Abstract
Native mass spectrometry, or as is sometimes called “native electrospray (ESI)” allows proteins in their native or near-native protein in solution to be introduced into gas phase and interrogated by MS. This approach is now a powerful tool to investigate protein complexes. This article reviews the background of native MS of protein complexes and describes its strengths, taking photosynthetic pigment-protein complexes as examples. Native MS can be utilized in combination with other MS-based approaches to obtain complementary information to that provided by tools such as X-ray crystallography and NMR spectroscopy to understand the structure-function relationships of protein complexes. When additional information beyond that provided by native MS is required, other MS-based strategies can be successfully applied to augment the results of native MS.
Keywords: ESI-MS, Native ESI, photosynthesis, pigment-protein complex, top-down
1. Introduction
Many functional units in biology are high-order complexes formed of proteins and ligands [1]. As an example, the multi-subunit pigment-protein complexes in photosynthetic systems are important and timely examples [2]. Native mass spectrometry (MS), in addition to the traditional tools for protein structural studies, X-ray crystallography and NMR spectroscopy, is emerging as a complementary tool to interrogate protein complexes in their near native states [3,4] and to provide insights that complement those from traditional biology approaches [5].
Efforts to study the relationship between protein structure and function [6] focus on higher order protein complexes [7]. In photosynthesis, these complexes are composed of proteins and cofactors, usually pigments. In photosynthesis, light energy is absorbed by pigment-protein complexes in an antenna complex and is funneled to the reaction center complex. This process relies on the light-absorbing pigment molecules, that are arranged at well-defined positions and angles in the pigment-protein complexes to facilitate energy transfer [8]. Motivated by their importance, traditional and novel structural biology methods continue to be developed and applied to these complexes [1]. High-resolution methods (e.g., X-ray crystallography and NMR spectroscopy [9]), and low-resolution methods (e.g., cryo-electron microscopy [10] and small-angle X-ray scattering (SAXS) [11]) are the principal tools. In addition, MS is providing an important complementary approach [12]. Although it does not yet provide high resolution data, it has advantages that protein complexes can be interrogated in their near-native state with consumption of relatively small amounts of sample and with fast turnaround. One strategy in the MS “tool box” is native MS (the definition is given in 2.2), which is a fast-growing approach to address questions in the characterization of protein complexes [13]. A summary of native MS based studies, from fundamental MS to critical applications in photosynthetic pigment-protein complexes, is presented here.
2. Mass spectrometry of protein complexes
2.1 Generating ions of protein complexes
The first step in an MS-based study of protein complexes is generating ions of the intact complexes. It was not possible before the 1980s to generate ions in any routine way from nonvolatile and labile biomolecules, much less from large bio-molecular complexes. Traditional ionization methods (e.g., electron ionization (EI) and chemical ionization (CI)) are suitable for volatile small molecules, and Fast Atom Bombardment (FAB), the first routine approach to nonvolatile biomolecules, is inappropriate for large biomolecules. The introduction of electrospray ionization (ESI) and matrix assisted laser desorption ionization (MALDI) positioned MS to evolve into a more important method for macromolecular studies owing to both its sensitivity and tolerance of sample heterogeneity. Proteins can be analyzed in solution by ESI [14] or as solids formed by co-crystallization with UV-absorbing organic acids by using MALDI [15]. Protein complexes can also be approached using both ionization methods [16,17], although most efforts directed at protein complexes come from using ESI-MS [18]. For MALDI, preserving non-covalent interactions of protein complexes in the solid phase is problematic, and the singly-charged large protein ions have m/z values usually beyond the optimum range of most commercial instruments. One prospect to overcome this is to combine cross-linking with MALDI [19] to determine the stoichiometry, composition of protein complexes and cross-linking yield.
The focus of this review is ESI. A standard setup involves flowing an analyte solution to the end of a capillary held at high electrical potential (Figure 1A) [20]. A parallel gas flow surrounds the capillary to aid nebulization of the emerging analyte solution. Because the electrical potential is high, positive ions (in the positive-ion mode of ESI) accumulate at the tip of the solution, causing the solution to form a “Taylor cone” [21]. The stream of solution is drawn out as small, charged droplets that move away each other owing to electrostatic repulsion of the excess positive (or negative) ions within each droplet. According to the charge-residue mechanism [22] of ESI, solvent evaporation continually reduces the size of charged droplet until the Coulombic repulsions between crowded positive ions overcome the droplet surface tension (called the Rayleigh limit). Droplets undergo fission and form even smaller droplets [23]. Evaporation and fission are repeated in several cycles until non-solvated gas-phase ions are produced.
Figure 1.
Native MS of protein complexes. A) Electron spray (ESI) process; B) The comparison between native ESI and regular ESI; C) Regular ESI and native ESI spectra of Fenna-Matthews-Olson (FMO) antenna protein samples.
2.2 Comparing regular ESI and Native ESI (Native MS)
Several factors, including pH of the solution, concentration of non-volatile salts, and fraction of organic solvent affect the protein in ESI. Using a typical ESI solvent (50% Acetonitrile, 50% Water, 0.1% Formic Acid, pH = 1~2), causes the majority of proteins to be in a denatured state, where the proteins are largely unfolded and have their basic side chains exposed to solvent [24] (Figure 1B). The ESI spectra of denatured proteins show a broad charge-state distribution (charge envelope) centered at low m/z (Figure 1C). Because non-covalent interactions between proteins and pigments/ligands are usually destroyed in their denatured state, preservation of the native state of a protein and the even more demanding maintenance of a non-covalent protein complex are problematic when using typical ESI solvents.
To preserve the native state and the noncovalent interactions in a protein complex, it must be in aqueous solution at physiological pH and appropriate ionic strength. An ESI approach called native MS (or native ESI) meets these requirements [25,26]. Proteins are placed in a solution containing a volatile salt (e.g., ammonium acetate) and directly sprayed by using ESI. In the gas phase, the native-like proteins carry fewer charges than denatured or unfolded states owing to the fewer exposed basic residues in the folded form. As a consequence, the protein ions exhibit a narrow spread of a charge envelope or distribution; the m/z of the protein in this case falls at a higher value (Figure 1C). Non-covalent interactions are thus preserved.
Another advance in ESI that benefits studies of protein complex is nano ESI (nESI) [27]. nESI generates smaller droplets than does regular ESI by spraying the sample through a capillary with a smaller diameter (e.g., ~ 1 μm inner diameter) than normally used for ESI. Ionization conditions (spray voltage, capillary temperature, nebulizer gas flow) are gentler for evaporating smaller droplets than the larger drops of conventional ESI [28]. nESI also dramatically reduces the amount of protein sample (e.g., from ~50 to 1–3 μL) required for MS analysis.
