Abstract
Objective
To compare the uptake of three mailed high-sensitivity fecal occult blood tests (FOBTs).
Methods
We conducted a parallel 3-arm randomized controlled trial in an integrated healthcare delivery system in Washington State. From January 2010 through February 2011, automated data were used to identify potentially eligible patients aged 50-74 due for colorectal cancer screening. Participants were mailed one of three FOBT kits (1-sample OC-Auto® fecal immunochemical test [FIT], 2-sample InSure® FIT, or 3-sample guaiac Hemoccult SENSA®), instructions, and a postage-paid return envelope. We performed a modified intent-to-treat analysis with return of any FOBT within 6 months of randomization as the primary outcome.
Results
Of the 9922 people invited, 2873 returned surveys, 2263 were randomized, and 2234 were analyzed. FOBTs were returned by 1431 participants. At 6 months post randomization, the proportions screened by any FOBT were 0.69 (95% confidence interval [CI] 0.66-0.72) for the OC-Auto arm, 0.64 (95% CI: 0.61-0.68) for the InSure arm, and 0.61 (95% CI: 0.58-0.65) for the Hemoccult SENSA arm (P < 0.001 for any difference). Pairwise comparisons showed significant differences between the OC-Auto group and each of the other groups after correction for multiple comparisons.
Conclusion
Uptake of mailed FOBT kits varies by kit type.
INTRODUCTION
Healthcare providers and delivery systems face challenges in deciding how to increase screening for colorectal cancer (CRC). The United States Preventive Services Task Force (USPSTF) recommends either annual high-sensitivity fecal occult blood test (FOBT), sigmoidoscopy every five years with FOBT every three years, or colonoscopy every ten years (U. S. Preventive Services Task Force, 2008). Some studies have shown that patients prefer colonoscopy to FOBT (Powell, et al., 2009) or that preferences are similar for the two tests (Hawley, et al., 2008, Hawley, et al., 2012). However, other recent studies suggest that patients are more willing to screen using FOBT (Lisi, et al., 2010, Inadomi, et al., 2012, Khalid-de Bakker, et al., 2011, Green, et al., 2012, Gupta, et al., 2013). Regardless of test, both the USPSTF (U. S. Preventive Services Task Force, 2008) and the United States Multi-Society Task Force on Colorectal Cancer (USMSTF) (Levin, et al., 2008) recognize that uptake and adherence are important for CRC screening regimens.
Studies comparing high-sensitivity guaiac FOBT (gFOBT) to fecal immunochemical tests (FIT) find generally higher adherence to FIT, which has fewer dietary and other restrictions than gFOBT (Vart, et al., 2012). However, data comparing adherence to and accuracy of different FITs is lacking (Whitlock, et al., 2008). Understanding the difference in acceptability and accuracy of different high-sensitivity FOBTs is important for comparing their effectiveness and for healthcare systems facing choices about how to provide different options for CRC screening. We therefore conducted a randomized controlled trial (RCT) to compare the uptake and positive predictive value (PPV) of three different high-sensitivity FOBTs (two FITs and one gFOBT).
METHODS
Design Overview
We conducted a 3-arm parallel RCT to compare uptake of three different high-sensitivity FOBTs. Participants were allocated to the three arms in equal proportions. PPVs were calculated by abstracting medical records of patients with positive tests to determine if cancer or advanced neoplasm was present. The Group Health Institutional Review Board approved all study activities. This study was registered with ClinicalTrials.gov (identifier NCT01052922) (Green, et al., 2012, Green, et al., 2010).
Setting and Participants
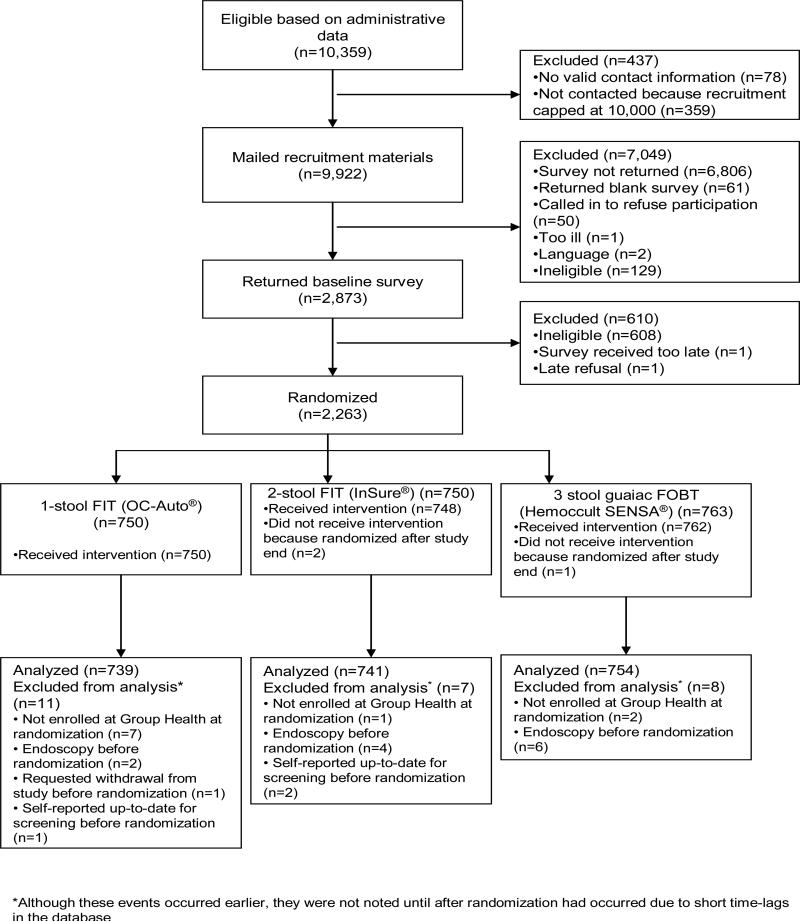
Participants were members of Group Health, an integrated health insurance and care delivery system in Washington State (Group Health Cooperative, 2012). Only Group Health members who received their primary care at one of 21 Western Washington Group Health-owned medical centers were eligible. We obtained a waiver of consent to use administrative and clinical data systems to identify plan members aged 50-74 years who were due for CRC screening, defined as not having had a colonoscopy in the past 9 years, flexible sigmoidoscopy within 4 years, or FOBT in the past 8 months. We mailed an invitation letter, baseline survey, $2, and consent checklist to 9,922 Group Health patients. Potential participants indicated their willingness to participate in the trial by returning the baseline survey (Figure 1). The baseline survey collected information on demographics (including race/ethnicity), past CRC screening, family history of CRC, screening knowledge, preferences, self-efficacy, and general health. Reminder letters were sent to nonresponders after 4-5 days, followed by a second survey packet 4 weeks after the initial survey to those who still had not responded. Completed surveys were scanned into the database within 2 days of receipt and eligibility was checked. Ineligibility criteria were past CRC, ulcerative colitis, Crohn's disease, colostomy, hereditary polyposis, family history of CRC in a first-degree relative younger than age 60, or serious chronic diseases (e.g., heart failure, dementia), all determined from clinical data systems or survey responses. We excluded potential participants whose survey response reported colonoscopy in the past 9 years. Recruitment occurred from January 2010 through February 2011.
