Abstract
Cognitive impairment and especially memory disruption is a major complicating feature of the epilepsies. In this review we begin with a focus on the problem of memory impairment in temporal lobe epilepsy. We start with a brief overview of the early development of knowledge regarding the anatomic substrates of memory disorder in temporal lobe epilepsy, followed by discussion of the refinement of that knowledge over time as informed by the outcomes of epilepsy surgery (anterior temporal lobectomy) and the clinical efforts to predict those patients at greatest risk of adverse cognitive outcomes following epilepsy surgery. These efforts also yielded new theoretical insights regarding the function of the human hippocampus and a few examples of these insights are touched on briefly. Finally, the vastly changing view of temporal lobe epilepsy is examined including findings demonstrating that anatomic abnormalities extend far outside the temporal lobe, cognitive impairments extend beyond memory function, with linkage of these distributed cognitive and anatomic abnormalities pointing to a new understanding of the anatomic architecture of cognitive impairment in epilepsy. Challenges remain in understanding the origin of these cognitive and anatomic abnormalities, their progression over time, and most importantly, how to intervene to protect cognitive and brain health in epilepsy.
Introduction
Epilepsy is a prevalent neurological disorder affecting an estimated 50 million people worldwide1. Although defined by the presence of recurrent seizures, epilepsy can exert an adverse impact on important aspects of day-today function including cognition, emotional-behavioral status, and social adaptive behaviors; these problems referred to as the comorbidities of epilepsy. At the recent conference sponsored by the National Institutes of Neurological Diseases and Stroke (Curing Epilepsy 2007: Translating Discoveries into Therapies), the prevention and reversal of the comorbidities of epilepsy were identified as a major new benchmark area for research and care. Here we will focus on arguably the most problematic of these comorbidities—cognitive impairment, and we will do so focusing on temporal lobe epilepsy (TLE), the most common form of focal epilepsy. The cognitive complications of epilepsy can be heterogeneous, but especially problematic is episodic memory impairment, a signature cognitive deficit in TLE. In this review we will first focus on the development of knowledge regarding memory impairment in epilepsy, then overview the effects of treatment including surgery on this cognitive system, and conclude with a review of recent insights into the underlying neurobiology of temporal lobe epilepsy and the implications of these findings for cognition and future research.
Epilepsy, memory and the hippocampus: early insights
The first empirical studies of cognition in epilepsy began to appear in the early 1900s with a focus on the relationship between intelligence and clinical characteristics of the patients’ epilepsy (e.g., age of onset, seizure frequency)2, 3. As methods of assessment and understanding of human cognition developed, interest in specific abilities such as memory ensued. Understanding of the neurobiology of disordered cognition and memory in epilepsy was accelerated by the development of organized epilepsy surgery programs. Collaboration with neuropsychology was key from the inception of these programs which involved Donald Hebb and Brenda Milner at the Montreal Neurological Institute, Ward Halstead at the University of Illinois in Chicago, and Victor Meyer at the Guy’s-Maudsley Hospital in London 4–8.
The earliest surgeries for temporal lobe epilepsy performed by Penfield and Jasper in Montreal and Bailey and Gibbs in Chicago largely avoided the hippocampal complex, in part due to the animal experiments of Kluver and Bucy demonstrating the deleterious behavioral consequences of bilateral temporal lobe resection. However, it became apparent that that the mesial temporal structures were critically involved in the epileptogenic network and in 1952 Penfield and Baldwin advised removal of the “deepest, most inferior and mesial portion” of the temporal lobe 5, 9 which became the accepted approach beginning in the 1950s 10.
At the time the temporal lobe was said to be concerned with “many known functions,” including hearing and sight, but the case of its mesial aspect involved “a host of unknowns” 11. As resection of the mesial temporal structures became a standard practice, the principal function of the hippocampus was elucidated8, 12, 13 due to two factors.
First was the unanticipated global amnesia suffered by a small number of patients following surgery. Milner and Penfield 14 described two cases that experienced a severe recent memory impairment following unilateral temporal lobectomy. They hypothesized that these patients had (undetected) contralateral (non-surgical) damage in the hippocampus, and the effect of resection of the ipsilateral epileptogenic hippocampus was to produce bilateral hippocampus damage. Consistent with this proposal, the serious memory consequences of bilateral temporal lobectomy was reported a few years later. Scoville & Milner15 presented the memory outcome findings for HM (and seven other patients) following bilateral temporal lobe resection. An extensive anterograde memory loss ensued with concomitant preservation of overall intellectual functioning and language ability. This profile became regarded as the prototypical presentation of an amnesic syndrome produced by bilateral temporal lobe damage. Extensive study of HM over the next 50 years produced important insights into the role of the hippocampus and temporal lobe for memory and a conceptual framework to understand the neural architecture of diverse memory systems16.
Second was the less severe but common problem of memory decline after anterior temporal lobectomy (ATL)17, 18, changes that remain a continuing concern. Milner19 described “material-specific” memory difficulties and the “clearest instance” (p. 175) was said to occur in left TLE patients in whom verbal memory could be impaired before surgery and became enduringly worse after left ATL, whereas so-called nonverbal memory was expected to be intact. A corresponding, if less robust, selective vulnerability to nonverbal memory impairment characterized patients with right TLE and temporal lobectomy. The impact of this early work was profound. The material-specific model of memory served as a foundation of research and practice far outside the narrow field of epilepsy and epilepsy surgery, influencing generations of investigators.
Refining understanding of memory change in epilepsy and epilepsy surgery
It is now generally acknowledged that 30–60% of left ATL patients experience a significant decline in verbal memory ability13, 20–24. But despite these robust trends before and especially after epilepsy surgery, a persisting finding has been variability in memory outcome following a standard surgical approach (Figure 125). While, on average, verbal memory outcome is worse following left compared to right ATL, many left ATL patients show no change or even postoperative improvement. In contrast, right ATL patients show postoperative improvement on average, but some exhibit decline as well. Similar variability in the context of a less robust effect for visual memory change following right ATL has been demonstrated as well26. Determining the factors that underlie this variability has been a critical issue in the role of ATL in treating patients with chronic TLE and the development of presurgical protocols to assess the risk of adverse memory outcome following surgery.
Figure 1.
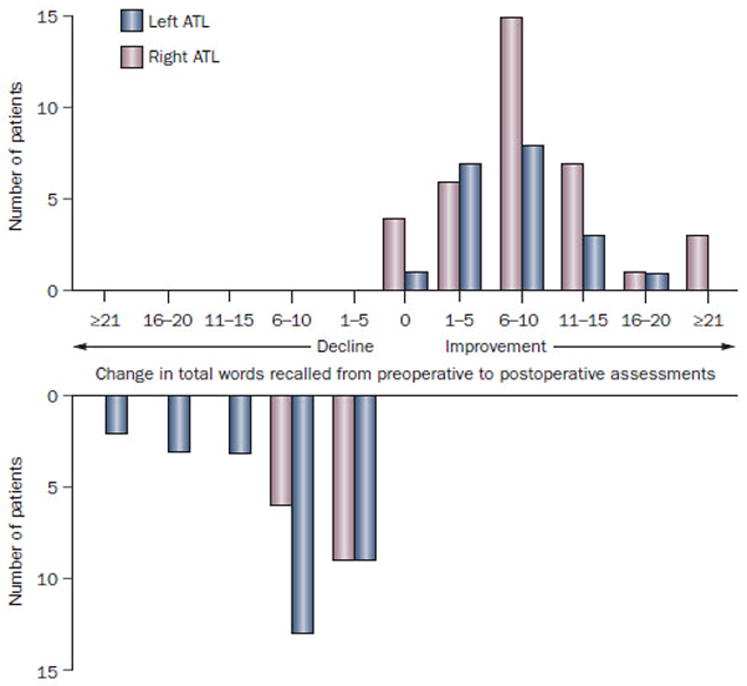
Variability in verbal memory change following left and right anterior temporal lobectomy. Preoperative to postoperative changes in verbal learning performance (total words recalled on California Verbal Learning Test) in 100 patients who underwent left or right anterior temporal lobectomy. The dependent variable is the number of words recalled from a 16-item word list across five learning trials. Abbreviation: ATL, anterior temporal lobectomy.
How memory is assessed makes a difference
One source of outcome variability relates to the heterogeneity of memory tests employed. As critically reviewed by Saling27, list learning, paragraph recall, and forming associations between related and unrelated word pairs differ in their semantic demands and the associated underlying neurobiology required for successful task performance and should not be considered equivalent measures of “verbal memory.” Even within similar tasks such as list learning, there are differences in semantic relationships among the words used as stimuli that are thought to contribute to different sensitivities to left temporal lobe dysfunction28–30.
What is resected makes a difference
Another possible cause of variable memory outcome was suggested by studies of the relationship between preoperative memory performance and the neuropathological status of the resected hippocampus. Rausch et al.31 found that the degree of neuron loss in the left hippocampus was associated with preoperative performance on an unrelated word paired-associate learning task and similar findings were reported by others32–33. Given that relationship, it was reasonable to expect that the integrity of the to-be-resected hippocampus would predict the risk of postoperative memory change—the risk greatest in those with less hippocampal cell loss and presumably a more functionally intact hippocampus, with less risk in those with the most cell loss and the least functionally intact hippocampus. Those assumptions were upheld. Findings from the early 1990’s confirmed that the risk of postoperative memory decline was associated with the structural integrity (or lack thereof) of the to-be-resected hippocampus34–37. The memory changes can be quite substantial. Figure 2 depicts the degree of change in rote verbal list learning performance apparent following resection of a left hippocampus with minimal or no sclerosis (top panel) compared to a left hippocampus with moderate to severe sclerosis (bottom panel). This relationship was diametrically opposed to the then conventional wisdom that the risk of postoperative memory decline was associated with the functional integrity of the contralateral hippocampus
Figure 2.
