Abstract
The past 5–10 years have brought significant advances in the identification and validation of novel biochemical biomarkers in the prevention and treatment of acute kidney injury (AKI). These biochemical biomarkers remain research tools but we anticipate that soon they will be employed in clinical practice. A Consensus Conference held by the Acute Dialysis Quality Initiative (ADQI) recently reviewed the evidence, and identified gaps and a research agenda. Furthermore, at this meeting was the birth of an initiative to comprehensively identify new opportunities to characterize the physiological changes during the course of AKI based upon a conceptual framework for the detection and monitoring of renal ischemia-reperfusion injury. This framework includes a transition from monitoring physiological biomarkers of adequate renal perfusion, to pathophysiologic biomarkers of renal hypoperfusion, and finally biomarkers of kidney cell structural injury/damage. Techniques to measure physiological changes in AKI include several physiological variables that might be used in an interactive way to supplement clinical information and biochemical damage biomarkers in the diagnosis and management of AKI. This review summarizes the spectrum of physiological parameters and potential new physiological methods that enable identification of high-risk patients for AKI, facilitate early diagnosis, and differential diagnosis to guide therapeutic management and prognostication. Finally, we propose a research agenda for the next 5 years to facilitate the development and validation of physiological biomarkers in AKI.
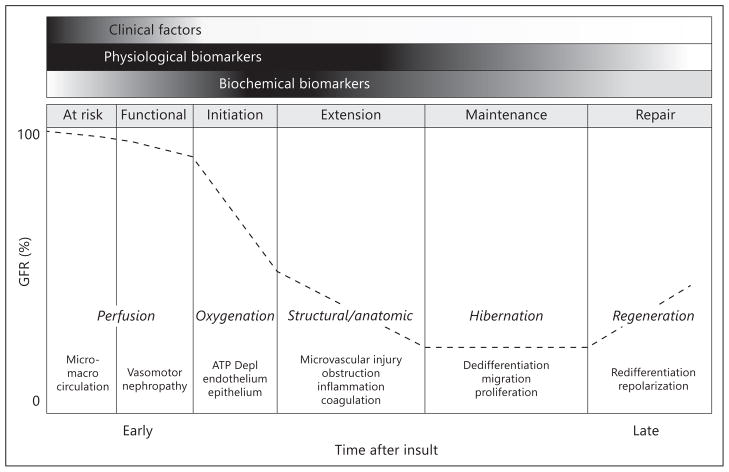
The discovery and validation of novel biomarkers of kidney damage over the past decade represent major advances in the field of acute kidney injury (AKI). The investment by major sponsors and investigators demonstrates a consensus that early biomarkers of AKI are needed. These damage biomarkers proved to be sensitive and specific in a number of small clinical studies, and their utility in diagnosing and prognosticating AKI awaits confirmation in larger studies. Damage biomarkers are biochemical markers of early cellular injury that can be measured upon release from cells into the plasma or urine. However, long before ischemic cellular injury, alterations in the renal microcirculation and tissue oxygenation occur, rendering epithelial cells susceptible to injury. These pathophysiological processes represent microcirculatory dysfunction ultimately leading to AKI. In addition, renal autoregulatory mechanisms are invoked in response to renal insults and play an important role in maintaining homeostasis. The underlying state of health of the kidney and the effective ‘renal reserve’ are important determinants of this autoregulatory response to modify renal function in response to injury. Currently, our ability to detect these changes in kidney function or structure has been limited to measurement of urine output and changes in serum creatinine reflecting alterations in the glomerular filtration rate (GFR). These measurements represent the physiological biomarkers to track the development and course of AKI and are the current parameters utilized for diagnosis and staging of AKI. However, several additional tools are now available to ascertain physiological response to kidney injury. Coupled with the emerging damage markers, these physiological biomarkers might help identify patients at risk, and provide mechanistic information and a window for therapeutic intervention to prevent AKI. As an example, we have developed a modified description of the clinical phases of ischemic AKI [1] that incorporates physiological parameters that complement the use of biochemical biomarkers and clinical characteristics for the study of AKI (fig. 1).
Fig. 1.
Physiological phases of AKI following ischemia-reperfusion injury. The conceptual framework of physiological biomarkers is superimposed upon the previously established concept of clinical phases of AKI. This figure illustrates progression from risk to prerenal AKI and represents experimental data from ischemia reperfusion but not necessarily other forms of AKI such as drug-induced direct nephrotoxicity. Thus physiological biomarkers are not only needed in the early phase of AKI but throughout the continuum of AKI. The ability to measure these physiological variables may lead to identification of patients at risk for AKI, early diagnosis of AKI and inform therapeutic decisions. These physiological processes represent an integrative environment for the interaction of inflammatory mediators, imbalance in the homeostasis of oxygen, nitric oxide and oxygen radicals causing microcirculatory dysfunction and impaired tissue oxygenation leading to AKI. Reproduced with permission from ADQI [58].
Methods
The 10th Acute Dialysis Quality Initiative (ADQI) Consensus Conference on ‘Acute Kidney Injury Biomarkers’ held in Dublin, Ireland, in August 2011 (www.adqi.net), was attended by an international group of experts, and focused on an objective scientific review of the current literature, developing a consensus of opinion, with evidence where possible, on best practice, and articulating a research agenda to address important unanswered questions. Consistent with previous ADQI meetings, a modified Delphi approach was followed. We performed a selected search and review of the available literature pre-conference, as described in detail elsewhere in this volume. We focused on the task of identifying various techniques to characterize the physiological changes that are encountered during the development and course of AKI and determine their utility as physiological biomarkers for patient management. Studies were identified via PubMed and Web of Science using the term ‘biomarker’, and acute kidney injury ‘(AKI)’ combined with ‘physiology’ and ‘functional’. The large body of literature retrieved generated a series of key questions, which were used to limit the scope of the review. Only representative publications are cited in this review.