While native ESI can introduce protein assemblies to the gas phase, the relationship between their structure in the gas-phase and in solution is of significance. Given that water molecules stabilize protein structure in solution, an ongoing question has been whether there is any similarity between the native-state protein structure in solution and its structure in the gas phase. The issue is whether the non-covalent interactions that join together the protein subunits can be preserved in the gas phase [29,30]. Of the two major types of noncovalent interactions involved in these protein assemblies, electrostatic and hydrophobic, the electrostatic interactions are strengthened in the gas phase in the absence of solvent, whereas hydrophobic interactions are weakened by dehydration and introduction into the gas phase [31]. Ion mobility (IM) measurements can provide critical structural insights on this question of protein assemblies in the gas phase [32,33]. One remarkable example is the study of trp RNA binding protein, TRAP, that forms a ring structure with 11 subunits. Evidence from IM measurements indicate that the ring structures are preserved in the absence of bulk solvent [34]. Klassen and coworkers reported supporting evidence that comes from the measurement of hydrophobic interaction kinetics in the gas phase [35]. Outcomes from these and other experiments indicate strongly that the same solution-phase interactions as well as some protein complexes with near-native structures can be preserved in the gas phase at least in the short timeframe of mass spectrometric observation [25].
2.3 Analyzing protein complex ions in the gas phase
After successfully generating ions of protein complexes, transmitting and mass analyzing these ions become the next challenges for MS. One major advance is the “collisional focusing” (or “collisional cooling”) for transmitting large protein complex ions in the source and ion-guide regions [36]. In this breakthrough, it was demonstrated that increasing pressure of the source region in the first vacuum stage resulted in signal improvement of native-like protein complex ions at high m/z [37]. Similar “collisional focusing” effects are used in the later stages of mass spectrometers (quadrupole and collision cell regions). Carefully adjusting the pressure during ion transmission is an essential step for MS-based studies of protein complexes [38]. Another issue is that protein complexes with molecular weights over 60 kDa form ions having an m/z greater than 4000. Thus, the quadrupole analyzer, which is a major ion transmission component in hybrid mass spectrometers [39], must be able to transmit the ions. When a quadrupole is operating in the RF-only mode, ions can be focused by RF frequency and transmitted to the detector, but the maximum m/z ions that can be transmitted are determined by the RF amplitude, inner radius of the quadrupole, and RF frequency, which can be modified or adjusted to increase the upper m/z limit. In practice, usually the RF frequency is reduced to fulfill this purpose [40,41]. Reports show that “hybrid” quadrupole time-of-flight (Q-TOF) instruments equipped with low-frequency quadrupoles can achieve extended mass ranges above m/z 20,000 [38,42].
Two major mass analyzers can satisfy the analysis of high m/z ions of protein complexes: time-of-flight (TOF) and Fourier transform ion cyclotron resonance (FTICR) although orbitraps are improving in this area. Very recently, a modified Orbitrap mass analyzer was used to measure protein assemblies of molecular weights approaching one megadalton with sensitivity down to the detection of single ions and outstanding mass-spectral resolution [43]. Given that the TOF mass analyzer has a relatively simple design and high sensitivity, the majority of protein-complex studies have been conducted with these instruments, which can transmit and mass analyze large protein complexes of MDa masses [42]. The mass measurement accuracy achievable to ions of protein complexes depends more on complete ion desolvation than on instrument mass resolving power and accuracy. Even the high resolving power provided by FTICR MS is less important than the quality of ionization and the ability to remove all solvent and counterions from the intact protein complexes without destroying them. Advantages of FTICR MS also include the availability of various dissociation approaches that have important applications for the study of protein complexes [44].
2.4 Manipulating ions of protein complexes
The observed mass of a complex from native ESI is usually higher than that calculated based on the protein sequence. Because desolvation is incomplete, extra solvent molecules or other small MW substances in the solution can reside in the final droplet along with the protein ions [45]. These species associate with the protein, resulting in broadened protein peaks in the native ESI mass spectrum. Desolvation can be improved by applying collision energy in the source or collision-cell regions [46]. The collision energy used in “cleaning up” native ESI mass spectra, however, needs to be tuned carefully to avoid complex unfolding and dissociation [47,48]. In practice, the most accurate mass assignments for protein complexes come from the spectra acquired under conditions just below the dissociation energy of the complexes whereby most small molecules have been “shaken off” [49], but the complex remains largely intact.
Dissociation of the complex can be achieved by tandem MS [44]. Protein complexes gain internal energy during collisions with an inert gas in the ion-source or collision-cell region. When the overall internal energy is sufficient to break non-covalent interactions, the protein complex often releases some subunits [50]. Light-Wahl et al. first reported the release of a single monomer from the tetrameric concanavalin A [51]. Interestingly, the charge partitioning between ejected monomer and the rest of complex is asymmetric based on the charge per mass [52]. Systematic studies of structure and charge effects on protein complex dissociation are now being conducted [53,54], and new evidence shows that the loss of a highly charged monomer is not the only pathway for dissociation of protein assemblies [55].
Although the majority of tandem MS experiments are conducted by using collision-induced dissociation (CID), new dissociation techniques, including blackbody infrared radiative dissociation (BIRD) and surface-induced dissociation (SID), are becoming important [44]. Slow heating of a protein complex trapped in the FTICR cell by absorption of blackbody photons (via BIRD) is a unique approach in studies of protein-ligand interactions [56].
Jones et al. [57] first reported SID as an extension of CID. Compared with CID, SID provides a large-mass collision partner (a solid surface instead of small inert gas molecule) for protein complexes, thus increasing in principle the center-of-mass energy. In the subsequent fragmentation, symmetric charge partitioning occurs [58]. In addition to the dissociation methods in the hybrid Q-TOF instruments, electron capture dissociation (ECD) can be used to dissociate the protein complex in an FTICR mass spectrometer [59]. ECD and electron transfer dissociation (ETD) are now feasible on a Q-TOF instrument [60,61]. The application to protein complexes is expected to appear in the new future.
2.5 Sequencing the flexible region with ECD
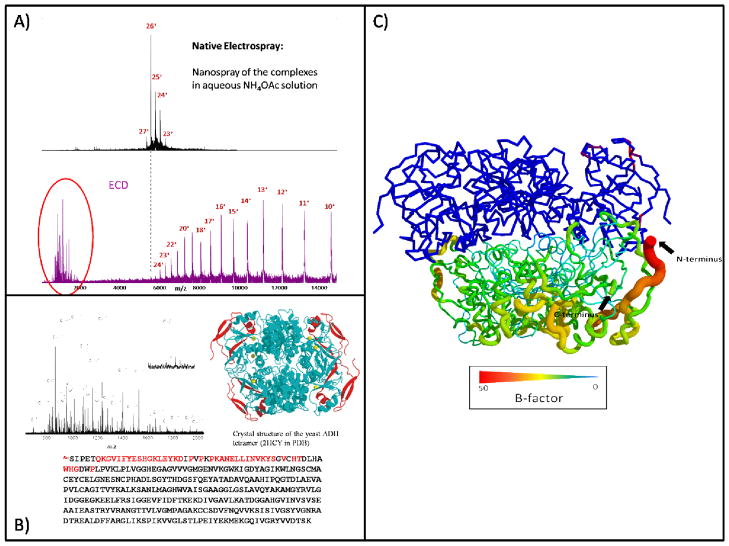
ECD is an established tool for top-down sequencing and for determining post-translational modifications (PTMs) [62]. Proteins of MW up to 200 kDa and non-covalent protein-ligand complexes can be sequenced [63,64]. Although the combination of ECD and FTICR MS is an appealing approach to study large protein complexes, only recently have results been obtained that show that ECD has the potential to fragment the protein complex without dissociating the subunits. The high mass resolving power of the FTICR instrument can also improve the confidence in identifying fragment ions in complicated ion mixtures. For example, we reported that a large number of consecutive backbone cleavages occur for the 147 kDa yeast alcohol dehydrogenase (ADH) tetramer upon ECD in a 12 T FTICR mass spectrometer [65] (Figure 2). The ECD spectrum contains two sets of product ions: those fragment ions of m/z < 2000 and the expected array of charge-reduced precursor ions at higher m/z than that of the precursor. Remarkably, no subunit dissociation was observed in the ECD top-down experiment (Figure 2A). The intact ADH complex is presumably preserved in the gas phase by the many salt bridges that become stronger in the gas phase.
