Abstract
Visit-to-visit variability (VVV) of clinic systolic blood pressure (SBP) has been associated with cardiovascular disease risk. Given the need for obtaining blood pressure (BP) at multiple visits to calculate VVV, substituting BP variability from ambulatory blood pressure monitoring (ABPM) may be a practical alternative. We assessed the correlation between VVV of BP and BP variability from ABPM using data from 146 untreated, mostly normotensive participants (mean age 47.9 years) in a substudy of the ongoing Masked Hypertension Study. VVV of SBP and diastolic blood pressure (DBP) was estimated by the standard deviation (SDvvv) and average real variability (ARVvvv) from 6 study visits over a median of 216 days. ABPM data were used to calculate the day-night SD (SDdn) and the ARV of SBP and DBP over 24 hours (ARV24). For SBP, the mean SDvvv and SDdn were 6.3 (SD=2.5) and 8.8 (SD=1.8) mmHg, respectively, and mean ARVvvv and ARV24 were 7.2 (SD=3.2) and 8.4 (SD=2.1) mmHg, respectively. The Spearman correlation coefficient between SDvvv and SDdn of SBP was rs=0.25 and between ARVvvv and ARV24 was rs=0.17. Participants in the highest quartile of SDdn of SBP were 1.66 (95% CI: 0.93 – 2.75) times more likely to be in the highest quartile of SDvvv of SBP. The observed-to-expected ratio between the highest quartiles of ARVvvv and ARV24 of SBP was 0.89 (95% CI: 0.41 – 1.69). The correlations for SDvvv and SDdn and ARVvvv and ARV24 of DBP were minimal. These data suggest VVV and 24-hour variability are weakly correlated and not interchangeable.
Keywords: Blood pressure variability, reliability, ambulatory blood pressure monitoring, blood pressure measurement, methods
Ambulatory blood pressure monitoring (ABPM) is considered to provide a more accurate estimate of a patient’s mean systolic and diastolic blood pressure (SBP and DBP) compared to clinic measurements.1, 2 Also, mean blood pressure (BP) from ABPM is a stronger risk factor for cardiovascular disease (CVD) compared to mean clinic BP.3–5 The value of ABPM for the measurement of BP is highlighted in the 2011 NICE guidelines which recommend using it to confirm the diagnosis of hypertension for patients with an SBP or DBP ≥ 140 or 90 mmHg, respectively.6, 7
Several recent studies have reported strong associations between visit-to-visit variability (VVV) of clinic SBP and the occurrence of stroke, coronary heart disease and all-cause mortality.8, 9 This has created substantial interest in BP variability as a novel risk factor and possible target for interventions to reduce CVD risk.10, 11 However, calculating VVV of BP requires multiple visits and therefore is not currently done in clinical practice. Substituting BP variability from ABPM for VVV of BP may be a practical alternative. However, a meta-analysis of 8,938 individuals demonstrated weak or no associations between measures of BP variability from ABPM (e.g., day-night standard deviation, SDdn, and average real variability over 24 hours, ARV24) and CVD incidence and all-cause mortality.12 While these data suggest that VVV of BP and ABPM measures of BP variability represent different underlying constructs, few data are available on the relationship of BP variability derived from ABPM to that derived across multiple clinic visits.
The goal of the current study was to assess the association between VVV and ABPM measures of BP variability. Additionally, we determined correlates of VVV and ABPM variability of BP. To accomplish these goals, we analyzed data on SBP and DBP from a substudy of the Masked Hypertension Study.
METHODS
The Masked Hypertension Study, an ongoing study of the prevalence, predictors, and prognosis of masked hypertension, is comprised of employees recruited from Stony Brook University, Stony Brook University Hospital, Columbia University Medical Center, and a private hedge fund management organization in New York. The study was restricted to individuals ≥ 18 years of age who were not taking antihypertensive or other medications that are known to affect blood pressure. Additionally, participants were deemed ineligible if they had a history of CVD or major arrhythmias, evidence of secondary hypertension other than a history of pregnancy-induced hypertension, a serum creatinine >1.6 mg/dL, liver disease, adrenal disease, or thyroid disease. Based on the average of their second and third BP measurements during an initial screening visit prior to enrollment, participants were required to have a clinic SBP <160 mmHg and DBP <105 mmHg.
Of relevance to the current analysis, eligible participants attended 5 visits over a 4-week period. During each of the first 3 visits (Visit 1–3), which were scheduled to occur over a 3-week period, clinic BP was measured. At the conclusion of Visit 3, the participant was fitted with an ABPM device for BP monitoring over the subsequent 24 hours. At Visit 4, the next day, participants returned the ABPM device and were given a plastic container and instructions for the collection of an overnight urine sample. At Visit 5, CVD risk factor measures including anthropometrics were obtained. As part of a validation substudy, a randomly selected subset of approximately 20% of Masked Hypertension Study participants recruited at Stony Brook University or Stony Brook University Hospital completed a second set of four visits (Visits 6–9) an average of 7 months (median of 6 months) after their visit 5. For these participants, during Visits 6–8, which again were scheduled to occur over a 3-week period, clinic BP was once again measured. The median duration between visits with clinic BP measurements was 7, 11, 165, 7 and 9 days for visits 1 to 2, 2 to 3, 3 to 6, 6 to 7 and 7 to 8, respectively. Overall, the median duration between visits 1 and 8 was 216 days.