Results
The ADQI breakout groups identified important new clinical needs in AKI including: (1) Do physiological biomarkers improve the risk assessment of AKI? If so, what set or group of physiological biomarkers is essential for assessment of the functional state of the kidney? (2) Do real-time physiological biomarkers improve the early diagnosis and aid in the differential diagnosis of AKI? (3) What is the interplay or added value of using physiological biomarkers along with biochemical markers of AKI and clinical characteristics in the diagnostic and prognostic assessment of AKI? (4) Can real-time monitoring of physiological biomarkers be used to guide therapy? These questions cannot be answered at this time, but it is our strong belief that in the next 5 years, we will begin to answer some of these important questions.
In this brief review, we summarize the current-state-of-the-art of available techniques to monitor physiological biomarkers, and propose a road map aimed at providing a reliable and quantifiable platform for the measurement of renal physiological biomarkers. Since this is an emerging area, techniques are currently limited and under development.
Renal Blood Flow, Tissue Oxygenation and Microcirculatory Function: Critical Physiological Biomarkers
Adequate parenchymal oxygen is an essential physiological requirement for sustaining kidney cell viability. In the kidney, 90% of oxygen consumption sustains oxidative phosphorylation for ATP production and sodium reabsorption [2]. Since sodium reabsorption is intrinsically dependent on sodium load, renal oxygen consumption is in turn highly dependent on the GFR and renal blood flow (RBF) [3]. RBF, blood flow distribution between cortex and medulla and microcirculatory blood flow may change prior to changes in damage biomarkers and may serve as one of the earliest indicators of altered renal function and complement damage biomarkers. Hyperfiltration and an increase in RBF [4] may reflect differences in ‘renal reserve’ and assessment of this parameter may provide information on risk of AKI. Autoregulation maintains normal GFR and RBF when mean arterial pressures drop to ~80 mm Hg. This is accomplished through a decrease in afferent arteriolar tone mediated by vasodilators such as prostaglandins and increase in efferent arteriolar tone mediated by angiotensin II. Prior to tubular injury, alterations in RBF may represent the earliest measurable physiological changes.
In the absence of adequate oxygen supply, the kidney is at risk for failure [5]. Pathological processes such as AKI disrupt the balance between tissue oxygen, nitric oxide and radical oxygen species [6].
Existing and developing non-invasive techniques to measure physiological parameters of kidney function in AKI are listed in table 1. The known clinical characteristics of each of these techniques are included in table 2. For example, contrast-enhanced ultrasound (CEU) may be used to determine ‘renal reserve’ by measuring changes in RBF in response to a protein load [7]. This maneuver could potentially identify subjects who lack reserve capacity and may be at higher risk for AKI. Near infrared spectroscopy or blood oxygenation level-dependent (BOLD) magnetic resonance imaging (MRI) may be used for detection of changes in oxygenation of the kidney. Detection of changes in oxygenation could identify those individuals at risk for AKI.
Table 1.
Physiological biomarkers for assessment of kidney function in AKI
Glomerular filtration rate/urine flow monitoring
|
Renal perfusion
|
Renal oxygenation
|
Other complimentary markers
|
Table 2.
Characteristics of physiological biomarkers of AKI
| Physiological biomarker | Parameter | Unit | Bedside | Invasive | Continuous | Clinical performance | Cost |
|---|---|---|---|---|---|---|---|
| Urine indices | Urine Na, FeNa, Feurea osmolality | mEq/l, AU, mosm/kg H2O | + | − | − | + | + |
| Serial serum creatinine | Estimated GFR | mg/dl | + | − | − | ? | + |
| Real-time measured GFR | Measured GFR | ml/min | + | + | − | ? | +++ |
| Continuous urine flow | Urine output | ml/kg/h | + | − | + | ? | + |
| Doppler ultrasound | Macrocirculation | resistive index | + | − | − | ? | ++ |
| Contrast-enhanced ultrasound | Macro/microcirculation | AU | + | + | − | ? | +++ |
| Urine pO2 | Renal medullary O2 tension | mm Hg | + | − | + | ? | ++ |
| Bladder pO2 | Tissue O2 tension | mm Hg | + | + | + | ? | ++ |
| BOLD MRI | Renal O2 availability | Hb O2/Hb | − | − | − | ? | ++++ |
| Positron emission tomography | O2 uptake/renal metabolism | mCi/μg | − | + | − | ? | ++++ |
| Near infrared spectroscopy | Renal O2 availability | Hb O2/Hb | + | − | + | ? | ++++ |
| Bioelectrical impedance analysis | Renal tissue perfusion | AU | + | − | + | ? | + |
Glomerular Filtration Rate, Continuous Urine Output Measurement, and Urine Indices
Real-Time GFR Measurement
Determination of GFR is of utmost importance for evaluating kidney function in patients with kidney disease and for appropriate drug dosing. The clearance of an ideal filtration marker such as inulin, iothalamate or iohexol, is the gold standard for measuring GFR [8]. However, these cumbersome, time-consuming techniques are not practical for use in clinical practice. A transcutaneously remote real-time analysis of FITC-sinistrin excretion has been used to determine GFR in healthy rats and rats with kidney disease [9]. In these studies, comparative measurements of transcutaneous and plasma elimination kinetics of FITC-sinistrin were compared, and highly comparable GFR values were demonstrated in all animal groups.
