Abstract
Context
Constipation is often inadequately assessed and underdiagnosed in patients with advanced cancer. Many studies use patient-reported constipation as an outcome.
Objectives
The aim was to compare the accuracy of patient-reported constipation as compared with the modified Rome III (ROME) criteria and to determine the agreement between patient-reported constipation, physician assessment of constipation, and objective assessment of constipation by modified ROME criteria among outpatients with advanced cancer.
Methods
Patients with advanced cancer attending a supportive care clinic were screened. Constipation was assessed using the modified ROME criteria, patient report (yes or no and rated 0-10; 10 = worst possible symptom), and physician assessments (yes or no and rated 0-10).
Results
One hundred patients were enrolled and 50 of 100 (50%) patients met the modified ROME criteria for constipation. Disagreement between ROME criteria and the patient report (yes/no) was found in 33 patients (33%), and between ROME criteria and the physician assessment (yes/no) in 39 (39%). The best combination of sensitivity (0.84) and specificity (0.62) was found with scores ≥ 3/10 for patient-reported constipation.
Conclusions
We found a high frequency of constipation. The limited agreement with modified ROME criteria suggests that a patient’s self-report as yes or no is not useful for clinical practice. Patient self-rating on a 0 to 10 scale (score of three or greater) seems to be the best tool for constipation screening among this population. More research is needed to identify the best way to assess constipation in advanced cancer patients
Keywords: Constipation, assessment, Rome criteria, advanced cancer, palliative care
Introduction
Constipation can be defined as infrequent or difficult defecation with a reduced number of bowel movements, which may or may not be abnormally hard, and increased difficulty or discomfort [1]. Constipation is a frequent and distressing symptom in patients with advanced cancer and may affect 40% of patients referred to palliative care and up to 90% of patients treated with opioids [2-5]. Cancer patients experience episodes of chronic constipation from many causes, including medications (e.g., opioids, antiemetics, antidepressants), tumor compression/neural plexus invasion, dehydration, poor oral intake, immobility, metabolic disorders (such as hypercalcemia, hypokalemia, or hypothyroidism), and/or autonomic failure [6, 7]. Untreated constipation may lead to distressing symptoms such as abdominal pain and distention, nausea and/or vomiting, anorexia, urinary retention, mental status changes, and delirium [1, 8]. Severe constipation may lead to obstipation and subsequent life-threatening complications of bowel obstruction or perforation [9].
Constipation is inadequately assessed [10] and underdiagnosed [11]. Assessment of constipation by self-report has been proposed and used by a number of authors [11-15]. Preliminary studies have observed limited correlation between patient-reported outcomes and radiological diagnosis of constipation [3, 16]. The Rome criteria are widely accepted and considered to be the most valid tool for the evaluation of constipation in non-cancer patients [17]. Despite the high frequency of and significant discomfort from constipation, the best practice to screen for constipation in advanced cancer patients has not been established.
The primary purpose of this study was to compare the accuracy of patient-reported constipation with the accuracy of the modified Rome III (ROME) criteria and to determine the agreement between patient-reported constipation (by a 0-10 numeric rating score, a report of yes or no, and five questions about constipation-related symptoms scored 0-10) and objective assessment of constipation using the modified ROME criteria among outpatients with advanced cancer. The secondary aims were to determine the associations among constipation, symptom burden (assessed by the Edmonton Symptom Assessment System [ESAS]) and quality of life and to explore the agreement between patient-reported constipation and palliative care specialists’ assessment of constipation.
Methods
The Institutional Review Board at The University of Texas M. D. Anderson Cancer Center approved this study and all patients gave written informed consent.
Patients who attended the Supportive Care Clinic at M. D. Anderson Cancer Center between December 6, 2010 and March 28, 2011 were screened and invited to participate if eligible for this study. Patients aged 18 years or older with advanced cancer were included. Exclusion criteria included patients: with impaired cognition; those who did not speak English; with a diagnosis of inflammatory bowel disease (i.e., Crohn’s disease or ulcerative colitis) or constant diarrhea; with complete or partial bowel obstruction as determined by the palliative care physician; or with a bowel ostomy.