Overall, 886 participants were enrolled between February 2005 and August 2011. For the current analysis, we included 174 (19.6%) participants who completed the validation substudy visits and, thus, could have 6 clinic visits with BP measurements. Of these participants, 16 did not have BP measurements at all six study visits, 10 were missing nighttime SD from ABPM, and 2 participants had < 80% valid ABPM measurements and were excluded from all analyses. After excluding these participants, there were 146 participants available for the current analysis. The Masked Hypertension Study and validation substudy were approved by the Institutional Review Boards at the participating institutions and all participants provided written informed consent.
Of relevance to the current study, information on demographics (age, race, ethnicity, and gender) was collected by standardized questionnaire. At Visit 5, waist circumference was measured mid-way between the lowest rib and the iliac crest with the participant standing. Also at Visit 5, fasting blood samples were drawn from participants and glycosylated hemoglobin (HbA1c) was measured using high pressure liquid chromatography. Diabetes was defined as a fasting HbA1c ≥ 6.5%, current use of insulin or oral hypoglycemics, or a patient-reported diagnosis of diabetes by a physician.13 Among participants not using insulin or oral hypoglycemics and without a history of diabetes, impaired fasting glucose was defined as an HbA1c of 5.7% – 6.4%, and normal glucose was defined as an HbA1c < 5.7%. High-sensitivity C-reactive protein (hsCRP) was measured by nephelometry and levels > 3 mg/L were defined as elevated.13 Using the urine collected the night before Visit 5, urinary albumin and urinary creatinine were measured by nephelometry and albuminuria was calculated as the albumin-to-creatinine ratio (ACR).
Blood Pressure Measurements
During Visits 1–3 and Visits 6–8, clinic BP was measured in a standardized manner consistent with published recommendations.14 Prior to clinic BP measurements being obtained, participants were asked to sit at rest for 5 minutes or longer. Arm circumference was measured, and appropriate-sized cuffs were utilized for BP assessment. A trained research nurse/technician obtained three sitting clinic BP readings, at 1–2 minute intervals, using a Baum mercury sphygmomanometer (W.A. Baum, Copiague, NY) and stethoscope. For each visit, the three measurements were averaged and the six visit averages were used for all subsequent calculations and analyses.
At the conclusion of visit 3, participants were fit with an appropriate-sized arm cuff for the Spacelabs ABPM (Model 90207; Spacelabs, Redmond, WA). ABP readings were taken at 28-minute intervals throughout the following 24 hours. Recordings were analyzed to obtain average awake and sleep SBP and DBP levels, based on times defined by data obtained from an actigraphy monitor worn on the wrist (ActiWatch; Phillips Respironics, Murrayville, PA) and supplemented by diary reports of the times participants woke up and went to sleep.
Derivation of BP Variability Measures
Two measures of VVV and ABPM variability were calculated for the current study. For VVV, we calculated the intra-individual SD and the ARV across the six visits. ARV takes into account the order in which the BP measurements were obtained and is a measure of differences between adjacent visits.15 For ABPM, we calculated the SDdn and ARV24. The SD for daytime measurements and, separately for nighttime measurements were calculated based on when participants were awake and sleeping and the SDdn calculated as the weighted mean of these SDs.16 Weights were calculated as the duration of time participants were awake and sleeping. This approach is considered advantageous compared to calculating a single SD over 24 hours because it eliminates the influence of the day-night change in BP. ARV24 was calculated as the average absolute difference between consecutive readings.17 The ARV24 accounts for the order of BP measurements over the ABP monitoring period. Day-night changes in SBP and DBP (i.e., dipping) were calculated as the ratio of mean sleep-to-awake SBP and DBP, respectively.
Statistical methods
Participant characteristics were calculated as mean (95% confidence interval) or percentage as appropriate. Given its non-Gaussian distribution, albuminuria is presented as geometric mean (95% confidence interval). Paired t-tests were used to compare the mean levels of clinic and ambulatory measures of mean BP and BP variability. Below we describe the analyses conducted for SDvvv and SDdn of SBP. Identical analyses were conducted for SDvvv and SDdn of DBP and for ARVVVV and ARV24 of SBP and DBP. Scatterplots between SDVVV and SDdn of SBP were created and Spearman correlation coefficients were calculated. SDvvv and SDdn of SBP were divided into quartiles based on the distribution in the study sample. Weighted Kappa statistics were used to calculate the agreement between quartiles.18 Among individuals in the highest quartile of SDdn, we calculated the observed and expected number of participants in the highest quartile of SDvvv. Next, using linear regression we identified factors associated with SDvvv and, separately, SDdn. Factors investigated included age, gender, race, ethnicity, diabetes status, abdominal obesity, elevated hsCRP, albuminuria, and mean SBP across study visits for SDVVV and over 24 hours for SDdn. Two sensitivity analyses were conducted. First, the correlation between daytime SD (SDday) with SDvvv of SBP and DBP was assessed. Additionally, the agreement between SDday and SDvvv of SBP and DBP was calculated. Second, the correlation between sleep-to-awake ratio of SBP and DBP with SDVVV was determined. Additionally, the mean SDVVV was calculated for extreme dippers, dippers and non-dippers (sleep-to-awake ratio of SBP and DBP < 0.8, 0.8 to ≤ 0.9 and > 0.9, respectively).19 P-values < 0.05 were considered statistically significant. Analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
The mean age of the 146 study participants included in the current analyses was 47.9 years, 35.6% were men, 6.2% were black and 6.2% were Hispanic (Table 1). The mean SBP from clinic and 24-hour ABPM measurements was 115.2 mmHg (SD=10.9) and 117.6 mmHg (SD=9.5), respectively (p<0.001). The mean DBP was 74.7 mmHg (SD=7.3) for clinic measurements and 72.3 mmHg (SD=7.0) for the 24-hour ABPM measurements, respectively (p<0.001).