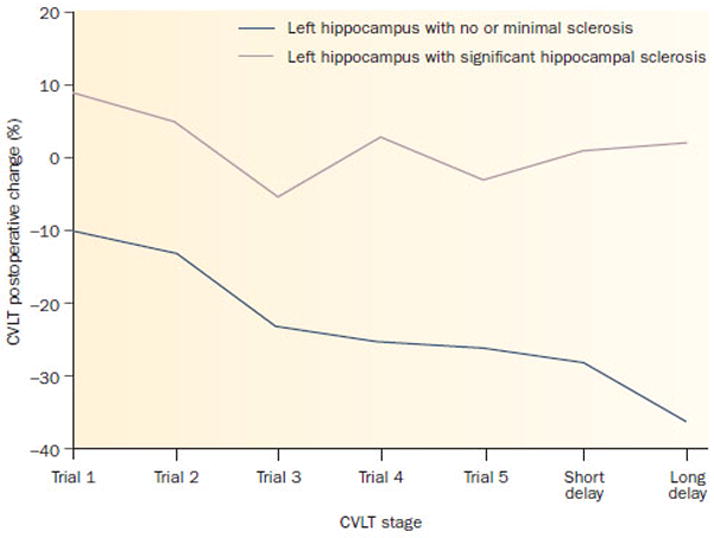
Verbal memory change following left anterior temporal lobectomy in relation to hippocampal pathology. Resection of left hippocampus with no or minimal sclerosis results in significant preoperative to postoperative decline in trial-to-trial learning. Long-delay recall is ≈35% lower compared with preoperative performance. Resection of left hippocampus with significant hippocampal sclerosis has a minimal effect on postoperative trial-to-trial learning compared to preoperative performance. All patients were confirmed to be left hemisphere speech dominant by the Wada test. Abbreviation: CVLT, California Verbal Learning Test.
A paradigm shift
In a major theoretical contribution, Chelune20 integrated the findings to date and contrasted a new model of ipsilateral hippocampal adequacy versus the classic model of contralateral functional reserve. The hippocampal adequacy model inferred that the functional status of the surgical hemisphere and hippocampus prior to surgery was critical to determining memory outcome. Individuals with a more intact hippocampus were at greater risk for memory decline because (relatively) functional tissue has been removed. In contrast, the functional reserve model emphasized the status of the contralateral non-surgical hippocampus. An intact contralateral hippocampus capable of subserving memory could offset the impact of resection of the ipsilateral (surgical) hippocampus. A wide range of findings have been found to be consistent with the hippocampal adequacy model.
Wada Test
In response to the concern about producing a severe global memory impairment following unilateral ATL, Milner, Branch, & Rausmussen38 developed intracarotid amobarbitol testing as a means of assessing the memory ability of the contralateral hemisphere. The test, developed by Juhn Wada39, was already used to determine language dominance, and this approach was extended to assessing memory ability before surgery. The Wada Test provides an opportunity to assess the functional status of both the ipsilateral and contralateral hippocampus independently by transient hemispheric anesthesia.
The presence or absence of a marked memory asymmetry score is a clear predictor of verbal memory outcome after left ATL 40, 41,42,43. Preoperative Wada Test memory asymmetry (impaired ipsilateral and intact contralateral memory) has been found to be associated with side of ictal EEG onset 44, hippocampal atrophy on MRI45, 46, and neuronal loss in resected hippocampus33. However, there are factors that can affect this relationship including atypical language representation 47,48,49, the types of memory stimuli used50, and the type of neuropsychological memory outcome measure [better for predicting list learning than prose recall change51. The Wada Test has been less useful for predicting non-verbal memory outcome following right ATL 52, in part a likely reflection of the difficulty finding memory measures linked to a consistent right hemisphere effect 53,54.
Age of onset of recurrent seizures
Several studies have identified age of recurrent seizure onset to be a predictor of ATL memory outcome. Earlier age of seizure onset is associated with poorer memory before ATL and less decline after ATL, while later onset of epilepsy is associated with better preoperative memory and a greater postoperative decline 55. The reason for this relationship is most likely due to the increased probability of hippocampal pathology with earlier onset of epilepsy 56, which in turn is related to functional adequacy.
Preoperative memory performance
Patients with better preoperative memory performance show greater decline in memory following ATL 57,25, this is believed to reflect the association with the degree of hippocampal sclerosis—less underlying sclerosis associated with better preoperative performance and thus greater risk of postoperative decline. TLE subjects without hippocampal sclerosis are more likely to have a functional hippocampus which subserves stronger presurgical memory performance. Resection will remove functional tissue leading to a significant memory decline.
Neuroimaging predictors
The advent and refinement of neuroimaging techniques over the past 20 years (MRI, PET, fMRI) has provided a new opportunity to identify alternative approaches for predicting memory outcome after ATL58.
Hippocampal atrophy
The absence of hippocampal atrophy on MRI is significantly associated with better pre-ATL verbal memory performance and poorer pre-post ATL verbal memory outcome 59, 60.
FDG-PET hypometabolism
Only a few studies have examined pre to postoperative memory outcome using FDG-PET. Griffith et al.61 found that absence of preoperative left temporal lobe hypometabolism was associated with poorer verbal memory outcome following left ATL. However a recent study failed to find a significant relationship between preoperative FDG-PET hippocampus asymmetry and memory outcome 62.
fMRI
The rationale underlying the use of fMRI is that the degree of presurgical hippocampal activation reflects the functional adequacy of the structure. Richardson et al.63 showed left TLE patients with hippocampal sclerosis to demonstrate less activation in the region of the left hippocampus than controls during a verbal encoding task. Subsequent studies showed that increased activation in the ipsilateral hippocampus before surgery or asymmetry in activity between the left and right hippocampus was associated with a poorer memory outcome64–67. Bonelli et al.68 recently reported that increased left hippocampal activity in left TLE was associated with better pre-surgery memory performance and greater decline following ATL on a word list learning task. For the right TLE group, increased right hippocampal activity for a face encoding task was associated with better pre-surgical memory performance on learning a set of designs and more decline following ATL. However, hippocampal activation may not necessarily be the best verbal memory outcome predictor, with language lateralization superior to scene encoding in a large and carefully investigated series69, 70.
Multiple methodological issues remain to be resolved including the optimal activation tasks to use, which fMRI activation measure is the best predictor, the impact of hippocampus pathology, and the predictive incremental value of fMRI activity in relation to other predictor variables.
Mutivariate prediction
Many studies examined the impact of a single predictor in relation to memory outcome. Informative are investigations using a multivariate approach that makes use of multiple, non-redundant sources of information71. Stroup et al.72 reported that a combination of factors including side of resection, baseline memory performance, extent of hippocampal sclerosis and Wada Test performance all provided independent information regarding prediction of memory outcome. Lineweaver73 found that MRI volumes and baseline memory performance, but not Wada Test performance, significantly predicted memory outcome. Baxendale74 also found that preoperative memory level emerged as the most reliable predictor of memory outcome when side of resection, amount of cortical dysgenesis, chronological age, and IQ were also entered into a prediction model.
Binder et al.66 examined 60 left ATL patients who underwent preoperative language mapping with fMRI, preoperative intracarotid amobarbital (Wada) testing for language and memory lateralization, and pre- and postoperative neuropsychological testing. Demographic, historical, neuropsychological, and imaging variables were examined for their ability to predict pre- to postoperative memory change. Verbal memory decline was observed in over 30% of patients. Predictive of memory decline were good preoperative performance, late age at onset of epilepsy, left dominance on fMRI, and left dominance on the Wada test. Preoperative performance and age at onset together accounted for roughly 50% of the variance in memory outcome and fMRI explained an additional 10% of this variance. Neither Wada memory asymmetry nor Wada language asymmetry added additional predictive power beyond these noninvasive measures.
Binder et al.66 also used an interesting multivariate approach to predict verbal memory outcome following left ATL. Order of entry of prediction variables was based on risk and cost. Age of onset and preoperative memory performance were entered first and predicted about 50% of the variance for memory outcome, the fMRI language index (not memory) was added next and added 10% of the variance in predicting outcome. The fMRI laterality index still added significant predictive values after Wada Test language and memory scores were considered.
These predictive models would have more utility if the surgical resection were standard across centers. This of course is far from the case and the predictive models apply to surgery as performed at the reporting center. Growing clinical evidence documents the impact of various surgical approaches to ATL and the variable cognitive outcomes that may follow (see 75 for review), although it should be appreciated that the number of randomized clinical trials comparing different surgical approaches is extremely small. For example, Helmstaedter 76 compared pre- and postoperative verbal learning and memory performance in left temporal lobe epilepsy patients with hippocampal pathology who underwent anterior temporal lobectomy (ATL) or selective amygdalohippocampectomy (SAH), as well as patients with left lateral temporal lobe lesions who underwent cortical lesionectomy. All three groups exhibited similarly impaired preoperative verbal learning and memory performance compared to controls. Postoperatively, long term memory declines were similar for the ATL and SAH groups, but the ATL group also exhibited decline in new verbal learning efficiency, presumably due to resection of functional left lateral temporal neocortex. The left cortical lesionectomy group showed minimal pre- to postoperative verbal learning and memory change.