Lately, a point-of-care bedside fluorescence-based measured GFR (mGFR) assay has been developed experimentally for the rapid measurement of GFR [9, 10], which has potential applicability in AKI. In the main descriptive study, fluorescent conjugates of a small freely filterable reporter (inulin) and a large non-filterable marker (500 kDa dextran) were infused as a bolus, and the in vivo fluorescent signals were detected and quantified by the ratiometric optical fiber system [10]. The plasma volume was estimated by dilution of the large dextran molecule and the GFR was determined using the ratiometric two-compartment method 60 min after the injection. This method presented excellent agreement with the concurrent 6-hour iohexol-based GFR measurement technique.
In summary, fully developed real-time transcutaneous or intravascular monitoring of GFR may prove to be an ideal bedside tool for risk stratification, early diagnosis, prognosis and therapy guidance in AKI.
Serial Creatinine Measurement
Serum creatinine is an inexpensive and readily available physiological biomarker of kidney function and remains the ‘gold standard’ test for diagnosing AKI [11, 12], however it is non-specific for the nature and extent of injury and is influenced by several factors including the volume of distribution requiring adjustments for the level of fluid accumulation (prior to achievement of steady-state conditions) [13]. For these reasons, it is considered a suboptimal GFR marker [14] and a late biomarker of AKI when both damage and physiological changes occur together. More frequent measurements might allow earlier detection of AKI and could provide valuable insight into the mechanism and pathways to track the course of AKI. This hypothesis needs to be formally tested.
Continuous Urine Output Measurement
The importance of urine output in the detection of AKI [11] has recently been preliminarily verified [15], providing support for continuously monitoring urine output. A recent study assessed the use of an electronic urine output monitoring device in 20 critically ill patients [16]. Urine volume was measured using a drip detector, based on infrared detection. In this study, hourly urine output was measured with both traditional urinometer and continuous urine output monitoring device; both validated by cylinder measurements. Positive predictive value for AKI of a urine output of 40 ml/h was 91% with the continuous urine output monitoring device, and 77% with the urinometer. Additional studies are needed to further validate the performance of this user-friendly system and to prospectively evaluate whether urine output combined with serial creatinine measurements provide reliable, robust predictive tools in the setting of AKI.
Fractional Excretion of Sodium and Urea, and Urine Osmolality
Urine sodium and osmolality have been standard urine indices used in differentiating pre-renal AKI from acute tubular necrosis for decades. In a small study of patients with various forms of AKI, the fractional excretion of sodium (FeNa) proved to be useful. Although FeNa correctly diagnosed 86 of 87 patients, and urinary osmolality and urinary sodium correctly diagnosed AKI in 46 and 60% of patients, respectively [17], numerous exceptions limit the value of these clinical tests [18].
The measurement of the fractional excretion of urea (Feurea) may be useful in patients with AKI. Studies have documented an Feurea of 50–65% in patients with acute tubular necrosis compared to values below 35% in patients with pre-renal AKI. Although this urine index may be more useful than Feurea in patients who have received diuretics, it should not be used in isolation [19]. The traditional use of FeNa and Feurea will need to be reconsidered given the growing concept that pure functional loss may also be associated with damage and altered tubular function. For example, we will need to understand the diagnostic and prognostic significance of FeNa and Feurea of <1% and 35%, respectively, when damage biomarkers are positive.
Assessment of Renal Tissue Perfusion
Doppler Ultrasound
Using 2D ultrasonography and color Doppler, systolic and diastolic velocities of interlobar arteries can be measured. The Doppler resistive index (RI) described by Pourcelot ((peak systolic velocity − end diastolic velocity)/peak systolic velocity) is the traditional index used as a measure of renal vascular resistance. A value of 0.60 is considered to be a normal value for renal RI, whereas 0.70 is usually considered to be the upper threshold of normal RI in adults [20]. This index has been proposed for the quantification of the changes in renal vascular resistance but may be affected by other factors such as central hemodynamics (especially arterial stiffness, pulse pressure and heart rate), interstitial renal pressure, intra-abdominal pressure and other factors including age and underlying kidney disease. The RI has been found to reflect the degree of parenchymal damage in chronic kidney disease [21, 22] or hypertension [23], predict long-term kidney function in transplanted patients [24] and the diagnosis of obstructive uropathy [25].
Renal Doppler ultrasound has been used to predict AKI [26, 27]. In 37 critically patients with septic shock, the RI predicted the development of AKI 5 days after presentation (RI of 0.77 vs. 0.68 in AKI vs. non-AKI, respectively) [26]. These findings were recently confirmed in mechanically ventilated patients [27]. The RI was found to be higher in patients with acute tubular necrosis (0.85) than in pre-renal AKI (0.67), and better than urinary indices in the diagnosis of persistent AKI [28]. Finally, the RI might be useful in the assessment of renal circulation during the initial resuscitation in sepsis [29]. These studies indicate that Doppler is a simple, non-invasive tool that may be of significant utility in the setting of AKI (table 3).
Table 3.
Utility of physiological biomarkers in AKI
| Physiological biomarker | Risk assessment | Early diagnosis | Differential diagnosis | Prognosis | Therapy guidance |
|---|---|---|---|---|---|
| Urine indices | – | ✓ | ✓ | – | ✓ |
| Serial serum creatinine | ✓ | ✓ | ✓ | ✓ | ✓ |
| Real-time measured GFR | ✓ | ✓ | ✓ | ✓ | ✓ |
| Continuous urine flow | ✓ | ✓ | ✓ | ✓ | ? |
| Doppler ultrasound | ✓ | ✓ | ✓ | ? | ✓ |
| Contrast-enhanced ultrasound | ✓ | ✓ | ✓ | ? | ✓ |
| Urine pO2 | ✓ | ✓ | ? | ? | ✓ |
| Bladder pO2 | ✓ | ✓ | ✓ | ? | ✓ |
| BOLD MRI | ? | ? | ✓ | ✓ | ? |
| Positron emission tomography | ? | ? | ✓ | ✓ | ? |
| Near infrared spectroscopy | ? | ? | ✓ | ✓ | ? |
| Bioelectrical impedance analysis | – | ✓ | – | ✓ | ✓ |
Contrast-Enhanced Ultrasound
The gold standard measurement of RBF is p-aminohippurate (PAH) clearance. Although relatively accurate, this method has the disadvantage of being time-consuming and expensive. Furthermore, decreased renal tubular extraction in AKI and other high-risk settings (cardiopulmonary bypass, critical illness) largely invalidates this method of RBF measurement in such patients.