Figure 2.
The ECD top-down experiment of ADH tetramer. A) Native MS and ECD top-down spectra of ADH tetramer. The sequence c ions were circled. B) N-terminal sequence coverage of c ions in ECD top-down experiment. D) The B-factor (flexibility) plots of ADH tetramer.
The low m/z fragment ions were searched against the database (NCBI) and 39 c-type ions up to the 55th residue from the N terminus of ADH and N-terminal acetylated serine were identified (Figure 2B). In this case, fragment ions from the ECD top-down experiment are sufficient in number to identify the protein subunit. The ECD top-down experiment does not disrupt the ADH assembly but rather provides sequence information. Moreover, the origin of fragments is from a flexible region of the protein as determined by the B-factor—a measure of flexibility—in the X-ray crystal structure. The B-factor shows that the N-terminus is free and available for fragmentation, whereas the C terminus is buried at the interface (Figure 2C). N-terminal residues, up to 55th in the polypeptide sequence, are not involved in the interface of ADH complex and ready for ECD fragmentation.
Two advantages make the dissociation methods attractive complements for native MS of protein complexes. First, the sequence identification of subunits from protein complexes may be obtainable in a top-down manner. In a native MS experiment, the MW of a complex is directly measured, but sequence identification still relies on traditional bottom-up proteomics, whereby the protein complex is denatured and loaded onto SDS-PAGE. Each gel band is proteolytically digested and analyzed by LCMS to provide the sequence information. Thus, elucidating the nature and stoichiometry of a protein complex require two independent experiments, native MS and bottom-up LCMS proteomics [66]. The application of ECD (and possibly ETD, although no experimental data yet) to the protein complex ion can generate sequence-specific fragmentation and allow integration of protein identification with native MS in a single experiment. The second advantage is that some structural analysis of the protein complex is achievable (e.g., location of flexible regions of the protein assembly). Other activation approaches (e.g., CID) identify subunit interactions among protein complexes by dissociating the protein complexes. Furthermore, by using Q-TOF and IM technology, the architecture of protein complexes can be established based on the dissociation pathways and from information from ion mobility [4]. ECD now joins the other dissociation methods, CID, BIRD, IRMPD and SID, as reviewed above and in other publications [44] to open new sources of information about protein complexes.
2.6 Measuring protein complexes by IM
A fast-growing area for protein-complex studies is the coupling of IM with MS [67]. In IM, ions from ion complexes are injected into a region containing neutral gas molecules in the presence of an electric field. Driven by this electric field, the ions move and are separated based on their shape [68]. Large ions experience more collisions and take more time to arrive at a detector than do smaller ions or ones of smaller cross sections. The collision cross sections (CCS) of ions can be calculated based on their drift times and charge state, which are now measured in instruments in which MS and IM are concatenated [69]. The shape information provided by an IM measurement is now an essential component in the native MS studies of protein complexes and can be integrated with structural information from other MS-based methods.
2.7 Characterizing protein complexes by native MS
Native MS allows characterization of protein complexes, from measurements of stoichiometry to those of dynamics and structural features and topology of protein complexes. The ability to introduce non-covalent protein-ligand complexes in the gas phase was the promising starting point of native MS applications [17,70,71], but native MS quickly found a role in the field of protein-protein complexes [72] (descriptions of pioneering studies are available in reviews [30]). Early examples of success by native MS are intact Escherichia coli ribosomes (2.3 MDa for 70S ribosome) and vanillyl-alcohol oxidase (> 1 MDa), large fully functional biological protein complexes [73,74]. Functional biological protein complexes dynamically undergo assembly, disassembly, and even subunit exchange [75]. Native MS not only can determine the stoichiometry but also monitor protein complex dynamics including the assembly and disassembly pathways as exemplified by studies of the chaperone complex MtGimC [76]. Water-insoluble protein complexes, which are embedded in the cell membrane, remain a challenge for native MS. One promising strategy is to use gas-phase micelles [77]. Membrane-embedded protein complexes, recently large intact V-type ATPases, can be preserved by micelles in native MS [78,79]. Applications using amphipathic polymers and nanodiscs to stabilize membrane protein complexes also demonstrate the potential of extending native MS to membrane protein complexes [80]. Information from native MS can also benefit the modeling of protein complex architecture [81,82]. The ultimate goal is to establish the best model of protein complexes by integrating data from all MS-based approaches: native MS, protein footprinting (hydrogen/deuterium exchange [83], hydroxyl radical footprinting [84]), limited-proteolysis [85], and cross-linking [86].
3. Native MS of photosynthetic pigment-protein complexes
Understanding proteins in photosynthesis presents a new challenge to MS-based approaches. In photosynthesis, protein assemblies/complexes capture sunlight and convert solar energy to redox energy [87]. Those units contain light-absorbing molecules (chromophores/pigments) that vary in size, shape, energy gap, absorption strength, and spectral features [2]. Photon absorption, energy transfer from the antenna to the reaction center, and charge separation within the reaction center are all mediated by pigments and other cofactors in those pigment-protein complexes [88]. Understanding how those complexes direct and regulate excitation-energy flow not only is important in biochemistry and photosynthesis but also can suggest strategies for designing artificial light-harvesting systems that may contribute to meeting the world’s future energy needs [89].
3.1 The Fenna-Matthews-Olson (FMO) antenna protein as a model complex
We have begun studying photosynthetic pigment-protein complexes by using the well-known Fenna-Matthews-Olson (FMO) antenna complex from green sulfur bacteria [90] as a benchmark to develop the technology. This example is one of many pigment-protein complexes from photosynthetic systems (for reviews, see [2,91–93]). The FMO protein was the first pigment-protein complex whose structure was determined by X-ray crystallography [94,95]. FMO forms a water-soluble trimeric complex that functions as both photosynthetic light-harvesting antenna and as an energy-transfer intermediate, coupling the flow of electronic energy between the peripheral antenna chlorosomes to the reaction centers [96,97] (Figure 3A–B). All the FMO trimeric complexes from Chlorobaculum tepidum [94], Prosthecochloris aestuarii 2K [95] and Pelodictyon phaeum [98] share a similar structure, in which three subunits form a trimeric complex with a three-fold rotational axis of symmetry.
Figure 3.
The photosynthetic system from green sulfur bacteria. A) The components of the green sulfur bacterial photosystem; B) X-ray crystal model of FMO protein complex. Three subunits are colored blue, yellow and gray. The BChl a pigments are colored red; C) BChl a pigments of one subunit; D) BChl a molecular structure.
The pigment inside FMO is bacteriochlorophyll (BChl) a, a highly colored pigment that contains a porphyrin-like ring structure (Figure 3D). Each subunit is formed mainly by β sheet, secondary structure, like a compact “taco shell” containing α helices and loops on the open end, which encloses a central core of seven BChl a pigments,. The energy-delocalization process within FMO exhibits quantum-coherence effects, which were elucidated by using multidimensional coherent spectroscopy [99]. The pigment-pigment and pigment-protein coupling during the energy transfer can also be described by theoretical calculations [97]. The seven BChl a pigments should be arranged in an orientation that facilitates electronic-energy transfer [100].