Table 1.
Characteristics of participants in the Masked Hypertension Study.
| Characteristics | Mean (SD) or percentage* (n=146) |
|---|---|
| Age, years | 47.9 (9.2) |
| Men | 35.6% |
| Black | 6.2% |
| Hispanic | 6.2% |
| Diabetes status | |
| Normal fasting glucose | 58.6% |
| Impaired fasting glucose | 36.6% |
| Diabetes | 4.8% |
| Abdominal obesity | 32.9% |
| Elevated C-reactive protein | 19.2% |
| Albumin-to-creatinine ratio, mg/g | 4.4 (2.3 – 8.5) |
| Clinic SBP, mmHg | |
| Mean | 115.2 (10.9) |
| Standard deviation (SDvvv) | 6.3 (2.5) |
| Average real variability (ARVvvv) | 7.2 (3.2) |
| Clinic DBP, mmHg | |
| Mean | 74.7 (7.3) |
| Standard deviation (SDvvv) | 4.6 (1.7) |
| Average real variability (ARVvvv) | 5.2 (2.3) |
| Ambulatory SBP, mmHg | |
| Mean – 24-hour | 117.6 (9.5) |
| Mean - awake | 122.5 (9.8) |
| Mean - sleep | 105.0 (10.3) |
| Standard deviation (SDdn) | 8.8 (1.8) |
| Average real variability (ARV24) | 8.4 (2.1) |
| Ambulatory DBP, mmHg | |
| Mean – 24-hour | 72.3 (7.0) |
| Mean - awake | 76.7 (7.5) |
| Mean - sleep | 61.0 (7.6) |
| Standard deviation (SDdn) | 7.4 (1.5) |
| Average real variability (ARV24) | 7.1 (1.5) |
SD – standard deviation, SBP – systolic blood pressure, DBP – diastolic blood pressure
Except albuminuria which is geometric mean (95% confidence interval)
Impaired fasting glucose defined as hemoglobin A1c of 5.7% to <6.5% and diabetes was defined as ≥ 6.5% or anti-diabetes medication use; abdominal obesity is defined as a waist circumference > 35 inches in women or > 40 inches in men; elevated C-reactive protein defined as levels > 3 mg/L
Visit-to-visit and day-night standard deviation of blood pressure
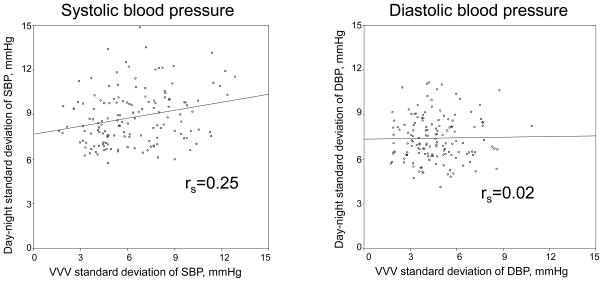
For both SBP and DBP, the SDvvv was lower than the SDdn (SDvvv and SDdn SBP: 6.3 and 8.8 mmHg, respectively, and SDvvv and SDdn DBP: 4.6 and 7.4 mmHg, respectively; each p<0.001). The Spearman correlation of SDvvv and SDdn was 0.25 for SBP and 0.02 for DBP (Figure 1). The weighted kappa for the concordance of quartiles of SDvvv and SDdn of SBP was 0.17 (Table 2, top panel). The analogous weighted kappa statistic for DBP was 0.01 (Table 2, bottom panel). Participants in the highest quartile of SDdn of SBP were more likely to be in the highest quartile of SDvvv of SBP (observed-to-expected ratio =1.66, 95% CI: 0.93 – 2.75). For DBP, the observed-to-expected ratio for being in the highest quartile of SDdn and SDvvv was 1.11 (95% CI: 0.56 – 1.98).
Figure 1.
Scatterplot of the visit-to-visit standard deviation (SDVVV) vs. day-night standard deviation (SDdn) from ambulatory blood pressure monitoring for systolic blood pressure (left panel) and diastolic blood pressure (right panel).
SBP – systolic blood pressure
DBP – diastolic blood pressure
Table 2.
