Abstract
Treatment with combination antiretroviral therapy (cART) regimens with a high capacity to penetrate the blood-brain barrier has been associated with lower levels of human immunodeficiency virus (HIV) in the central nervous system (CNS). This study examined neurocognitive performance among a sample of 118 HIV+ substance dependent individuals (SDIs) and 310 HIV− SDIs. HIV+ participants were prescribed cART regimens with varying capacity to penetrate the CNS as indexed by the revised CNS penetration effectiveness (CPE) scale. Participants completed the Rotary Pursuit Task (RPT) and the Weather Prediction Task (WPT)--two measures of procedural learning (PL) with known sensitivity to HIV infection--and a control task of sustained attention. HIV+ SDIs prescribed cART with relatively high CNS penetrance performed significantly more poorly on both tasks than HIV− controls. Task performance of HIV+ SDIs prescribed cART with relatively low CNS penetrance did not differ significantly from either HIV− controls or the HIV+/High CPE group, although a trend towards lower RPT performance relative to HIV− participants was observed. Between-group differences were not seen on a control task of motor impulsivity (Immediate Memory Task), indicating that the observed deficits among HIV+/High CPE SDIs may have some specificity.
Keywords: HIV, neurocognition, substance abuse, antiretroviral therapy, addiction, procedural learning, CPE
Introduction
While the prevalence of human immunodeficiency virus (HIV) associated dementia has decreased in the era of combination antiretroviral therapy (cART), rates of milder neurocognitive impairment have remained stable. Investigators have speculated that detectable virus may persist in the brain despite successful peripheral viral suppression due to the limited capacity of some antiretroviral compounds to penetrate the central nervous system (CNS). The CNS penetration effectiveness (CPE) scale introduced by Letendre and colleagues (2008) provides a quantitative index of the relative capacity for an antiretroviral drug to cross the blood brain barrier. Evidence of the scale's validity has been demonstrated by the finding that cerebrospinal fluid levels of HIV are significantly lower among HIV-seropositive (HIV+) individuals on cART regimens with higher CPE scores than among HIV+ individuals on CPE regimens with lower CPE scores (Letendre et al., 2008; Marra et al., 2009).
Although higher CPE scores are predictive of decreased CNS viral burden, investigations of the relationship between antiretroviral CPE and neurocognitive performance have yielded inconsistent findings. Prospective studies have reported a range of outcomes associated with CPE in HIV+ subjects, including an association between highly neuropenetrant cART regimens and improved neurocognitive performance (Cysique et al., 2009; Cysique, Maruff, & Brew, 2006; Letendre et al., 2004); no relationship (Simioni et al., 2010); or a negative relationship (Marra et al., 2009) between CPE scores and neurocognition. The latter finding raises the possibility that cART regimens with high CPE scores may be associated with neurocognitive deficits in some HIV+ individuals, possibly due to neurotoxic injury (Robertson et al., 2010; Schweinsburg et al., 2005). Similar findings suggesting that high CPE drugs may exert unique neurocognitive deficits were observed by Robertson (2010), when interruption of cART treatment in medically stable patients led to significant improvement in neurocognitive functioning that remained stable for 96 weeks. A recent cross-sectional examination found that a revised version of the CPE scale (Letendre et al., 2010) has significantly improved association with neurocognitive performance relative to the original CPE scale, with evidence indicating that higher revised CPE scores and higher cART adherence were associated with lower risk of neurocognitive dysfunction (Ciccarelli et al., 2013). Therefore research utilizing the revised CPE scale may provide more consistent findings on the relationship between CPE scores and neurocognition.
Since cART CPE has been inconsistently associated with neurocognitive effects, more specific disease or comorbid subject characteristics might interact with cART CPE levels and should therefore be identified. For example, one study demonstrated a positive association between CPE scores and neurocognition limited to a subset of HIV+ participants with neurocognitive impairment at baseline (Cysique, Maruff & Brew, 2004), indicating that premorbid clinical status prior to initiation of cART may be an important determinant of the relationship between CPE and neurocognitive status. Similarly, Tozzi et al. (2007) reported persistent neurocognitive deficits correlated with baseline neurocognitive impairment in a HIV+ cART-treated sample. Cysique, Marruff & Brew (2006) observed a relationship between lower nadir CD4 and long-term neurocognitive decline in cART-treated subjects, suggesting that history of immunosuppression may also be prognostically relevant in addition to baseline neurocognitive impairment. Additionally, findings from the AIDS Clinical Trials Group (Robertson et al., 2007) indicated that neurocognitive impairment persisted in a sample of previously significantly immunosuppressed (i.e. nadir CD4 count < 200) HIV+ subjects despite treatment with cART, further indicating that cART may be ineffective for ameliorating HIV-associated neurocognitive deficits in immunosuppressed individuals. Although these findings offer promising evidence for identifying relevant participant characteristics that influence the relationship of cART CPE and neurocognition, to our knowledge no studies have previously examined the role of comorbid substance dependence.
Comorbid substance misuse is common among HIV+ individuals (Martin-Thormeyer & Paul, 2009) and neurocognitive impairment is often observed among both HIV-seronegative (HIV−) and HIV+ substance dependent individuals (SDIs). Additive effects of HIV and substances of abuse on neurocognitive performance have been reported (Jernigan et al., 2005; Schulte, Muller-Oehring, Sullivan, & Pfefferbaum, 2011), raising the possibility that a relationship between CPE scores and neurocognition in HIV+ individuals may be influenced by comorbid substance dependence. Previously, our research group has demonstrated selective deficits among HIV+ SDIs compared with matched HIV− SDIs in neurocognitive functions critically dependent on prefrontal-striatal integrity including decision-making and prospective memory (Gonzalez et al., 2005; E.M. Martin et al., 2007). Most recently, we reported selective deficits in nondeclarative or procedural learning (PL) tasks critically dependent on the integrity of basal ganglia among HIV+ SDIs compared with HIV− controls (Gonzalez et al., 2008; E. Martin, Gonzalez, Vassileva, & Maki, 2011).