Figure 1.
Participant recruitment and randomization for trial of fecal occult blood test uptake, Group Health, Washington State 2010-2012.
Randomization and Interventions
Randomization
Randomization was stratified by clinic, age (50-64 years, ≥65 years), and gender. Participants were allocated equally to the three study arms. Lists of treatment group assignments were created for each of 84 potential strata for 21 clinics, 2 age groups, and 2 gender groups. Each list was formed by stacking 166 blocks of length 6, where each block was a random permutation of the three treatment assignments, each represented twice. In this way, we created randomization lists sufficient for the number of anticipated participants for each stratum. The study database automatically checked daily for newly entered baseline surveys. After these eligibility checks, the database assigned eligible patients to the next available randomization slot in their stratum. This process was concealed from the study investigators and all assignments were final.
Interventions
Participants received one of three different FOBT kits by mail: 1-sample OC-Auto® FIT, 2-sample InSure® FIT, or 3-sample gFOBT Hemoccult SENSA®, with instructions and a postage-paid returned envelope. FOBT intervention characteristics, including number of samples required, sampling methods, and testing restrictions, are in Table 1. We used the manufacturers’ recommendations for number of samples and sampling instructions. A research assistant mailed the assigned FOBT to the participant. If no kit was returned within three weeks, the patient was sent a reminder letter. FOBT kits were re-mailed only in cases of address change or by patient request after a lost kit.
Table 1.
Characteristics of fecal occult blood tests mailed to participants in different study arms
| Test | OC-Auto® | InSure® | Hemoccult SENSA® |
|---|---|---|---|
| Manufacturera | Eiken | Enterix | Beckman Coulter |
| Type | FIT | FIT | Guaiac |
| Number of stools | 1 | 2 | 3 |
| Number of samples per stool | 1 | 1 | 2 |
| Sampling method | Use stick to scrape stool surface, put in liquid in tube | Use brush to brush surface of stool, smear on dry cards | Use stick to sample surface of stool, smear on dry cards |
| Restrictions | Not during menstruation | Not during menstruation | No red meat or NSAIDs. Limit aspirin and vitamin C. |
| Cut-off for positive test | 100 ng/cc | 75 ng/cc | Not applicable |
Abbreviations: FIT, fecal immunochemical test; NSAID, nonsteroidal anti-inflammatory drug.
Eiken Chemical Company, LDT (Tokyo, Japan); distributed by Polymedco Cancer Diagnostic Products, LLC (Cortland Manor, New York); Enterix (Edison, New Jersey); Beckman Coulter (Fullerton, California).
As with routine Group Health laboratory tests, study FOBT results were recorded in the patient's electronic health record (EHR). For positive FOBTs, a notice was sent to the inbox of the patient's physician, who ordered follow-up diagnostic colonoscopies as usual for a positive FOBT. Participants also received the results and instructions to call their doctor, if they had not already discussed the results. A study nurse tracked colonoscopy completion. The nurse communicated with the physician, patient, and principal investigator if follow-up was not completed. The nurse assured EHR documentation for those not completing colonoscopy (e.g., patient too ill or refused after a discussion with physician).
Blinding
Participants were not blinded to the FOBT they received. The chart abstractor who recorded colonoscopy findings following positive FOBTs was blinded to intervention group status, but the study nurse who ensured that follow-up colonoscopies were complete was not blinded. The outcome of test return was obtained from administrative data sources using the same process and codes for all three intervention groups. Investigators were not blinded; however, they were not involved in data collection or management.
Outcomes and Follow-up
FOBT uptake
Primary
Our primary outcome was return of any FOBT kit within 6 months of a participant's randomization date. Information on test return was collected from Group Health electronic laboratory files using laboratory test codes designated specifically for the study.
Secondary
In secondary analyses, we examined FOBT return within 3 months of randomization, return of only those FOBTs that were mailed to participants as part of this study (determined based on laboratory code), and receipt of any CRC testing (FOBT, flexible sigmoidoscopy, or colonoscopy) within 3 or 6 months of randomization. We obtained colonoscopy and flexible sigmoidoscopy completion data using procedure codes from claims and EHR data.
FOBT positive predictive value
PPV was a planned secondary outcome in this study. For screening tests, the PPV is the probability that the condition of interest is present when the test is positive. For this study, the condition of interest was CRC or a high-risk finding requiring increased surveillance as defined by the USMSTF: ≥3 adenomas, an adenoma >1 cm (we used ≥ 1 cm, as recommended by our gastroenterologist advisors), an adenoma with villous features, an adenoma with high-grade dysplasia, or sessile adenomas removed piecemeal (Levin, et al., 2008). If any of these conditions were found within one year after the positive FOBT, we considered that FOBT to be a true positive. We allowed a full year to ensure adequate time for follow-up colonoscopy in all patients. We ascertained the presence of CRC or high-risk adenomas through medical records abstraction. For each FOBT positive participant, a trained chart abstractor, blinded to study randomization arm, reviewed endoscopy and pathology reports for cancer and number of polyps, as well as polyp size, location, pathology, and degree of dysplasia.
Harms
Automated data was used to track any hospitalizations within 30 days of a colonoscopy and participant deaths. The project manager and/or the principal investigator reviewed all occurrences to determine whether they were related to study procedures.
Statistical analysis
FOBT uptake
We compared FOBT uptake in the three study arms using a modified intent-to-treat approach (Figure 1). The three trial arms were compared descriptively for patient demographics, healthcare utilization before baseline, and patient-reported beliefs about CRC screening and readiness to be screened. In the primary analysis of uptake of any FOBT, we considered colonoscopy and flexible sigmoidoscopy to be competing risks (Lau, et al., 2009), meaning events that would prevent a person from completing an FOBT as the first CRC screening event after randomization, which was our outcome of interest. In the secondary analysis of uptake of study FOBT, we considered colonoscopy, flexible sigmoidoscopy, or any non-study FOBT kit completion as competing risks. Finally, for the secondary analysis of uptake of any CRC test, the event of interest was use of any testing modality, so no competing risks were considered. In each analysis, participants who self-reported disenrolling from Group Health,reported being up to date for screening or otherwise ineligible, or who chose to withdraw from the study were censored at the time of the event. For each analysis, we compared estimates of the cumulative incidence of FOBT completion across treatment groups using Gray's test (Gray, 1988). In secondary analyses, we performed pair-wise comparisons for the three arms using Gray's tests with a Bonferroni correction to account for multiple comparisons (Hsu, 1996). P values < 0.017 (0.05/3) were considered significant. All tests compared cumulative incidence between randomization and 6 months.