Insights into human hippocampal function
In addition to providing important information about the cognitive complications of epilepsy surgery, careful pre to postoperative studies have provided unique information about the function of the human hippocampus. Paradoxically, this information comes from those persons who experienced the poorest cognitive outcomes, that is, those with resection of hippocampus with minimal or no hippocampal sclerosis. The following lessons have been learned.
Serial position curve
Classic learning studies demonstrated that when humans are presented with a supraspan list of words to learn and remember, in free recall there is preferential recall of words from the beginning (primacy) and end (recency) of the list compared to words from the middle—this pattern referred to as the serial position curve. The primacy portion of the list, and to some degree the middle portion, reflects the operation of so-called secondary or long term memory, while the recency portion has been thought to reflect primary or immediate memory. Examining patterns of free word list recall before and after ATL, resection of a minimally sclerotic left hippocampus selectively affected the primacy and middle portions of the list demonstrating reliance on hippocampus, while the recency portion of the list remained unaffected, thus independent of the hippocampus (Figure 3). 77.
Figure 3.
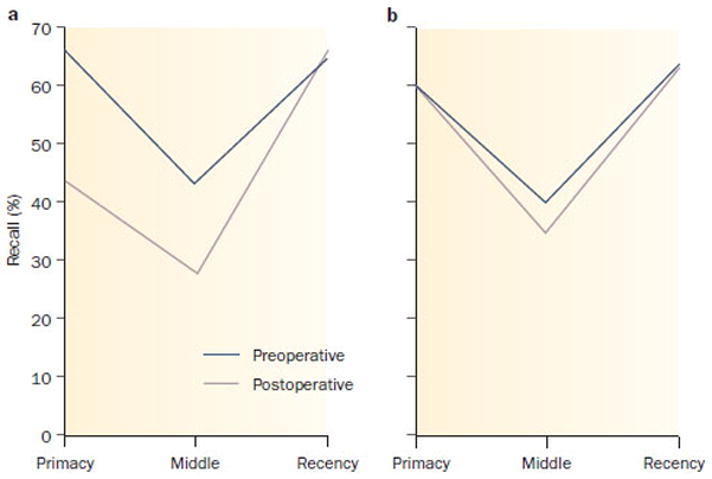
Change in the serial position curve following left anterior temporal lobectomy as a function of left hippocampal pathology. a | Resection of left hippocampus with no or minimal sclerosis results in significant preoperative to postoperative alteration of the serial position curve with decreased recall of primacy and middle portions of the list. b | Resection of left hippocampus with notable hippocampal Sclerosis has no effect on the serial position curve. The data are derived from the patient’s free recall of a supraspan word list.
Semantic encoding
While there is decline in verbal list learning ability following resection of nonscleotic left hippocampus, it is possible that the inability to freely recall words is due to retrieval difficulties. That is, there may be degraded access to newly learned information. If true, recognition testing via yes-no or multiple choice testing might normalize performance. In addition, by presenting both target words (words from the list) as well as foils (words not from the list), error patterns would be informative. If patients misidentified words with certain attributes (semantic, phonological, prototypical), then a specific encoding contribution of hippocampus would be inferred. In point of fact, support for the retrieval hypothesis was not obtained—recognition testing did not significantly facilitate performance. Moreover, patients who underwent resection of nonsclerotic left hippocampus showed a selectively increased error rate for semantically related words78.
Semantic knowledge
Classically the human hippocampus is viewed as having a time limited role in memory encoding, with newly acquired episodic information eventually stored independent of the hippocampus. However, recent findings show that at the hippocampus plays an ongoing role in at least one class of semantic information—visual object naming. Significant declines in confrontation naming ability are seen following resection of nonsclerotic left hippocampus with a tendency for recall failures to affect words acquired comparatively later in life (yet many years prior to surgery), suggesting a temporal gradient79, 80. Further, there appear to be differences in the risk to semantic memory systems (both naming and recognition) based upon different semantic categories. Existing studies highlight the importance of the temporal lobes in recognition and naming of several object categories81–84 while deficits in recognition and familiarity judgment are common occurrences following nondominant ATL resection85.
The changing view of temporal lobe epilepsy
At the second Palm Desert International Conference on the Surgical treatment of the Epilepsies, focus was placed on “surgically remediable syndromes”, among which mesial temporal lobe epilepsy was prominent 86. This conceptualization facilitated increasingly careful characterization of the syndromes of localization related temporal lobe epilepsy (mesial TLE, lesional TLE, and so called MRI-negative, paradoxical or cryptogenic TLE). The primary cognitive signature of mesial TLE (mTLE) was viewed to be material-specific memory impairment demonstrated either through formal neuropsychological assessment or the Wada Test. A stated contraindication to mTLE was the presence of generalized cognitive compromise—all reasonable characterizations at the time86. However, later studies examining the full range of cognitive abilities found that patients with neuropathologically confirmed mesial TLE exhibited a pattern of generalized cognitive disruption—an unanticipated finding 87. One possible cause was that structural abnormality may also extend beyond the confines of the mesial temporal lobe and that these extratemporal abnormalities may have additional cognitive consequences.
Widespread anatomic abnormalities beyond the epileptogenic hippocampus
In the 1990s, the examination of structural abnormalities in people with mTLE was extended beyond the epileptogenic hippocampus. Using quantitative MRI tools that focused on manually delineated volumes to assess brain size, investigators first probed hippocampal related structures and found atrophy in neocortical temporal lobes 59, entorhinal cortex 88, 89, fornix 90, parahippocampal gyrus 89, and amygdala 89, 91. Quantitative volumetric studies were also applied to other subcortical structures and yielded abnormalities in the basal ganglia 92, 93, thalamus 92, 94, 95, and cerebellum 96, 97. Thus, these studies demonstrated that anatomical abnormalities in mTLE were certainly not limited to the epileptogenic hippocampus. However, these early volumetric techniques only permitted examinations of one, or a limited number of predetermined structures, rather than simultaneously characterizing a broad range of deficits throughout the entire brain.
The first glimpse of the distributed nature of structural abnormalities in mTLE came from Sisodiya and colleagues 98. Instead of manually tracing structures with defined borders, they divided each hemisphere into blocks and quantified the amount of cortical gray matter and white matter throughout the entire brain. Each anatomical block of a patient with mTLE was compared to normal controls in order to assess for disproportional distribution of gray and white matter. Indeed, a majority of patients with hippocampal sclerosis had diffuse abnormalities throughout the cerebral cortex, but the exact location could not be elucidated from this technique.
With the emergence of automated analysis tools such as voxel based morphometry (VBM), the whole brain now can be scrutinized voxel by voxel in the same patient group to more precisely localize brain regions that are affected in mTLE. This unbiased examination of the entire brain facilitated appreciation of the distribution and relative degree of structural burden carried by many patients. In that regard, a very helpful summary of the presence and distribution of structural abnormalities associated with TLE is provided by Keller and Roberts 99. They summarized 18 VBM studies and found 26 brain regions that showed reduced volumes in TLE, compared to healthy controls. The distribution of abnormalities was widespread, involving mesial, extramesial temporal lobe, subcortical and extratemporal lobe cortical regions.
Although VBM studies provide an anatomical profile of the extent of abnormalities, the pathological nature of these changes was uncertain 99. Gray matter measurements in VBM are sensitive to both losses in gray matter as well as increases in CSF volume, as well as differences in cortical surface curvature, which cannot be distinguished from each other. These limitations provided the impetus to examine changes in other brain features such as indices of gyrification, cortical thickness, and surface area. Lin and colleagues examined cortical thickness in a group of mTLE patients with pathologically confirmed hippocampal sclerosis and found that these patients to have up to a 30% decrease in cortical thickness, with significant thinning of frontal poles, frontal operculum, orbitofrontal, lateral temporal, and occipital regions (Figure 4, a and b). Interestingly, reductions in cortical thickness were evident in bilateral cerebral hemispheres, despite unilateral seizure onset 100. Other investigators have also reported bilateral cortical mantel thinning in select regions throughout the entire cerebral cortex, but most consistently in the frontal, central and temporal regions101–103. Widespread abnormalities in gyrification patterns were found in multiple cortical regions, both ipsilateral and contralateral
Figure 4.