In the current cross-sectional study, we investigated PL performance among HIV+ SDIs on cART regimens with varying level of CPE scores (indexed by the revised CPE scale), a topic which has received little attention to date despite its high clinical relevance. We compared two groups of HIV+ SDIs prescribed cART regimens with a variety of CPE scores on tasks of motor skill and probability learning with known sensitivity to HIV-associated neurocognitive impairment, and on a control go-no go task. Additionally, we compared task performance of the HIV+ groups with a well-matched HIV− group of SDIs in order to verify PL task deficits among the HIV+ group. For the purposes of statistical analyses, HIV+ participants were classified as belonging to either High CPE or Low CPE groups using a median split of CPE scores. Given the paucity of literature on antiretroviral neuropenetrance and substance abuse, we espoused the more conservative hypothesis that any significant performance differences between HIV+ groups on cART regimens with high or low CPE scores would be most apparent on the PL tasks due to the critical importance of neostriatal integrity for successful performance of these tasks.
Methods
Participants
Participants included 118 HIV+ and 310 HIV− adults enrolled in a larger ongoing NIH-funded study of neurocognitive effects of HIV and substance dependence. Participants were recruited from HIV/AIDS and substance dependence treatment programs at UIC, the Jesse Brown VA and the Chicago community via flyers and word-of-mouth. Informed consent was obtained from all study participants in accordance with protocols approved by the University of Illinois at Chicago Institutional Review Board and the Jesse Brown VA R & D Committee. HIV and hepatitis C serostatus were verified by ELISA for all participants. Inclusion criteria included a history of dependence on cocaine or opioids as assessed by the Substance Abuse Module of the Structured Clinical Interview for DSM-IV (SCID-SAM; First, Spitzer, Gibbon, & Williams, 2002). Exclusion criteria included a history of neurological disorder (e.g. stroke, seizures, dementia), closed-head injury with loss of consciousness exceeding 30 minutes, open head injury of any kind, schizophrenia, or current neuroleptic use. All participants received breathalyzer testing for alcohol intoxication (Intoxilyzer, CMI Inc., Owensborough, KY) and rapid urine toxicology screening for cocaine, amphetamines, methamphetamines, MDMA, cannabis, opiates and hallucinogens (Visualine, Sun Biomedical, Blackwood, NJ) upon arrival. If either test was positive or the participant appeared acutely intoxicated or presented with withdrawal symptoms, the visit was terminated and the appointment was rescheduled. Participant premorbid intelligence (IQ) was estimated via the Wechsler Test of Adult Reading (WTAR, Holdnack, 2001). Participant demographics, psychiatric comorbidities and substance use histories are presented on Table 1.
Table 1.
Participant demographics, psychiatric comorbidities and substance dependence characteristics
| Demographics | HIV− | HIV+ Low CPE | HIV+ High CPE | Significance Testing |
|---|---|---|---|---|
| n | 310 | 62 | 56 | -- |
| Age (M, SD) | 42.2 (8.5) | 42.6 (6.2) | 44.4 (6.9) | F = 1.9 |
| Years Education (M, SD) | 11.6 (1.7) | 11.3 (2.1) | 12.0 (2.0) | F = 2.3 |
| Estimated IQ (M, SD) | 87.5 (9.9) | 87.8 (10.1) | 86.1 (8.9) | F = 0.6 |
| % Female | 26.8 | 38.7 | 32.1 | χ2 = 3.8 |
| % African American | 82.3 | 82.3 | 95.4 | χ2 = 5.3 |
| % Hepatitis C Seropositive | 13.9† | 30.6 | 32.7 | χ2 = 17.7** |
| % Undetectable HIV RNA | -- | 59 | 68 | χ2 = 1.2 |
| CPE Scale (M, SD) | -- | 6.6 (0.7) | 9.1 (1.2) | F = 214.7** |
| Psychiatric Comorbidities (M, SD) | ||||
| Beck Depression Inventory-II | 10.5† (8.6) | 15.9 (11.8) | 12.2 (9.0) | F = 9.0** |
| STAI-State | 32.6† (10.2) | 36.9 (13.3) | 35.6 (11.6) | F = 4.8** |
| Wender Utah Rating Scale | 31.7 (18.7) | 34.6 (18.1) | 35.8 (24.3) | F = 1.4 |
| PTSD Checklist-Civilian | 36.2 (12.8) | 40.4 (14.6) | 35.3 (15.3) | F = 2.9 |
| Self-Report Psychopathy | 51.5 (10.5) | 53.9 (9.9) | 52.8 (10.3) | F = 1.6 |
| Sensation-Seeking Scale V | 14.8 (6.0) | 14.2 (5.7) | 14.7 (5.8) | F = 0.3 |
| Lifetime Substance Dependence (%) | ||||
| Alcohol | 67.1 | 77.4 | 75.0 | χ2 = 3.5 |
| Cannabis | 61.6 | 51.6 | 50.0 | χ2 = 4.1 |
| Cocaine | 74.8† | 85.5 | 87.5 | χ2 = 6.8* |
| Opioids | 54.2†† | 37.1 | 35.7 | χ2= 10.8** |
| Kreek-McHuah-Schluaer-Kelloaa Scale (M, SD) | ||||
| Alcohol | 10.5 (2.2) | 10.7 (2.1) | 10.6 (2.0) | F = 0.4 |
| Cocaine | 13.0 (3.0) | 13.4 (2.7) | 13.8 (3.0) | F = 2.0 |
| Opiates | 10.0 (2.8) | 9.8 (2.8) | 10.0 (3.3) | F = 0.1 |
| Days since last use (Mdn, IQR) | ||||
| Alcohol | 197 (1,015) | 184 (2,042) | 730 (2,743) | H = 4.2 |
| Cannabis | 2,555 (7,060) | 2,986 (7,177) | 5,657.5 (8,304) | H = 1.5 |
| Cocaine | 187 (1,090) | 280 (2,302) | 518 (1,205) | H = 2.7 |
| Opiates | 183 (1,160) | 295 (2,253) | 518 (932) | H = 3.2 |
Note.