The study coordinator collected the following patient data: age, sex, ethnicity, religious affiliation, marital status, educational level, type of cancer, date of diagnosis and Eastern Cooperative Oncology Group (ECOG) performance status.
Measures
Constipation was assessed using the modified ROME criteria, the patient’s report and the physician’s assessment.
Modified ROME Criteria
A recent paper from Larkin et al. [11] suggests that, as the evidence base is poor and that there are limited data on many aspects of the assessment, diagnosis and management of constipation in palliative care, recommendations should be based on expert clinical opinion or relevant research findings from other settings. We decided to better explore the problem of constipation in advanced cancer by looking at paradigms that already exist in gastroenterology. Several articles regarding constipation in advanced cancer patients or palliative care patients suggest the use of the ROME criteria for the assessment of constipation in this field [7, 10, 18-22]. The ROME criteria also were used to build a questionnaire to screen for constipation in palliative care [8].
The ROME criteria diagnose functional constipation and provide severity indicators [17]. This questionnaire comprises 17 questions relating to abdominal pain or discomfort (questions 1-8), frequency of bowel movements (question 9), stool consistency (questions 10 and 17), ease of defecation (questions 11-15), and the onset of constipation symptoms (question 16) (Appendix I). Most questions have five possible responses ranging from “never/rarely” to “always.” Question 1 has six possible responses ranging from “never” to “every day.” Answers for questions 2, 3, and 16 are “yes” or “no.” In an effort to better adapt the criteria to the dynamic nature of cancer-related constipation, it was necessary to make some minimal changes to the timeline to make it applicable to cancer patients. We increased the time frame for the irritable bowel syndrome (IBS) questions (question 1) from “the last 3 months” to “one year prior to the diagnosis of cancer.” We similarly decreased the interval for the constipation questions (questions 9-15, 17) from “the last 3 months” to “the last 2 weeks.” Since most cancer patients are already on laxatives, we added the phrase “without the use of laxatives” to the loose-stool question (question 17). All these changes were discussed, reviewed and edited for appropriateness by two medical oncologists and two palliative care physicians.
In palliative care patients, it is recommended to take a full patient history to distinguish current and normal pre-illness bowel disorders [11]. As the ROME criteria propose that patients with IBS be considered separately, we report our results in both ways, for the complete cohort and after removing those patients who screened positive for IBS. In each cohort, we assessed the presence of constipation (ROME criteria, yes/no, 0-10) and the severity of constipation was determined as the sum of the scores for questions 9 to 14 (ROME severity).
Patient-Reported Constipation and Related Symptoms
We collected patient-reported constipation data using a numeric rating score ranging from 0 (no symptom) to 10 (worst possible symptom) (PRC 0-10), and a report of yes or no (PRC yes/no). Patients were asked to rate five constipation-related symptoms using an 11-point numeric rating scale ranging from 0 (no symptom) to 10 (worst possible symptom): crampy abdominal pain, abdominal bloating/fullness, belching, gas feeling, and early satiety. These symptoms have been described in the literature as associated with constipation [1, 16, 18, 20, 23-25].
For the PRC 0-10, PRC yes/no and the five related symptoms, we asked patients to describe their symptoms for the past two weeks to coincide with the ROME assessment for stool frequency, consistency, and discomfort. Specifically, the patient would need a two-week window to assess stool frequency to meet the ROME criterion of “fewer than 3 defecations per week.”
Symptom burden was assessed using the ESAS, a validated self-assessment tool that is one of the most commonly used tools for clinical care in cancer [26]. The ESAS was used to measure the intensity of 10 symptoms experienced in the previous 24 hours (pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, lack of appetite, feeling of well-being, and sleep disturbances) [27-29]. Quality of life was assessed using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) [30, 31].
When the interview was finished, patients were asked to complete a questionnaire about their opioid and laxative consumption. The estimated morphine equivalent daily dose was calculated using an equianalgesic conversion table. We calculated the laxatives’ mean equivalent daily dose using the number of laxatives and the dose (e.g., a patient receiving the minimal dose of one laxative would have a score of 1; a patient receiving the minimal dose of two laxatives or twice the minimal dose of one laxative would have a score of 2).