3.2 Determining the number of pigments in FMO antenna protein complexes
Taking a lead from high-resolution structures of FMO protein that suggested the presence of an eighth pigment BChl a located in the connection region between the open end of “taco shell” FMO and the antenna chlorosomes, we began a program to apply native MS to photosynthetic complexes [98,101]. In these structures, the electron density of the putative eighth BChl a pigment is weak and incomplete owing to partial occupancy in the binding site. Does the partial occupancy of the eighth BChl a observed in X-ray experiments represent the state of the intact FMO in vivo or is some pigment lost during protein purification or analysis? This could be the case if the eighth BChl a occupies an exposed binding position on the surface of the intact FMO complex.
One answer to the question can come from the time-honored role of MS to solve stoichiometry problems by measuring molecular weight. In this problem, however, we needed native MS to examine the pigment stoichiometry of this sensitive FMO antenna protein in its native state [102]. After buffer exchanging with ammonium acetate the purified intact FMO antenna protein complexes from Chlorobaculum tepidum (TFMO) and Prosthecochloris aestuarii (AFMO), we utilized native ESI of the intact complexes and observed three to four charge states at high m/z range (Figure 1C, 4A), allowing the molecular weight (MW) of intact FMO complexes to be determined. The MW of FMO from native MS is ~141 kDa, which is greater than the total MW of three polypeptide chain subunits (39.8 kDa each). Clearly, the intact FMO complex containing three subunits can be preserved in the gas phase, but can we find evidence for the seven BChl a molecules inside each subunit as well as the eighth putative BChl a on the bridge region? We desolvated further the intact FMO complex ions in the gas phase by applying CID to yield multiple species in each charge state of intact FMO complex (Figure 4A). The mass differences between neighboring peaks are ~630 Da, which is less than the mass of the full BChl a pigment (910 Da), but close to the mass of bacteriochlorophyllide (632 Da), which lacks the isoprenoid phytyl tail found in the intact pigment.
Figure 4.
The native MS and ECD top-down results of FMO antenna complex. A) Native MS of intact FMO protein complex; B) ECD top-down spectrum of FMO complexes. The native MS spectrum of FMO protein complex is highlighted in the black box; C) The B-factor plot of FMO complex and the fragment coverage of FMO protein sequence.
Because subsequent HPLC analysis of BChl a from FMO gave no evidence of a BChl a pigment without its “tail”, we examined the prospects that the eighth BChl is lost in the purification and that the “tail” is lost as part of the CID used in the native MS desolvation. The eighth BChl may be particularly vulnerable to loss and to fragmentation because it is on the surface (bridge region) of the FMO complex. Indeed, isolated BChl a readily fragments in a mass spectrometer by losing its phytyl chain [103]. Furthermore, the partial occupancy of the eighth BChl a causes multiple peaks in each charge state of intact FMO ions, confirming the presence of an eighth BChl a pigment. This conclusion finds support by application of different purification methods (“strong” vs. “soft”) of the FMO complex; “stronger” purification gave less intense signals for the eighth BChl a pigment, indicating that the partial occupancy of the eighth BChl a is a consequence of protein purification. Thus, the intact FMO trimer complex in vivo contains three extra BChl a molecules (one for each subunit). The native MS provided the first experimental evidence of the existence of this eighth BChl a pigment in stoichiometric quantities [102].
3.3 Locating flexible regions of FMO complex by ECD and CID
We also submitted the intact FMO antenna complex introduced by native ESI to a FTICR trap to ECD and top-down analysis [104]. Direct ECD of intact FMO only generated charge-reduced precursor ions, strongly suggesting that FMO forms a compact complex in the gas phase, with unexposed N- and C-termini. We hypothesized earlier in our study of the ADH tetramer that ECD of protein complexes leads to fragmentation of flexible regions of the complex. Although ECD may reveal terminal region(s) of high flexibility [65], it may be unable to sample a flexible region in the middle of a protein sequence. Thus, we attempted to “unfold” any flexible regions of the protein complex by applying collision energy prior to ECD. The anticipation was that a combination of CID and ECD will provide more structural information.
With the collision energy applied in the source region (IS-CID), ECD produced z-type ions from FMO C-terminal region in the top-down experiment (Figure 4B). The C-terminal region of the FMO complex is closer to a flexible region than is the N-terminus. The application of collision energy extends the flexible region to the C-terminus of FMO, positioning it for ECD fragmentation. We anticipated that the collision energy applied to protein complexes must be adjusted because flexible regions will vary in nature and position from protein to protein. When we adjusted the collision energy applied to FMO complexes, an unusual set of fragment ions of the FMO complex with charge states from 4+ to 8+ were observed without ECD. A database search indicates the fragment ion is not from the N- or C- terminal regions. Using the high resolving power and accurate mass measurement capabilities of FTICR MS, we identified the fragment to be from the middle region (peptide 201–295, MW = 10160.26 Da) of the FMO protein sequence.
These unique results obtained from native MS provide new insights on intact FMO complexes [94,98]. For perspective, in the “taco shell” structure of intact FMO complex, a series of beta sheets form two parallel walls holding the seven BChl a pigments. The C-terminus of FMO is at the bottom of complex, whereas the N-terminus is in the middle of beta sheet structure forming the side wall of the “taco shell” structure. The region observed in the CID experiment contains a loop on the top of the FMO complex. The region undergoing fragmentation in CID is one of the most flexible regions in the FMO complex (Figure 4C). Moreover, this CID fragment region is overlapped with the ECD fragmented C-terminal region (compare to the flexible region of ADH in Figure 2C). Given our supposition that the most flexible region starts unfolding upon CID, the flexible region of the protein becomes extended to the C-terminal region, making it susceptible to ECD fragmentation.
ECD and CID incorporated in a native MS platform afford new structural information of intact FMO complexes. Protein sequence, complex stoichiometry, and structural insights pinpointing flexible regions can be obtained to complement information from X-ray and NMR, and from other MS-based protein footprinting, as will be discussed in the next section.
3.4 Augmenting information from native MS by protein footprinting
With the help of native MS, it is clear that each subunit of FMO contains eight pigments with seven located in the hydrophobic core and the eighth on the outside. Among the seven, the pigment with the lowest site energy is predicted to be BChl a #3 on the basis of coupling with the dipoles of adjacent alpha helices. On a simple energetic basis, this pigment is expected to be physically closest to the reaction center [105]. However, a structural study of isolated FMO suggested that BChl a #1 was physically closest to the reaction center [94]. At this point, native MS falls short of providing an answer.
MS-based protein footprinting using carboxyl group labeling solved this question by examining the orientation of FMO between the antenna chlorosomes and the membrane-associated reaction center. The strategy was to prepare samples in three conditions including the FMO only, FMO attached to the membrane, and FMO “sandwiched” between the chlorosome and the membrane holding the reaction center. We then labeled acidic aspartic and glutamic residues by glycine ethyl ester (GEE) to reveal those residues that become less protected as the membrane or chlorosome are removed. Employing standard bottom-up proteomics method, we identified and compared the peptides in the same region of FMO but under different preparation states described above. We could conclude that the open end (BChl a #1 side) of “taco shell” FMO interacts with the antenna chlorosomes, while the side that contains both the N-and C-terminal ends (BChl a #3 side) of FMO interacts with the reaction center [106] (Figure 3C).