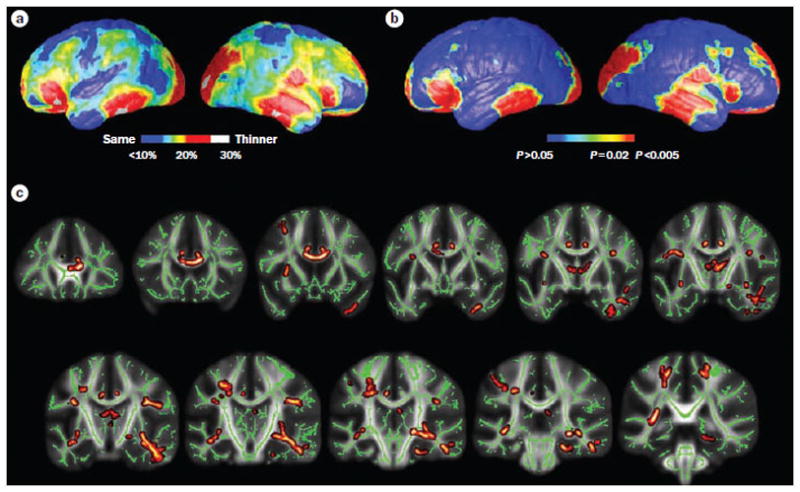
Reduced gray matter thickness and white matter integrity in left MTLE. a | Mean percent reduction in cortical thickness as a percentage of control average. Red areas in the bilateral in the frontal poles, frontal operculum, orbitalfrontal, lateral temporal and occipital regions, and the right angular gyrus and primary sensorimotor cortex surroundings the central sulcus denote ≤30% decrease in thickness, on average, compared with corresponding areas in controls. b | Significance of these changes shown as a map of P values. c | Reductions in white matter integrity, measured by decreased fractional anisotropy, were evident in mesial and lateral temporal lobe, limbic system and extratemporal lobe regions, particularly ipsilateral to the side of seizure onset. Yellow and dark red regions indicate white matter tracts with decreased fractional anisotropy. Green regions indicate areas not notably different from controls. Only left MTLE patients are presented here, although similar gray and white matter abnormalities—albeit to a lesser degree—were evident in right MTLE. Parts a and b are modified, with permission from Oxford University Press © Lin, J. J. et al. Cereb. Cortex 17, 2007–2018 (2007). Part c is modified with permission from Elsevier Ltd © Focke, N. K. et al. Neuroimage 40, 728–737 (2008). Abbreviation: MTLE, mesial temporal lobe epilepsy.
In addition to gray matter abnormalities, aberrant white matter tracts and connections are present in chronic TLE. The advent of diffusion tensor imaging (DTI) techniques has allowed investigators to measure white matter tract integrity by assessing magnetic resonance signal of water diffusion and its directionality in three-dimensional space. Parallel to early quantitative gray matter volumetric studies, initial DTI studies also focused on the limbic system and found diffusion abnormalities in the bilateral of fornix and cingulum 104. Postulating on a more diffuse epileptogenic network in TLE, other investigations extended this initial finding to frontal-temporal (uncinate fasciculus and arcuate fasciculus) 105–107, temporal-occipital (inferior longitudinal fasciculus)108, frontal-occipital (inferior frontal occipital fasciculus)108 and interhemispheric connections (corpus callosum) 109–111. More recently, whole brain voxelwise analysis techniques have mapped white matter profiles and delineated systemic differences between TLE patients and healthy individuals, without a priori bias for specific tracts or brain regions. Focke and colleagues (2008) used such a voxelwise technique to evaluate diffusion abnormalities in patients with mTLE and found that reduced white matter integrity was present in mesial and lateral temporal lobe, limbic system (thalamus, fornix and cingulum), and extratemporal regions (arcuate fasciculus, external capsule and corpus collosum), The white matter changes were more pronounced ipsilateral to side of seizure onset (Figure 4, c)112. Other studies have also showed demonstrated extensive bilateral white matter diffusion abnormalities, particularly in the temporal and frontal lobes ipsilateral to the side of seizure onset 113–115.
In summary, there is converging evidence that while the primary epileptic zone may be contained within the confines of the hippocampus, considerable anatomic abnormality exists outside this region, affecting a myriad of cortical, subcortical, and cerebellar regions and their direct and indirect connectivity.
Widespread anatomic abnormalities are linked to distributed cognitive impairments
In concert with the extensive anatomical abnormalities, mTLE patients also exhibited a pattern of distributed cognitive impairments, affecting not only memory, but also a broad array of cognitive areas including IQ, executive functions, language and sensorimotor skills. 87, 116, 117. A cumulative literature has now emerged, linking structural changes with cognitive performances. In the cortical regions, specific one-to-one structural-functional association in TLE has been sparse and is primarily limited to the frontal and neocortical temporal lobes. For example, reduced volumes in specific sub-regions of the prefrontal cortex have been related to poor executive functioning 118 and impaired memory 119, while left neocortical temporal lobe volume predicted confrontation naming ability 120. When examining anatomical features of the entire cerebral cortex, only global indices of structural integrity, such as overall gyrification 121, whole brain volumes 122–124, and disproportionate distribution of white and gray matter volumes 125, have been related cognitive performances. Indeed, a VBM study failed to associate localized gray matter changes with material-specific neuropsychological deficits 124. Thus, structural-functional correlations in the cortical regions are more evident at a global level than local level, implying that the distributed nature of cognitive impairment in TLE involves a widespread network.
The link between subcortical atrophy and cognition in TLE further highlights the importance of this integrated network. Subcortical structures such as the thalamus, basal ganglia, and cerebellum are critical nodes in the cortico-subcortical circuits that are involved in the transfer, convergence, and processing of cognitive information. To this end, thalamic volumes have been correlated with IQ, memory 126 and executive function 127; basal ganglia changes have been related to negative symptoms in TLE patients 128; and cerebellar abnormalities have been associated with impaired procedural memory 129 as well as executive function 115. When combining the degree of cortical thinning with volume loss in these subcortical regions, the collective structural abnormality has been found to be closely associated with patterns of cognitive impairment (or cognitive phenotypes) observed among patients with temporal lobe epilepsy 130.
Another facet of the coordinated network in TLE is derived from the link between white matter tract integrity and cognitive ability. White matter fiber tracts that connect cortical to cortical, cortical to subcortical, and interhemispheric regions have been associated with specific cognitive deficits in memory and language (see Table 1). These studies have led to a unifying hypothesis that disconnection between important cortical and subcortical regions would impair information transfer and thus contribute to cognitive impairments in TLE.
Table 1.
Abnormal white matter tract connections and associated cognitive deficits in TLE
| Tracts | Connections | Cognitive deficits in TLE |
|---|---|---|
| Arcuate fasciculus | Connects perisylvian frontal, parietal and temporal cortex | Confrontational naming131 |
| Inferior longitudinal fasciculus | Connecting temporal lobe to the occipital lobe | Delayed memory 115 |
| Inferior fronto-occipital fasciculus | Connecting frontal lobe to occipital lobe | Delayed memory 131 |
| Uncinate fasciculus | Connections between the mesial temporal structures (uncus and amygdala) and mesial frontal region. | Immediate memory, delayed memory and confrontational naming115, 131, 132 |
|
| ||
| Parahippocampal cingulum | Connects the uncus and parahippocampal gyrus to subrostral areas of the frontal region | Delayed memory 131 |
| Fornix | Connects hippocampus to other limbic regions | Immediate memory 115 |
| Corpus callosum | Major connection between the two hemispheres | Psychomotor speed and executive function133 |
In summary, there is now substantial evidence that cognitive impairment in TLE is a result of disrupted network rather than specific damage to a certain brain structure. Importantly, the sum of these distributed structural abnormalities may result in a cumulative cognitive and behavioral burden that may be substantial in TLE patients.
Focal epilepsy and abnormal organization of higher cognitive functions
A defining characteristic of mesial TLE is childhood/early adolescent onset, often with an early initial precipitating injury. This hallmark characteristic is important as the timing and nature of the initial precipitating injury and recurrent seizures could directly affect organization of higher cognitive abilities—both within and between cerebral hemispheres.
Evidence of altered cerebral organization is now substantial. Increased rates of right hemisphere or bilateral language dominance has been frequently observed among patients with left TLE 134–136, and partial transfer of language dominance occurs more frequently in the presence of early onset epilepsy and left hippocampal sclerosis137. Intrahemisphere reorganization of language has been demonstrated by intra- and extraoperative speech mapping where early onset epilepsy is associated with more relocation of visual and auditory naming sites, especially more posteriorally 137–139,140
Functional neuroimaging studies demonstrate abnormal organization of memory. Using fMRI, Powell et al.141 showed that compared to controls, both right and left TLE patients showed less ipsilateral than contralateral hippocampal activation while viewing word, pictures, and face stimuli. In addition, increased activation in the ipsilateral hippocampus was negatively correlated with verbal memory in left TLE and non-verbal memory in right TLE. In contrast, greater contralateral hippocampus activity was correlated with poorer memory performance. They also suggested that reorganization of memory ability to contralateral hippocampus and MTL structures may not lead to effective memory performance.
This abnormal organization may also be responsible in part for the distributed cognitive compromise that may be observed. In individuals with early-onset epilepsy, the degree of language shift to the right hemisphere was correlated with poorer performance in language, executive function and memory142. In addition, shifting of language to the right hemisphere was associated with deficits in non-verbal cognitive tasks, suggesting that reorganization of language may “crowd” out normal right hemisphere functions 143. Shifting of language to the right hemisphere alters normal language networks, resulting in adverse cognitive outcome. It should be remembered that most of these studies are cross-sectional in nature and, as such, do not address the important question of when and how the cognitive deficits develop or even whether they antedate the onset of temporal lobe epilepsy.