Physicians’ Assessment
Based on his or her best clinical judgment, the palliative care specialist taking care of each patient assessed the patient’s constipation using an 11-point numeric rating scale ranging from 0 (no symptom) to 10 (worst possible symptom) (MDAC 0-10), and a report of yes or no (MDAC yes/no). For both assessments, physicians were allowed to answer “I do not know.”
Statistical Analyses
We report categorical variables as numbers and percentages. We tested the distributions of the continuous variables using the Kolmogorov-Smirnov test and found most to be non-normally distributed. Therefore, we analyzed the data using nonparametric methods, and report continuous variables by their median, with the interquartile range.
To determine the prognostic value of the PRC 0-10 item score for detecting patients with constipation according to the ROME criteria, we summarized sensitivity, specificity, positive and negative predictive value. We used simple unweighted kappa coefficients to test for agreement between PRC Yes/No, PRC 0-10 (using a cutoff of ≥3), MDAC Yes/No, and MDAC 0-10 (using a cutoff of ≥3), with the yes/no outcome of constipation using the ROME criteria. We examined the correlations between the continuous score given by the ROME criteria and the score given by PRC 0-10, MDAC 0-10, and opioid and laxative intake by calculating Spearman correlation coefficients. To determine whether patients with constipation, as defined by the ROME criteria, have a higher symptom burden and worse quality of life than patients without constipation, we used a nonparametric Wilcoxon two-sample test to examine the associations between constipation diagnosis according to the ROME criteria and ESAS symptoms (each rated 0-10), five constipation-related symptoms (rated 0-10), the EORTC QLQ-C30, opioid intake, and laxative intake.
For all our statistical analyses, a P-value of <0.05 was considered to be statistically significant.
Results
A total of 148 patients were eligible and were approached for the study, and 100 were enrolled (Fig. 1). Patient demographics are summarized in Table 1. Forty-six of the 100 (46%) patients enrolled had been diagnosed with cancer at least two years previously, and 41 of 93 (44%) had a performance status score of 0 or 1.
Fig. 1.
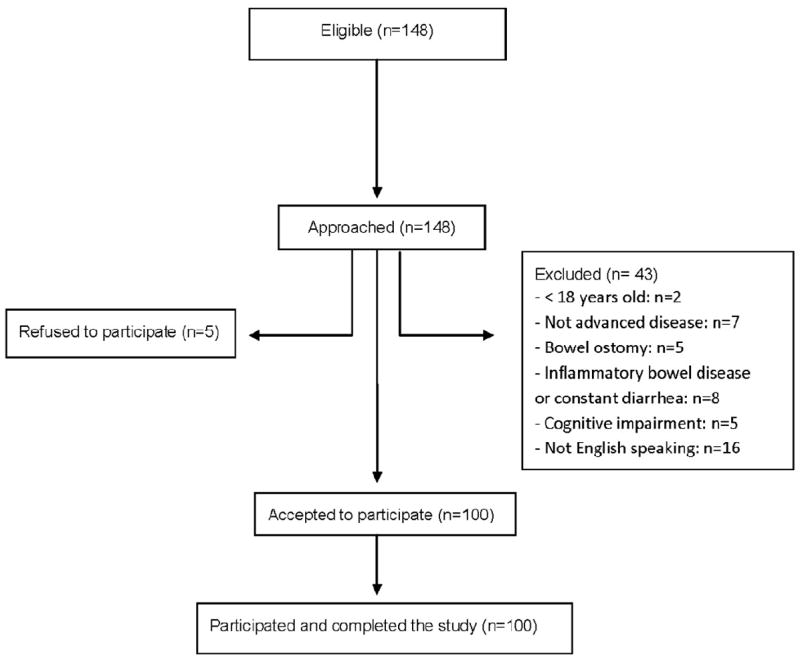
Flowchart of the patient selection process.
Table 1.