More recently, the interaction between chlorosomal baseplate protein and open end region of “taco shell’ FMO was investigated by another MS-based protein footprinting method, hydrogen-deuterium exchange (HDX), and this orientation was confirmed [107]. All evidence taken together shows that the eighth BChl a pigment is located near the chlorosome baseplate and probably represents the entry point for excitations into the FMO protein from the chlorosome. Thus, a more comprehensive picture of the larger scale architecture of the photosynthetic membrane and how the FMO is positioned to achieve highly efficient excitation energy transfer can be achieved by using native MS and footprinting together.
4 Future native MS studies of photosynthetic pigment-protein complex
There is a large family of photosynthetic pigment-protein complexes that contains various pigment-protein complexes involved in sunlight capture, energy transfer and final conversion of electronic excitation energy to separated charges. Detailed structural understanding of those pigment-protein complexes will contribute to an overall picture of photosynthesis and will benefit future designs of artificial light-harvesting devices. Native MS, a relatively new approach in structural biology, especially in photosynthetic research, will continue to provide new insights into these systems and complement. traditional structural biology approaches. We anticipate that more researchers will integrate native MS into their “tool box” for characterization of protein complexes.
In our laboratory, different types of pigment-protein complexes are under investigation by native MS. Two aspects will be highlighted in the near future: membrane embedded pigment-protein complexes and IM measurements for structural information. We also expect that the use of lipid nanodiscs with native MS [108] and footprinting will become a promising platform to investigate membrane-associated protein complexes.
Acknowledgments
The research reviewed here was supported by the Photosynthetic Antenna Research Center, an Energy Frontier Research Center funded by the U.S. DOE, Office of Basic Energy Sciences (Grant No. DE-SC 0001035 to R.E.B), and grants from the National Institute of General Medical Sciences (8 P41 GM103422-35) of the NIH and by the NSF (IBDR 0964199 to M.L.G.).
References
- 1.Ban N, Egelman EH. Structure and function of large cellular assemblies. Curr Opin Struct Biol. 2010;20:207–9. doi: 10.1016/j.sbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Scholes GD, Fleming GR, Olaya-Castro A, van Grondelle R. Lessons from nature about solar light harvesting. Nat Chem. 2011;3:763–74. doi: 10.1038/nchem.1145. [DOI] [PubMed] [Google Scholar]
- 3.Winston RL, Fitzgerald MC. Mass spectrometry as a readout of protein structure and function. Mass Spectrom Rev. 1997;16:165–79. doi: 10.1002/(SICI)1098-2787(1997)16:4<165::AID-MAS1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Benesch JL, Ruotolo BT. Mass spectrometry: come of age for structural and dynamical biology. Curr Opin Struct Biol. 2011;21:641–9. doi: 10.1016/j.sbi.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton GR, Benesch JL. Two decades of studying non-covalent biomolecular assemblies by means of electrospray ionization mass spectrometry. J R Soc Interface. 2012;9:801–16. doi: 10.1098/rsif.2011.0823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Congreve M, Murray CW, Blundell TL. Structural biology and drug discovery. Drug Discov Today. 2005;10:895–907. doi: 10.1016/S1359-6446(05)03484-7. [DOI] [PubMed] [Google Scholar]
- 7.Robinson CV, Sali A, Baumeister W. The molecular sociology of the cell. Nature. 2007;450:973–82. doi: 10.1038/nature06523. [DOI] [PubMed] [Google Scholar]
- 8.Blankenship RE. Molecular mechanisms of photosynthesis. Blackwell; 2002. [Google Scholar]
- 9.Sjolin L. 3D-structural elucidation of biologically important macromolecules. Drug Des Discov. 1993;9:261–76. [PubMed] [Google Scholar]
- 10.Henderson R. Realizing the potential of electron cryo-microscopy. Q Rev Biophys. 2004;37:3–13. doi: 10.1017/s0033583504003920. [DOI] [PubMed] [Google Scholar]
- 11.Mertens HD, Svergun DI. Structural characterization of proteins and complexes using small-angle X-ray solution scattering. J Struct Biol. 2010;172:128–41. doi: 10.1016/j.jsb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Cowieson NP, Kobe B, Martin JL. United we stand: combining structural methods. Curr Opin Struct Biol. 2008;18:617–22. doi: 10.1016/j.sbi.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Heck AJ. Native mass spectrometry: a bridge between interactomics and structural biology. Nat Methods. 2008;5:927–33. doi: 10.1038/nmeth.1265. [DOI] [PubMed] [Google Scholar]
- 14.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 15.Stults JT. Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) Curr Opin Struct Biol. 1995;5:691–8. doi: 10.1016/0959-440x(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 16.Kiselar JG, Downard KM. Preservation and detection of specific antibody--peptide complexes by matrix-assisted laser desorption ionization mass spectrometry. J Am Soc Mass Spectrom. 2000;11:746–50. doi: 10.1016/S1044-0305(00)00144-6. [DOI] [PubMed] [Google Scholar]
- 17.Ganem B, li Y-T, Henion JD. Observation of Noncovalent Enzyme-Substrate and Enzyme-Product Complexes by Ion-Spray Mass Spectrometry. J Am Chem Soc. 1991:7818–7819. [Google Scholar]
- 18.Lorenzen K, Duijn EV. Current Protocols in Protein Science. In: Coligan JE, Dunn BM, Speicher DW, Wingfield PT, editors. UNIT 17.12 Native Mass Spectrometry as a Tool in Structural Biology. John Wiley & Sons, Inc; 2010. [DOI] [PubMed] [Google Scholar]
- 19.Mädler S, Barylyuk K, Erba EB, Nieckarz RJ, Zenobi R. Compelling Advantages of Negative Ion Mode Detection in High-Mass MALDI-MS for Homomeric Protein Complexes. J Am Soc Mass Spectrom. 2012;23:213–224. doi: 10.1007/s13361-011-0274-x. [DOI] [PubMed] [Google Scholar]
- 20.Kebarle P, Verkerk UH. Electrospray: from ions in solution to ions in the gas phase, what we know now. Mass Spectrom Rev. 2009;28:898–917. doi: 10.1002/mas.20247. [DOI] [PubMed] [Google Scholar]
- 21.Wilm MS, Mann M. Electrospray and Taylor-cone theory, Dole’s beam of macromolecules at last? Int J Mass Spectrom Ion Process. 1994;136:167–180. [Google Scholar]
- 22.Dole M, Mack LL, Hines RL, Mobley RC, Ferguson LD, Alice MB. Molecular Beams of Macroions. J Chem Phys. 1968;49:2240–2249. [Google Scholar]