.05 ≥ p
.01 > p
HIV+ > HIV−
HIV− > HIV+
STAI = State-Trait Anxiety Inventory
CNS penetration effectiveness
All HIV+ participants were currently prescribed cART. Each HIV+ participant's cART regimen was reviewed and scored using the most recent version of the CNS Penetration Effectiveness (CPE) Scale (Letendre et al., 2010). The scoring system assigns a ranking to each antiretroviral compound using a four-point scale, with 1 indicating the lowest and 4 indicating the highest relative CNS penetration capability. CPE scores for HIV+ participant cART regimens ranged from 4-12, M = 7.8, SD = 3.6. The modal CPE score for the sample was 7, n = 43 (36.4%). Additionally, a CPE score of 7 was also the median for the sample, CPE score ≤ 7, n = 62 (52.5%). CPE scores were non-normally distributed, Shapiro-Wilk test statistic (S-W) = .93, df = 118, p < .001, and were skewed rightward (see Figure 1). Several data transformations failed to yield a normal distribution of CPE scores, including log10, S-W = .94, p < .001; natural logarithm, S-W = .94, p < .001; or square root transformations, S-W = .94, p < .001. Self-reported medication adherence over the past four days as indexed by the self-report Adult AIDS Clinical Trials Group Adherence Questionnaire (Chesney et al., 2000) was not found to differ across HIV+ participant groups, Mann-Whitney U(115) = 1630, p = .84, abs(r) = .02, nor was time since last missed medication dose, U(115) = 1644, p = .97, abs(r) = .02.
Figure 1.
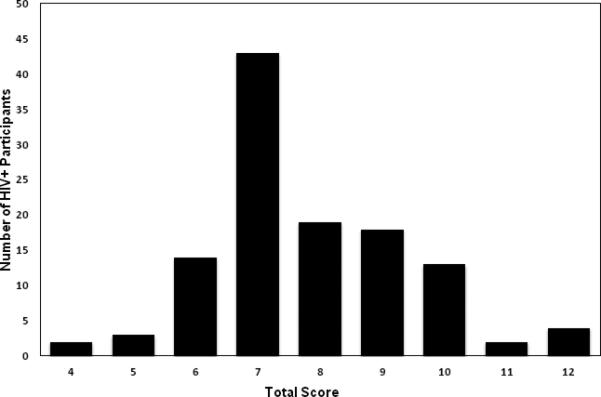
Distribution of total cART regimen CPE scores for HIV+ participants.
Substance dependence
Diagnoses of current and lifetime substance use disorder were obtained via the SCID-SAM. A majority of participants met lifetime diagnostic criteria for more than one form of substance dependence, including alcohol (70%), cannabis (59%), cocaine (78%), and opiates (49%). Past methamphetamine dependence among this study population was relatively low (l0%). Severity of cocaine, alcohol and heroin use was indexed using the Kreek-McHugh-Schluger-Kellogg scale (KMSK; Kellogg et al., 2003), a self-report scale that queries the amount, frequency, duration of use and amount of money spent on drugs during the participant's heaviest period of use. Possible KMSK scores for peak use range from 0-16 for cocaine and from 0-13 for both alcohol and heroin. Participants also reported the time in days since they had last used alcohol, cannabis, cocaine and opiates. Three-way comparisons of HIV− , HIV+/High CPE, and HIV+/Low CPE participants revealed no group differences in time since last use of alcohol, Kruskal-Wallis H(2) = 4.19, p = .12, η2 = .02; cannabis, H(2) = 1.51, p = .47; η2 = .01, cocaine; H(2) = 2.68, p = .26, η2 = .01; or heroin, H(2) = 3.20, p = .20, η2 = .01.
Psychiatric comorbidities
All participants completed paper and pencil measures of psychiatric conditions that are frequently comorbid with substance dependence and may potentially confound neurocognitive performance. These measures were later examined as potential covariates and included the Beck Depression Inventory-II (Beck, Steer, Ball, & Ranieri, 1996); the Wender Utah Rating Scale of childhood ADHD symptoms (WURS; Ward, Wender, & Reimherr, 1993); the “state” portion of the State-Trait Anxiety Inventory (STAI; Spielberger, Lushene, Vagg, & Jacobs, 1983); the Self-Report Psychopathy Scale (SRPS; Levenson, Kiehl & Fitzpatrick, 1995); the PTSD Checklist-Civilian version (PCL-C; Weathers, Litz, Herman, Huska, & Keane, 1993); and the Sensation-Seeking Scale-V (SSS-V; Zuckerman, 1996).
Neurocognitive tasks
All participants completed two PL tasks that are dependent on neostriatal integrity. The Rotary Pursuit Task (RPT; Lafayette Instruments, Model 30014A) was administered as a measure of motor skills learning (Weickert et al., 2002). The task requires the participant to hold a stylus directly over a patch of light that rotates around the circumference of a circular disk at a set speed (55 RPM). The task consists of ten 20-second trials. Participants are allowed to rest for approximately 20 seconds between each trial, and are given a 15-minute break halfway through the task. The dependent variable is the total time the stylus was kept on target.