Patient Characteristics (N=100)
| Patient Characteristics | n (%) |
|---|---|
| Female | 63 (63) |
| Married | 77 (77) |
| Highest education level | |
| High school or below | 34 (34) |
| Any college undergraduate education | 54 (54) |
| Any advanced postgraduate education | 11 (11) |
| Missing | 1(1) |
| Age (years), median (Q1-Q3) | 57 (49-65) |
| Cancer diagnosis | |
| Breast | 15 (15) |
| Dermatologic | 15 (15) |
| Gastrointestinal | 12 (12) |
| Genitourinary | 10 (10) |
| Gynecologic | 9 (9) |
| Head and neck | 4 (4) |
| Hematologic | 6 (6) |
| Respiratory | 18 (18) |
| Other | 11 (11) |
| ECOG performance status | |
| 0 | 3 (3) |
| 1 | 38 (41) |
| 2 | 35 (38) |
| 3 | 17 (18) |
| Missing | 7 |
| ESAS items [median (Q1-Q3)] | |
| Pain | 5.0 (2.3-7.0) |
| Fatigue | 5.0 (3.0-7.0) |
| Nausea | 1.0 (0.0-3.8) |
| Depression | 2.0 (0.0-4.0) |
| Anxiety | 2.0 (0.0-5.8) |
| Drowsiness | 3.0 (1.0-6.0) |
| Shortness of breath | 1.0 (0.0-4.0) |
| Lack of appetite | 5.0 (2.0-7.0) |
| Feeling of well-being | 4.0 (3.0-7.0) |
| Sleep | 4.0 (2.0-7.0) |
| Opioid MEDD median (Q1-Q3) | 60 (25-190) |
Q1-Q3 = first and third quartiles; ECOG = Eastern Cooperative Oncology Group; ESAS = Edmonton Symptom Assessment System; MEDD = mean equivalent daily dose.
Fifty of the 100 (50%) patients met the ROME criteria for constipation. (Table 2). Fifteen of the 100 patients met criteria for IBS and when these patients were excluded, 38 (45%) of the remaining 85 patients met the criteria for constipation. Sixty-three of the 100 (63%) patients had laxatives prescribed, 24 (38%) of whom were receiving two or more laxatives simultaneously. The most commonly prescribed laxatives were senna (n=54) and polyethylene glycol (n=23).
Table 2.
Agreement Between Modified Rome III Criteria, Patient-Reported Constipation (PRC 0-10; PRC yes/no) and Physician Assessment of Constipation (MDAC 0-10; MDAC yes/no).
| Complete Cohort (N=100) | Cohort Excluding Patients with IBS (N=85) | |||||
|---|---|---|---|---|---|---|
| Variables | Rome III No Constipation n = 50 (50%) | Rome III Constipation n = 50 (50%) | Cohen’s Unweighted Kappa | Rome III No constipation n = 47 (55%) | Rome III Constipation n = 38 (45%) | Cohen’s Unweighted Kappa |
| PRC (≥3) (n=61) |
19 (38%) | 42 (84%) | 0.46 | 18 (38%) | 33 (87%) | 0.47 |
| PRC (yes) (n=49) |
16 (32%) | 33 (66%) | 0.34 | 14 (30%) | 28 (74%) | 0.43 |
| MDAC (≥3) a (n=49) |
19(39%) | 30(61%) | 0.22 | 17 (37%) | 23(62%) | 0.25 |
| MDAC (yes)b (n=59) |
25 (52%) | 34 (71%) | 0.19 | 22 (49%) | 25 (69%) | 0.20 |
IBS = irritable bowel syndrome; PRC = patient-reported constipation; MDAC = physician-assessed constipation.
Two patients were assessed as “I do not know.”
Four patients were assessed as “I do not know.”
Evaluation of PRC 0-10 Accuracy
Table 3 shows the sensitivity and specificity of different cutoff scores for PRC 0-10. The cutoff score ≥3/10 achieved the best combination of sensitivity (0.84) and specificity (0.62), positive predictive value (0.69) and negative predictive value (0.79).
Table 3.