- 23.Iribarne JV, Thomson BA. On the evaporation of small ions from charged droplets. J Chem Phys. 1976;64:2287–2294. [Google Scholar]
- 24.Smith RD, Loo JA, Edmonds CG, Barinaga CJ, Udseth HR. New developments in biochemical mass spectrometry: electrospray ionization. Anal Chem. 1990;62:882–99. doi: 10.1021/ac00208a002. [DOI] [PubMed] [Google Scholar]
- 25.Benesch JL, Ruotolo BT, Simmons DA, Robinson CV. Protein complexes in the gas phase: technology for structural genomics and proteomics. Chem Rev. 2007;107:3544–67. doi: 10.1021/cr068289b. [DOI] [PubMed] [Google Scholar]
- 26.van den Heuvel RH, Heck AJ. Native protein mass spectrometry: from intact oligomers to functional machineries. Curr Opin Chem Biol. 2004;8:519–26. doi: 10.1016/j.cbpa.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Wilm M, Mann M. Analytical properties of the nanoelectrospray ion source. Anal Chem. 1996;68:1–8. doi: 10.1021/ac9509519. [DOI] [PubMed] [Google Scholar]
- 28.Wahl JH, Goodlett DR, Udseth HR, Smith RD. Use of small-diameter capillaries for increasing peptide and protein detection sensitivity in capillary electrophoresis-mass spectrometry. Electrophoresis. 1993;14:448–57. doi: 10.1002/elps.1150140170. [DOI] [PubMed] [Google Scholar]
- 29.Breuker K, McLafferty FW. Stepwise evolution of protein native structure with electrospray into the gas phase, 10(-12) to 10(2) s. Proc Natl Acad Sci U S A. 2008;105:18145–18152. doi: 10.1073/pnas.0807005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loo JA. Studying noncovalent protein complexes by electrospray ionization mass spectrometry. Mass Spectrom Rev. 1997;16:1–23. doi: 10.1002/(SICI)1098-2787(1997)16:1<1::AID-MAS1>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 31.Meyer T, de la Cruz X, Orozco M. An atomistic view to the gas phase proteome. Structure. 2009;17:88–95. doi: 10.1016/j.str.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Shelimov KB, Clemmer DE, Hudgins RR, Jorrold MF. Protein structure in vacuo: gas-phase confirmations of BPTI and cytochrome c. J Am Chem Soc. 1997;119:2240. [Google Scholar]
- 33.Badman ER, Hoaglund-Hyzer CS, Clemmer DE. Monitoring structural changes of proteins in an ion trap over approximately 10–200 ms: unfolding transitions in cytochrome c ions. Anal Chem. 2001;73:6000–7. doi: 10.1021/ac010744a. [DOI] [PubMed] [Google Scholar]
- 34.Ruotolo BT, Giles K, Campuzano I, Sandercock AM, Bateman RH, Robinson CV. Evidence for macromolecular protein rings in the absence of bulk water. Science. 2005;310:1658–61. doi: 10.1126/science.1120177. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Bagal D, Kitova EN, Schnier PD, Klassen JS. Hydrophobic protein-ligand interactions preserved in the gas phase. J Am Chem Soc. 2009;131:15980–15981. doi: 10.1021/ja9060454. [DOI] [PubMed] [Google Scholar]
- 36.Douglas DJ, French JB. Collisional focusing effects in radio-frequency quadrupoles. J Am Soc Mass Spectrom. 1992;3:398–408. doi: 10.1016/1044-0305(92)87067-9. [DOI] [PubMed] [Google Scholar]
- 37.Krutchinsky AN, Chernushevich IV, Spicer VL, Ens W, Standing KG. Studies of Noncovalent Complexes in an Electrospray Ionization/time-of-flight Mass Spectrometer. J Am Soc Mass Spectrom. 1998;9:569–579. [Google Scholar]
- 38.Sobott F, Hernandez H, McCammon MG, Tito MA, Robinson CV. A tandem mass spectrometer for improved transmission and analysis of large macromolecular assemblies. Anal Chem. 2002;74:1402–7. doi: 10.1021/ac0110552. [DOI] [PubMed] [Google Scholar]
- 39.Verentchikov AN, Ens W, Standing KG. Reflecting time-of-flight mass spectrometer with an electrospray ion source and orthogonal extraction. Anal Chem. 1994;66:126–33. doi: 10.1021/ac00073a022. [DOI] [PubMed] [Google Scholar]
- 40.Doy M, Labastie P. A high mass range quadrupole spectrometer for cluster studies. Int J Mass Spectrom Ion Process. 1989;91:105–112. [Google Scholar]
- 41.Collings BA, Douglas DJ. An extended mass range quadrupole for electrospray mass spectrometry. Int J Mass Spectrom Ion Process. 1997;162:121–127. [Google Scholar]
- 42.van den Heuvel RH, et al. Improving the performance of a quadrupole time-of-flight instrument for macromolecular mass spectrometry. Anal Chem. 2006;78:7473–83. doi: 10.1021/ac061039a. [DOI] [PubMed] [Google Scholar]
- 43.Rose RJ, Damoc E, Denisov E, Alexander M, Heck AJR. High-sensitivity Orbitrap mass analysis of intact macromolecular assemblies. Nature Methods. 2012;9:1084–1086. doi: 10.1038/nmeth.2208. [DOI] [PubMed] [Google Scholar]
- 44.Benesch JL. Collisional activation of protein complexes: picking up the pieces. J Am Soc Mass Spectrom. 2009;20:341–8. doi: 10.1016/j.jasms.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 45.McKay AR, Ruotolo BT, Ilag LL, Robinson CV. Mass measurements of increased accuracy resolve heterogeneous populations of intact ribosomes. J Am Chem Soc. 2006;128:11433–42. doi: 10.1021/ja061468q. [DOI] [PubMed] [Google Scholar]
- 46.Shukla AK, Futrell JH. Tandem mass spectrometry: dissociation of ions by collisional activation. J Mass Spectrom. 2000;35:1069–90. doi: 10.1002/1096-9888(200009)35:9<1069::AID-JMS54>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 47.Tolic LP, Bruce JE, Lei QP, Anderson GA, Smith RD. In-trap cleanup of proteins from electrospray ionization using soft sustained off-resonance irradiation with fourier transform ion cyclotron resonance mass spectrometry. Anal Chem. 1998;70:405–8. doi: 10.1021/ac970828c. [DOI] [PubMed] [Google Scholar]
- 48.El-Faramawy A, Guo Y, Verkerk UH, Thomson BA, Siu KW. Infrared irradiation in the collision cell of a hybrid tandem quadrupole/time-of-flight mass spectrometer for declustering and cleaning of nanoelectrosprayed protein complex ions. Anal Chem. 2010;82:9878–84. doi: 10.1021/ac102351m. [DOI] [PubMed] [Google Scholar]
- 49.Ruotolo BT, Robinson CV. Aspects of native proteins are retained in vacuum. Curr Opin Chem Biol. 2006;10:402–8. doi: 10.1016/j.cbpa.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Ruotolo BT, Hyung SJ, Robinson PM, Giles K, Bateman RH, Robinson CV. Ion mobility-mass spectrometry reveals long-lived, unfolded intermediates in the dissociation of protein complexes. Angew Chem Int Ed Engl. 2007;46:8001–4. doi: 10.1002/anie.200702161. [DOI] [PubMed] [Google Scholar]
- 51.Light-Wahl KJ, Schaedler TA, Smith RD. Observation of the Noncovalent Quaternary Associations of Proteins by Electrospray Ionization Mass Spectrometry. J Am Chem Soc. 1994;116:5271–5278. [Google Scholar]