Participants also completed the Weather Prediction Task (WPT, (Knowlton, Mangels, & Squire, 1996), a computerized measure of probabilistic learning. Test stimuli consist of a set of four cards with non-identical geometric patterns. On each trial, a combination of up to three different cards is displayed on the computer monitor. The participant is told that some card combinations predict sunny weather and others predict rain. They are instructed to press a key labeled “sun” or a key labeled “rain” to register their prediction for each card display. Participants are told that they may need to guess at first. No other instructions are provided. After each key-press, the computer presents the participant with visual and auditory feedback indicating if their prediction is correct or incorrect. Participants are administered 200 consecutive trials. For 50% of trials the correct outcome is “sun” and for 50% the correct outcome is “rain.” Each of the four possible cards that can be shown to participants is associated with a fixed probability of predicting “rain” or “sun.” The test employs a total of 14 different card patterns, each with a specific probability of occurring across the 200 trials. “Optimal” responses are those when a participant chooses the most likely outcome associated with a particular combination of cards, regardless of the feedback provided by the computer on that given trial. Task performance is measured by the percent of optimal responses, overall and for each of eight 25-trial blocks.
Finally, all participants were administered the Immediate Memory Task (IMT), a go-no go measure of motor impulsivity and sustained attention that is typically performed abnormally by SDIs and individuals with other disinhibitory behavior disorders (Dougherty, Marsh, & Mathias, 2002). On each trial a five-digit cue number appears for 500ms; 500ms after the cue stimulus offset, a second target number appears. Participants are instructed to press a response key if the target and the cue stimulus display are identical. Positive responses to target stimuli are recorded as correct detections and positive responses to non-identical targets are recorded as commission errors. The dependent variable is d′, an index of overall discriminability reflecting both correct detections and false positives. The IMT was administered for comparison purposes so that any group differences on the RPT or WPT could not be attributed to a more generalized performance deficit.
Statistical procedures
For our primary data analyses we dichotomized the 118 HIV+ participants into two groups: HIV+/Low CPE, CPE score range = 4-7, n = 62 (52.2%); and HIV+/High CPE, CPE score range = 8-12, n = 56 (48.8%). Ciccarelli and colleagues (2013) recently demonstrated that a categorical cutoff of revised CPE score ≥ 6 was effective in identifying neuroeffective doses associated with lower neurocognitive dysfunction in a sample of HIV+ individuals. We chose to utilize a cutoff score of 7 for the HIV+/Low CPE group from the present sample due to the relatively limited number (see Figure 1) of HIV+ participants with CPE scores ≤ 6, n = 14 (11.6%). Mixed-design analysis of variance (ANCOVA) was used to investigate between-group differences in performance of the neurocognitive tasks. This analytical approach allowed a between-group comparison of HIV+ participants on cART regimens with relatively lower and higher CPE scores, and also enabled the comparison of both HIV+ participant groups to HIV− controls with comparable histories of substance dependence.
In order to select covariates for use in ANCOVA models, we examined potential differences in demographic, psychiatric, and substance use characteristics across all groups using chi-square tests and ANOVAs. Variables with nonsignificant differences between groups were then tested with follow-up Pearson correlations examining the relationship between these variables and neurocognitive performance, with significantly correlating variables selected as covariates. Measures of effect size for ANOVAs were obtained via calculation of partial eta squared (ηp2) while effect sizes of follow-up t-tests were measured by calculating Cohen's d. For chi-square analyses, effect sizes were quantified via Cramer's V. Statistical significance for all analyses was set at p ≤ .05.
For all ANCOVA models, a three-level Group variable (HIV−, HIV+/Low CPE, HIV+/High CPE) was used as the between-participants factor. For both PL task ANCOVA models, Trial Blocks were used as the within-participants factor. Huynh-Feldt corrections were applied to violations of sphericity for within-subjects analyses. Fisher's Least Significant Difference (LSD) post-hoc tests were used to follow up significant ANCOVA effects. We hypothesized that PL task performance would be highest among HIV− participants. Additionally, we conducted a series of correlational analyses of data from the HIV+ groups only to determine whether a direct relationship of CPE scores to neurocognitive performance could be detected within the subsample of HIV+ participants.
Results
HIV− vs. HIV+ group demographics
Table 1 shows demographic data for the three groups. No significant group differences were observed for mean age, F(2, 425) = 1.85, p = .16, ηp2 = .01; years of education, F(2, 425) = 2.29, p = .10, ηp2 = .01; or estimated IQ from the WTAR, F(2, 424) = 0.55, p = .58, ηp2 = .003. Additionally, there were no significant group differences in sex, χ2(2) = 3.83, p = .15, V = .10; or race, χ2(2) = 5.46, p = .24, V = .11. There was a significant difference between the groups in prevalence of positive hepatitis C virus serostatus, χ2(2) = 17.67, p < .001, V = .20. A between-group comparison indicated that this difference was driven by significantly higher rates of hepatitis C among HIV+ groups (i.e. HIV+/Low CPE and HIV+/High CPE) compared with HIV− participants, χ2(1) = 17.58, p < .001, V = .20. The two HIV+ groups did not differ significantly in the proportion of participants with undetectable HIV RNA levels, χ2(1) = 1.21. p = .28, V = .10.