Cross tabulation of quartiles of visit-to-visit standard deviation (SDVVV) and day-night standard deviation (SDdn) of systolic blood pressure (top panel) and diastolic blood pressure (bottom panel).
| Quartile of day-night standard deviation of SBP from ambulatory monitoring (range in mmHg) | |||||
|---|---|---|---|---|---|
| 1 (<7.45) | 2 (7.45 – 8.49) | 3 (8.50 – 9.93) | 4 (≥ 9.94) | ||
| Quartile of VVV standard deviation of SBP (range in mmHg) | 1 (<4.41) | 12 | 11 | 9 | 4 |
| 2 (4.41 – 5.95) | 10 | 10 | 10 | 7 | |
| 3 (5.96 – 7.99) | 10 | 7 | 10 | 10 | |
| 4 (≥ 8.00) | 4 | 9 | 8 | 15 | |
| Quartile of day-night standard deviation of DBP from ambulatory monitoring (range in mmHg) | |||||
|---|---|---|---|---|---|
| 1 (<6.36) | 2 (6.36 – 7.10) | 3 (7.11 – 8.39) | 4 (≥ 8.40) | ||
| Quartile of VVV standard deviation of DBP (range in mmHg) (range in mmHg) | 1 (<3.40) | 9 | 7 | 12 | 8 |
| 2 (3.40 – 4.36) | 8 | 12 | 6 | 11 | |
| 3 (4.37 – 5.62) | 11 | 8 | 11 | 7 | |
| 4 (≥ 5.63) | 8 | 10 | 8 | 10 | |
Numbers in table represent number of study participants
Weighted Kappa statistic = 0.17
Observed-to-expected ratio for highest quartile: 1.66 (95% CI: 0.93 – 2.75)
Numbers in table represent number of study participants
Weighted Kappa statistic = 0.01
Observed-to-expected ratio for highest quartile: 1.11 (95% CI: 0.56 – 1.98)
Calculated via: http://www.sph.emory.edu/~cdckms/exact-midP-SMR.html
VVV – visit-to-visit variability
After multivariable adjustment for age, gender, race, ethnicity, and mean clinic SBP, higher levels of albuminuria were associated with higher SDvvv of SBP (Table 3). Although not statistically significant after further adjustment for diabetes status, abdominal obesity and elevated CRP, each doubling of albuminuria was associated with a 0.43 (standard error = 0.22, p=0.06) mmHg higher SDvvv of SBP. Mean clinic SBP was associated with higher SDvvv after multivariable adjustment. After multivariable adjustment, older age, being male, and higher mean 24-hour SBP were each independently associated with higher SDdn of SBP. Only higher mean clinic DBP was associated with higher SDvvv of DBP. After adjustment for age, race, ethnicity, sex and mean 24-hour DBP from ABPM, abdominal obesity was associated with higher SDdn of DBP. After multivariable adjustment, older age was associated with higher SDdn of DBP.
Table 3.
Adjusted differences in visit-to-visit and day-night standard deviation of systolic blood pressure (top panel) and diastolic blood pressure (bottom panel) associated with participant characteristics.
| Systolic blood pressure | ||||
|---|---|---|---|---|
| Age, gender, race, ethnicity, and mean SBP adjusted | Fully adjusted | |||
| Visit-to-visit SD β (SE) | Day-night SD β (SE) | Visit-to-visit SD β (SE) | Day-night SD β (SE) | |
| Age, per 10 years | 0.28 (0.23) | 0.59 (0.15)*** | 0.23 (0.24) | 0.50 (0.16)** |
| Men | −0.45 (0.46) | 0.64 (0.30)* | −0.31 (0.51) | 0.70 (0.32)* |
| Black | −0.62 (0.83) | −0.69 (0.57) | −0.62 (0.89) | −0.45 (0.60) |
| Hispanic | 1.44 (0.83) | −0.59 (0.56) | 1.63 (0.84) | −0.53 (0.58) |
| Diabetes status | ||||
| Normal fasting glucose | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| Impaired fasting glucose | 0.37 (0.44) | 0.45 (0.30) | 0.27 (0.45) | 0.36 (0.30) |
| Diabetes | 0.99 (0.93) | 0.23 (0.63) | 0.86 (0.96) | 0.15 (0.65) |
| Abdominal obesity | 0.07 (0.46) | 0.39 (0.30) | −0.01 (0.52) | 0.44 (0.34) |
| Elevated c-reactive protein | −0.12 (0.52) | −0.09 (0.35) | −0.32 (0.59) | −0.36 (0.40) |
| Albuminuria, per doubling of level | 0.47 (0.21)* | 0.22 (0.15) | 0.43 (0.22) | 0.17 (0.15) |
| Mean clinic SBP, per 10 mmHg | 0.85 (0.22)*** | 0.84 (0.24)*** | ||
| Mean 24-hour SBP, per 10 mmHg | 0.48 (0.16)** | 0.43 (0.17)** | ||
| Diastolic blood pressure | ||||
| Age, gender, race, ethnicity, and mean DBP adjusted | Fully adjusted | |||
| Visit-to-visit SD | Day-night SD | Visit-to-visit SD | Day-night SD | |
| β (SE) | β (SE) | β (SE) | β (SE) | |
| Age, per 10 years | −0.16 (0.15) | 0.35 (0.13)** | −0.16 (0.16) | 0.29 (0.14)* |
| Men | −0.09 (0.31) | 0.22 (0.27) | −0.11 (0.34) | 0.36 (0.28) |
| Black | 0.66 (0.58) | 0.22 (0.51) | 0.64 (0.63) | 0.12 (0.54) |
| Hispanic | 0.24 (0.59) | 0.19 (0.51) | 0.13 (0.60) | 0.17 (0.52) |
| Diabetes status | ||||
| Normal fasting glucose | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| Impaired fasting glucose | −0.07 (0.31) | 0.25 (0.27) | −0.02 (0.32) | 0.14 (0.28) |
| Diabetes | 0.66 (0.67) | 0.89 (0.58) | 0.83 (0.69) | 0.68 (0.59) |
| Abdominal obesity | 0.10 (0.33) | 0.67 (0.26)* | 0.24 (0.37) | 0.58 (0.30) |
| Elevated c-reactive protein | −0.14 (0.37) | 0.46 (0.31) | −0.30 (0.42) | 0.04 (0.36) |
| Albuminuria, per two fold higher level | −0.17 (0.15) | 0.15 (0.13) | −0.20 (0.16) | 0.07 (0.14) |
| Mean clinic DBP, per 10 mmHg | 0.66 (0.21)** | 0.63 (0.23)** | ||
| Mean 24-hour DBP, per 10 mmHg | 0.12 (0.18) | 0.10 (0.18) | ||
(SE) is the difference in the standard deviation (standard error) from linear regression models.