- 52.Jurchen JC, Williams ER. Origin of asymmetric charge partitioning in the dissociation of gas-phase protein homodimers. J Am Chem Soc. 2003;125:2817–26. doi: 10.1021/ja0211508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanasundara SN, Thachuk M. Theoretical investigations of the dissociation of charged protein complexes in the gas phase. J Am Soc Mass Spectrom. 2007;18:2242–53. doi: 10.1016/j.jasms.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 54.Benesch JL, Aquilina JA, Ruotolo BT, Sobott F, Robinson CV. Tandem mass spectrometry reveals the quaternary organization of macromolecular assemblies. Chem Biol. 2006;13:597–605. doi: 10.1016/j.chembiol.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Boeri Erba E, Ruotolo BT, Barsky D, Robinson CV. Ion mobility-mass spectrometry reveals the influence of subunit packing and charge on the dissociation of multiprotein complexes. Anal Chem. 2010;82:9702–10. doi: 10.1021/ac101778e. [DOI] [PubMed] [Google Scholar]
- 56.Felitsyn N, Kitova EN, Klassen JS. Thermal decomposition of a gaseous multiprotein complex studied by blackbody infrared radiative dissociation. Investigating the origin of the asymmetric dissociation behavior. Anal Chem. 2001;73:4647–61. doi: 10.1021/ac0103975. [DOI] [PubMed] [Google Scholar]
- 57.Jones CM, Beardsley RL, Galhena AS, Dagan S, Cheng G, Wysocki VH. Symmetrical gas-phase dissociation of noncovalent protein complexes via surface collisions. J Am Chem Soc. 2006;128:15044–5. doi: 10.1021/ja064586m. [DOI] [PubMed] [Google Scholar]
- 58.Blackwell AE, Dodds ED, Bandarian V, Wysocki VH. Revealing the quaternary structure of a heterogeneous noncovalent protein complex through surface-induced dissociation. Anal Chem. 2011;83:2862–5. doi: 10.1021/ac200452b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geels RB, van der Vies SM, Heck AJ, Heeren RM. Electron capture dissociation as structural probe for noncovalent gas-phase protein assemblies. Anal Chem. 2006;78:7191–6. doi: 10.1021/ac060960p. [DOI] [PubMed] [Google Scholar]
- 60.Voinov VG, Deinzer ML, Beckman JS, Barofsky DF. Electron Capture, Collision-Induced, and Electron Capture-Collision Induced Dissociation in Q-TOF. J Am Soc Mass Spectrom. 2011;22:607–611. doi: 10.1007/s13361-010-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsybin YO, Fornelli L, Stoermer C, Luebeck M, Parra J, Nallet S, Wurm FM, Hartmer R. Structural Analysis of Intact Monoclonal Antibodies by Electron Transfer Dissociation Mass Spectrometry. Anal Chem. 2011;83:8919–8927. doi: 10.1021/ac201293m. [DOI] [PubMed] [Google Scholar]
- 62.Zubarev RA, Kelleher NL, McLafferty FW. Electron capture dissocation of multiply charged protein cations. A nonergodic process. J Am Chem Soc. 1998;120:3265–3266. [Google Scholar]
- 63.Xie Y, Zhang J, Yin S, Loo JA. Top-down ESI-ECD-FT-ICR mass spectrometry localizes noncovalent protein-ligand binding sites. J Am Chem Soc. 2006;128:14432–3. doi: 10.1021/ja063197p. [DOI] [PubMed] [Google Scholar]
- 64.Han X, Jin M, Breuker K, McLafferty FW. Extending top-down mass spectrometry to proteins with masses greater than 200 kilodaltons. Science. 2006;314:109–12. doi: 10.1126/science.1128868. [DOI] [PubMed] [Google Scholar]
- 65.Zhang H, Cui W, Wen J, Blankenship RE, Gross ML. Native electrospray and electron-capture dissociation in FTICR mass spectrometry provide top-down sequencing of a protein component in an intact protein assembly. J Am Soc Mass Spectrom. 2010;21:1966–8. doi: 10.1016/j.jasms.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou M, Robinson CV. When proteomics meets structural biology. Trends Biochem Sci. 2010;35:522–9. doi: 10.1016/j.tibs.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Uetrecht C, Rose RJ, van Duijn E, Lorenzen K, Heck AJ. Ion mobility mass spectrometry of proteins and protein assemblies. Chem Soc Rev. 2010;39:1633–55. doi: 10.1039/b914002f. [DOI] [PubMed] [Google Scholar]
- 68.Kanu AB, Dwivedi P, Tam M, Matz L, Herbert HHJ. Ion mobility-mass spectrometry. J Mass Spectrom. 2008;43:1–22. doi: 10.1002/jms.1383. [DOI] [PubMed] [Google Scholar]
- 69.Ruotolo BT, Benesch JL, Sandercock AM, Hyung SJ, Robinson CV. Ion mobility-mass spectrometry analysis of large protein complexes. Nat Protoc. 2008;3:1139–52. doi: 10.1038/nprot.2008.78. [DOI] [PubMed] [Google Scholar]
- 70.Ganem B, li YT, Henion JD. Detection of noncovalent receptor ligand complexes by mass spectrometry. J Am Chem Soc. 1991;113:6294–6296. [Google Scholar]
- 71.Katta V, Chait BT. Observation of the heme-globin complex in native myoglobin by electrospray-ionization mass-spectrometry. J Am Chem Soc. 1991;113:8534–8535. [Google Scholar]
- 72.Baca MH, KSB Direct observation of a ternary complex between the dimeric enzyme HIV-1 protease and a substrate-based inhibitor. J Am Chem Soc. 1992;114:3992–3993. [Google Scholar]
- 73.van Berkel WJ, van den Heuvel RH, Versluis C, Heck AJ. Detection of intact megaDalton protein assemblies of vanillyl-alcohol oxidase by mass spectrometry. Protein Sci. 2000;9:435–9. doi: 10.1110/ps.9.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rostom AA, Fucini P, Benjamin DR, Juenemann R, Nierhaus KH, Hartl FU, Dobson CM, Robinson CV. Detection and selective dissociation of intact ribosomes in a mass spectrometer. Proc Natl Acad Sci U S A. 2000;97:5185–90. doi: 10.1073/pnas.97.10.5185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ben-Nissan G, Sharon M. Capturing protein structural kinetics by mass spectrometry. Chem Soc Rev. 2011;40:3627–37. doi: 10.1039/c1cs15052a. [DOI] [PubMed] [Google Scholar]
- 76.Fandrich M, Tito MA, Leroux MR, Rostom AA, Hartl FU, Dobson CM, Robinson CV. Observation of the noncovalent assembly and disassembly pathways of the chaperone complex MtGimC by mass spectrometry. Proc Natl Acad Sci U S A. 2000;97:14151–5. doi: 10.1073/pnas.240326597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sharon M, Ilag LL, Robinson CV. Evidence for micellar structure in the gas phase. J Am Chem Soc. 2007;129:8740–6. doi: 10.1021/ja067820h. [DOI] [PubMed] [Google Scholar]
- 78.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Micelles protect membrane complexes from solution to vacuum. Science. 2008;321:243–6. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 79.Zhou M, et al. Mass spectrometry of intact V-type ATPases reveals bound lipids and the effects of nucleotide binding. Science. 2011;334:380–5. doi: 10.1126/science.1210148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Popot JL. Amphipols, nanodiscs, and fluorinated surfactants: three nonconventional approaches to studying membrane proteins in aqueous solutions. Annu Rev Biochem. 2010;79:737–75. doi: 10.1146/annurev.biochem.052208.114057. [DOI] [PubMed] [Google Scholar]