Conclusions and Future Directions
TLE is far more than a localization-related form of epilepsy with a primary and limited impact on episodic memory. Depending on the specific syndrome and its associated underlying characteristics, the impact of “temporal lobe epilepsy” on brain structure and function can be widespread, impacting brain and cognitive development, invoking compensatory processes including reorganization of function, and altering the landscape of brain-behavior relationships. Despite the significant progress made regarding the broader understanding of cognitive comorbidities in TLE, specific biomarkers that predict development of cognitive deficits have not been identified and there are relatively few strategies that identify individuals at risk for cognitive dysfunction. Thus the current state of knowledge highlights the need for longitudinal studies across the life span in order to identify brain-based predictors of cognitive comorbidities and target candidates for effective cognitive intervention.
While cognitive reorganization has a fortuitous benefit for those who undergo ATL by adventitiously preserving language and memory function postoperatively, the broader implications of these diverse impacts remain uncertain, including brain and cognitive health in older age. While there are divergent views regarding the primary adverse influence on life course (early neurodevelopmental impact vs. progressive decline vs. mixed neurodevelopmental and degenerative) 144–147, all views agree in predicting worse cognitive function in elder years compared to population based norms—an outcome that deserves much closer scrutiny148.
While it is clear that much has been learned about memory and other cognitive processes in persons with temporal lobe epilepsy over the years, much remains to be clarified. As can be appreciated, the opportunity to carefully study persons with temporal lobe epilepsy who are candidates for epilepsy surgery has provided investigators with unparalleled opportunities to learn more about the effects of epilepsy on cognition and brain structure. However, these patients are among the most intractable to medication treatment and therefore not representative of the larger population of people with this form of epilepsy. Population based investigations would inform a more representative picture of the consequences for neurobehavioral status and brain structure.
Supplementary Material
Key points.
Examination of patients following epilepsy surgery has contributed significantly to understanding the neuroanatomy of human memory.
There is wide variability in the impact of anterior temporal lobectomy on postoperative memory function.
The cause(s) of this variability is now better understood leading to improved ability to identify patients at greatest risk of adverse cognitive outcomes.
Recent findings demonstrate that cognitive morbidity in temporal lobe epilepsy can extend beyond memory function and that anatomical abnormalities can extend far beyond the temporal lobe.
These distributed cognitive abnormalities are being linked to anatomic abnormalities outside the temporal lobe, providing a new neurobiological understanding of the neuropsychology of temporal lobe epilepsy.
Acknowledgments
We thank Drs. David Loring and John Langfitt for their critical review of earlier versions of this manuscript. Preparation of this paper was supported in part by NINDS RO1-44351 (BH, MS) and K-23 NS060993 (JL).
Biographies
Brian D. Bell, PhD is an Associate Professor and Clinical Neuropsychologist at the Matthews Neuropsychology Lab in the Department of Neurology at the University of Wisconsin School of Medicine and Public Health in Madison, Wisconsin. His clinical and research interests include memory and language functioning in temporal lobe epilepsy patients.
Jack J. Lin, MD is Associate Professor and Director of the Clinical Neurophysiology Fellowship Program at the University of California, Irvine. He is a neurologist specializing in clinical epileptology at the UC Irvine Medical Center Comprehensive Epilepsy Program. He is a member of the Epilepsy Foundation Mood Disorders Initiative and has contributed to the National Institutes of Health Epilepsy Research Benchmark. His research interests include the anatomic consequences of epilepsy and neuroimaging biomarkers of cognitive impairment in epilepsy.
Michael Seidenberg, PhD is Professor in the Department of Psychology at the Rosalind Franklin University of Medicine and Science in North Chicago IL. His primary research interest has been the neuropsychological features of childhood and adult epilepsies.
Bruce Hermann, PhD is Professor and Director of the Matthews Neuropsychology Lab in the Department of Neurology at the University of Wisconsin School of Medicine and Public Health in Madison, Wisconsin. His clinical and research interests have focused on the cognitive and behavioral aspects of the epilepsies and the outcomes of epilepsy surgery in children and adults. Most recently his interests have concentrated on the natural history of the neurobehavioral comorbidities of epilepsy and especially the effects of epilepsy on cognitive and brain development in children with new onset epilepsy.
References
- 1.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: a systematic review. Bull World Health Organ. 2010;88:260–6. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallin JEW. Eight months of psycho-clinical research at the new jersey state village for epileptics, with some results from the binet-simon testing. Epilepsia. 1912;A3:366–380. [Google Scholar]
- 3.Fox JT. The response of epileptic children to mental and educational tests. British Journal of Medical Psychology. 1924;4:235–248. [Google Scholar]
- 4.Bladin PF. Murray Alexander Falconer and the Guy’s-Maudsley Hospital seizure surgery program. J Clin Neurosci. 2004;11:577–83. doi: 10.1016/j.jocn.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Feindel W, Leblanc R, de Almeida AN. Epilepsy surgery: historical highlights 1909–2009. Epilepsia. 2009;50 (Suppl 3):131–51. doi: 10.1111/j.1528-1167.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- 6.Hermann BP, Stone JL. A historical review of the epilepsy surgery program at the University of Illinois Medical Center: The contributions of Bailey, Gibbs, and collaborators to the refinement of anterior temporal lobectomy. J Epilepsy. 1989;2:155–163. [Google Scholar]
- 7.Loring DW. History of neuropsychology through epilepsy eyes. Arch Clin Neuropsychol. 2010;25:259–73. doi: 10.1093/arclin/acq024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Almeida AN, Teixeira MJ, Feindel WH. From lateral to mesial: The quest for a surgical cure for temporal lobe epilepsy. Epilepsia. 2008;49:98–107. doi: 10.1111/j.1528-1167.2007.01321.x. [DOI] [PubMed] [Google Scholar]
- 9.Penfield W, Baldwin M. Temporal lobe seizures and the technic of subtotal temporal lobectomy. Ann Surg. 1952;136:625–34. [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen I. Temporal lobe surgery around the world. Results, complications, and mortality. Acta Neurol Scand. 1975;52:354–73. doi: 10.1111/j.1600-0404.1975.tb05831.x. [DOI] [PubMed] [Google Scholar]
- 11.Bailey P, Gibbs FA. The surgical treatment of psychomotor epilepsy. J Am Med Assoc. 1951;145:365–70. doi: 10.1001/jama.1951.02920240001001. [DOI] [PubMed] [Google Scholar]
- 12.Green JR. Temporal lobectomy, with special reference to selection of epileptic patients. J Neurosurg. 1967;26:585–93. [PubMed] [Google Scholar]
- 13.Baxendale S. Amnesia in temporal lobectomy patients: historical perspective and review. Seizure. 1998;7:15–24. doi: 10.1016/s1059-1311(98)90003-6. [DOI] [PubMed] [Google Scholar]
- 14.Milner B, Penfield W. The effect of hippocampal lesions on recent memory. Trans Am Neurol Assoc. 1955;80:42–8. [PubMed] [Google Scholar]
- 15.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squire LR. Memory and Brain Systems: 1969–2009. J Neurosci. 2009;29:12711–12716. doi: 10.1523/JNEUROSCI.3575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer V, Yates AJ. Intellectual changes following temporal lobectomy for psychomotor epilepsy. Journal of Neurology, Neurosurgery & Psychiatry. 1955;18:44–52. doi: 10.1136/jnnp.18.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milner B. Psychological defects produced by temporal lobe excision. Res Publ Assoc Res Nerv Ment Dis. 1958;36:244–57. [PubMed] [Google Scholar]
- 19.Milner B. Disorders of memory after brain lesions in man: Preface: Material-specific and generalized memory loss. Neuropsychologia. 1968;6:175–179. [Google Scholar]
- 20.Chelune GJ. Hippocampal adequacy versus functional reserve: predicting memory functions following temporal lobectomy. Arch Clin Neuropsychol. 1995;10:413–32. [PubMed] [Google Scholar]
- 21.Martin RC, et al. Individual memory change after anterior temporal lobectomy: a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39:1075–82. doi: 10.1111/j.1528-1157.1998.tb01293.x. [DOI] [PubMed] [Google Scholar]