FOBT positive predictive value
A positive FOBT was defined as ≥100 ng/mL fecal hemoglobin for OC-Auto, ≥ 75 ng/mL on at least one of two samples for InSure, or a positive result for any of the six samples for Hemoccult SENSA (which tests 2 samples on each of 3 stools).
We computed PPV among study participants who had a positive FOBT within 6 months of randomization and who received a colonoscopy to investigate the positive result. Because this was an analysis of test performance, data were analyzed according to test received rather than test assigned. In the main PPV analysis, we excluded patients who did not have a colonoscopy within one year of the positive FOBT because their true status could not be known (N = 4). We computed exact binomial confidence intervals for each proportion. Given the small number of positive tests, we did not use regression modeling to obtain adjusted results. In a secondary analysis, we did not classify sessile adenomas removed piecemeal as positive. We also computed PPV separately for any adenoma or cancer.
Analyses were conducted in Stata 12 (College Station, TX), and Gray's testing of cumulative incidence was conducted in R, version 2.15.1 (R Core Team, 2012).
Sample size
Assuming 45% uptake of Hemoccult SENSA, a sample size of 600 patients per group with 10% loss to follow-up would provide 85% power to detect a 10% difference in screening adherence comparing the 1-sample OC-Auto FIT with the 3-sample Hemoccult SENSA and 85% power to detect a 10% difference in screening adherence comparing the 2-sample InSure with the 3-sample Hemoccult SENSA. Under these assumptions, we also had 82% power to detect a 10% difference between any of the 3 arms.
Role of funding source
The funder played no role in the study.
RESULTS
From 10,359 potential participants identified through automated data systems, 9922 were mailed recruitment materials, and 2263 (22% of 10,359) were randomized to one of the three study arms (Figure 1). Three people were inadvertently randomized after the end of recruitment; they did not receive an intervention and were not included in the analysis. We excluded persons who, after randomization, were found to have disenrolled from the health plan before randomization (n = 10), been screened before randomization date (n = 15), or requested withdrawal (n = 1). A total of 2234 participants remained for analysis.
FOBT uptake
Participants in the three arms of the study were similar demographically and in their views on CRC screening (Table 2). The average participant age was 58 years; the majority were white and had at least some college education. Slightly more than half (58%) were women. Most participants believed that receiving CRC screening in the next 6 months was somewhat or very important; about half were thinking of or taking steps to be screened.
Table 2.
Baseline characteristics of analyzed participants by randomization group, Group Health, Washington State 2010-2012
| OC-Auto® N = 739 | InSure® N = 741 | Hemoccult SENSA® N = 754 | |
|---|---|---|---|
| Mean age, years (SD) | 59 (6) | 59 (6) | 58 (6) |
| 50–64 years, No. (%) | 605 (81.9) | 613 (82.7) | 619 (82.1) |
| ≥65 years, No. (%) | 134 (18.1) | 128 (17.3) | 135 (17.9) |
| Female, No. (%) | 424 (57.4) | 438 (59.1) | 433 (57.4) |
| Race/Ethnicity, No. (%)a | |||
| Hispanic | 21 (2.9) | 24 (3.3) | 20 (2.7) |
| Non-Hispanic | |||
| Black | 22 (3.1) | 18 (2.5) | 17 (2.3) |
| Asian/Pacific Islander | 38 (5.3) | 45 (6.2) | 62 (8.4) |
| Other | 33 (4.6) | 29 (4.0) | 31 (4.2) |
| White | 603 (84.1) | 605 (83.9) | 606 (82.3) |
| (Missing) | 22 | 20 | 18 |
| Education, No. (%) | |||
| High school graduate, GED or less | 120 (16.3) | 115 (15.7) | 95 (12.7) |
| Some college, no degree | 245 (33.3) | 259 (35.3) | 280 (37.4) |
| Bachelor's degree or higher | 371 (50.4) | 359 (49) | 373 (49.9) |
| (Missing) | 3 | 8 | 6 |
| Marital status, No. (%) | |||
| Not married or living with partner | 172 (24.7) | 174 (25.1) | 180 (25.4) |
| Married or living with partner | 524 (75.3) | 520 (74.9) | 529 (74.6) |
| (Missing) | 43 | 47 | 45 |
| Employment status, No. (%) | |||
| Full or part time | 518 (70.7) | 505 (68.9) | 532 (71.0) |
| Retired | 159 (21.7) | 165 (22.5) | 164 (21.9) |
| Other | 56 (7.6) | 63 (8.6) | 53 (7.1) |
| (Missing) | 6 | 8 | 5 |
| Importance of CRC screening in the next 6 months, No. (%) | |||
| Very | 125 (17.2) | 122 (16.8) | 140 (18.9) |
| Somewhat | 321 (44.2) | 314 (43.2) | 321 (43.3) |
| Not very | 212 (29.2) | 203 (27.9) | 216 (29.1) |
| Not at all | 68 (9.4) | 88 (12.1) | 65 (8.8) |
| (Missing) | 13 | 14 | 12 |
| CRC screening plans in the next 6 months, No. (%) | |||
| Not planning on screening in the next 6 months | 187 (26.1) | 212 (29.5) | 217 (29.6) |
| Thinking about it, but not in next 6 months | 151 (21.1) | 118 (16.4) | 131 (17.9) |
| Thinking of screening in next 6 months | 299 (41.7) | 312 (43.5) | 313 (42.7) |
| Already taking steps to be screened in next 6 months | 71 (9.9) | 65 (9.1) | 67 (9.1) |
| Already completed CRC screening within last 6 months | 9 (1.3) | 11 (1.5) | 5 (0.7) |
| (Missing) | 22 | 23 | 21 |
| Screening is too embarrassing: agree or strongly agree, No. (%) | 129 (17.7) | 106 (14.5) | 123 (16.6) |
| (Missing) | 10 | 14 | 10 |
| Blood stool test might be disgusting: agree or strongly agree, No. (%) | 103 (14.1) | 88 (12.1) | 80 (10.8) |
| (Missing) | 9 | 16 | 13 |
| Screening preference, No. (%) | |||
| FOBT | 349 (47.8) | 337 (46.1) | 357 (48.0) |
| Flexible sigmoidoscopy | 20 (2.7) | 24 (3.3) | 20 (2.7) |
| Colonoscopy | 158 (21.6) | 153 (20.9) | 159 (21.4) |
| FOBT and flexible sigmoidoscopy | 49 (6.7) | 50 (6.8) | 60 (8.1) |
| No test | 11 (1.5) | 26 (3.6) | 16 (2.2) |
| Don't know | 17 (2.3) | 13 (1.8) | 7 (0.9) |
| Other | 126 (17.3) | 128 (17.5) | 124 (16.7) |
| (Missing) | 9 | 10 | 11 |
Abbreviations: CRC, colorectal cancer; FOBT, fecal occult blood test; GED, graduate equivalency degree; SD, standard deviation.
