culture plastic; all data were normalized to this condition. Asterisks denote statistical significance (p < 0.05) by comparison with the other fiber substrates, as indicated. Bars represent mean ± standard deviation (n =3).
4. Conclusions
This study demonstrated that a combination of the chemical features and the topography of electrospun nanofibers influence the fate of neuronal precursor cells. From a chemical point of view, many polysaccharide candidates for construction of electrospun nanofibers suffer from limitations. For example, cellulose (e.g., CA used in this study) is an excellent structural material (indeed, is one of the most abundant structural biomacromolecules used throughout nature and industry) but lacks amino groups found in most mammalian glycosoaminoglycans (GAGs) and other polysaccharides such as polysialic acid (PSA). PSA in particular is an intriguing amino-bearing polysaccharide shown to have benefits for neural guidance in tissue engineering applications (Haile et al., 2006), but PSA fibers have poor stability in aqueous milieu limiting their use to hydrogel formats (Haile et al., 2008) or immobilization on glass (Steinhaus et al., 2010). Similarly other GAGs, without substantial chemical modification such as the introduction of stabilizing crosslinkers, are not suitable for nanofiber construction.
Based on these considerations, CS – which is an abundant natural material – is one of the few facile options for creating electrospun nanofibers from amine-bearing polysaccharides. This study verifies that the desirable attributes of CS in other formats (e.g., hydrogels) are maintained, and indeed can be enhanced further for nerve cell engineering, when using this polysaccharide in the form of electrospun nanofibers. An important benefit for the CS-derived scaffolds compared to either CA or PES is that they support a combined increase in both proliferation and differentiation during the early stages of neuronal differentiation as shown in this study. Furthermore this effect was qualitatively observed across species, occurring in both rat and human cells thereby indicating that the utilization of CS polysaccharides in electrospun nanofibers has broad potential for tissue engineering purposes.
Highlights for review.
Prepared nature polysaccharides nanofibers and synthetic polymer polyethersulfone nanofibers with similar diameters
Compared the biological properties between nature and synthetic polymers
Characterized neural differentiations for human neural stem cell and rat PC12 cell lines on different nanofiber scaffolds
The cell fate was determined by both the chemical nature of the material and the 3D structural architecture
Amino-bearing polysaccharide shown to have benefits for neural guidance in tissue engineering applications
Acknowledgments
Funding for this study was provided by the National Institute for Biomedical Imaging and Bioengineering (EB 005692).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ao Q, Wang A, Cao W, Zhang L, Kong L, He Q, Gong Y, Zhang X. Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro. Journal of Biomedical Material Research Part A. 2006;77A(1):11–18. doi: 10.1002/jbm.a.30593. [DOI] [PubMed] [Google Scholar]
- Campbell CT, Sampathkumar S-G, Weier C, Yarema KJ. Metabolic oligosaccharide engineering: perspectives, applications, and future directions. Molecular Biosystems. 2007;3(3):187–194. doi: 10.1039/b614939c. [DOI] [PubMed] [Google Scholar]
- Christopherson GT, Song H, Mao H-Q. The influence of fiber diameter of electrospun substrates on neural stem cell differentiation and proliferation. Biomaterials. 2009;30(4):556–564. doi: 10.1016/j.biomaterials.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Cooke MJ, Zahir T, Phillips SR, Shah DS, Athey D, Lakey JH, Shoichet MS, Przyborski SA. Neural differentiation regulated by biomimetic surfaces presenting motifs of extracellular matrix proteins. Journal of Biomedical Material Research Part A. 2010;93(3):824–832. doi: 10.1002/jbm.a.32585. [DOI] [PubMed] [Google Scholar]
- Das KP, Freudenrich TM, Mundy WR. Assessment of PC12 cell differentiation and neurite growth: a comparison of morphological and neurochemical measures. Neurotoxicology and Teratology. 2004;26(3):397–406. doi: 10.1016/j.ntt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Du J, Che P-L, Wang Z-Y, Aich U, Yarema KJ. Designing a binding interface for control of cancer cell adhesion via 3D topography and metabolic oligosaccharide engineering. Biomaterials. 2011;32(23):5427–5437. doi: 10.1016/j.biomaterials.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Hsieh Y-L. Cellulose/chitosan hybrid nanofibers from electrospinning of their ester derivatives. Cellulose. 2009;16(2):247–260. [Google Scholar]
- Du J, Yarema KJ. Carbohydrate engineered cells for regenerative medicine. Advanced Drug Delivery Reviews. 2010;62(7–8):671–682. doi: 10.1016/j.addr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freier T, Koh HS, Kazazian K, Shoichet MS. Controlling cell adhesion and degradation of chitosan films by N-acetylation. Biomaterials. 2005;26(29):5872–5878. doi: 10.1016/j.biomaterials.2005.02.033. [DOI] [PubMed] [Google Scholar]
- Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Bio-functionalized PCL nanofibrous scaffolds for nerve tissue engineering. Materials Science and Engineering: C. 2010;30(8):1129–1136. [Google Scholar]
- Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proceedings of the National Academy of Science of the United States of America. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile Y, Berski S, Dräger G, Nobre A, Stummeyer K, Gerardy-Schahn R, Grothe C. The effect of modified polysialic acid based hydrogels on the adhesion and viability of primary neurons and glial cells. Biomaterials. 2008;29(12):1880–1891. doi: 10.1016/j.biomaterials.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Haile Y, Haastert K, Cesnulevicius K, Stummeyer K, Timmer M, Berski S, Dräger G, Gerardy-Schahn R, Grothe C. Culturing of glial and neuronal cells on polysialic acid. Biomaterials. 2006;28(6):1163–1173. doi: 10.1016/j.biomaterials.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Hakomori S-I. The glycosynapse. Proceedings of the National Academy of Science of the United States of America. 2002;99(1):225–232. doi: 10.1073/pnas.012540899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AJ, Tudor D, Nowell MA, Caterson B, Hughes CE. Chondroitin sulfate sulfation motifs as putative biomarkers for isolation of articular cartilage progenitor cells. Journal of Histochemistry & Cytochemistry. 2008;56:125–138. doi: 10.1369/jhc.7A7320.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Liao S, Quan D, Ma K, Chan C, Ramakrishna S, Lu J. Synergistic effects of electrospun PLLA fiber dimension and pattern on neonatal mouse cerebellum C17.2 stem cells. Acta Biomaterialia. 2010;6(8):2960–2969. doi: 10.1016/j.actbio.2010.02.039. [DOI] [PubMed] [Google Scholar]
- Hu N, Wu H, Xue C, Gong Y, Wu J, Xiao Z, Yang Y, Ding F, Gu X. Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials. 2013;34(1):100–111. doi: 10.1016/j.biomaterials.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Hurtado A, Cregg JM, Wang HB, Wendell DF, Oudega M, Gilbert RJ, McDonald JW. Robust CNS regeneration after complete spinal cord transection using aligned poly-L-lactic acid microfibers. Biomaterials. 2011;32(26):6068–6079. doi: 10.1016/j.biomaterials.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim IY, Seo SJ, Moon HS, Yoo MK, Park IY, Kim BC, Cho CS. Chitosan and its derivatives for tissue engineering applications. Biotechnology Advances. 2008;26(1):1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kim Y-t, Haftel VK, Kumar S, Bellamkond RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117–3127. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29(26):3574–3582. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- Krick K, Tammia M, Martin R, Höke A, Mao H-Q. Signaling cue presentation and cell delivery to promote nerve regeneration. Current Opinion in Biotechnology. 2011;22(5):741–746. doi: 10.1016/j.copbio.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Kumar MNVR. A review of chitin and chitosan applications. Reactive & Functional Polymers. 2000;46(1):1–27. [Google Scholar]
- Leach JB, Brown XQ, Jacot JG, A DP, Wong JY. Neurite outgrowth and branching of PC12 cells on very soft substrates sharply decreases below a threshold of substrate rigidity. Journal of Neural Engineering. 2007;4(2):26–34. doi: 10.1088/1741-2560/4/2/003. [DOI] [PubMed] [Google Scholar]
- Lim SH, Mao H-Q. Electrospun scaffolds for stem cell engineering. Advanced Drug Delivery Reviews. 2009;61(12):1084–1096. doi: 10.1016/j.addr.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Lin K, Chua K-N, Christopherson GT, Lim S, Mao H-Q. Reducing electrospun nanofiber diameter and variability using cationic amphiphiles. Polymer. 2007;48(21):6384–6394. [Google Scholar]
- Pfister LA, Papaloïzos M, Merkle HP, Gander B. Hydrogel nerve conduits produced from alginate/chitosan complexes. Journal of Biomedical Materials Research Part A. 2007;80A(4):932–937. doi: 10.1002/jbm.a.31052. [DOI] [PubMed] [Google Scholar]
- Prabhakaran MP, Venugopal JR, Chyan TT, Hai LB, Chan CK, Lim AY, Ramakrishna S. Electrospun biocomposite nanofibrous scaffolds for neural tissue engineering. Tissue Engineering Part A. 2008;14(11):1787–1797. doi: 10.1089/ten.tea.2007.0393. [DOI] [PubMed] [Google Scholar]
- Sangsanoh P, Supapho P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules. 2006;7(10):2710–2714. doi: 10.1021/bm060286l. [DOI] [PubMed] [Google Scholar]
- Scanga VI, Goraltchouk A, Nussaiba N, Shoichet MS, Morshead CM. Biomaterials for neural-tissue engineering — Chitosan supports the survival, migration, and differentiation of adult-derived neural stem and progenitor cells. Canadian Journal of Chemistry. 2010;88(3):277–287. [Google Scholar]
- Steinhaus S, Stark Y, Bruns S, Haile Y, Scheper T, Grothe C, Behrens P. Polysialic acid immobilized on silanized glass surfaces: a test case for its use as a biomaterial for nerve regeneration. Journal of Material Science: Materials in Medicine. 2010;21(4):1371–1378. doi: 10.1007/s10856-009-3981-0. [DOI] [PubMed] [Google Scholar]
- Supaphol P, Sangsanoh P. Stability improvement of electrospun chitosan nanofibrous membranes in neutral or weak basic aqueous solutions. Biomacromolecules. 2006;7(10):2710–2714. doi: 10.1021/bm060286l. [DOI] [PubMed] [Google Scholar]
- Tirtsa Ehrenfreund-Kleinman JG, Domb AJ. Polysaccharides scaffolds for tissue engineering. In: Ma X, Peter JE, editors. Scaffolding in Tissue Engineering. Boca Raton, Florida: Taylor & Francis; 2006. [Google Scholar]
- Wang Z, Che P-L, Du J, Ha B, Yarema KJ. Static magnetic field exposure reproduces cellular effects of the Parkinson's disease drug candidate ZM241385. PLoS ONE. 2010;5(11):e13883. doi: 10.1371/journal.pone.0013883. [DOI] [PMC free article] [PubMed] [Google Scholar]