- 22.Baxendale SA. The hippocampus: functional and structural correlations. Seizure. 1995;4:105–17. doi: 10.1016/s1059-1311(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 23.Bell BD, Davies KG. Anterior temporal lobectomy, hippocampal sclerosis, and memory: recent neuropsychological findings. Neuropsychol Rev. 1998;8:25–41. doi: 10.1023/a:1025679122911. [DOI] [PubMed] [Google Scholar]
- 24.Hamberger MJ, Drake EB. Cognitive functioning following epilepsy surgery. Curr Neurol Neurosci Rep. 2006;6:319–26. doi: 10.1007/s11910-006-0025-8. [DOI] [PubMed] [Google Scholar]
- 25.Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Relationship of age at onset, chronologic age, and adequacy of preoperative performance to verbal memory change after anterior temporal lobectomy. Epilepsia. 1995;36:137–45. doi: 10.1111/j.1528-1157.1995.tb00972.x. [DOI] [PubMed] [Google Scholar]
- 26.Powell GE, Polkey CE, McMillan T. The new Maudsley series of temporal lobectomy. I: Short-term cognitive effects. Br J Clin Psychol. 1985;24 (Pt 2):109–24. doi: 10.1111/j.2044-8260.1985.tb01321.x. [DOI] [PubMed] [Google Scholar]
- 27.Saling MM. Verbal memory in mesial temporal lobe epilepsy: beyond material specificity. Brain. 2009;132:570–82. doi: 10.1093/brain/awp012. [DOI] [PubMed] [Google Scholar]
- 28.Loring DW, et al. Differential neuropsychological test sensitivity to left temporal lobe epilepsy. J Int Neuropsychol Soc. 2008;14:394–400. doi: 10.1017/S1355617708080582. [DOI] [PubMed] [Google Scholar]
- 29.Helmstaedter C, Gleissner U, Di Perna M, Elger CE. Relational verbal memory processing in patients with temporal lobe epilepsy. Cortex. 1997;33:667–78. doi: 10.1016/s0010-9452(08)70724-x. [DOI] [PubMed] [Google Scholar]
- 30.Helmstaedter C, Wietzke J, Lutz MT. Unique and shared validity of the “Wechsler logical memory test”, the “California verbal learning test”, and the “verbal learning and memory test” in patients with epilepsy. Epilepsy Res. 2009;87:203–12. doi: 10.1016/j.eplepsyres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Rausch R. Anatomical substrates of interictal memory deficits in temporal lobe epileptics. Int J Neurol. 1987;21–22:17–32. [PubMed] [Google Scholar]
- 32.Saling MM, et al. Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J Clin Exp Neuropsychol. 1993;15:608–18. doi: 10.1080/01688639308402582. [DOI] [PubMed] [Google Scholar]
- 33.Sass KJ, et al. Specificity in the correlation of verbal memory and hippocampal neuron loss: dissociation of memory, language, and verbal intellectual ability. J Clin Exp Neuropsychol. 1992;14:662–72. doi: 10.1080/01688639208402854. [DOI] [PubMed] [Google Scholar]
- 34.Sass KJ, et al. Verbal memory impairment correlates with hippocampal pyramidal cell density. Neurology. 1990;40:1694–7. doi: 10.1212/wnl.40.11.1694. [DOI] [PubMed] [Google Scholar]
- 35.Martin R, et al. Determining reliable cognitive change after epilepsy surgery: development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43:1551–8. doi: 10.1046/j.1528-1157.2002.23602.x. [DOI] [PubMed] [Google Scholar]
- 36.Rausch R, Babb TL. Hippocampal neuron loss and memory scores before and after temporal lobe surgery for epilepsy. Arch Neurol. 1993;50:812–7. doi: 10.1001/archneur.1993.00540080023008. [DOI] [PubMed] [Google Scholar]
- 37.Hermann BP, Wyler AR, Somes G, Berry AD, 3rd, Dohan FC., Jr Pathological status of the mesial temporal lobe predicts memory outcome from left anterior temporal lobectomy. Neurosurgery. 1992;31:652–6. doi: 10.1227/00006123-199210000-00006. discussion 656–7. [DOI] [PubMed] [Google Scholar]
- 38.Milner B, Branch C, Rassmussen T. Study of short-term memory after intracarotid injection of sodium amytal. Transactions of American Neurological Association. 1962;87:224–226. [Google Scholar]
- 39.Wada J. A new method for the determination of the side of the cerebral speech dominance: A preliminary report on the intracarotid injection of Sodium Amytal in man. Medicine and Biology. 1949;14:221–222. [Google Scholar]
- 40.Wyllie E, et al. Intracarotid amobarbital procedure: I. Prediction of decreased modality-specific memory scores after temporal lobectomy. Epilepsia. 1991;32:857–64. doi: 10.1111/j.1528-1157.1991.tb05542.x. [DOI] [PubMed] [Google Scholar]
- 41.Rausch R, Babb TL, Engel J, Jr, Crandall PH. Memory following intracarotid amobarbital injection contralateral to hippocampal damage. Arch Neurol. 1989;46:783–8. doi: 10.1001/archneur.1989.00520430077022. [DOI] [PubMed] [Google Scholar]
- 42.Loring DW, et al. Wada memory asymmetries predict verbal memory decline after anterior temporal lobectomy. Neurology. 1995;45:1329–33. doi: 10.1212/wnl.45.7.1329. [DOI] [PubMed] [Google Scholar]
- 43.Kneebone AC, Chelune GJ, Dinner DS, Naugle RI, Awad IA. Intracarotid amobarbital procedure as a predictor of material-specific memory change after anterior temporal lobectomy. Epilepsia. 1995;36:857–65. doi: 10.1111/j.1528-1157.1995.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 44.Loring DW, Bowden SC, Lee GP, Meador KJ. Diagnostic utility of Wada Memory Asymmetries: sensitivity, specificity, and likelihood ratio characterization. Neuropsychology. 2009;23:687–93. doi: 10.1037/a0016528. [DOI] [PubMed] [Google Scholar]
- 45.Cohen-Gadol AA, Westerveld M, Alvarez-Carilles J, Spencer DD. Intracarotid Amytal memory test and hippocampal magnetic resonance imaging volumetry: validity of the Wada test as an indicator of hippocampal integrity among candidates for epilepsy surgery. J Neurosurg. 2004;101:926–31. doi: 10.3171/jns.2004.101.6.0926. [DOI] [PubMed] [Google Scholar]
- 46.Loring DW, et al. Wada memory testing and hippocampal volume measurements in the evaluation for temporal lobectomy. Neurology. 1993;43:1789–93. doi: 10.1212/wnl.43.9.1789. [DOI] [PubMed] [Google Scholar]
- 47.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Right hemisphere restitution of language and memory functions in right hemisphere language-dominant patients with left temporal lobe epilepsy. Brain. 1994;117 (Pt 4):729–37. doi: 10.1093/brain/117.4.729. [DOI] [PubMed] [Google Scholar]
- 48.Glosser G, Saykin AJ, Deutsch GK, O’Connor MJ, Sperling MR. Neural organization of material-specific memory functions in temporal lobe epilepsy patients as assessed by the intracarotid amobarbital test. Neuropsychology. 1995;9:449–456. [Google Scholar]
- 49.Rausch R, Boone K, Ary CM. Right-hemisphere language dominance in temporal lobe epilepsy: clinical and neuropsychological correlates. J Clin Exp Neuropsychol. 1991;13:217–31. doi: 10.1080/01688639108401039. [DOI] [PubMed] [Google Scholar]
- 50.Lee GP, Park YD, Westerveld M, Hempel A, Loring DW. Effect of Wada methodology in predicting lateralized memory impairment in pediatric epilepsy surgery candidates. Epilepsy Behav. 2002;3:439–447. doi: 10.1016/s1525-5050(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 51.Sabsevitz DS, Swanson SJ, Morris GL, Mueller WM, Seidenberg M. Memory Outcome after Left Anterior Temporal Lobectomy in Patients with Expected and Reversed Wada Memory Asymmetry Scores. doi: 10.1046/j.1528-1157.2001.38500.x. [DOI] [PubMed] [Google Scholar]
- 52.Chiaravalloti ND, Glosser G. Material-specific memory changes after anterior temporal lobectomy as predicted by the intracarotid amobarbital test. Epilepsia. 2001;42:902–11. doi: 10.1046/j.1528-1157.2001.02500.x. [DOI] [PubMed] [Google Scholar]
- 53.Barr WB, et al. The use of figural reproduction tests as measures of nonverbal memory in epilepsy surgery candidates. J Int Neuropsychol Soc. 1997;3:435–43. [PubMed] [Google Scholar]
- 54.Helmstaedter C, Pohl C, Elger CE. Relations between verbal and nonverbal memory performance: evidence of confounding effects particularly in patients with right temporal lobe epilepsy. Cortex. 1995;31:345–55. doi: 10.1016/s0010-9452(13)80367-x. [DOI] [PubMed] [Google Scholar]
- 55.Saykin AJ, Gur RC, Sussman NM, O’Connor MJ, Gur RE. Memory deficits before and after temporal lobectomy: effect of laterality and age of onset. Brain Cogn. 1989;9:191–200. doi: 10.1016/0278-2626(89)90029-8. [DOI] [PubMed] [Google Scholar]
- 56.Davies KG, et al. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996;24:119–26. doi: 10.1016/0920-1211(96)00008-3. [DOI] [PubMed] [Google Scholar]
- 57.Chelune GJ, Naugle RI, Luders H, Awad IA. Prediction of cognitive change as a function of preoperative ability status among temporal lobectomy patients seen at 6-month follow-up. Neurology. 1991;41:399–404. doi: 10.1212/wnl.41.3.399. [DOI] [PubMed] [Google Scholar]
- 58.McDonald CR. The use of neuroimaging to study behavior in patients with epilepsy. Epilepsy Behav. 2008;12:600–11. doi: 10.1016/j.yebeh.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lencz T, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992;31:629–37. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- 60.Trenerry MR, et al. MRI hippocampal volumes and memory function before and after temporal lobectomy. Neurology. 1993;43:1800–5. doi: 10.1212/wnl.43.9.1800. [DOI] [PubMed] [Google Scholar]
- 61.Griffith HR, et al. Preoperative FDG-PET temporal lobe hypometabolism and verbal memory after temporal lobectomy. Neurology. 2000;54:1161–5. doi: 10.1212/wnl.54.5.1161. [DOI] [PubMed] [Google Scholar]