Contrast-enhanced ultrasonography, although relatively new in assessing renal perfusion, has been used extensively to assess myocardial perfusion during echocardiography. Contrast agents used for ultrasonography are gas-filled microbubbles (1–6 μm in diameter) which scatter ultrasound very effectively and are coated with a protein, lipid or polymer layer [30]. Second-generation microbubbles use inert, poorly soluble perfluorinated gases stabilized by phospholipids or albumin [30]. The small size allows microbubbles to provide information on small capillary beds. Although CEU has been used safely in humans, there are few studies on its use in AKI. In healthy volunteers, use of drugs to block the renin-angiotensin system produced measurable changes in microbubble perfusion indices that correlated with PAH clearance [31]. CEU has been used to diagnose renal artery stenosis, and was found to be more sensitive and specific in comparison to color Doppler ultrasound and similar to angiography [32]. In healthy volunteers, CEU detected a 43% increase in RBF following a high protein meal [7]. The ability to determine renal reserve could be useful in identifying patients who are at risk for AKI. Lastly, in kidney transplantation, CEU has been used to identify perfusion abnormalities helpful in the diagnosis of acute rejection [33] and chronic allograft nephropathy [34].
Bioelectrical Impedance Analysis (BIA)
BIA is a safe non-invasive, rapid and inexpensive technique to measure fluid compartment volume. It measures electrical properties of the body by measuring resistance, reactance and phase angle [35]. This method has been used in assessment nutritional status, urea distribution or dry weight during dialysis. It also has been used to assess intradialytic hypotension, as well as in AKI during CRRT. The use of BIA with serum creatinine is supported by recent data suggesting that further accuracy can be gained if serum creatinine is corrected for fluid accumulation [13]. The current method to assess fluid accumulation is through daily assessment of fluid balance. The use of BIA can give accurate assessment of total body water within minutes and can provide rapid correction of serum creatinine.
Assessment of Kidney Oxygenation and Metabolism
Positron Emission Tomography (PET)
PET is an imaging technique that produces three-dimensional images of physiological activity in the body, by detection of pairs of gamma rays emitted by a positron-emitting radionuclide tracer. The tracer is administered on a biologically active molecule, which tracks to specific areas of physiological activity and quantifies processes of biological importance, in an anatomical context. In the context of the kidney, PET scanning provides the ability to non-invasively quantify both RBF and kidney metabolism. PET measurement of RBF using 15O-labeled water (H215O) has been validated using radiolabelled microspheres in pigs [36]. Similar analyses in humans with and without kidney disease demonstrate that PET with H215O provides a non-invasive method for quantitative mapping of RBF [37]. PET imaging has also been used to evaluate kidney metabolism. In one such study, PET with 18F-fluorodeoxyglucose was used to assess acute rejection in a rat renal transplantation model [38]. Further prospective studies are needed to determine the utility of PET scanning as a tool in the setting of AKI.
Optical Spectroscopy
Optical spectroscopy provides a non-invasive method of determining the oxygenation of blood in tissues. Optical spectroscopy uses differing optical absorbance of light in the near infrared (NIR) region (650–900 nm) between oxy- and deoxyhemoglobin to assess the oxygenation status in various vascular compartments. NIR spectroscopy devices entail an emitting light source directing light into tissues, and one or more sensors placed cutaneously measuring the returned light at several wavelengths. Currently, NIR spectroscopy devices for monitoring the kidney have only been used in children due to the relative accessibility of the pediatric kidney to NIR light in comparison to that in adults. NIR spectroscopy was first used to non-invasively monitor kidney oxygenation transcutaneously in mechanically ventilated children [39]. This study was able to monitor the effects of spontaneous episodes of oxygen desaturation on the kidney and determine the renal oxygen extraction during these episodes. Another study of NIR spectroscopy in children with heart failure was able to demonstrate a strong correlation between renal NIR spectroscopy values measured at the flank and renal vein saturation [40]. NIR spectroscopy has been used to demonstrate the ability of blood transfusions to effectively oxygenate the kidney in anemic children [41]. This use is of special importance due to the sensitivity of the kidney to hypoxemia and anemia [5]. A study in children undergoing cardiac surgery demonstrated a strong correlation between the low kidney oxygen saturation level and the development of AKI [42].
Direct access to the kidney could provide more direct and detailed measurements. Direct observation of the human renal microcirculation for this purpose has been accomplished by the use of hand-held microscopes [43]. Direct NIR spectroscopy measurement for assessment of renal cortex microcirculatory oxygenation has been combined with a miniature laser Doppler device to measure microcirculatory oxygenation and perfusion of the kidney cortex for assessing the success of transplantation [44]. Although the current applications are still sparse, it is expected that technical improvements will permit a comprehensive assessment of renal perfusion and oxygenation and provide a robust clinical platform to monitor the progress, response to therapy and resolution of AKI at the bedside.