p<0.05,
p<0.01,
p<0.001
DBP – diastolic blood pressure, SD – standard deviation, SBP – systolic blood pressure
Fully adjusted includes all variables listed.
Average real variability
The ARVVVV was lower than the ARV24 for SBP (7.2 and 8.4 mmHg, respectively; p<0.001) and DBP (5.2 and 7.1 mmHg, respectively; p<0.001). The Spearman correlation between ARVVVV and ARV24 was 0.17 for SBP and −0.13 for DBP (Supplemental Figure 1). The weighted Kappa statistic for quartiles of ARVVVV and ARV24 were 0.08 for SBP and −0.13 for DBP (Supplemental Table 1). For SBP, there was no association between being in the highest quartile of ARVVVV conditional on being in the highest quartile of ARV24 (observed-to-expected ratio = 0.89, 95% CI: 0.41 – 1.69). For DBP, individuals in the highest quartile of ARV24 were less likely to be in the highest quartile of ARVVVV (0.44, 95% CI: 0.14 – 1.07). Hispanics had higher ARVVVV of SBP than whites and elevated CRP was associated with higher ARV24 of SBP (Table 4). Mean clinic SBP was associated with higher ARVVVV and mean 24-hour SBP was associated with higher ARV24. None of the factors investigated were associated with ARVVVV or ARV24 of DBP.
Table 4.
Adjusted differences in visit-to-visit and 24-hour average real variability of systolic blood pressure (top panel) and diastolic blood pressure (bottom panel) associated with participant characteristics.
| Systolic blood pressure | ||||
|---|---|---|---|---|
| Age, gender, race, ethnicity, and mean SBP adjusted | Fully adjusted | |||
| ARVVVV β (SE) | ARV24 β (SE) | ARVVVV β (SE) | ARV24 β (SE) | |
| Age, per 10 years | 0.34 (0.29) | 0.22 (0.19) | 0.30 (0.31) | 0.19 (0.20) |
| Men | −0.58 (0.59) | 0.46 (0.37) | −0.57 (0.65) | 0.59 (0.40) |
| Black | −0.74 (1.08) | −0.74 (0.71) | −1.02 (1.15) | −0.46 (0.74) |
| Hispanic | 2.32 (1.07)* | 0.27 (0.70) | 2.40 (1.09)* | 0.21 (0.71) |
| Diabetes status | ||||
| Normal fasting glucose | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| Impaired fasting glucose | 0.40 (0.56) | 0.53 (0.37) | 0.38 (0.58) | 0.39 (0.37) |
| Diabetes | 1.47 (1.20) | 0.35 (0.79) | 1.38 (1.24) | −0.13 (0.81) |
| Abdominal obesity | −0.36 (0.60) | 0.30 (0.38) | −0.64 (0.68) | −0.16 (0.42) |
| Elevated c-reactive protein | 0.03 (0.66) | 0.89 (0.43)* | 0.04 (0.76) | 0.98 (0.49)* |
| Albuminuria, per two fold higher level | 0.42 (0.28) | 0.31 (0.18) | 0.38 (0.29) | 0.29 (0.19) |
| Mean clinic SBP, per 10 mmHg | 0.96 (0.28)*** | 1.04 (0.30)*** | ||
| Mean 24-hour SBP, per 10 mmHg | 0.55 (0.20)** | 0.49 (0.21)* | ||
| Diastolic blood pressure | ||||
| Age, gender, race, ethnicity, and mean SBP adjusted | Fully adjusted | |||
| ARVVVV | ARV24 | ARVVVV | ARV24 | |
| β (SE) | β (SE) | β (SE) | β (SE) | |
| Age, per 10 years | −0.22 (0.21) | 0.09 (0.14) | −0.23 (0.22) | 0.09 (0.15) |
| Men | 0.22 (0.43) | −0.05 (0.29) | 0.25 (0.47) | 0.11 (0.30) |
| Black | 0.86 (0.80) | 0.08 (0.54) | 0.84 (0.86) | 0.09 (0.58) |
| Hispanic | 0.30 (0.80) | 0.31 (0.54) | 0.29 (0.83) | 0.32 (0.56) |
| Diabetes status | ||||
| Normal fasting glucose | 0 (ref) | 0 (ref) | 0 (ref) | 0 (ref) |
| Impaired fasting glucose | −0.04 (0.43) | 0.05 (0.29) | −0.02 (0.44) | −0.06 (0.30) |
| Diabetes | 0.98 (0.91) | 0.66 (0.62) | 1.16 (0.94) | 0.35 (0.63) |
| Abdominal obesity | 0.09 (0.45) | 0.40 (0.28) | 0.29 (0.51) | 0.19 (0.32) |
| Elevated c-reactive protein | −0.35 (0.50) | 0.58 (0.33) | −0.62 (0.58) | 0.44 (0.39) |
| Albuminuria, per two fold higher level | 0.0 (0.21) | 0.19 (0.14) | −0.03 (0.22) | 0.16 (0.15) |
| Mean clinic DBP, per 10 mmHg | 0.38 (0.29) | 0.35 (0.31) | ||
| Mean 24-hour DBP, per 10 mmHg | −0.11 (0.19) | −0.14 (0.20) | ||
(SE) is the difference in the standard deviation (standard error) from linear regression models.