- 62.Leeman BA, Leveroni CL, Johnson KA. Does hippocampal FDG-PET asymmetry predict verbal memory dysfunction after left temporal lobectomy? Epilepsy Behav. 2009;16:274–80. doi: 10.1016/j.yebeh.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 63.Richardson MP, Strange BA, Duncan JS, Dolan RJ. Preserved verbal memory function in left medial temporal pathology involves reorganisation of function to right medial temporal lobe. Neuroimage. 2003;20 (Suppl 1):S112–9. doi: 10.1016/j.neuroimage.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Rabin ML, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–98. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- 65.Richardson MP, et al. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004;127:2419–26. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- 66.Binder JR, et al. Use of preoperative functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepsia. 2008;49:1377–94. doi: 10.1111/j.1528-1167.2008.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Powell HW, et al. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry. 2008;79:327–30. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonelli SB, et al. Imaging memory in temporal lobe epilepsy: predicting the effects of temporal lobe resection. Brain. 2010;133:1186–99. doi: 10.1093/brain/awq006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binder JR. Functional MRI is a valid noninvasive alternative to Wada testing. Epilepsy Behav. 2010 doi: 10.1016/j.yebeh.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Binder JR, et al. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy: language lateralization versus hippocampal activation asymmetry. Epilepsia. 2010;51:618–26. doi: 10.1111/j.1528-1167.2009.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chelune GJ, Najm IM. In: Epilepsy Surgery. Luders HO, Comair Y, editors. Lippencott-Raven; Philadelphia: 2000. pp. 497–504. [Google Scholar]
- 72.Stroup E, et al. Predicting verbal memory decline following anterior temporal lobectomy (ATL) Neurology. 2003;60:1266–73. doi: 10.1212/01.wnl.0000058765.33878.0d. [DOI] [PubMed] [Google Scholar]
- 73.Lineweaver TT, et al. Evaluating the contributions of state-of-the-art assessment techniques to predicting memory outcome after unilateral anterior temporal lobectomy. Epilepsia. 2006;47:1895–903. doi: 10.1111/j.1528-1167.2006.00807.x. [DOI] [PubMed] [Google Scholar]
- 74.Baxendale S, Thompson P, Harkness W, Duncan J. Predicting memory decline following epilepsy surgery: a multivariate approach. Epilepsia. 2006;47:1887–94. doi: 10.1111/j.1528-1167.2006.00810.x. [DOI] [PubMed] [Google Scholar]
- 75.Helmstaedter C. In: Neuropsychology and its role in the care of people with epilepsy. Arzimanoglou A, Helmstaedter C, Hermann B, Lassonde M, editors. John Libby Ltd; In press. [Google Scholar]
- 76.Hermann BP, et al. The effects of human hippocampal resection on the serial position curve. Cortex. 1996;32:323–34. doi: 10.1016/s0010-9452(96)80054-2. [DOI] [PubMed] [Google Scholar]
- 77.Seidenberg M, et al. Hippocampal sclerosis and verbal encoding ability following anterior temporal lobectomy. Neuropsychologia. 1996;34:699–708. doi: 10.1016/0028-3932(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 78.Bell BD, Davies KG, Hermann BP, Walters G. Confrontation naming after anterior temporal lobectomy is related to age of acquisition of the object names. Neuropsychologia. 2000;38:83–92. doi: 10.1016/s0028-3932(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 79.Yucus CJ, Tranel D. Preserved proper naming following left anterior temporal lobectomy is associated with early age of seizure onset. Epilepsia. 2007;48:2241–52. doi: 10.1111/j.1528-1167.2007.01211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380:499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- 81.Chao LL, Haxby JV, Martin A. Attribute-based neural substrates in temporal cortex for perceiving and knowing about objects. Nat Neurosci. 1999;2:913–9. doi: 10.1038/13217. [DOI] [PubMed] [Google Scholar]
- 82.Grabowski TJ, et al. Residual naming after damage to the left temporal pole: a PET activation study. Neuroimage. 2003;19:846–60. doi: 10.1016/s1053-8119(03)00109-5. [DOI] [PubMed] [Google Scholar]
- 83.Strauss E, et al. Left anterior lobectomy and category-specific naming. Brain Cogn. 2000;43:403–6. [PubMed] [Google Scholar]
- 84.Drane DL, et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46:1242–55. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Engel J., Jr Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992) Neurology. 1993;43:1612–7. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- 86.Hermann BP, Seidenberg M, Schoenfeld J, Davies K. Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch Neurol. 1997;54:369–76. doi: 10.1001/archneur.1997.00550160019010. [DOI] [PubMed] [Google Scholar]
- 87.Bernasconi N, et al. Entorhinal cortex in temporal lobe epilepsy: a quantitative MRI study. Neurology. 1999;52:1870–6. doi: 10.1212/wnl.52.9.1870. [DOI] [PubMed] [Google Scholar]
- 88.Bernasconi N, et al. Mesial temporal damage in temporal lobe epilepsy: a volumetric MRI study of the hippocampus, amygdala and parahippocampal region. Brain. 2003;126:462–9. doi: 10.1093/brain/awg034. [DOI] [PubMed] [Google Scholar]
- 89.Kuzniecky R, et al. Quantitative MRI in temporal lobe epilepsy: evidence for fornix atrophy. Neurology. 1999;53:496–501. doi: 10.1212/wnl.53.3.496. [DOI] [PubMed] [Google Scholar]
- 90.Salmenpera T, Kalviainen R, Partanen K, Pitkanen A. Hippocampal and amygdaloid damage in partial epilepsy: a cross-sectional MRI study of 241 patients. Epilepsy Res. 2001;46:69–82. doi: 10.1016/s0920-1211(01)00258-3. [DOI] [PubMed] [Google Scholar]
- 91.DeCarli C, Hatta J, Fazilat S, Gaillard WD, Theodore WH. Extratemporal atrophy in patients with complex partial seizures of left temporal origin. Ann Neurol. 1998;43:41–5. doi: 10.1002/ana.410430110. [DOI] [PubMed] [Google Scholar]
- 92.Dreifuss S, et al. Volumetric measurements of subcortical nuclei in patients with temporal lobe epilepsy. Neurology. 2001;57:1636–41. doi: 10.1212/wnl.57.9.1636. [DOI] [PubMed] [Google Scholar]
- 93.Szabo CA, et al. MR imaging volumetry of subcortical structures and cerebellar hemispheres in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2006;27:2155–60. [PMC free article] [PubMed] [Google Scholar]
- 94.Natsume J, Bernasconi N, Andermann F, Bernasconi A. MRI volumetry of the thalamus in temporal, extratemporal, and idiopathic generalized epilepsy. Neurology. 2003;60:1296–300. doi: 10.1212/01.wnl.0000058764.34968.c2. [DOI] [PubMed] [Google Scholar]
- 95.Sandok EK, O’Brien TJ, Jack CR, So EL. Significance of cerebellar atrophy in intracn temporal lobe epilepsy: a quantitative MRI study. Epilepsia. 2000;41:1315–20. doi: 10.1111/j.1528-1157.2000.tb04611.x. [DOI] [PubMed] [Google Scholar]
- 96.Hermann BP, Bayless K, Hansen R, Parrish J, Seidenberg M. Cerebellar atrophy in temporal lobe epilepsy. Epilepsy Behav. 2005;7:279–87. doi: 10.1016/j.yebeh.2005.05.022. [DOI] [PubMed] [Google Scholar]
- 97.Sisodiya SM, et al. Correlation of widespread preoperative magnetic resonance imaging changes with unsuccessful surgery for hippocampal sclerosis. Ann Neurol. 1997;41:490–6. doi: 10.1002/ana.410410412. [DOI] [PubMed] [Google Scholar]
- 98.Keller SS, Roberts N. Voxel-based morphometry of temporal lobe epilepsy: an introduction and review of the literature. Epilepsia. 2008;49:741–57. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 99.Eriksson SH, et al. Quantitative grey matter histological measures do not correlate with grey matter probability values from in vivo MRI in the temporal lobe. J Neurosci Methods. 2009;181:111–8. doi: 10.1016/j.jneumeth.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin JJ, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 101.McDonald CR, et al. Regional neocortical thinning in mesial temporal lobe epilepsy. Epilepsia. 2008;49:794–803. doi: 10.1111/j.1528-1167.2008.01539.x. [DOI] [PubMed] [Google Scholar]
- 102.Bernhardt BC, et al. Mapping limbic network organization in temporal lobe epilepsy using morphometric correlations: insights on the relation between mesiotemporal connectivity and cortical atrophy. Neuroimage. 2008;42:515–24. doi: 10.1016/j.neuroimage.2008.04.261. [DOI] [PubMed] [Google Scholar]
- 103.Oyegbile T, et al. Quantitative measurement of cortical surface features in localization-related temporal lobe epilepsy. Neuropsychology. 2004;18:729–37. doi: 10.1037/0894-4105.18.4.729. [DOI] [PubMed] [Google Scholar]
- 104.Lee JW, et al. Morphometric analysis of the temporal lobe in temporal lobe epilepsy. Epilepsia. 1998;39:727–36. doi: 10.1111/j.1528-1157.1998.tb01158.x. [DOI] [PubMed] [Google Scholar]
- 105.Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005;57:188–96. doi: 10.1002/ana.20334. [DOI] [PubMed] [Google Scholar]
- 106.Rodrigo S, et al. Uncinate fasciculus fiber tracking in mesial temporal lobe epilepsy. Initial findings. Eur Radiol. 2007 doi: 10.1007/s00330-006-0558-x. [DOI] [PubMed] [Google Scholar]