No significant group differences were observed in prevalence of lifetime alcohol dependence, χ2(2) = 3.48, p = .18, V = .09; and lifetime cannabis dependence, χ2(2) = 4.12, p = .13, V = .09. There were significant group differences in prevalence of lifetime cocaine dependence, χ2(2) = 6.78, p = .03, V = .13; and lifetime opiate dependence, χ2(2) = 10.80, p = .005, V = .16. Follow-up tests revealed that lifetime cocaine dependence was significantly more common among HIV+ participants than HIV− participants, χ2(1) = 6.71, p = .01, V = .13; while lifetime opioid dependence was significantly more prevalent among HIV− participants than HIV+ participants, χ2(1) = 10.78, p = 0.001, V = .16. No significant group difference were observed on KMSK indices of severity of peak alcohol use, F(2,412) = 0.39, p = .69, ηp2 = .002; peak cocaine use, F(2,378) = 2.0, p = .14, ηp2 = .01; or peak opiate use F(2,200) = 0.06, p = .94; ηp2 = .001.
Between-group differences were observed in recent symptoms of depression, F(2,426 = 9.30, p < .0001, ηp2 = .04; and state anxiety, F(2,403) = 4.83, p = .01, ηp2 = .02. Follow-up comparisons indicated that relative to the HIV− control group, HIV+ participants reported significantly more symptoms of depression, t(425) = 3.65, p < .001, d = 0.37; and state anxiety, t(404) = 3.04, p = .002, d = 0.32. There were no significant group differences in reported symptoms of PTSD, F(2,426) = 2.87, p = .06, ηp2 = .01; or ADHD, F(2,426) = 1.41, p = .25, ηp2 = .01; or in degree of psychopathy, F(2,426) = 1.59, p = .21, ηp2 = .01; or sensation-seeking, F(2,426) = 0.30, p = .74, ηp2 = .001.
Selection of covariates for between-group analyses
In order to select appropriate covariates for ANCOVAs examining between-group differences in neurocognitive performance, we computed Pearson's correlations between the neurocognitive test scores and demographic variables, psychiatric comorbidities, and lifetime substance dependence characteristics that did not systematically vary between groups. Results of correlation analyses of all potential covariates for the full sample of participants are presented in Table 2. Mean RPT performance correlated inversely and significantly with age, r = −.18, p < .001; gender, r = −.28, p < .001 (indicating that female gender showed a small correlation with lower RPT performance); ethnicity, r = −.16, p = .001 (indicating that African-American ethnicity was weakly associated with lower RPT performance relative to non-African-American ethnicity); and sensation-seeking, r = .11, p = .03. Performance on the WPT showed small but statistically significant inverse relationships with age, r = −.19, p < .001; gender, r = −.14, p < .001; ethnicity, r = −.16, p = .001; and small direct relationships with years of education, r = .18, p < .001, and sensation-seeking, r = .17, p < .001. IMT d′ scores showed small correlations with education, r = .22, p < .001; age, r = −.12, p = .02; and scores on the PCL-C, r = −.12, p = .01; WURS, r = −.19, p = .001; and SRPS, r = −.24, p < .001.
Table 2.
Correlations of potential covariates and neurocognitive performance across all participants and among HIV+ participants only.
| Full sample (n = 428) |
HIV+ Participants (n = 118) |
|||||
|---|---|---|---|---|---|---|
| RPT | WPT | IMT d' | RPT | WPT | IMT d' | |
| Age | −.18** | −.19** | −.12* | −.28** | −.10 | −.15 |
| Education | .09 | .18** | .22** | .19* | .21* | .26** |
| WTAR IQ | .20** | .24** | .40** | .28** | .27** | .42** |
| Gender | −.28** | −.14** | .03 | −.28** | −.03 | .07 |
| Ethnicity | −.16** | −.16** | .09 | −.17 | −.19** | −.07 |
| Hepatitis C Status | -- | -- | -- | −.10 | −.01 | −.20* |
| WURS | −.05 | .05 | .19** | −.09 | −.16 | −.29 |
| PCL-C | −.02 | .001 | .12* | .02 | −.01 | −.21* |
| SRPS | −.08 | −.08 | .24** | −.13 | −.07 | −.38** |
| SSS-V | .11* | .17** | .01 | .15 | .18* | −.02 |
| BDI-II | -- | -- | -- | −.16 | .01 | −.11 |
| STAI-State | -- | -- | -- | −.16 | .10 | −.19* |
| Lifetime Substance Dependence | ||||||
| Alcohol | .03 | .06 | .04 | .11 | .13 | .06 |
| Cannabis | .03 | .06 | −.05 | .05 | −.09 | −.02 |
| Cocaine | -- | -- | -- | −.01 | .004 | .03 |
| Opiates | -- | -- | -- | −.11 | −.02 | −.04 |
| Kreek-McHugh-Schluger-Kellogg Scale | ||||||
| Alcohol | .07 | .02 | −.001 | .05 | .05 | .03 |
| Cocaine | −.07 | −.01 | −.10 | −.09 | −.10 | −.19* |
| Opiates | −.05 | −.07 | −.05 | −.12 | −.04 | −.12 |
Note.