p<0.05,
p<0.01,
p<0.001
SD – standard deviation, SBP – systolic blood pressure
Fully adjusted includes all variables listed.
Visit-to-visit and day-time standard deviation of blood pressure
The Spearman correlation of SDvvv and SDday was 0.21 for SBP and 0.06 for DBP. The weighted Kappa statistic between SDvvv and SDday was 0.05 for SBP and between SDvvv and SDday was 0.08 for DBP. The multivariable adjusted association of awake mean SBP with SDdn of SBP was similar to that observed for mean 24-hour SBP described above (data not shown). After age, gender, race, ethnicity and full multivariable adjustment, awake mean DBP from ABPM was not associated with SDdn of DBP (data not shown).
Visit-to-visit standard deviation and day-night change in blood pressure
The Spearman correlation of SDvvv and sleep-to-awake ratio in SBP and DBP was 0.07 and 0.05, respectively. There were 19 (13%), 98 (67%), and 29 (20%) participants with a sleep-to-awake ratio in SBP <0.8 (extreme dippers), 0.8 to ≤ 0.9 (dippers), and > 0.9 (non-dippers), respectively. The average SDvvv of SBP was 6.3 (SD 2.2) mm Hg, 6.3 (SD 2.6) mm Hg and 6.4 (SD 2.5) mm Hg for participants with a sleep-to-awake SBP ratio < 0.8, 0.8 to ≤ 0.9, and > 0.9, respectively (p-trend=0.91). For DBP, 78 (53%), 54 (37%), and 14 (10%) of participants had a sleep-to-awake ratio < 0.8, 0.8 to ≤ 0.9, and > 0.9, respectively. The average SDvvv of DBP was 4.5 (SD 1.5) mm Hg, 4.9 (SD 2.1) mm Hg and 4.1 (SD 1.4) mm Hg for participants with a sleep-to-awake DBP ratio < 0.8, 0.8 to ≤ 0.9, and > 0.9, respectively (p-trend=0.77). After multivariable adjustment, no association was present between SDvvv and sleep-to-awake ratio for SBP or DBP (data not shown).
DISCUSSION
In this study of people not taking antihypertensive medication, VVV of clinic BP and 24-hour variability of BP on ABPM were only weakly correlated. The lack of a strong association between these two measures of BP variability was consistent for SBP and DBP and using SD and ARV as measures of variability. Furthermore, when VVV of BP and 24-hour variability were grouped into quartiles, the degree of agreement between the levels of these markers was not significantly better than chance.18 Similarly low correlations were present between visit-to-visit variability and awake BP variability and also day-night changes in BP. These data suggest that BP variability over 24 hours from a single ABPM is not a proxy marker for VVV of BP obtained across multiple clinic visits and that the mechanisms responsible for these indices of variability differ.
Studies have reported a strong association between VVV of SBP and stroke events.8 For example, comparing the highest to lowest deciles of the standard deviation (SD) of SBP over seven visits in the UK-TIA trial, the hazard ratio for stroke was 6.22 (95% CI: 4.16 – 9.29) after multivariable adjustment including mean SBP. The associations of VVV of SBP with both coronary heart disease (CHD) and all-cause mortality are also well established.8, 9, 20, 21 Among 956 normotensive US adults with BP measured three times over two months in NHANES III, the multivariable adjusted hazard ratios for all-cause mortality associated with a SD of SBP of either 4.80 – 8.34 mmHg or ≥8.35 mmHg, versus <4.80 mmHg, were 1.57 (95% CI: 1.07–2.18) and 1.50 (95% CI: 1.03–2.18), respectively.9 In contrast, using data pooled from 11 studies on 8,938 adults, Hansen and colleagues found a weak association (hazard ratios < 1.2) between BP variability (e.g., SDdn and ARV24) from ABPM and CVD outcomes.12 The discrepancy between VVV of BP, which maintains a strong association with outcomes, and shorter term BP variability from ABPM is consistent with the findings of our study, which suggests that these two phenotypes may have a different underlying pathophysiology.