Multiple endorsed race/ethnicity items were categorized in this order: Hispanic, black, Asian, other, and white. No response to the Hispanic item was combined with not Hispanic. No endorsement of Hispanic ethnicity or any race category was coded as missing.
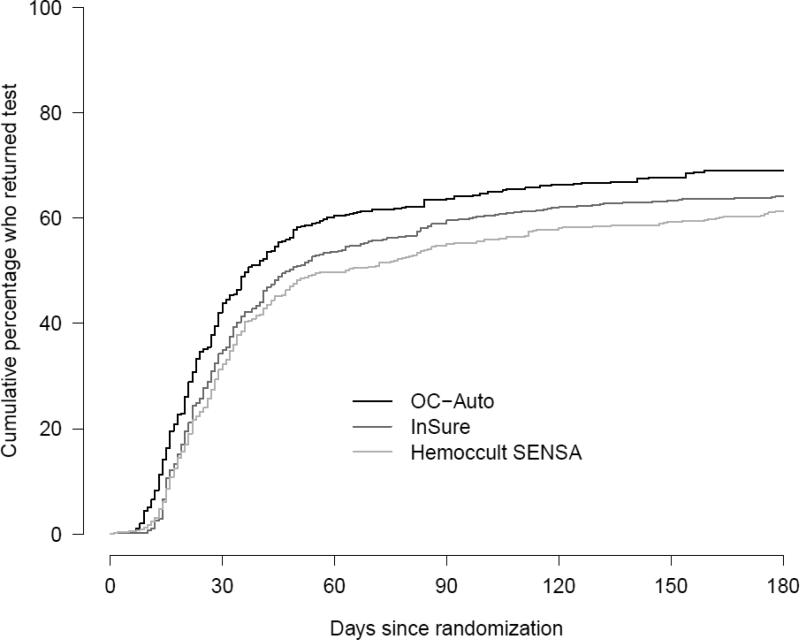
Table 3 shows the follow-up status of participants in each group at six months. For our primary outcome of return of any FOBT, 1431 (64%) of 2234 eligible participants returned any FOBT kit within 6 months of randomization and before any censoring events. As shown in Table 4, after accommodating loss to follow-up and competing endoscopy events, the proportions screened by any FOBT within 6 months of randomization were 0.69 (95% confidence interval [CI]: 0.66-0.72) for the OC-Auto FIT arm, 0.64 (95% CI: 0.61-0.68) for the InSure FIT arm, and 0.61 (95% CI: 0.58-0.65) for the gFOBT Hemoccult SENSA arm. Differences in FOBT return between randomization and 6 months were significant (P < 0.001) (Figure 2). Pairwise comparisons showed significant differences between the OC-Auto group and each of the other groups after correction for multiple comparisons (Table 4). Differences between the InSure and SENSA groups were not significant. Similar results emerged for our secondary outcomes of uptake of study FOBT and receipt of any CRC test (Table 4).
Table 3.
Follow-up status of participants at 180 days by randomization group, Group Health, Washington State 2010-2012
| OC-Auto® | InSure® | Hemoccult SENSA® | |
|---|---|---|---|
| N = 739 | N = 741 | N = 754 | |
| FOBTa, No. (%) | |||
| Completed assigned study FOBT | 473 (64.0%) | 445 (60.1%) | 403 (53.4%) |
| Completed non-study FOBT | 31 (4.2%) | 26 (3.5%) | 53 (7.0%) |
| Endoscopy, No. (%) | |||
| Colonoscopy | 13 (1.8%) | 18 (2.4%) | 18 (2.4%) |
| Flexible Sigmoidoscopy. | 2 (0.3%) | 2 (0.3%) | 0 (0%) |
| Still at risk for screening at 180 days | 203 (27.5%) | 236 (31.8%) | 260 (34.5%) |
| Withdrawal, No. (%) | |||
| Self-report ineligible | 1 (0.1%) | 0 (0%) | 0 (0%) |
| Self-report up to date | 4 (0.5%) | 1 (0.1%) | 5 (0.7%) |
| Request termination | 0 (0%) | 0 (0%) | 1 (0.1%) |
| Disenrolled from GH, No. (%) | 12 (1.6%) | 13 (1.8%) | 14 (1.9%) |
Abbreviations: FOBT, fecal occult blood test.
Percents do not exactly match those in Table 4, which were derived from a competing risks analysis which allowed the denominator to change over time based on who was still “at risk” of screening. This table uses a fixed denominator for each column (i.e., the number of participants eligible for analysis assigned to that group).
Table 4.
Proportion of analyzed participants returning mailed FOBT, by randomization group, Group Health, Washington State 2010-2012a
| OC-Auto® | InSure® | Hemoccult SENSA® | Omnibusb | Posthoc pairwise testsc | |||
|---|---|---|---|---|---|---|---|
| N = 739 | N = 741 | N = 754 | Gray's test | OC-Auto® vs. InSure® | OC-Auto® vs. Hemoccult SENSA® | InSure® vs. Hemoccult SENSA® | |
| Any FOBT returned | P < 0.001 | P = 0.005 | P < 0.001 | P = 0.176 | |||
| by 3 months | 0.64 (0.60, 0.67) | 0.60 (0.56, 0.63) | 0.55 (0.52, 0.59) | ||||
| by 6 months | 0.69 (0.66, 0.72) | 0.64 (0.61, 0.68) | 0.61 (0.58, 0.65) | ||||
| Study FOBT returned | P < 0.001 | P = 0.016 | P < 0.001 | P = 0.008 | |||
| by 3 months | 0.61 (0.57, 0.64) | 0.57 (0.54, 0.61) | 0.50 (0.46, 0.53) | ||||
| by 6 months | 0.65 (0.61, 0.68) | 0.61 (0.57, 0.64) | 0.54 (0.51, 0.58) | ||||
| Any CRC testing completed | P < 0.001 | P = 0.006 | P < 0.001 | P = 0.155 | |||
| by 3 months | 0.65 (0.62, 0.69) | 0.61 (0.57, 0.64) | 0.56 (0.53, 0.60) | ||||
| by 6 months | 0.71 (0.68, 0.74) | 0.67 (0.64, 0.70) | 0.64 (0.60, 0.67) | ||||
Abbreviations: CRC, colorectal cancer; FOBT, fecal occult blood test.