BOLD MRI
BOLD MRI is a rapid non-invasive method for assessing renal tissue oxygen bioavailability, relying on the paramagnetic properties of deoxyhemoglobin [45]. The concentration of deoxyhemoglobin increases with rising oxygen consumption, leading to a decreasing T2 relaxation time of the surrounding tissue. Increased R2 (the rate of spin dephasing; equal to 1/T2 relaxation time) levels therefore imply increased deoxyhemoglobin (decreased oxyhemoglobin) and decreased partial pressures of oxygen (PaO2) in renal tissues [45]. The capability of BOLD MRI to evaluate parenchymal oxygen bioavailability in many renal settings has been reported both experimentally and clinically [46–50]. There are fewer studies utilizing BOLD MRI in AKI [51], in acute rejection compared to kidney transplant recipients with prerenal azotemia or acute tubular necrosis [52].
Urine Oxygen (O2) Tension
O2 tension is the local partial pressure of O2 in the urine (PuO2). The pO2 of urine entering the calyces reflects oxygen tension of the renal medulla, and is determined by O2 supply, renal medullary blood flow, and oxygen consumption and may be the most reliable quantitative index of renal medullary perfusion.
Real-time PuO2 has been measured in anesthetized dogs using the polarographic method [53], which is costly and cumbersome. A newer more promising and less expensive dynamic fluorescence quenching method relies on the collision of an O2 molecule with a fluorophore in its excited state, which leads to a non-radiative transfer of energy [53]. MRI has also been used to quantify PuO2 [54].
In studies of anesthetized human volunteers [55], the PuO2 is fairly constant under normal conditions. The PuO2 decreases in states of volume depletion and increases following volume re-expansion. In hemorrhagic shock, there is a sharp decline in PuO2. Bladder PuO2 has also been measured in settings of critical illness [56], cardiac arrest [55], cardiogenic shock [55], and following on-pump cardiac surgery [57], and has been shown to improve by the use of mannitol [55] and fenoldopam [56]. In a study of 90 patients undergoing cardiopulmonary bypass surgery, the postoperative serum creatinine level was significantly higher in patients whose PuO2 decreased after surgery, as compared with those whose PuO2 remained constant or increased [57]. This study presents the possibility of using bladder PuO2 monitoring for the early detection of postoperative AKI.
Application of Physiological Biomarkers for Assessment and Management of AKI
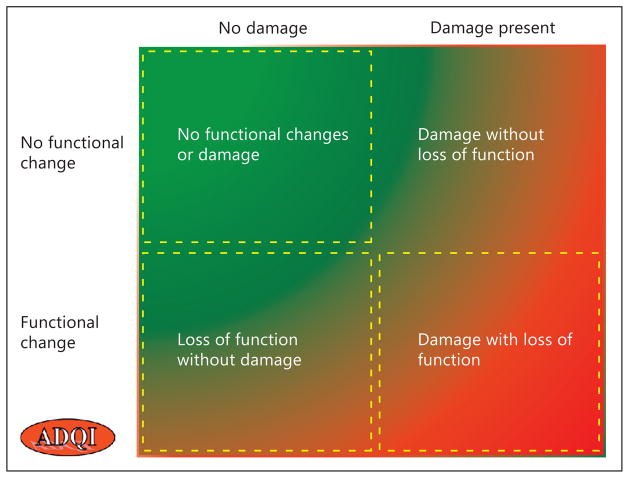
The emergence of novel markers of kidney function offers the promise to provide clinicians with a set of tools to efficiently and more accurately assess the underlying processes resulting from kidney injury and determine the course and time points for intervention. As illustrated in figure 2, we anticipate that the physiological biomarkers identified in this review will be combined with damage markers to classify patients into one of the four AKI categories at presentation, which could be used to track potential transitions from one category to another over time. The choice of physiological biomarkers for diagnosis and staging, differential diagnosis, and prognosis of AKI will need to be determined in prospective studies. We believe that an expanded tool kit of physiological biomarkers might significantly enhance our capabilities to recognize AKI early, and provide a better understanding of the pathophysiology of this heterogeneous condition.
Fig. 2.
Application of physiological biomarkers in the spectrum of AKI. Physiological biomarkers including imaging and other novel markers of kidney function can be used across the quadrants to establish normal or abnormal kidney physiological state. The choice of physiological biomarkers would be based on the contributing factors and correlated with clinical and laboratory findings. The physiological biomarkers would be utilized to determine transitions across these groups with sequential measurements. Which physiological biomarkers are used and the thresholds applied will need to be defined further in prospective studies. Reproduced with permission from ADQI [58].
Conclusions
New methods to assess physiological variables are needed to integrate with existing biomarkers for the assessment of AKI. Many of the concepts discussed in this review are supported by animal studies and early human studies. The challenge will be to identify a minimum set of essential physiological parameters related to renal function, integrate these with the measurement of chemical biomarkers measured at relevant intervals and identify therapeutic/preventive modalities in relation to the physiological state of the kidney monitored at the bedside. Table 4 lists a research agenda to guide investigation for the next 5 years. It is expected that the integration of these techniques into a comprehensive monitoring environment will provide the needed physiological characterization of the kidney together with chemical biomarkers and to monitor to characterize progress and resolution of AKI.
Table 4.
Research agenda on the potential role of physiological biomarkers in AKI
| 1. Physiological biomarkers are valid, testable markers of kidney function and they can provide normal kidney function variables during AKI |
| 2. To test the hypothesis that physiological biomarkers are able to guide titration of fluid management |
| 3. To test the hypothesis that physiological biomarkers are able to determine renal functional reserve |
| 4. To test the hypothesis that physiological biomarkers improve precision of clinical and biochemical markers |
| 5. To test the hypothesis that bioelectrical impedance analysis is valid for assessing volume status |
| 6. To test the hypothesis that bioelectrical impedance analysis to correct for fluid over-load in using serum creatinine improves precision over using serum creatinine alone |
Acknowledgments
This work was the result of a two and half day meeting of the 10th Acute Dialysis Quality Initiative Consensus Conference held in Dublin, Ireland, August 30, 2011 to September 1, 2011.