At least three recent studies have reported the correlation between VVV of BP and 24-hour BP variability.8, 22, 23 In the BP lowering arm of the Anglo-Scandinavian Cardiac Outcomes Trial, the correlation between SD of daytime BP on ABPM and SDVVV for SBP was 0.26.8 The authors of this study also reported that the coefficient of variation of daytime SBP was not correlated with SDVVV. In the European Lacidipine Study on Atherosclerosis, the Pearson correlation coefficients between SDVVV of BP from ≥ 7 visits and ABPM SD of BP were 0.20 and 0.15 for SBP and DBP, respectively.22 A similar correlation was reported between VVV of SBP and sleep and awake ABP variability (r=0.19 and 0.20, respectively) in a study by Eguchi and colleagues.23 These modest correlations between VVV and 24-hour variability are consistent with the current study. In contrast to prior studies, for the current study we conducted a more in-depth study of the relationship between VVV and 24-hour variability. The association was weak between being in the highest quartiles of SDdn and SDVVV as well as for the highest quartiles of ARVVVV and ARV24. These data suggest that BP variability on ABPM does not discriminate VVV of BP. The current study extends the prior studies of VVV and variability on ABPM to a multi-ethnic cohort of individuals without hypertension and the consistency of the findings is noteworthy.
The low correlation between VVV of BP and 24-hour BP variability is not entirely surprising given the hypothesized differences in their underlying mechanisms.23, 24 There is a substantial amount of data showing physical and emotional stimulation result in BP variability over the course of a day.24 In contrast, it is recommended that clinic BP be measured in a controlled setting limiting the influence of external stimuli.14 At each of the six clinic visits used in the current study, BP was measured following the same study protocol and quality control was monitored. There have been additional biologic underpinnings (e.g., impaired sympathetic function, arterial stiffness, and inflammation) hypothesized to affect both VVV of BP and 24-hour BP variability.10, 25, 26 However, there is a paucity of data on these mechanisms and whether they have a similar effect on VVV of BP and 24-hour BP variability.
While mean SBP was associated with both SDVVV and SDdn, a few factors were not associated with both outcomes. The higher 24-hour BP variability, but not VVV of BP, among older participants and men may be an indication that these participants experience greater variability in, or a heightened response to, physical activity, mental stress, or physical, behavioral, or emotional factors over the ambulatory monitoring period. While the differential effects of C-reactive protein and albuminuria on VVV of BP and 24-hour BP variability is interesting, we urge caution in making definitive conclusions given the modest sample size of the current study. Future studies are needed to evaluate the different mechanisms that may underlie higher levels of VVV of BP and 24-hour BP variability.
The results from the current study should be interpreted in the context of known and potential limitations. Although 886 participants completed the Masked Hypertension Study, only 20% of participants were invited to complete the validation substudy. The duration of time between visits with BP measurements varied substantially, ranging from a median of 7 days between visits 1 and 2 to 165 days between visits 3 and 6. While a longer duration of time between visits 3 and 6 was planned, this may have affected the correlation between VVV of BP and 24-hour variability of BP. Despite these limitations, the current study has several strengths. These include high quality control for the measurement of BP; the availability of three BP measurements per clinic visit; and the collection of a broad array of potential correlates of high BP variability.
The correlation between VVV of BP and 24-hour BP variability from ABPM was low in the current study. As VVV of BP requires multiple visits and may not be practical, or in some cases feasible, data from the current study suggest that using BP variability from ABPM will not provide similar information. Future studies are needed to evaluate practical approaches to measuring VVV of BP across multiple clinic visits.
Supplementary Material
Acknowledgments
We are indebted to the study participants and research staff of the Masked Hypertension Study, without whose cooperation and dedication this study would not have been possible.