Data are proportion (95% confidence interval).
Omnibus Gray's test is for differences in cumulative incidence estimates across all three none, with follow-up truncated at 6 months. Estimates of cumulative incidence accommodate competing risks, as described in the text.
Posthoc pairwise Gray's tests are significant when P is below 0.05/3 = 0.0167, based on a Bonferroni correction.
Figure 2.
Test return within 6 months by randomization group. Blue, OC-Auto; red, InSure; green, Hemoccult SENSA, Group Health, Washington State 2010-2012.
Positive predictive value
Among the 1431 participants who returned FOBT kits within 6 months of randomization, 2.3% (N=51) had positive tests: 2.6% (N=19) in the OC-Auto FIT arm (three of the returned test were the standard Group Health gFOBT instead of the study FIT, 2.8% (N=21) in the InSure FIT arm, and 1.5% (N=11) in the Hemoccult SENSA arm ). All participants who tested positive by the InSure or Hemoccult SENSA FOBT received a colonoscopy; however, four people in the OC-Auto test group who tested positive did not complete a colonoscopy within one year of their positive FOBT. No cancers were detected in any participants; however, of the 47 who received a colonoscopy, 28% (13/47) had a high-risk finding. PPVs for the FOBTs were 33% for OC-Auto, 24% for InSure, and 29% for Hemoccult SENSA. Table 5 shows results by FOBT group, including alternate outcome definitions.
Table 5.
Positive predictive value of three different FOBTs, Group Health, Washington State 2010-2012a
| OC-Auto® | InSure® | Hemoccult SENSA® | |
|---|---|---|---|
| FOBT positive, No. | 16 | 21 | 14 |
| Followed-up by colonoscopy, No. | 12 | 21 | 14 |
| High-risk finding per modified USMSTF guidelinesa | |||
| No. | 4 | 5 | 4 |
| PPV (95% confidence interval) | 33% (10%-65%) | 24% (8%-47%) | 29% (8%-58%) |
| High-risk finding per adapted USMSTF guidelinesa, excluding sessile adenomas removed piecemeal | |||
| No. | 2 | 5 | 2 |
| PPV (95% confidence interval) | 17% (2%-48%) | 24% (8%-47%) | 14% (2%-42%) |
| Any adenoma or cancerb | |||
| No. | 7 | 8 | 7 |
| PPV (95% confidence interval) | 58% (28%-85%) | 38% (18%-62%) | 50% (23%-77%) |
Abbreviations: FOBT, fecal occult blood test; PPV: positive predictive value; USMSTF: United States Multi-Society Task Force.
USMSTF defines high risk as cancer, ≥3 adenomas, adenoma >1 cm (we used ≥1 cm), adenoma with villous features, adenoma with high-grade dysplasia, or sessile adenomas removed piecemeal.
All were adenomas. PPVs were computed based on test returned rather than randomization group. Three of the participants assigned to the OC-Auto FIT test had completed a standard Group Health gFOBT instead of the study FIT.
Harms
No deaths or hospitalizations within one month of colonoscopy were attributable to the study.
DISCUSSION
Identifying approaches to improve adherence to CRC screening is critical for providers and systems trying to improve patient outcomes; FOBT shows promise as an acceptable test to patients. However, much remains unknown about which FOBT strategies yield the highest adherence. Our RCT found that completion and return of mailed FOBTs depended on test type. More patients returned the 1-sample OC-Auto test than the 2-sample InSure or 3-sample Hemoccult SENSA tests. The greater uptake we observed for FIT vs. gFOBT is consistent with patient-reported preferences in a Dutch population (Hol, et al., 2010). The FITs we evaluated did not have the dietary and other restrictions of gFOBTs. However, we do not know if stool-sampling method, dietary restrictions, or number of required stool samples accounted for the differences in test uptake. In any case, the overall effect on adherence is the most relevant result for providers and healthcare systems deciding which tests to offer.
Our uptake results are consistent with other RCTs that found higher uptake of FIT than gFOBT (Cole, et al., 2003, Hoffman, et al., 2010, Hol, et al., 2010, Hughes, et al., 2005, van Rossum, et al., 2008). In the one RCT that did not observe a difference in uptake between mailed FIT and gFOBT, the FIT procedure was equally if not more demanding than the gFOBT, with the FIT requiring 3 stool samples (similar to the guaiac test) and sample refrigeration (Birkenfeld, et al., 2011). Percent uptake differs considerably among trials, ranging from 30-40% in an Australian study to 50-70% in a Veterans Affairs study. A possible reason our rates were relatively high is that all participants agreed to participate in the study before being randomized. International studies of screening programs that simultaneously delivered an invitation to participate and the intervention observed slightly lower uptake (Cole, et al., 2003, Hol, et al., 2010, van Rossum, et al., 2008).
Most RCTs to date compare uptake of FIT to gFOBT but do not compare adherence to different FITs (Vart, et al., 2012). However, Cole et al. randomized 1818 people in an urban region of Australia to receive Hemoccult SENSA (dietary and medication restrictions, 3-stool sample, spatula collection), FlexSure OBT (no dietary or medication restrictions, 3-stool sample, spatula collection), and InSure (no dietary or medication restrictions, 2-stool sample, brush collection) (Cole, et al., 2003). They found that both removing dietary restrictions and simplifying sampling increased screening participation. Uptake of InSure was 39.6% compared to 30.5% for FlexSure OBT and 23.4% for SENSA. A Dutch study found comparable uptake rates (approximately 61%) in groups mailed 1-sample and 2-sample OC-Micro tests (van Roon, et al., 2011). Differences between their results and ours might be attributable to: different study populations, differences between the sampling procedures of the 1- and 2- stool tests in our study, or confounding by calendar time. The Dutch study was not a randomized controlled trial, with the 2-stool group mailed test kits approximately two years after the 1-stool group, when screening rates may have been higher overall.