References
- 1.Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial injury and dysfunction during ischemic acute renal failure. Kidney Int. 2002;62:1539–1549. doi: 10.1046/j.1523-1755.2002.00631.x. [DOI] [PubMed] [Google Scholar]
- 2.Welch WJ. Intrarenal oxygen and hypertension. Clin Exp Pharmacol Physiol. 2006;33:1002–1005. doi: 10.1111/j.1440-1681.2006.04478.x. [DOI] [PubMed] [Google Scholar]
- 3.Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiology. 2008;295:F1259–F1270. doi: 10.1152/ajprenal.90230.2008. [DOI] [PubMed] [Google Scholar]
- 4.Hostetter TH. Human renal response to meat meal. Am J Physiol. 1986;250:F613–F618. doi: 10.1152/ajprenal.1986.250.4.F613. [DOI] [PubMed] [Google Scholar]
- 5.Legrand M, Mik EG, Balestra GM, Lutter R, Pirracchio R, Payen D, Ince C. Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology. 2010;112:119–127. doi: 10.1097/ALN.0b013e3181c4a5e2. [DOI] [PubMed] [Google Scholar]
- 6.Le Dorze M, Legrand M, Payen D, Ince C. The role of the microcirculation in acute kidney injury. Curr Opin Crit Care. 2009;15:503–508. doi: 10.1097/MCC.0b013e328332f6cf. [DOI] [PubMed] [Google Scholar]
- 7.Kalantarinia K, Belcik JT, Patrie JT, Wei K. Real-time measurement of renal blood flow in healthy subjects using contrast-enhanced ultrasound. Am J Physiol Renal Physiol. 2009;297:F1129–F1134. doi: 10.1152/ajprenal.00172.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagher PC, Herget-Rosenthal S, Ruehm SG, Jo SK, Star RA, Agarwal R, Molitoris BA. Newly developed techniques to study and diagnose acute renal failure. J Am Soc Nephrol. 2003;14:2188–2198. doi: 10.1097/01.asn.0000079790.91292.4a. [DOI] [PubMed] [Google Scholar]
- 9.Schock-Kusch D, Xie Q, Shulhevich Y, Hesser J, Stsepankou D, Sadick M, Koenig S, Hoecklin F, Pill J, Gretz N. Transcutaneous assessment of renal function in conscious rats with a device for measuring FITC-sinistrin disappearance curves. Kidney Int. 2011;79:1254–1258. doi: 10.1038/ki.2011.31. [DOI] [PubMed] [Google Scholar]
- 10.Wang E, Meier DJ, Sandoval RM, Von Hendy-Willson VE, Pressler BM, Bunch RM, Alloosh M, Sturek MS, Schwartz GJ, Molitoris BA. A portable fiberoptic ratiometric fluorescence analyzer provides rapid point-of-care determination of glomerular filtration rate in large animals. Kidney Int. 2012;81:112–117. doi: 10.1038/ki.2011.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure – definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A Acute Kidney Injury Network. Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;2007:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macedo E, Bouchard J, Soroko SH, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL. Fluid accumulation, recognition and staging of acute kidney injury in critically ill patients. Crit Care. 2010;14:R82. doi: 10.1186/cc9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function – measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 15.Macedo E, Malhotra R, Claure-Del Granado R, Fedullo P, Mehta RL. Defining urine output criterion for acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2011;26:509–515. doi: 10.1093/ndt/gfq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hersch M, Einav S, Izbicki G. Accuracy and ease of use of a novel electronic urine output monitoring device compared with standard manual urinometer in the intensive care unit. J Crit Care. 2009;24:629, e613–e627. doi: 10.1016/j.jcrc.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Espinel CH, Gregory AW. Differential diagnosis of acute renal failure. Clin Nephrol. 1980;13:73–77. [PubMed] [Google Scholar]
- 18.Zarich S, Fang LS, Diamond JR. Fractional excretion of sodium. Exceptions to its diagnostic value. Arch Intern Med. 1985;145:108–112. [PubMed] [Google Scholar]
- 19.Pepin MN, Bouchard J, Legault L, Ethier J. Diagnostic performance of fractional excretion of urea and fractional excretion of sodium in the evaluations of patients with acute kidney injury with or without diuretic treatment. Am J Kidney Dis. 2007;50:566–573. doi: 10.1053/j.ajkd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Keogan MT, Kliewer MA, Hertzberg BS, DeLong DM, Tupler RH, Carroll BA. Renal resistive indexes: variability in Doppler US measurement in a healthy population. Radiology. 1996;199:165–169. doi: 10.1148/radiology.199.1.8633141. [DOI] [PubMed] [Google Scholar]
- 21.Splendiani G, Parolini C, Fortunato L, Sturniolo A, Costanzi S. Resistive index in chronic nephropathies: predictive value of renal outcome. Clin Nephrol. 2002;57:45–50. doi: 10.5414/cnp57045. [DOI] [PubMed] [Google Scholar]
- 22.Sugiura T, Nakamori A, Wada A, Fukuhara Y. Evaluation of tubulointerstitial injury by Doppler ultrasonography in glomerular diseases. Clin Nephrol. 2004;61:119–126. doi: 10.5414/cnp61119. [DOI] [PubMed] [Google Scholar]