Sensitivity and Specificity of Cutoff Points for Patient-Reported Constipation on a Scale of 0-10
| Cutoff Score | Sensitivity | Specificity | PPV | NPV | Number of False Negatives |
|---|---|---|---|---|---|
| ≥ 1 | 0.92 | 0.40 | 0.61 | 0.83 | 4 |
| ≥ 2 | 0.92 | 0.50 | 0.65 | 0.86 | 4 |
| ≥ 3 | 0.84 | 0.62 | 0.69 | 0.79 | 8 |
| ≥ 4 | 0.74 | 0.68 | 0.70 | 0.72 | 13 |
| ≥ 5 | 0.68 | 0.72 | 0.71 | 0.69 | 16 |
| ≥ 6 | 0.44 | 0.84 | 0.73 | 0.60 | 28 |
| ≥ 7 | 0.38 | 0.86 | 0.73 | 0.58 | 31 |
PPV = positive predictive value; NPV = negative predictive value.
Agreement Between PRC, MDAC and ROME Criteria
Table 2 summarizes the agreement between the yes/no outcome of constipation according to the ROME criteria and the patient- and physician-reported assessments. In both the overall cohort and the cohort excluding patients with IBS, moderate agreement (>0.40) was only observed for PRC ≥3/10 and not for PRC yes/no, MDAC ≥3/10, or MDAC yes/no. One exception was that moderate agreement also was observed for PRC yes/no for the IBS excluded cohort.
We found significant correlation between ROME severity and PRC 0-10 (r= 0.61; P< 0.001). The correlation between ROME severity and MDAC 0-10 was much lower (r=0.34; P= 0.001). There was a significant correlation between ROME and opioid intake (r=0.25; P= 0.015). Although there was a significant association between PRC and ROME severity, the use of PRC ≥3/10 resulted in 38% false positive and 16% false negative rates, and PRC yes/no resulted in 33% false positive and 33% false negative rates, respectively (Table 2).
The correlation between ROME severity and patient-reported outcomes was stronger than the correlation between ROME severity and physicians’ assessment. Those results suggest that patients’ reports of constipation are more reliable than the physicians’ opinion.
Factors Associated With Constipation According to the ROME Criteria
There was no significant association between the ESAS score and the presence of constipation according to the ROME criteria. Table 4 shows that three of the five constipation-related symptoms were significantly more severe among constipated patients and there was a non-significant trend toward more severity for the two remaining symptoms (abdominal bloating and early satiety).
Table 4.
Constipation-Related Symptom Intensity in Patients With and Without Constipation According to the Modified Rome III Criteria
| Variables (scored 0-10) | No Constipation n = 50 Median (Q1-Q3) |
Constipation n = 50 Median (Q1-Q3) |
Pa |
|---|---|---|---|
| Abdominal bloating/fullness | 1.0 (0-4.0) | 2.5 (0.0-5.0) | 0.20 |
| Burping/ belching | 1.0 (0-3.0) | 3.0 (0.0-5.0) | 0.044 |
| Crampy abdominal pain | 0.0 (0.0-3.0) | 3.0 (0.0-6.0) | 0.011 |
| Gassy feeling | 2.0 (0.0-5.0) | 4.5 (2.0-6.0) | 0.027 |
| Early satiety | 1.5 (0.0-6.0) | 4.0 (00-7.0) | 0.09 |
Q1-Q3 = first and third quartiles.
Wilcoxon two-sample test.
The EORTC QLQ-C30 subscale scores did not differ between non-constipated and constipated patients except for cognitive functioning, for which the median scores were 83, interquartile range [IQR] 67-100 for non-constipated patients and 67 (IQR 50-83) for constipated patients (P= 0.05), and financial difficulties, for which the median scores were 33 (IQR 0-67) for non-constipated patients and 67 (IQR 33-75) for constipated patients (P= 0.04). As expected, there was a significant difference in scores for the constipation question, for which the median scores were 0 (range 0-33) for non-constipated patients and 33 (range 33-67) for constipated patients (P < 0.001).
There was a non-significant trend suggesting higher levels of fatigue (EORTC QLQ-C30) for the constipated group; the median score for non-constipated patients was 44 (IQR 22-67) compared with 56 (IQR 33-78) for constipated patients (P= 0.09).
Table 5 shows that both the opioid and the laxative mean adjusted doses were significantly higher for patients with constipation than for those with no constipation.
Table 5.