.05 ≥ p
.01 > p
WURS = Wender Utah Rating Scale; PCL-C = PTSD Checklist-Civilian Version; SRPS = Self-Report Psychopathy Scale; SSS-V = Sensation Seeking Scale-V; BDI-II = Beck Depression Inventory-II; STAI = State-Trait Anxiety Inventory
Between-group comparisons of neurocognitive performance
Rotary Performance Task
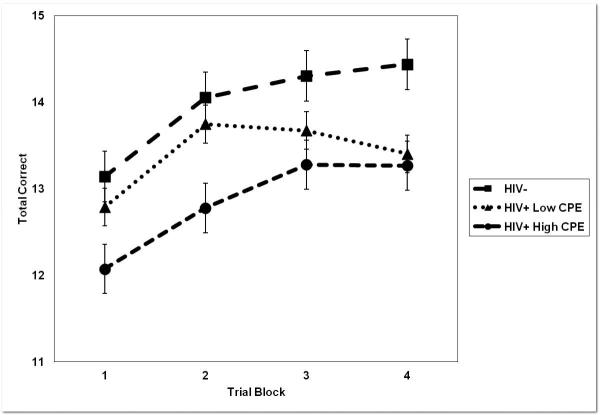
Individual scores from the ten RPT trial blocks were collapsed to five blocks, each representing average performance across two consecutive trials. A 3 × 5 (Group × Block) ANCOVA controlling for age, gender, ethnicity, and sensation-seeking revealed a significant main effect for Block, F(3.41, 5467.71) = 7.71, p = .001, ηp2 = .02, and a significant main effect for Group, F(2, 398) = 4.98, p = .01, ηp2 = .02. In accordance with Cohen (1988), the effect sizes of both Block and Group were considered small. No significant Block × Group interaction was observed, F(6.83, 5467.71) = 1.02, p = .40, ηp2 = .01. Post-hoc Fisher's LSD comparisons based on estimated marginal means indicated that the HIV+/High CPE group (M = 8.69, SE = .57) showed lower performance on the RPT than the HIV− group (M = 10.41, SE = .24), p = .01. No significant difference in RPT performance was observed between the HIV+/Low CPE participants (M = 9.40, SE = .53) and the HIV+/High CPE Group, p = .36. A nonsignificant trend was observed towards lower RPT performance among HIV+/Low CPE participants in comparison to the HIV− group, p = .08 (see Figure 2).
Figure 2.
Mean time on target during the Rotary Pursuit Task (RPT) by HIV-seronegative, HIV+ Low CPE and HIV+ High CPE participants.
Note. Error bars represent SEM.
Weather Prediction Task
Scores on the eight WPT trials were reduced to four blocks, each representing average performance across fifty consecutive trials. Data were analyzed with a 3 × 4 (Group × Block) mixed design ANCOVA controlling for age, gender, ethnicity, sensation-seeking and education. The Block effect was statistically significant, F(2.88, 1201.50) = 3.40, p = .02, ηp2 = .01, as was the main effect for Group, F(2, 417) = 4.73, p = .01, ηp2 = .02. No significant Block × Group interaction was observed, F(5.76, 5589.60) = 0.80, p = .56, ηp2 = .004. Planned Fisher's LSD comparisons of estimated marginal means indicated that HIV+/High CPE participants (M = 13.01, SE = .30) performed significantly more poorly on the WPT than HIV− controls, (M = 13.94, SE = .13), p = .002. Post-hoc comparisons indicated no significant group differences in WPT performance between the HIV+/Low CPE group (M = 13.47, SE = .28) and the HIV+/High CPE group, p = .26, or between the HIV+/Low CPE Group and the HIV− controls, p = .13 (see Figure 3). Although decrease in performance was observed among the HIV+/Low CPE Group across trials 3 and 4, this decrement did not reach trend levels of statistical significance.
Figure 3.
Mean percentage of correct responses on the Weather Prediction Task (WPT) by HIV-seronegative, HIV+ Low CPE and HIV+ High CPE participants.
Note. Error bars represent SEM.
Immediate Memory Task
A univariate ANCOVA of the IMT d’ index controlling for education, PCL-C, WURS and SRPS scores showed no statistically significant Group effect, F(2, 421) = 0.93, p = .39, ηp2 = .004.
Post-hoc analyses of power indicated that the between-group ANCOVAs were sufficiently powered (Cohen, 1988) to detect significant effects of Block and Group for both PL tasks at α = .05. Observed power for Block effects ranged from .78-.99 while observed power for Group effects ranged from .79-.80. In contrast, the repeated-measures ANCOVA models examining PL task performance were not sufficiently powered to detect Block × Group interactions, with observed power ranging from .31 to .44. The univariate ANCOVA was not sufficient to detect significant effects of Group on IMT performance, observed power = .21.
Effects of HIV serostatus on neurocognitive performance
As a validity check for the effects of HIV infection on performance of PL tasks, we recalculated the three ANCOVA models above with the three groups replaced by a two-level HIV serostatus grouping variable (i.e. HIV+ vs. HIV−) in order to verify that HIV+ subjects performed the two PL tasks significantly more poorly than HIV− controls. As expected, the HIV+ group performed significantly more poorly than the HIV− group on both the RPT, F(1, 399) = 8.75, p = .003, ηp2 = .02, observed power = .84, and on the WPT, F(1, 418) = 8.20, p = .004, ηp2 = .02, observed power = .82. No significant effect of HIV serostatus on IMT d′ was observed, F(1, 422) = 1.69, p = .19, ηp2 = .004, observed power = .26.
Analysis of monotonic relationships of CPE and neurocognitive performance among HIV+ SDIs
We performed an additional series of partial correlation analyses using data from the HIV+ subjects only in order to investigate potential monotonic relationships between CPE scores and neurocognitive performance. In order to select appropriate covariates, Pearson correlations of task performance and demographic, psychiatric and substance use measures were recalculated for the HIV+ participants only. Results of these analyses appear in Table 2.
The presence of monotonic relationships between cART CPE scores and neurocognitive performance was assessed using partial Spearman rank correlations controlling for the covariates previously identified (see Table 2). A partial Spearman's ρ between CPE scores and RPT controlling for age, education, gender and PCL-C scores was non-significant, ρp = −.01, p = .91. Similarly, CPE scores and WPT were not correlated significantly when controlling for age, ethnicity, education and sensation-seeking, ρp = −.11, p = .26. Finally, CPE scores and IMT d′ scores were also uncorrelated when controlling for education, IQ, hepatitis C serostatus, severity of lifetime cocaine dependence, and symptoms of PTSD, psychopathy and anxiety, ρp = −.04, p = .70.