Rehydrated guaiac FOBT have generally been found to have lower specificity than FITs (U. S. Preventive Services Task Force, 2008, Whitlock, et al., 2008, Allison, et al., 2007). Since PPV is strongly influenced by specificity, FITs would be expected to have higher PPV than Hemoccult SENSA. PPV for FITs in screening populations ranges from 5%-15% for cancer and 10%-50% for high-risk findings (van Rossum, et al., 2008, Allison, et al., 2007, Grazzini, et al., 2009, Fenocchi, et al., 2006, Castiglione, et al., 2002, Dancourt, et al., 2008, Hol, et al., 2009, Faivre, et al., 2012, Faivre, et al., 2012, Chiang, et al., 2011, Hundt, et al., 2009, Levi, et al., 2011, Smith, et al., 2006, Park, et al., 2010, Parra-Blanco, et al., 2010, Crotta, et al., 2012, Levi, et al., 2007, de Wijkerslooth, et al., 2012) compared to 2%-10% and 10-40% for gFOBT (Allison, et al., 2007, Dancourt, et al., 2008, Hol, et al., 2009, Faivre, et al., 2012, Faivre, et al., 2012, Hundt, et al., 2009, Levi, et al., 2011, Smith, et al., 2006, Park, et al., 2010, Kershenbaum, et al., 2012); however, these numbers vary based on number of tests, cutoffs, and populations. In a direct comparison of InSure vs. Hemoccult SENSA using data from individuals who completed both tests, Smith et al. reported positive test rates of 6.7% for InSure and 4.2% for Hemoccult SENSA with comparable PPVs (26.0% and 20.2% for cancer and significant adenoma combined, and 41.9% and 40.4% for all neoplasias) (Smith, et al., 2006). The absence of cancer findings might have been because we studied a relatively low-risk population: the average age was <60 years, we excluded patients with a family history of CRC in a first-degree relative younger than age 60, and some had been screened previously. Because of the small number of positive tests in our study, we were unable to detect significant difference in PPV among study groups or determine whether test characteristics, such as the number of specimens or cut-off values, improved performance.
Our study has several strengths and limitations. To our knowledge, this is the second published trial, and the first in the United States, to compare uptake of different FITs to each other. The 3-arm RCT design controlled for confounding and facilitated direct comparison of different FOBT strategies. The trial was pragmatic and the interventions (mailed FOBT kits) were incorporated into routine healthcare within a population-based healthcare delivery setting. However, participants had to consent to be in the study, and we previously observed differences in people who chose not to participate in a study of CRC screening (Green, et al., 2012). The results from this study might not be generalizable to other populations. Participants who chose not to participate in the study were less likely to report CRC screening as important; they therefore might not have been as influenced by a test's convenience. Conversely, it is also possible that in a population with lower screening prevalence due to access, test convenience could be even more important and result in more pronounced differences than observed in the present study. Another limitation of the study was that we were not able to evaluate tests’ sensitivity or long-term outcomes.
CONCLUSIONS
Our study suggests that uptake of mailed FOBT varies by test type. The test that required the fewest samples had the highest uptake. Although we were unable to separate effects from dietary restrictions, sampling methods, or sample number, our results provide important comparative data on different testing strategies as they would be offered in clinical practice. Our findings—along with information on other factors affecting uptake such as invitation method (Van Roosbroeck, et al., 2012, Libby, et al., 2011, Cole, et al., 2007), patient instructions from physicians (Bapuji, et al., 2012), physician recommendation (Inadomi, et al., 2012, Subramanian, et al., 2004), and level of support (Green, et al., 2013)—are important considerations for providers and healthcare delivery systems seeking to increase CRC screening compliance.
Supplementary Material
Presentations.
Some of these data have been presented in a poster at the annual meeting of the American Society of Preventive Oncology (March 5, 2012; Washington DC) and the biennial meeting of the International Cancer Screening Network (ICSN) (October 24, 2012; Sydney, Australia).
Highlights.
FOBT uptake is important for attaining high rates of colorectal cancer screening
Mailed FOBT tests with fewer samples and dietary restrictions had higher uptake
Healthcare systems can use this information to increase colorectal cancer screening
ACKNOWLEDGMENTS
Dr. Green had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health as an American Recovery and Reinvestment Act supplemental award to the Systems of Support to Increase Colorectal Cancer Screening and Follow-up Trial (R01CA121125-03S1). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The funder was not involved in any of the following: the conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Polymedco Cancer Diagnostic Products, LLC provided the OC-AUTO Micro 80 instrument and service (training and support); the study purchased the reagents and patient collection packs.
The authors would like to thank Dr. John Inadomi, MD and Dr. Cynthia Ko, MD for assistance classifying high risk findings; Dr. James Allison, MD for assistance selecting the FITs and providing us with contacts; Dr. Ching-Yu Wang, PhD for assistance obtaining funding and statistical support; Ms. Kathryn Horner, MS and Ms. Jacquelyn St. John, BS for project management; Ms. Mary Lyons for study implementation; and Ms. Leslie Nemerever Sizemore, BFA for chart abstraction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.U. S. Preventive Services Task Force Screening for colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 2.Powell AA, Burgess DJ, Vernon SW, Griffin JM, Grill JP, Noorbaloochi S, Partin MR. Colorectal cancer screening mode preferences among US veterans. Preventive Medicine. 2009;49:442–448. doi: 10.1016/j.ypmed.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Hawley ST, Volk RJ, Krishnamurthy P, Jibaja-Weiss M, Vernon SW, Kneuper S. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care. 2008;46:S10–16. doi: 10.1097/MLR.0b013e31817d932e. [DOI] [PubMed] [Google Scholar]