- 23.Derchi LE, Leoncini G, Parodi D, Viazzi F, Martinoli C, Ratto E, Vettoretti S, Vaccaro V, Falqui V, Tomolillo C, Deferrari G, Pontremoli R. Mild renal dysfunction and renal vascular resistance in primary hypertension. Am J Hypertens. 2005;18:966–971. doi: 10.1016/j.amjhyper.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 24.Akgul A, Ibis A, Sezer S, Basaran C, Usluogullari A, Ozdemir FN, Arat Z, Haberal M. Early assessment of renal resistance index and long-term renal function in renal transplant recipients. Ren Fail. 2009;31:18–24. doi: 10.1080/08860220802546347. [DOI] [PubMed] [Google Scholar]
- 25.Platt JF, Rubin JM, Ellis JH. Acute renal obstruction: evaluation with intrarenal duplex Doppler and conventional US. Radiology. 1993;186:685–688. doi: 10.1148/radiology.186.3.8430174. [DOI] [PubMed] [Google Scholar]
- 26.Lerolle N, Guerot E, Faisy C, Bornstain C, Diehl JL, Fagon JY. Renal failure in septic shock: predictive value of Doppler-based renal arterial resistive index. Intensive Care Med. 2006;32:1553–1559. doi: 10.1007/s00134-006-0360-x. [DOI] [PubMed] [Google Scholar]
- 27.Darmon M, Schortgen F, Vargas F, Liazydi A, Schlemmer B, Brun-Buisson C, Brochard L. Diagnostic accuracy of Doppler renal resistive index for reversibility of acute kidney injury in critically ill patients. Intensive Care Med. 2011;37:68–76. doi: 10.1007/s00134-010-2050-y. [DOI] [PubMed] [Google Scholar]
- 28.Platt JF, Rubin JM, Ellis JH. Acute renal failure: possible role of duplex Doppler US in distinction between acute prerenal failure and acute tubular necrosis. Radiology. 1991;179:419–423. doi: 10.1148/radiology.179.2.2014284. [DOI] [PubMed] [Google Scholar]
- 29.Deruddre S, Cheisson G, Mazoit JX, Vicaut E, Benhamou D, Duranteau J. Renal arterial resistance in septic shock: effects of increasing mean arterial pressure with norepinephrine on the renal resistive index assessed with Doppler ultrasonography. Intensive Care Med. 2007;33:1557–1562. doi: 10.1007/s00134-007-0665-4. [DOI] [PubMed] [Google Scholar]
- 30.Klibanov AL. Ultrasound contrast agents: development of the field and current status. Top Curr Chem. 2002;222:73–106. [Google Scholar]
- 31.Kishimoto N, Mori Y, Nishiue T, Nose A, Kijima Y, Tokoro T, Yamahara H, Okigaki M, Kosaki A, Iwasaka T. Ultrasound evaluation of valsartan therapy for renal cortical perfusion. Hypertens Res. 2004;27:345–349. doi: 10.1291/hypres.27.345. [DOI] [PubMed] [Google Scholar]
- 32.Ciccone MM, Cortese F, Fiorella A, Scicchitano P, Cito F, Quistelli G, Pertosa G, D’Agostino R, Guida P, Favale S. The clinical role of contrast-enhanced ultrasound in the evaluation of renal artery stenosis and diagnostic superiority as compared to traditional echo-color-Doppler flow imaging. Int Angiol. 2011;30:135–139. [PubMed] [Google Scholar]
- 33.Benozzi L, Cappelli G, Granito M, Davoli D, Favali D, Montecchi MG, Grossi A, Torricelli P, Albertazzi A. Contrast-enhanced sonography in early kidney graft dysfunction. Transplant Proc. 2009;41:1214–1215. doi: 10.1016/j.transproceed.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 34.Schwenger V, Korosoglou G, Hinkel UP, Morath C, Hansen A, Sommerer C, Dikow R, Hardt S, Schmidt J, Kucherer H, Katus HA, Zeier M. Real-time contrast-enhanced sonography of renal transplant recipients predicts chronic allograft nephropathy. Am J Transplant. 2006;6:609–615. doi: 10.1111/j.1600-6143.2005.01224.x. [DOI] [PubMed] [Google Scholar]
- 35.Di Iorio BR, Scalfi L, Terracciano V, Bellizzi V. A systematic evaluation of bioelectrical impedance measurement after hemodialysis session. Kidney Int. 2004;65:2435–2440. doi: 10.1111/j.1523-1755.2004.00660.x. [DOI] [PubMed] [Google Scholar]
- 36.Juillard L, Janier MF, Fouque D, Lionnet M, Le Bars D, Cinotti L, Barthez P, Gharib C, Laville M. Renal blood flow measurement by positron emission tomography using 15O-labeled water. Kidney Int. 2000;57:2511–2518. doi: 10.1046/j.1523-1755.2000.00110.x. [DOI] [PubMed] [Google Scholar]
- 37.Alpert NM, Rabito CA, Correia DJ, Babich JW, Littman BH, Tompkins RG, Rubin NT, Rubin RH, Fischman AJ. Mapping of local renal blood flow with PET and H215O. J Nucl Med. 2002;43:470–475. [PubMed] [Google Scholar]
- 38.Reuter S, Schnockel U, Schroter R, Schober O, Pavenstadt H, Schafers M, Gabriels G, Schlatter E. Non-invasive imaging of acute renal allograft rejection in rats using small animal F-FDG-PET. PLoS One. 2009;4:e5296. doi: 10.1371/journal.pone.0005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petrova A, Mehta R. Near-infrared spectroscopy in the detection of regional tissue oxygenation during hypoxic events in preterm infants undergoing critical care. Pediatr Crit Care Med. 2006;7:449–454. doi: 10.1097/01.PCC.0000235248.70482.14. [DOI] [PubMed] [Google Scholar]