Discussion
The present cross-sectional study explored neurocognitive performance among HIV+ SDIs prescribed cART regimens with relatively high or low CNS penetrance as indicated by scores on the revised CPE scale. To our knowledge, no previous studies have addressed the relationship of CPE scores and neurocognition within an explicitly identified sample of HIV+ individuals with lifetime substance dependence. Additionally, the present study addressed the association of cART CPE scores and PL, a domain of neurocognitive functioning which has high relevance for individuals living with HIV-associated neurocognitive impairment that, to our knowledge, has not previously been utilized in studies examining CPE scores and neurocognition.
We compared the neurocognitive performance of HIV+ SDIs prescribed cART regimens with relatively high CPE scores with well-matched groups of HIV+ SDIs prescribed cART regimens with relatively low CPE scores and HIV− SDIs. High and Low CPE groups were defined via a median split of CPE scores, resulting in cutoff scores approximating recently published cutoff recommendations (Ciccarelli et al., 2013). Our results showed that the groups’ performance of each task improved significantly across trial blocks, indicating that participants were able to learn the task. There were no significant interactions between Group and Trial Block, indicating that rates of learning did not differ between HIV− and HIV+ SDI, consistent with previous findings (Gonzalez et al., 2008). HIV+ SDIs prescribed High CPE regimens performed both PL tasks significantly more poorly than HIV− SDIs. In contrast, task performance of HIV+ SDIs prescribed Low CPE regimens did not differ significantly from that of either group, although a trend was observed for lower RPT performance among HIV+/Low CPE participants relative to controls. IMT task performance was comparable between groups, suggesting that observed RPT and WPT deficits cannot be attributed to generalized performance deficits within the HIV+/High CPE participants.
While the cross-sectional nature of our study does not permit direct inferences regarding potential neurotoxic effects of cART, our findings of lower performance among HIV+/High CPE participants relative to HIV− controls but no differences between HIV+/Low CPE participants and controls are compatible with studies suggesting that antiretrovirals with relatively high CPE may have a deleterious impact on neurocognitive functioning (Ciccarelli et al., 2011; Marra et al., 2009), although the effects observed in the present sample were relatively small. An emerging body of literature has found evidence of multiple cellular abnormalities consistent with potential neurotoxic effects of antiretroviral compounds. For example, zidovudine and indinavir in combination damage human blood-brain barrier endothelial cells (Manda, Banerjee, Banks, & Ercal, 2011). Additionally, therapeutic doses of the protease inhibitors nelfinavir and saquinavir have been shown to inhibit human proteasome function (Piccinini et al., 2005). Further, magnetic resonance spectroscopy data have shown evidence of mitochondrial disruption in frontal white matter of HIV+ subjects treated with the nucleoside reverse transcriptase inhibitors didanosine and stavudine (Schweinsburg et al., 2005). Most recently, Robertson, Liner, & Meeker (2012) reported that fifteen commonly prescribed antiretrovirals are neurotoxic to fetal rat neuronal cultures, although neurotoxic effects were modest at clinically relevant concentrations and no additive neurotoxic effects of multiple compounds were observed. HIV and drugs of abuse frequently interact synergistically at the cellular level (for review see Martin-Thormeyer & Paul, 2009), suggesting the possibility that CPE-associated neurotoxicity might be more prevalent among SDIs, although future studies are needed to address this question.
In addition to our comparisons of HIV− SDIs and HIV+ participants, we assessed for the presence of a direct monotonic relationship between cART CPE scores and neurocognitive performance on tasks of PL and a control task of impulsivity among HIV+ SDIs. We found no evidence of these relationships for any neurocognitive task. It is possible that specific sample characteristics limited the capacity to detect such relationships i.e. these data were obtained from a non-treatment sample of SDIs using a cross-sectional design. Additionally, the range of CPE scores obtained was limited, and this restriction could mask an underlying monotonic relationship. While the between-group ANCOVA enabled comparison of two groups of HIV+ SDIs representing broad levels of cART neuropenetrance to HIV− SDI, the precision of available information on cART components (e.g., dosing information) was considerably lower than data available from large clinical trials, and reliability of subjects’ self-reported level of adherence is questionable.
Our study design was cross-sectional and does not permit causal inferences. Additionally, we speculate that neurocognitive impairment may have been more evident among the HIV+ individuals prescribed high CPE regimens prior to initiation of therapy. There were potentially significant group differences in potentially confounding conditions, include psychological distress and hepatitis C serostatus. These cross-sectional findings demand further longitudinal investigation before questions of the effects of pre-existing neurocognitive impairment, disease course, and CPE levels on neurocognitive performance can be addressed. Finally, we note that despite significant serostatus effects on both PL tasks, no group differences were detected on a measure of motor impulsivity that is commonly impaired among SDIs, suggesting that neurocognitive impairment among HIV+ SDIs is selective rather than generalized.
Acknowledgments
We thank Leslie Ladd and Sarah Wicks for data collection, and Dr. Robin Mermelstein for helpful comments on a prior version of this manuscript.