- 4.Hawley ST, McQueen A, Bartholomew LK, Greisinger AJ, Coan SP, Myers R, Vernon SW. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer. 2012;118:2726–2734. doi: 10.1002/cncr.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lisi D, Hassan C, Crespi M, Group AS. Participation in colorectal cancer screening with FOBT and colonoscopy: an Italian, multicentre, randomized population study. Dig Liver Dis. 2010;42:371–376. doi: 10.1016/j.dld.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 6.Inadomi JM, Vijan S, Janz NK, Fagerlin A, Thomas JP, Lin YV, Munoz R, Lau C, Somsouk M, El-Nachef N, Hayward RA. Adherence to Colorectal Cancer Screening: A Randomized Clinical Trial of Competing Strategies. Arch Intern Med. 2012;172:575–582. doi: 10.1001/archinternmed.2012.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalid-de Bakker C, Jonkers D, Smits K, Mesters I, Masclee A, Stockbrugger R. Participation in colorectal cancer screening trials after first-time invitation: a systematic review. Endoscopy. 2011;43:1059–1086. doi: 10.1055/s-0031-1291430. [DOI] [PubMed] [Google Scholar]
- 8.Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. Systems of Support to Increase Colorectal Cancer Screening (SOS): A 2-Year Randomized Trial of an Automated Intervention with Stepped Increases of Support to Increase Uptake of Colorectal Cancer Screening. Ann Intern Med. 2012;158:301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: A randomized clinical trial. JAMA Internal Medicine. 2013 doi: 10.1001/jamainternmed.2013.9294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR, Pickhardt P, Rex DK, Thorson A, Winawer SJ. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: A joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130–160. doi: 10.3322/CA.2007.0018. [DOI] [PubMed] [Google Scholar]
- 11.Vart G, Banzi R, Minozzi S. Comparing participation rates between immunochemical and guaiac faecal occult blood tests: A systematic review and meta-analysis. Prev Med. 2012;55:87–92. doi: 10.1016/j.ypmed.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Whitlock EP, Lin JS, Liles E, Beil TL, Rongwei F. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638–658. doi: 10.7326/0003-4819-149-9-200811040-00245. [DOI] [PubMed] [Google Scholar]
- 13.Green BB, Wang CY, Horner K, Catz S, Meenan RT, Vernon SW, Carrell D, Chubak J, Ko C, Laing S, Bogart A. Systems of support to increase colorectal cancer screening and follow-up rates (SOS): Design, challenges, and baseline characteristics of trial participants. Contemp Clin Trials. 2010;31:589–603. doi: 10.1016/j.cct.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Group Health Cooperative Fast Facts About Group Health. 2012 [Google Scholar]
- 15.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16:1141–1154. [Google Scholar]
- 17.Hsu J. Multiple Comparisons: Theory and Methods.) Chapman & Hall/CRC; Boca Raton, Florida: 1996. [Google Scholar]
- 18.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2012. [Google Scholar]
- 19.Hol L, de Jonge V, van Leerdam ME, van Ballegooijen M, Looman CW, van Vuuren AJ, Reijerink JC, Habbema JD, Essink-Bot ML, Kuipers EJ. Screening for colorectal cancer: comparison of perceived test burden of guaiac-based faecal occult blood test, faecal immunochemical test and flexible sigmoidoscopy. Eur J Cancer. 2010;46:2059–2066. doi: 10.1016/j.ejca.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 20.Cole SR, Young GP, Esterman A, Cadd B, Morcom J. A randomised trial of the impact of new faecal haemoglobin test technologies on population participation in screening for colorectal cancer. J Med Screen. 2003;10:117–122. doi: 10.1177/096914130301000304. [DOI] [PubMed] [Google Scholar]
- 21.Hoffman RM, Steel S, Yee EF, Massie L, Schrader RM, Murata GH. Colorectal cancer screening adherence is higher with fecal immunochemical tests than guaiac-based fecal occult blood tests: a randomized, controlled trial. Prev Med. 2010;50:297–299. doi: 10.1016/j.ypmed.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Hol L, van Leerdam ME, van Ballegooijen M, van Vuuren AJ, van Dekken H, Reijerink JC, van der Togt AC, Habbema JD, Kuipers EJ. Screening for colorectal cancer: randomised trial comparing guaiac-based and immunochemical faecal occult blood testing and flexible sigmoidoscopy. Gut. 2010;59:62–68. doi: 10.1136/gut.2009.177089. [DOI] [PubMed] [Google Scholar]
- 23.Hughes K, Leggett B, Del Mar C, Croese J, Fairley S, Masson J, Aitken J, Clavarino A, Janda M, Stanton WR, Tong S, Newman B. Guaiac versus immunochemical tests: faecal occult blood test screening for colorectal cancer in a rural community. Aust N Z J Public Health. 2005;29:358–364. doi: 10.1111/j.1467-842x.2005.tb00207.x. [DOI] [PubMed] [Google Scholar]
- 24.van Rossum LG, van Rijn AF, Laheij RJ, van Oijen MG, Fockens P, van Krieken HH, Verbeek AL, Jansen JB, Dekker E. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology. 2008;135:82–90. doi: 10.1053/j.gastro.2008.03.040. [DOI] [PubMed] [Google Scholar]
- 25.Birkenfeld S, Belfer RG, Chared M, Vilkin A, Barchana M, Lifshitz I, Fruchter D, Aronski D, Balicer R, Niv Y, Levi Z. Factors affecting compliance in faecal occult blood testing: a cluster randomized study of the faecal immunochemical test versus the guaiac faecal occult test. J Med Screen. 2011;18:135–141. doi: 10.1258/jms.2011.010147. [DOI] [PubMed] [Google Scholar]
- 26.van Roon AH, Wilschut JA, Hol L, van Ballegooijen M, Reijerink JC, t Mannetje H, Kranenburg LJ, Biermann K, van Vuuren AJ, Francke J, van der Togt AC, Habbema DJ, van Leerdam ME, Kuipers EJ. Diagnostic yield improves with collection of 2 samples in fecal immunochemical test screening without affecting attendance. Clin Gastroenterol Hepatol. 2011;9:333–339. doi: 10.1016/j.cgh.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Allison JE, Sakoda LC, Levin TR, Tucker JP, Tekawa IS, Cuff T, Pauly MP, Shlager L, Palitz AM, Zhao WK, Schwartz JS, Ransohoff DF, Selby JV. Screening for colorectal neoplasms with new fecal occult blood tests: update on performance characteristics. J Natl Cancer Inst. 2007;99:1462–1470. doi: 10.1093/jnci/djm150. [DOI] [PubMed] [Google Scholar]
- 28.Grazzini G, Visioli CB, Zorzi M, Ciatto S, Banovich F, Bonanomi AG, Bortoli A, Castiglione G, Cazzola L, Confortini M, Mantellini P, Rubeca T, Zappa M. Immunochemical faecal occult blood test: number of samples and positivity cutoff. What is the best strategy for colorectal cancer screening? Br J Cancer. 2009;100:259–265. doi: 10.1038/sj.bjc.6604864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenocchi E, Martinez L, Tolve J, Montano D, Rondan M, Parra-Blanco A, Eishi Y. Screening for colorectal cancer in Uruguay with an immunochemical faecal occult blood test. Eur J Cancer Prev. 2006;15:384–390. doi: 10.1097/00008469-200610000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Castiglione G, Grazzini G, Miccinesi G, Rubeca T, Sani C, Turco P, Zappa M. Basic variables at different positivity thresholds of a quantitative immunochemical test for faecal occult blood. J Med Screen. 2002;9:99–103. doi: 10.1136/jms.9.3.99. [DOI] [PubMed] [Google Scholar]