Comparison ofb Laxatives Between Patients With and Without Constipation According to the Modified Rome III Criteria
| No Constipation n = 50 | Constipation n = 50 | Pa | |
|---|---|---|---|
| Opioid MEDD median (range) | 45.0 (17.5-8) | 91.6 (40.0-330.0) | 0.0034 |
| Laxative EDD median (range) | 1.5 (0.0-3.0) | 3.0 (1.0-6.0) | 0.0098 |
MEDD = mean equivalent daily dose; EDD = equivalent daily dose.
Wilcoxon two-sample test.
Discussion
Among the patients’ and physicians’ subjective assessments of constipation that we evaluated, the self-reporting tool PRC 0-10 with a cutoff score ≥ 3 had the best combination of sensitivity, specificity, and predictive value. We found a high frequency of constipation (50%). Patients’ self-reported constipation as yes/no left almost one-third of patients undiagnosed. The limited sensitivity and specificity of patients’ self-reported constipation (yes/no) suggest that it is not useful for clinical practice.
Our findings are similar to those of Noguera et al. [8]. We found that the PRC 0-10 assessment with a cutoff score ≥3/10 had a better sensitivity than that reported by Noguera et al. This might reflect differences in the patient populations or in the treatments. However, both studies confirm the usefulness of a 0-10 patient-reported outcome for the assessment of constipation in advanced cancer patients. Our findings suggest that approximately 16% of constipated patients will not be identified by this assessment. Therefore, individualized assessment should always be conducted, particularly when there are multiple risk factors.
The modified ROME criteria appeared to be effective for detecting constipation because patients diagnosed with constipation according to these criteria had a higher severity of constipation-related symptoms, and received higher doses of laxatives and opioids. The laxative dose can be considered a measure of the diagnosis made by the clinicians, and in this case, there was a strong association between the modified ROME criteria diagnosis of constipation and the doses of laxatives, as well as of opioids.
This study was conducted in a supportive care outpatient center in a comprehensive cancer center specializing in the management of severe symptom distress. The board-certified palliative medicine specialists are highly trained in the evaluation of physical and psychosocial distress. It is likely that the assessment of constipation in this setting is superior to that in other settings because of the body of knowledge on constipation in palliative medicine [32]. Only eight of the 50 constipated patients were not treated with laxatives, suggesting a good rate of identification by the supportive care team. The results regarding the diagnosis and treatment of constipation in other clinical settings such as medical oncology or family practice may be significantly different. More research is needed in this area.
Because of the cross-sectional design of our research, we cannot differentiate patients with constipation that is refractory to laxative treatment from patients who have just started laxative treatment, nor can we differentiate patients without constipation because they were previously treated with laxatives from patients currently taking laxatives and without constipation.
More research is needed to validate the ROME component in cancer patients and to identify the best way to assess constipation in patients with advanced cancer in clinical practice. It is important that efforts to improve detection of constipation are accompanied by efforts to improve its treatment as well.
Acknowledgments
This study had no specific funding. Dr. E. Bruera is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA1222292.01, and R01CA124481-01.