- 40.Ortmann LA, Fontenot EE, Seib PM, Eble BK, Brown R, Bhutta AT. Use of near-infrared spectroscopy for estimation of renal oxygenation in children with heart disease. Pediatr Cardiol. 2011;32:748–753. doi: 10.1007/s00246-011-9960-5. [DOI] [PubMed] [Google Scholar]
- 41.Dani C, Pratesi S, Fontanelli G, Barp J, Bertini G. Blood transfusions increase cerebral, splanchnic, and renal oxygenation in anemic preterm infants. Transfusion. 2010;50:1220–1226. doi: 10.1111/j.1537-2995.2009.02575.x. [DOI] [PubMed] [Google Scholar]
- 42.Owens GE, King K, Gurney JG, Charpie JR. Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol. 2011;32:183–188. doi: 10.1007/s00246-010-9839-x. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto T, Hayashi K, Matsuda H, Tomura Y, Ogasawara Y, Hashimoto R, Tada T, Tanaka H, Kajiya F. Direct in vivo visualization of glomerular microcirculation by intravital pencil lens-probe CCD videomicroscopy. Clin Hemorheol Microcirc. 2000;23:103–108. [PubMed] [Google Scholar]
- 44.Scheeren TW, Martin K, Maruschke M, Hakenberg OW. Prognostic value of intraoperative renal tissue oxygenation measurement on early renal transplant function. Transpl Int. 2011;24:687–696. doi: 10.1111/j.1432-2277.2011.01258.x. [DOI] [PubMed] [Google Scholar]
- 45.Prasad PV, Chen Q, Goldfarb JW, Epstein FH, Edelman RR. Breath-hold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intra-renal oxygenation. J Magn Res Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 46.Djamali A, Sadowski EA, Muehrer RJ, Reese S, Smavatkul C, Vidyasagar A, Fain SB, Lip-scomb RC, Hullett DH, Samaniego-Picota M, Grist TM, Becker BN. BOLD-MRI assessment of intrarenal oxygenation and oxidative stress in patients with chronic kidney allograft dysfunction. Am J Physiol Renal Physiol. 2007;292:F513–F522. doi: 10.1152/ajprenal.00222.2006. [DOI] [PubMed] [Google Scholar]
- 47.Wang ZJ, Kumar R, Banerjee S, Hsu CY. Blood oxygen level-dependent (BOLD) MRI of diabetic nephropathy: preliminary experience. J Magn Res Imaging. 2011;33:655–660. doi: 10.1002/jmri.22501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thoeny HC, Zumstein D, Simon-Zoula S, Eisenberger U, De Keyzer F, Hofmann L, Vock P, Boesch C, Frey FJ, Vermathen P. Functional evaluation of transplanted kidneys with diffusion-weighted and BOLD MR imaging: initial experience. Radiology. 2006;241:812–821. doi: 10.1148/radiol.2413060103. [DOI] [PubMed] [Google Scholar]
- 49.Textor SC, Glockner JF, Lerman LO, Misra S, McKusick MA, Riederer SJ, Grande JP, Gomez SI, Romero JC. The use of magnetic resonance to evaluate tissue oxygenation in renal artery stenosis. J Am Soc Nephrol. 2008;19:780–788. doi: 10.1681/ASN.2007040420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pedersen M, Dissing TH, Morkenborg J, Stodkilde-Jorgensen H, Hansen LH, Pedersen LB, Grenier N, Frokiaer J. Validation of quantitative BOLD MRI measurements in kidney: application to unilateral ureteral obstruction. Kidney Int. 2005;67:2305–2312. doi: 10.1111/j.1523-1755.2005.00334.x. [DOI] [PubMed] [Google Scholar]
- 51.Juillard L, Lerman LO, Kruger DG, Haas JA, Rucker BC, Polzin JA, Riederer SJ, Romero JC. Blood oxygen level-dependent measurement of acute intra-renal ischemia. Kidney Int. 2004;65:944–950. doi: 10.1111/j.1523-1755.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 52.Han F, Xiao W, Xu Y, Wu J, Wang Q, Wang H, Zhang M, Chen J. The significance of BOLD MRI in differentiation between renal transplant rejection and acute tubular necrosis. Nephrol Dial Transplant. 2008;23:2666–2672. doi: 10.1093/ndt/gfn064. [DOI] [PubMed] [Google Scholar]
- 53.Shaw AD, Li Z, Thomas Z, Stevens CW. Assessment of tissue oxygen tension: comparison of dynamic fluorescence quenching and polarographic electrode technique. Crit Care. 2002;6:76–80. doi: 10.1186/cc1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang Z, Joe B, Coakley F, Zaharchuk G, Busse R, Yeh B. Urinary oxygen tension measurement in humans using magnetic resonance imaging. Acad Radiol. 2008;15:1467–1473. doi: 10.1016/j.acra.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leonhardt KO, Landes RR. Oxygen tension of the urine and renal structures. Preliminary report of clinical findings. N Engl J Med. 1963;269:115–121. doi: 10.1056/NEJM196307182690301. [DOI] [PubMed] [Google Scholar]
- 56.Morelli A, Rocco M, Conti G, Orecchioni A, Alberto De Blasi R, Coluzzi F, Pietropaoli P. Monitoring renal oxygen supply in critically-ill patients using urinary oxygen tension. Anesth Analg. 2003;97:1764–1768. doi: 10.1213/01.ANE.0000087037.41342.4F. [DOI] [PubMed] [Google Scholar]
- 57.Kainuma M, Yamada M, Miyake T. Continuous urine oxygen tension monitoring in patients undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:603–608. doi: 10.1016/s1053-0770(96)80137-6. [DOI] [PubMed] [Google Scholar]
- 58.Acute Dialysis Quality Initiative (ADQI) [accessed January 10, 2013]; www.ADQI.org.