- 31.Dancourt V, Lejeune C, Lepage C, Gailliard MC, Meny B, Faivre J. Immunochemical faecal occult blood tests are superior to guaiac-based tests for the detection of colorectal neoplasms. Eur J Cancer. 2008;44:2254–2258. doi: 10.1016/j.ejca.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 32.Hol L, Wilschut JA, van Ballegooijen M, van Vuuren AJ, van der Valk H, Reijerink JC, van der Togt AC, Kuipers EJ, Habbema JD, van Leerdam ME. Screening for colorectal cancer: random comparison of guaiac and immunochemical faecal occult blood testing at different cut-off levels. Br J Cancer. 2009;100:1103–1110. doi: 10.1038/sj.bjc.6604961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faivre J, Dancourt V, Denis B, Dorval E, Piette C, Perrin P, Bidan JM, Jard C, Jung S, Levillain R, Viguier J, Bretagne JF. Comparison between a guaiac and three immunochemical faecal occult blood tests in screening for colorectal cancer. Eur J Cancer. 2012;48:2969–2976. doi: 10.1016/j.ejca.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Faivre J, Dancourt V, Manfredi S, Denis B, Durand G, Gendre I, Bidan JM, Jard C, Levillain R, Jung S, Viguier J, Dorval E. Positivity rates and performances of immunochemical faecal occult blood tests at different cut-off levels within a colorectal cancer screening programme. Dig Liver Dis. 2012;44:700–704. doi: 10.1016/j.dld.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 35.Chiang T-H, Lee Y-C, Tu C-H, Chiu H-M, Wu M-S. Performance of the immunochemical fecal occult blood test in predicting lesions in the lower gastrointestinal tract. CMAJ. 2011;183:1474–1481. doi: 10.1503/cmaj.101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hundt S, Haug U, Brenner H. Comparative evaluation of immunochemical fecal occult blood tests for colorectal adenoma detection. Ann Intern Med. 2009;150:162–169. doi: 10.7326/0003-4819-150-3-200902030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Levi Z, Birkenfeld S, Vilkin A, Bar-Chana M, Lifshitz I, Chared M, Maoz E, Niv Y. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer. 2011;128:2415–2424. doi: 10.1002/ijc.25574. [DOI] [PubMed] [Google Scholar]
- 38.Smith A, Young GP, Cole SR, Bampton P. Comparison of a brush-sampling fecal immunochemical test for hemoglobin with a sensitive guaiac- based fecal occult blood test in detection of colorectal neoplasia. Cancer. 2006;107:2152–2159. doi: 10.1002/cncr.22230. [DOI] [PubMed] [Google Scholar]
- 39.Park DI, Ryu S, Kim YH, Lee SH, Lee CK, Eun CS, Han DS. Comparison of guaiac-based and quantitative immunochemical fecal occult blood testing in a population at average risk undergoing colorectal cancer screening. Am J Gastroenterol. 2010;105:2017–2025. doi: 10.1038/ajg.2010.179. [DOI] [PubMed] [Google Scholar]
- 40.Parra-Blanco A, Gimeno-Garcia AZ, Quintero E, Nicolas D, Moreno SG, Jimenez A, Hernandez-Guerra M, Carrillo-Palau M, Eishi Y, Lopez-Bastida J. Diagnostic accuracy of immunochemical versus guaiac faecal occult blood tests for colorectal cancer screening. J Gastroenterol. 2010;45:703–712. doi: 10.1007/s00535-010-0214-8. [DOI] [PubMed] [Google Scholar]
- 41.Crotta S, Segnan N, Paganin S, Dagnes B, Rosset R, Senore C. High Rate of Advanced Adenoma Detection in 4 Rounds of Colorectal Cancer Screening with the Fecal Immunochemical Test. Clin Gastroenterol Hepatol. 2012;10:633–638. doi: 10.1016/j.cgh.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Levi Z, Rozen P, Hazazi R, Vilkin A, Waked A, Maoz E, Birkenfeld S, Leshno M, Niv Y. A quantitative immunochemical fecal occult blood test for colorectal neoplasia. Ann Intern Med. 2007;146:244–255. doi: 10.7326/0003-4819-146-4-200702200-00003. [DOI] [PubMed] [Google Scholar]
- 43.de Wijkerslooth TR, Stoop EM, Bossuyt PM, Meijer GA, van Ballegooijen M, van Roon AH, Stegeman I, Kraaijenhagen RA, Fockens P, van Leerdam ME, Dekker E, Kuipers EJ. Immunochemical fecal occult blood testing is equally sensitive for proximal and distal advanced neoplasia. Am J Gastroenterol. 2012;107:1570–1578. doi: 10.1038/ajg.2012.249. [DOI] [PubMed] [Google Scholar]
- 44.Kershenbaum A, Flugelman A, Lejbkowicz F, Arad H, Rennert G. Excellent performance of Hemoccult Sensa in organised colorectal cancer screening. Eur J Cancer. 2012;49:923–930. doi: 10.1016/j.ejca.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Green BB, Bogart A, Chubak J, Vernon SW, Morales LS, Meenan RT, Laing SS, Fuller S, Ko C, Wang CY. Nonparticipation in a population-based trial to increase colorectal cancer screening. Am J Prev Med. 2012;42:390–397. doi: 10.1016/j.amepre.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Roosbroeck S, Hoeck S, Van Hal G. Population-based screening for colorectal cancer using an immunochemical faecal occult blood test: A comparison of two invitation strategies. Cancer Epidemiol. 2012;365:e317. doi: 10.1016/j.canep.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Libby G, Bray J, Champion J, Brownlee LA, Birrell J, Gorman DR, Crighton EM, Fraser CG, Steele RJ. Pre-notification increases uptake of colorectal cancer screening in all demographic groups: a randomized controlled trial. J Med Screen. 2011;18:24–29. doi: 10.1258/jms.2011.011002. [DOI] [PubMed] [Google Scholar]
- 48.Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP. An advance notification letter increases participation in colorectal cancer screening. J Med Screen. 2007;14:73–75. doi: 10.1258/096914107781261927. [DOI] [PubMed] [Google Scholar]
- 49.Bapuji SB, Lobchuk MM, McClement SE, Sisler JJ, Katz A, Martens P. Fecal occult blood testing instructions and impact on patient adherence. Cancer Epidemiol. 2012;36:e258–264. doi: 10.1016/j.canep.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 50.Subramanian S, Klosterman M, Amonkar MM, Hunt TL. Adherence with colorectal cancer screening guidelines: a review. Prev Med. 2004;38:536–550. doi: 10.1016/j.ypmed.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Green BB, Wang CY, Anderson ML, Chubak J, Meenan RT, Vernon SW, Fuller S. An automated intervention with stepped increases in support to increase uptake of colorectal cancer screening: a randomized trial. Ann Intern Med. 2013;158:301–311. doi: 10.7326/0003-4819-158-5-201303050-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.