- 107.Lin JJ, Riley JD, Juranek J, Cramer SC. Vulnerability of the frontal-temporal connections in temporal lobe epilepsy. Epilepsy Res. 2008;82:162–70. doi: 10.1016/j.eplepsyres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 108.Matsumoto R, et al. Hemispheric asymmetry of the arcuate fasciculus: a preliminary diffusion tensor tractography study in patients with unilateral language dominance defined by Wada test. J Neurol. 2008;255:1703–11. doi: 10.1007/s00415-008-0005-9. [DOI] [PubMed] [Google Scholar]
- 109.Ahmadi ME, et al. Side Matters: Diffusion Tensor Imaging Tractography in Left and Right Temporal Lobe Epilepsy. AJNR Am J Neuroradiol. 2009 doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Arfanakis K, et al. Diffusion tensor MRI in temporal lobe epilepsy. Magn Reson Imaging. 2002;20:511–9. doi: 10.1016/s0730-725x(02)00509-x. [DOI] [PubMed] [Google Scholar]
- 111.Gross DW, Concha L, Beaulieu C. Extratemporal white matter abnormalities in mesial temporal lobe epilepsy demonstrated with diffusion tensor imaging. Epilepsia. 2006;47:1360–3. doi: 10.1111/j.1528-1167.2006.00603.x. [DOI] [PubMed] [Google Scholar]
- 112.Concha L, Beaulieu C, Collins DL, Gross DW. White-matter diffusion abnormalities in temporal-lobe epilepsy with and without mesial temporal sclerosis. J Neurol Neurosurg Psychiatry. 2009;80:312–9. doi: 10.1136/jnnp.2007.139287. [DOI] [PubMed] [Google Scholar]
- 113.Thivard L, et al. Diffusion tensor imaging in medial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage. 2005;28:682–90. doi: 10.1016/j.neuroimage.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 114.Focke NK, et al. Voxel-based diffusion tensor imaging in patients with mesial temporal lobe epilepsy and hippocampal sclerosis. Neuroimage. 2008;40:728–37. doi: 10.1016/j.neuroimage.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 115.Schoene-Bake JC, et al. Widespread affections of large fiber tracts in postoperative temporal lobe epilepsy. Neuroimage. 2009;46:569–76. doi: 10.1016/j.neuroimage.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Riley JD, et al. Altered white matter integrity in temporal lobe epilepsy: association with cognitive and clinical profiles. Epilepsia. 2010;51:536–45. doi: 10.1111/j.1528-1167.2009.02508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Oyegbile TO, et al. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–42. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- 118.Marques CM, et al. Cognitive decline in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsy Behav. 2007;10:477–85. doi: 10.1016/j.yebeh.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 119.Keller SS, Baker G, Downes JJ, Roberts N. Quantitative MRI of the prefrontal cortex and executive function in patients with temporal lobe epilepsy. Epilepsy Behav. 2009;15:186–95. doi: 10.1016/j.yebeh.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 120.Bonilha L, et al. Extrahippocampal gray matter atrophy and memory impairment in patients with medial temporal lobe epilepsy. Hum Brain Mapp. 2007;28:1376–90. doi: 10.1002/hbm.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seidenberg M, Geary E, Hermann B. Investigating temporal lobe contribution to confrontation naming using MRI quantitative volumetrics. J Int Neuropsychol Soc. 2005;11:358–66. [PubMed] [Google Scholar]
- 122.Hermann B, et al. Extratemporal quantitative MR volumetrics and neuropsychological status in temporal lobe epilepsy. J Int Neuropsychol Soc. 2003;9:353–62. doi: 10.1017/S1355617703930013. [DOI] [PubMed] [Google Scholar]
- 123.Dow C, Seidenberg M, Hermann B. Relationship between information processing speed in temporal lobe epilepsy and white matter volume. Epilepsy Behav. 2004;5:919–25. doi: 10.1016/j.yebeh.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 124.Focke NK, Thompson PJ, Duncan JS. Correlation of cognitive functions with voxel-based morphometry in patients with hippocampal sclerosis. Epilepsy Behav. 2008;12:472–6. doi: 10.1016/j.yebeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 125.Baxendale SA, et al. Disproportion in the distribution of gray and white matter: neuropsychological correlates. Neurology. 1999;52:248–52. doi: 10.1212/wnl.52.2.248. [DOI] [PubMed] [Google Scholar]
- 126.Seidenberg M, et al. Thalamic atrophy and cognition in unilateral temporal lobe epilepsy. J Int Neuropsychol Soc. 2008;14:384–93. doi: 10.1017/S1355617708080399. [DOI] [PubMed] [Google Scholar]
- 127.Stewart CC, et al. Contributions of volumetrics of the hippocampus and thalamus to verbal memory in temporal lobe epilepsy patients. Brain Cogn. 2009;69:65–72. doi: 10.1016/j.bandc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Geary EK, Seidenberg M, Hermann B. Atrophy of basal ganglia nuclei and negative symptoms in temporal lobe epilepsy. J Neuropsychiatry Clin Neurosci. 2009;21:152–9. doi: 10.1176/appi.neuropsych.21.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hermann B, et al. Cerebellar atrophy in temporal lobe epilepsy affects procedural memory. Neurology. 2004;63:2129–31. doi: 10.1212/01.wnl.0000145774.89754.0c. [DOI] [PubMed] [Google Scholar]
- 130.Dabbs K, Jones J, Seidenberg M, Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15:445–51. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.McDonald CR, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 2008;71:1869–76. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Diehl B, et al. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 2008;49:1409–18. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- 133.Hermann B, Hansen R, Seidenberg M, Magnotta V, O’Leary D. Neurodevelopmental vulnerability of the corpus callosum to childhood onset localization-related epilepsy. Neuroimage. 2003;18:284–92. doi: 10.1016/s1053-8119(02)00044-7. [DOI] [PubMed] [Google Scholar]
- 134.Branch C, Milner B, Rasmussen T. Intracarotid Sodium Amytal for the Lateralization of Cerebral Speech Dominance; Observations in 123 Patients. J Neurosurg. 1964;21:399–405. doi: 10.3171/jns.1964.21.5.0399. [DOI] [PubMed] [Google Scholar]
- 135.Rausch R, Walsh GO. Right-hemisphere language dominance in right-handed epileptic patients. Arch Neurol. 1984;41:1077–80. doi: 10.1001/archneur.1984.04050210075018. [DOI] [PubMed] [Google Scholar]
- 136.Loring DW, et al. The intracarotid amobarbital procedure as a predictor of memory failure following unilateral temporal lobectomy. Neurology. 1990;40:605–10. doi: 10.1212/wnl.40.4.605. [DOI] [PubMed] [Google Scholar]
- 137.Springer JA, et al. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122 (Pt 11):2033–46. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- 138.Devinsky O, Perrine K, Llinas R, Luciano DJ, Dogali M. Anterior temporal language areas in patients with early onset of temporal lobe epilepsy. Ann Neurol. 1993;34:727–32. doi: 10.1002/ana.410340517. [DOI] [PubMed] [Google Scholar]
- 139.Hamberger MJ. Cortical language mapping in epilepsy: a critical review. Neuropsychol Rev. 2007;17:477–89. doi: 10.1007/s11065-007-9046-6. [DOI] [PubMed] [Google Scholar]
- 140.Hamberger MJ, McClelland S, 3rd, McKhann GM, 2nd, Williams AC, Goodman RR. Distribution of auditory and visual naming sites in nonlesional temporal lobe epilepsy patients and patients with space-occupying temporal lobe lesions. Epilepsia. 2007;48:531–8. doi: 10.1111/j.1528-1167.2006.00955.x. [DOI] [PubMed] [Google Scholar]
- 141.Powell HW, et al. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007;36:209–21. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- 142.Helmstaedter C, Kurthen M, Linke DB, Elger CE. Patterns of language dominance in focal left and right hemisphere epilepsies: relation to MRI findings EEG sex and age at onset of epilepsy. Brain Cogn. 1997;33:135–50. doi: 10.1006/brcg.1997.0888. [DOI] [PubMed] [Google Scholar]
- 143.Strauss E, Satz P, Wada J. An examination of the crowding hypothesis in epileptic patients who have undergone the carotid amytal test. Neuropsychologia. 1990;28:1221–7. doi: 10.1016/0028-3932(90)90057-u. [DOI] [PubMed] [Google Scholar]
- 144.Hermann BP, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60:80–7. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 145.Bernhardt BC, et al. Longitudinal and cross-sectional analysis of atrophy in pharmacoresistant temporal lobe epilepsy. Neurology. 2009;72:1747–54. doi: 10.1212/01.wnl.0000345969.57574.f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seidenberg M, Pulsipher DT, Hermann B. Cognitive progression in epilepsy. Neuropsychol Rev. 2007;17:445–54. doi: 10.1007/s11065-007-9042-x. [DOI] [PubMed] [Google Scholar]
- 147.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: a neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–30. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- 148.Hermann B, et al. Growing old with epilepsy: the neglected issue of cognitive and brain health in aging and elder persons with chronic epilepsy. Epilepsia. 2008;49:731–40. doi: 10.1111/j.1528-1167.2007.01435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