Sources of Funding: This work was supported by grants P01-HL47540 (PI: J Schwartz) and R24-HL076857 (PI: K Davidson) from the National Heart, Lung, and Blood Institute. The research was also supported in part by General Clinical Research Center Grant MO1-RR10710 (Stony Brook University) and Columbia University’s CTSA grant No. UL1 RR024156 from NCATS-NCRR/NIH. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Footnotes
Conflict of Interest(s) / Disclosure(s) Statement: None
References
- 1.Staessen JA, Beilin L, Parati G, Waeber B, White W. Task force iv: Clinical use of ambulatory blood pressure monitoring. Participants of the 1999 consensus conference on ambulatory blood pressure monitoring. Blood pressure monitoring. 1999;4:319–331. doi: 10.1097/00126097-199912000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Pickering TG, Shimbo D, Haas D. Ambulatory blood-pressure monitoring. N Engl J Med. 2006;354:2368–2374. doi: 10.1056/NEJMra060433. [DOI] [PubMed] [Google Scholar]
- 3.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 4.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E Office versus Ambulatory Pressure Study I. Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. The New England journal of medicine. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 5.Staessen JA, Thijs L, Fagard R, O’Brien ET, Clement D, de Leeuw PW, Mancia G, Nachev C, Palatini P, Parati G, Tuomilehto J, Webster J. Predicting cardiovascular risk using conventional vs ambulatory blood pressure in older patients with systolic hypertension. Systolic hypertension in europe trial investigators. JAMA. 1999;282:539–546. doi: 10.1001/jama.282.6.539. [DOI] [PubMed] [Google Scholar]
- 6.National institute for health and clinical excellence. Nice clinical guideline 127. Hypertension: Clinical management of primary hypertension in adults. 2011 [Google Scholar]
- 7.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, Hodgkinson J, Mant J, Martin U, Williams B, Wonderling D, McManus RJ. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: A modelling study. Lancet. 2011;378:1219–1230. doi: 10.1016/S0140-6736(11)61184-7. [DOI] [PubMed] [Google Scholar]
- 8.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlof B, Sever PS, Poulter NR. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 2010;375:895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 9.Muntner P, Shimbo D, Tonelli M, Reynolds K, Arnett DK, Oparil S. The relationship between visit-to-visit variability in systolic blood pressure and all-cause mortality in the general population: Findings from nhanes iii, 1988 to 1994. Hypertension. 2011;57:160–166. doi: 10.1161/HYPERTENSIONAHA.110.162255. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM. Limitations of the usual blood-pressure hypothesis and importance of variability, instability, and episodic hypertension. Lancet. 2010;375:938–948. doi: 10.1016/S0140-6736(10)60309-1. [DOI] [PubMed] [Google Scholar]
- 11.Oparil S. New challenges in blood pressure goals and assessment. Nat Clin Pract Cardiovasc Med. 2011:1–2. [Google Scholar]
- 12.Hansen TW, Thijs L, Li Y, Boggia J, Kikuya M, Bjorklund-Bodegard K, Richart T, Ohkubo T, Jeppesen J, Torp-Pedersen C, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA. Prognostic value of reading-to-reading blood pressure variability over 24 hours in 8938 subjects from 11 populations. Hypertension. 2010;55:1049–1057. doi: 10.1161/HYPERTENSIONAHA.109.140798. [DOI] [PubMed] [Google Scholar]
- 13.International Expert C. International expert committee report on the role of the a1c assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–1334. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the american heart association council on high blood pressure research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 15.Muntner P, Joyce C, Levitan EB, Holt E, Shimbo D, Webber LS, Oparil S, Re R, Krousel-Wood M. Reproducibility of visit-to-visit variability of blood pressure measured as part of routine clinical care. J Hypertens. 2011;29:2332–2338. doi: 10.1097/HJH.0b013e32834cf213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilo G, Giglio A, Styczkiewicz K, Caldara G, Maronati A, Kawecka-Jaszcz K, Mancia G, Parati G. A new method for assessing 24-h blood pressure variability after excluding the contribution of nocturnal blood pressure fall. J Hypertens. 2007;25:2058–2066. doi: 10.1097/HJH.0b013e32829c6a60. [DOI] [PubMed] [Google Scholar]
- 17.Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23:505–511. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 18.Landis JR, Koch GG. An application of hierarchical kappa-type statistics in the assessment of majority agreement among multiple observers. Biometrics. 1977;33:363–374. [PubMed] [Google Scholar]
- 19.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–135. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 20.Grove JS, Reed DM, Yano K, Hwang LJ. Variability in systolic blood pressure--a risk factor for coronary heart disease? Am J Epidemiol. 1997;145:771–776. doi: 10.1093/oxfordjournals.aje.a009169. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YT, Tu ST, Cho TJ, Chang SJ, Chen JF, Hsieh MC. Visit-to-visit variability in blood pressure strongly predicts all-cause mortality in patients with type 2 diabetes: A 5.5-year prospective analysis. European journal of clinical investigation. 2012;42:245–253. doi: 10.1111/j.1365-2362.2011.02574.x. [DOI] [PubMed] [Google Scholar]
- 22.Mancia G, Facchetti R, Parati G, Zanchetti A. Visit-to-visit blood pressure variability in the european lacidipine study on atherosclerosis: Methodological aspects and effects of antihypertensive treatment. J Hypertens. 2012;30:1241–1251. doi: 10.1097/HJH.0b013e32835339ac. [DOI] [PubMed] [Google Scholar]
- 23.Eguchi K, Hoshide S, Schwartz JE, Shimada K, Kario K. Visit-to-visit and ambulatory blood pressure variability as predictors of incident cardiovascular events in patients with hypertension. Am J Hypertens. 2012 doi: 10.1038/ajh.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancia G. Short- and long-term blood pressure variability: Present and future. Hypertension. 2012 doi: 10.1161/HYPERTENSIONAHA.112.194340. [DOI] [PubMed] [Google Scholar]
- 25.Tatasciore A, Zimarino M, Renda G, Zurro M, Soccio M, Prontera C, Emdin M, Flacco M, Schillaci G, De CR. Awake blood pressure variability, inflammatory markers and target organ damage in newly diagnosed hypertension. Hypertens Res. 2008;31:2137–2146. doi: 10.1291/hypres.31.2137. [DOI] [PubMed] [Google Scholar]
- 26.Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Brown MD. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in african americans. Hypertension research : official journal of the Japanese Society of Hypertension. 2012;35:55–61. doi: 10.1038/hr.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.