Appendix I
Rome Questionnaire – Constipation Module
| 1. In the last 3 months, how often did you have discomfort or pain anywhere in your abdomen? |
|
Skip to question 9 |
| 2. For women: Did this discomfort or pain occur only during your menstrual bleeding and not at other times? |
|
|
| 3. Have you had this discomfort or pain 6 months or longer? |
|
|
| 4. How often did this discomfort or pain get better or stop after you had a bowel movement? |
|
|
| 5. When this discomfort or pain started, did you have more frequent bowel movements? |
|
|
| 6. When this discomfort or pain started, did you have less frequent bowel movements? |
|
|
| 7. When this discomfort or pain started, were your stools (bowel movements) looser? |
|
|
| 8. When this discomfort or pain started, how often did you have harder stools? |
|
|
| 9. In the last 3 months, how often did you have fewer than three bowel movements (0-2) a week? |
|
|
| 10. In the last 3 months, how often did you have hard or lumpy stools? |
|
|
| 11. In the last 3 months, how often did you strain during bowel movements? |
|
|
| 12. In the last 3 months, how often did you have a feeling of incomplete emptying after bowel movements? |
|
|
| 13. In the last 3 months, how often did you have a sensation that the stool could not be passed, (i.e., blocked), when having a bowel movement? |
|
|
| 14. In the last 3 months, how often did you press on or around your bottom or remove stool in order to complete a bowel movement? |
|
|
| 15. In the last 3 months, how often did you have difficulty relaxing or letting go to allow the stool to come out during a bowel movement? |
|
|
| 16. Did any of the symptoms of constipation listed in questions 9-15 above begin more than 6 months ago? |
|
|
| 17. In the last 3 months, how often did you have loose, mushy or watery stools? |
|
Footnotes
Disclosures
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mancini I, Bruera E. Constipation in advanced cancer patients. Support Care Cancer. 1998;6(4):356–364. doi: 10.1007/s005200050177. [DOI] [PubMed] [Google Scholar]
- 2.Curtis EB, Krech R, Walsh TD. Common symptoms in patients with advanced cancer. J Palliat Care. 1991;7(2):25–29. [PubMed] [Google Scholar]
- 3.Bruera E, Suarez-Almazor M, Velasco A, et al. The assessment of constipation in terminal cancer patients admitted to a palliative care unit: a retrospective review. J Pain Symptom Manage. 1994;9(8):515–519. doi: 10.1016/0885-3924(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 4.Schuit KW, Sleijfer DT, Meijler WJ, et al. Symptoms and functional status of patients with disseminated cancer visiting outpatient departments. J Pain Symptom Manage. 1998;16(5):290–297. doi: 10.1016/s0885-3924(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 5.Riechelmann RP, Krzyzanowska MK, O’Carroll A, Zimmermann C. Symptom and medication profiles among cancer patients attending a palliative care clinic. Support Care Cancer. 2007;15(12):1407–1412. doi: 10.1007/s00520-007-0253-8. [DOI] [PubMed] [Google Scholar]
- 6.Sykes NP. The pathogenesis of constipation. J Support Oncol. 2006;4(5):213–218. [PubMed] [Google Scholar]
- 7.Clark K, Currow DC. Assessing constipation in palliative care within a gastroenterology framework. Palliat Med. 2011 Jul 20; doi: 10.1177/0269216311414756. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Noguera A, Centeno C, Librada S, Nabal M. Screening for constipation in palliative care patients. J Palliat Med. 2009;12(10):915–920. doi: 10.1089/jpm.2009.0054. [DOI] [PubMed] [Google Scholar]
- 9.Derby S, Portenoy RK. Assessment and management of opioid induced constipation. In: Portenoy RK, Bruera E, editors. Topics in palliative care. Vol. 1. New York: Oxford University Press; 1997. pp. 95–112. [Google Scholar]
- 10.Clark K, Urban K, Currow DC. Current approaches to diagnosing and managing constipation in advanced cancer and palliative care. J Palliat Med. 2010;13(4):473–476. doi: 10.1089/jpm.2009.0274. [DOI] [PubMed] [Google Scholar]
- 11.Larkin PJ, Sykes NP, Centeno C, et al. European Consensus Group on Constipation in Palliative Care. The management of constipation in palliative care: clinical practice recommendations. Palliat Med. 2008;22(7):796–807. doi: 10.1177/0269216308096908. [DOI] [PubMed] [Google Scholar]
- 12.Droney J, Ross J, Gretton S, et al. Constipation in cancer patients on morphine. Support Care Cancer. 2008;16(5):453–459. doi: 10.1007/s00520-007-0373-1. [DOI] [PubMed] [Google Scholar]