Supported by HHS R01 DA12828 to Eileen M. Martin
References
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG). AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Di Giambenedetto S, Fanti I, Baldonero E, Bracciale L, Silveri MC. Efavirenz associated with cognitive disorders in otherwise asymptomatic HIV-infected patients. Neurology. 2011;76:1403–1409. doi: 10.1212/WNL.0b013e31821670fb. [DOI] [PubMed] [Google Scholar]
- Ciccarelli N, Fabbiani M, Colafiqli M, Trecarichi E, Silveri M, Cauda R, Di Giambenedetto S. Revised central nervous system neuropenetration-effectiveness score is associated with cognitive disorders in HIV-infected patients with controlled plasma viraemia. Anitiviral Therapy. 2013;18:153–160. doi: 10.3851/IMP2560. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73:342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Variable benefit in neuropsychological function in HIV-infected HAART-treated patients. Neurology. 2006;66:1447–1450. doi: 10.1212/01.wnl.0000210477.63851.d3. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Antiretroviral therapy in HIV infection: Are neurologically active drugs important? Archives of Neurology. 2004;62:1699–1704. doi: 10.1001/archneur.61.11.1699. [DOI] [PubMed] [Google Scholar]
- Dougherty DM, Marsh DM, Mathias CW. Immediate and delayed memory tasks: A computerized behavioral measure of memory, attention, and impulsivity. Behavioral Research Methods, Instruments and Computers. 2002;34:391–398. doi: 10.3758/bf03195467. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometrics Research; New York, NY: 2002. [Google Scholar]
- Gonzalez R, Jacobus J, Amatya AK, Quartana PJ, Vassileva J, Martin EM. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV+ individuals with history of substance dependence. Neuropsychology. 2008;22:776–786. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Vassileva J, Bechara A, Grbesic S, Sworowski L, Novak RM, Martin EM. The influence of executive functions, sensation seeking, and HIV serostatus on the risky sexual practices of substance-dependent individuals. Journal of the International Neuropsychological Society. 2005;11:121–131. doi: 10.1017/s1355617705050186. [DOI] [PubMed] [Google Scholar]
- Holdnack JA. Wechsler Test of Adult Reading Manual. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Jernigan TL, Gamst AC, Archibald SL, Fennema-Notestine C, Mindt MR, Marcotte TD, Grant I. Effects of methamphetamine dependence and HIV infection on cerebral morphology. American Journal of Psychiatry. 2005;162:1461–1472. doi: 10.1176/appi.ajp.162.8.1461. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69:137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Letendre SE, Deutsch R, Clifford D, Marra C, McCutchan A, Morgello S, Grant I. Correlates of time-to-loss-of-viral response in CSF and plasma in the CHARTER cohort.. Paper presented at the 17th Conference on Retroviruses and Opportunistic Infections; 2010. [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Ellis RJ. Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Archives of Neurology. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, Ellis RJ. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Annals of Neurology. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- Levenson MR, Kiehl KA, Fitzpatrick CM. Assessing psychopathic attributes in a noninstitutionalized population. Journal of Personality and Social Psychology. 1995;68:151–158. doi: 10.1037//0022-3514.68.1.151. [DOI] [PubMed] [Google Scholar]
- Manda KR, Banerjee A, Banks WA, Ercal N. Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood–brain barrier endothelial cells. Free Radical Biology and Medicine. 2011;50:801–810. doi: 10.1016/j.freeradbiomed.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, Robertson K. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Thormeyer EM, Paul RH. Drug abuse and hepatitis C infection as comorbid features of HIV associated neurocognitive disorder: Neurocognitive and neuroimaging features. Neuropsychology Review. 2009;19:215–231. doi: 10.1007/s11065-009-9101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki P. HIV+ men and women show different performance patterns on procedural learning tasks. Journal of Clinical and Experimental Neuropsychology. 2011;33:112–120. doi: 10.1080/13803395.2010.493150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin EM, Nixon H, Pitrak DL, Weddington W, Rains NA, Nunnally G, Bechara A. Characteristics of prospective memory deficits in HIV-seropositive substance-dependent individuals: Preliminary observations. Journal of Clinical and Experimental Neuropsychology. 2007;29:496–504. doi: 10.1080/13803390600800970. [DOI] [PubMed] [Google Scholar]
- Piccinini M, Rinaudo MT, Anselmino A, Buccinnà B, Ramondetti C, Dematteis A, Tovo PA. The HIV protease inhibitors nelfinavir and saquinavir, but not a variety of HIV reverse transcriptase inhibitors, adversely affect human proteasome function. Antiviral Therapy. 2005;10:215–223. [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. Journal of Neurovirology. 2012;18:388–399. doi: 10.1007/s13365-012-0120-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, Skiest DJ. Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74:1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parson TD, Wu K, Bosch RJ, Wu J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21:1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Schulte T, Muller-Oehring EM, Sullivan EV, Pfefferbaum A. Disruption of emotion and conflict processing in HIV infection with and without alcoholism comorbidity. Journal of the International Neuropsychological Society. 2011;22:1–14. doi: 10.1017/S1355617711000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Grant I. Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. Journal of Neurovirology. 2005;11:356–364. doi: 10.1080/13550280591002342. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni J, Abraham AR, Bourquin I, Schiffer V, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA: 1983. [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. Journal of Acquired Immune Deficiency Symdromes. 2007;45:174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150:885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD checklist (PCL): Reliability, validity, and diagnostic utility.. Paper presented at the Annual Convention of the International Society for Traumatic Stress Studies; San Antonio, TX. October 1993. [Google Scholar]
- Weickert TW, Terrazas A, Bigelow LB, Malley JD, Hyde T, Egan MF, Goldberg TE. Habit and skill learning in schizophrenia: Evidence of normal striatal processing with abnormal cortical input. Learning and Memory. 2002;9:430–442. doi: 10.1101/lm.49102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman M. The psychobiological model for impulsive unsocialized sensation seeking: A comparative approach. Neuropsychobiology. 1996;34:125–129. doi: 10.1159/000119303. [DOI] [PubMed] [Google Scholar]