- 81.Pukala TL, Ruotolo BT, Zhou M, Politis A, Stefanescu R, Leary JA, Robinson CV. Subunit architecture of multiprotein assemblies determined using restraints from gas-phase measurements. Structure. 2009;17:1235–43. doi: 10.1016/j.str.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 82.Politis A, Park AY, Hyung SJ, Barsky D, Ruotolo BT, Robinson CV. Integrating ion mobility mass spectrometry with molecular modelling to determine the architecture of multiprotein complexes. PLoS One. 2010;5:e12080. doi: 10.1371/journal.pone.0012080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wales TE, Engen JR. Hydrogen exchange mass spectrometry for the analysis of protein dynamics. Mass Spectrom Rev. 2006;25:158–70. doi: 10.1002/mas.20064. [DOI] [PubMed] [Google Scholar]
- 84.Xu G, Chance MR. Hydroxyl radical-mediated modification of proteins as probes for structural proteomics. Chem Rev. 2007;107:3514–43. doi: 10.1021/cr0682047. [DOI] [PubMed] [Google Scholar]
- 85.Cohen SL. Domain elucidation by mass spectrometry. Structure. 1996;4:1013–6. doi: 10.1016/s0969-2126(96)00108-6. [DOI] [PubMed] [Google Scholar]
- 86.Leitner A, Walzthoeni T, Kahraman A, Herzog F, Rinner O, Beck M, Aebersold R. Probing native protein structures by chemical cross-linking, mass spectrometry, and bioinformatics. Mol Cell Proteomics. 2010;9:1634–49. doi: 10.1074/mcp.R000001-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ke B. Advances in Photosynthesis series. Vol. 10. Kluwer Academic; 2001. Photosynthesis: Photobiochemistry and Photobiophysics. [Google Scholar]
- 88.Scholes GD, Fleming GR. Energy transfer in photosynthesis. Adv Chem Phys. 2005;132:57–129. [Google Scholar]
- 89.Lewis NS, Nocera DG. Powering the planet: chemical challenges in solar energy utilization. Proc Natl Acad Sci U S A. 2006;103:15729–35. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Olson JM. The FMO protein. Photosynth Res. 2004;80:181–187. doi: 10.1023/B:PRES.0000030428.36950.43. [DOI] [PubMed] [Google Scholar]
- 91.Guldi DM. Fullerene-porphyrin architectures; photosynthetic antenna and reaction center models. Chem Soc Rev. 2002;31:22–36. doi: 10.1039/b106962b. [DOI] [PubMed] [Google Scholar]
- 92.Grossman AR, Bhaya D, Apt KE, Kehoe DM. Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu Rev Genet. 1995;29:231–88. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- 93.Cheng YC, Fleming GR. Dynamics of light harvesting in photosynthesis. Annu Rev Phys Chem. 2009;60:241–62. doi: 10.1146/annurev.physchem.040808.090259. [DOI] [PubMed] [Google Scholar]
- 94.Li YF, Zhou W, Blankenship RE, Allen JP. Crystal structure of the bacteriochlorophyll a protein from Chlorobium tepidum. J Mol Biol. 1997;271:456–471. doi: 10.1006/jmbi.1997.1189. [DOI] [PubMed] [Google Scholar]
- 95.Tronrud DE, Schmid MF, Matthews BW. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1. A resolution. J Mol Biol. 1986;188:443–54. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]
- 96.Muh F, Madjet Mel A, Adolphs J, Abdurahman A, Rabenstein B, Ishikita H, Knapp EW, Renger T. Alpha-helices direct excitation energy flow in the Fenna Matthews Olson protein. Proc Natl Acad Sci U S A. 2007;104:16862–7. doi: 10.1073/pnas.0708222104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adolphs J, Renger T. How proteins trigger excitation energy transfer in the FMO complex of green sulfur bacteria. Biophys J. 2006;91:2778–97. doi: 10.1529/biophysj.105.079483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Larson CR, Seng CO, Lauman L, Matthies HJ, Wen J, Blankenship RE, Allen JP. The three-dimensional structure of the FMO protein from Pelodictyon phaeum and the implications for energy transfer. Photosynth Res. 2011;107:139–50. doi: 10.1007/s11120-010-9604-2. [DOI] [PubMed] [Google Scholar]
- 99.Brixner T, Stenger J, Vaswani HM, Cho M, Blankenship RE, Fleming GR. Two-dimensional spectroscopy of electronic couplings in photosynthesis. Nature. 2005;434:625–628. doi: 10.1038/nature03429. [DOI] [PubMed] [Google Scholar]
- 100.Fenna RE, Matthews BW. Chlorophyll arrangement in a bacteriochlorophyll protein from Chlorobium limicola. Nature. 1975;258:573–577. [Google Scholar]
- 101.Tronrud DE, Wen J, Gay L, Blankenship RE. The structural basis for the difference in absorbance spectra for the FMO antenna protein from various green sulfur bacteria. Photosynth Res. 2009;100:79–87. doi: 10.1007/s11120-009-9430-6. [DOI] [PubMed] [Google Scholar]
- 102.Wen J, Zhang H, Gross ML, Blankenship RE. Native electrospray mass spectrometry reveals the nature and stoichiometry of pigments in the FMO photosynthetic antenna protein. Biochemistry. 2011;50:3502–11. doi: 10.1021/bi200239k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Airs RL, Keely BJ. Atmospheric pressure chemical ionisation liquid chromatography/mass spectrometry of bacteriochlorophylls from Chlorobiaceae: characteristic fragmentations. Rapid Commun Mass Spectrom. 2002;16:452–461. doi: 10.1002/rcm.598. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H, Cui W, Wen J, Blankenship RE, Gross ML. Native electrospray and electron-capture dissociation FTICR mass spectrometry for top-down studies of protein assemblies. Anal Chem. 2011;83:5598–606. doi: 10.1021/ac200695d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pearlstein R. Theory of the optical spctra of the bacteriochlorophyll a antenna protein trimer from Prosthecochloris aestuarii. Photosynth Res. 1992;31:213–226. doi: 10.1007/BF00035538. [DOI] [PubMed] [Google Scholar]
- 106.Wen J, Zhang H, Gross ML, Blankenship RE. Membrane orientation of the FMO antenna protein from Chlorobaculum tepidum as determined by mass spectrometry-based footprinting. Proc Natl Acad Sci U S A. 2009;106:6134–9. doi: 10.1073/pnas.0901691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang RY, Wen J, Blankenship RE, Gross ML. Hydrogen-deuterium exchange mass spectrometry reveals the interaction of Fenna-Matthews-Olson protein and chlorosome CsmA protein. Biochemistry. 2012;51:187–93. doi: 10.1021/bi201620y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Marty MT, Zhang H, Cui W, Blankenship RE, Gross ML, Sligar SG. Native Mass Spectrometry Characterization of Intact Nanodisc Lipoprotein Complexes. Anal Chem. 2012;84:8957–8960. doi: 10.1021/ac302663f. [DOI] [PMC free article] [PubMed] [Google Scholar]