- 13.McMillan SC. Presence and severity of constipation in hospice patients with advanced cancer. Am J Hosp Palliat Care. 2002;19(6):426–430. doi: 10.1177/104990910201900616. [DOI] [PubMed] [Google Scholar]
- 14.Garrigues V, Gálvez C, Ortiz V, et al. Prevalence of constipation: agreement among several criteria and evaluation of the diagnostic accuracy of qualifying symptoms and self-reported definition in a population-based survey in Spain. Am J Epidemiol. 2004;159(5):520–526. doi: 10.1093/aje/kwh072. [DOI] [PubMed] [Google Scholar]
- 15.Goodman M, Low J, Wilkinson S. Constipation management in palliative care: a survey of practices in the United kingdom. J Pain Symptom Manage. 2005;29(3):238–244. doi: 10.1016/j.jpainsymman.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Nagaviroj K, Yong WC, Fassbender K, Zhu G, Oneschuk D. Comparison of the constipation assessment scale and plain abdominal radiography in the assessment of constipation in advanced cancer patients. J Pain Symptom Manage. 2011;42(2):222–228. doi: 10.1016/j.jpainsymman.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Longstreth GF, Thompson WG, Chey WD, et al. Functional bowel disorders. Gastroenterology. 2006;130(5):1480–1491. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 18.Abernethy AP, Wheeler JL, Zafar SY. Detailing of gastrointestinal symptoms in cancer patients with advanced disease: new methodologies, new insights, and a proposed approach. Curr Opin Support Palliat Care. 2009;3(1):41–49. doi: 10.1097/SPC.0b013e32832531ce. [DOI] [PubMed] [Google Scholar]
- 19.Clemens KE, Klaschik E. Management of constipation in palliative care patients. Curr Opin Support Palliat Care. 2008;2(1):22–27. doi: 10.1097/SPC.0b013e3282f53146. [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Higginson IJ, Ripamonti C, von Gunten C, editors. Textbook of palliative medicine. London: Hodder Arnold Publishers; 2009. [Google Scholar]
- 21.Bruera E, Yennurajalingam S, editors. Oxford American handbook of hospice and palliative medicine. New York: Oxford University Press; 2011. [Google Scholar]
- 22.Hanks G, Cherny NI, Christakis NA, Fallon M, Kaasa S, Portenoy RK, editors. Oxford textbook of palliative medicine. 4. Oxford: Oxford University Press; 2011. [Google Scholar]
- 23.Norton C. Constipation in older patients: effects on quality of life. Br J Nurs. 2006;15(4):188–192. doi: 10.12968/bjon.2006.15.4.20542. [DOI] [PubMed] [Google Scholar]
- 24.Buddingh KT, Wieselmann E, Heineman E, Broens PM. Constipation and non-specific abdominal pain are the main causes of acute abdominal pain in teenage girls referred for emergency surgical consultation. J Pediatr Gastroenterol Nutr. 2012;54(5):672–676. doi: 10.1097/MPG.0b013e31823c253c. [DOI] [PubMed] [Google Scholar]
- 25.Fallon M, O’Neill B. ABC of palliative care. Constipation and diarrhoea. BMJ. 1997;315(7118):1293–1296. doi: 10.1136/bmj.315.7118.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings G, Biondo PD, Campbell D, et al. Can the global uptake of palliative care innovations be improved? Insights from a bibliometric analysis of the Edmonton Symptom Assessment System. Palliat Med. 2011;25(1):71–82. doi: 10.1177/0269216310381449. [DOI] [PubMed] [Google Scholar]
- 27.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 28.Chang VT, Hwang SS, Feuerman M. Validation of the Edmonton Symptom Assessment Scale. Cancer. 2000;88(9):2164–2171. doi: 10.1002/(sici)1097-0142(20000501)88:9<2164::aid-cncr24>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Carvajal A, Centeno C, Watson R, Bruera E. A comprehensive study of psychometric properties of the Edmonton Symptom Assessment System (ESAS) in Spanish advanced cancer patients. Eur J Cancer. 2011;47(12):1863–1872. doi: 10.1016/j.ejca.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Sprangers MA, Cull A, Groenvold M, et al. The European Organization for Research and Treatment of Cancer approach to developing questionnaire modules: an update and overview. EORTC Quality of Life Study Group. Qual Life Res. 1998;7(4):291–300. doi: 10.1023/a:1024977728719. [DOI] [PubMed] [Google Scholar]
- 31.Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Qual Life Res. 1993;2(4):287–295. doi: 10.1007/BF00434800. [DOI] [PubMed] [Google Scholar]
- 32.Bruera E, Fadul . Constipation and diarrhea. In: Bruera E, Higginson IJ, Ripamonti C, von Guten C, editors. Textbook of palliative medicine. London: Hodder Arnold Publishers; 2009. pp. 554–570. [Google Scholar]


