Abstract
SEP-225289 is a novel compound that, based on in vitro potencies for transporter function, potentially inhibits reuptake at dopamine, norepinephrine, and serotonin transporters. An open-label PET study was conducted during the development of SEP-225289 to investigate its dopamine and serotonin transporter occupancy.
Methods
Different single doses of SEP-225289 were administered to healthy volunteers in 3 cohorts: 8 mg (n = 7), 12 mg (n = 5), and 16 mg (n = 7). PET was performed before and approximately 24 h after oral administration of SEP-225289, to assess occupancy at trough levels. Dopamine and serotonin transporter occupancies were estimated from PET using 11C-N-(3-iodoprop-2E-enyl)-2β-carbomethoxy-3β-(4-methylphenyl) nortropane (11C-PE2I) and 11C-N,N-dimethyl-2-(2-amino-4-cyanophenylthio)benzylamine (11C-DASB), respectively. Plasma concentration of SEP-225289 was assessed before ligand injection, and subjects were monitored for adverse events.
Results
Average dopamine and serotonin transporter occupancies increased with increasing doses of SEP-225289. Mean dopamine and serotonin transporter occupancies were 33% ± 11% and 2% ± 13%, respectively, for 8 mg; 44% ± 4% and 9% ± 10%, respectively, for 12 mg; and 49% ± 7% and 14% ± 15%, respectively, for 16 mg. On the basis of the relationship between occupancy and plasma concentration, dopamine transporter IC50 (the plasma concentration of drug at 50% occupancy) was determined (4.5 ng/mL) and maximum dopamine transporter occupancy was extrapolated (85%); however, low serotonin transporter occupancy prevented similar serotonin transporter calculations. No serious adverse events were reported.
Conclusion
At the doses evaluated, occupancy of the dopamine transporter was significantly higher than that of the serotonin transporter, despite similar in vitro potencies, confirming that, in addition to in vitro assays, PET occupancy studies can be instrumental to the drug development process by informing early decisions about indication, dose, and therapeutic potential.
Keywords: dopamine, serotonin, occupancy, positron emission tomography, SEP-225289
Dysfunction in monoamine transmission has been implicated in the pathophysiology of several major neuropsychiatric disorders, including mood disorders, alcohol and drug abuse, pain disorders, eating disorders, and obesity. Although treatments for these conditions often involve blocking dopamine, norepinephrine, or serotonin reuptake, mounting evidence suggests that targeting multiple neurotransmitter systems simultaneously may increase or broaden efficacy.
For example, recent studies suggest that targeting dopamine or norepinephrine transmission, in addition to targeting transmission of serotonin, may improve treatment outcomes in major depression (1-3). Also, on the basis of the potential additional or differential benefits of dopamine reuptake inhibition in combination with serotonin or norepinephrine reuptake inhibition, triple-reuptake inhibitors have been studied for several years (4). These drugs have promise in the treatment of substance abuse (alcohol, nicotine, and cocaine) (5-7), chronic pain syndromes (8,9), and obesity and eating disorders (10,11).
On the basis of the numerous clinical applications of therapeutics targeting multiple monoamine systems, one such drug, SEP-225289, was developed by Sunovion Pharmaceuticals Inc. as a potential triple-reuptake inhibitor. Average in vitro potencies for transporter function inhibition by SEP-225289 have been measured (8 ± 7, 17 ± 3, and 10 ± 8 nM, for the dopamine, norepinephrine, and serotonin transporters, respectively) (12). However, a potentially more clinically valid gauge of a drug’s transporter binding profile is its in vivo occupancy, because differences in temperature, pH, concentration, or other unknown factors can generate discrepancies between in vitro and in vivo affinities (13-16).
In vivo drug occupancy can be assessed using PET. Numerous PET studies have been conducted on existing therapeutics to determine the threshold drug receptor (or transporter) occupancies necessary to achieve the therapeutic effect (17). Such studies have suggested that the minimum occupancy needed for selective serotonin reuptake inhibitors is approximately 80% of serotonin transporters (14,17), whereas the occupancy of dopamine transporters potentially associated with various clinical effects is less clear (18). PET occupancy studies are also becoming increasingly common in early drug development programs (16,17). However, despite this, few have examined drug occupancy at multiple monoamine neurotransmitter systems within the same subject, and those that have (13,15,19-22) were mostly restricted to neuroleptic compounds.
In vivo occupancy estimates of reuptake inhibitors with multiple monoamine targets are particularly important because occupancy at each transporter type must happen within a fairly narrow dose range, since adverse effects within one system (e.g., nausea and serotonin) may prevent the dose escalation needed to potentially engage additional systems. Consequently, supplementing in vitro, ex vivo, and preclinical target activity profiles of compounds with human imaging can provide information on potential efficacy, side effects, and therapeutic dose ranges, which may help to inform subsequent phases of development. Therefore, in this study, the multiple monoamine transporter occupancy of a nonneuroleptic compound (SEP-225289) was assessed in the same individual using PET and a reference region methodology.
MATERIALS AND METHODS
Subjects
All subjects were healthy volunteers who met the following inclusion criteria: age 18–50 y; body mass index between 16 and 32 kg/m2; capacity to provide written informed consent; negative results on urine screening for drugs; willingness to refrain from strenuous activity during the study; daily consumption of less than 180 mg of caffeine; and negative pregnancy test and use of an acceptable birth control method throughout the study for women of child-bearing potential. Exclusion criteria consisted of a significant medical illness; a major psychiatric illness, including mental retardation, drug or alcohol abuse or dependence; an axis I diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders: DSM-IV (23); use of psychotropic medications within 5 half-lives or 30 d; regular alcohol consumption exceeding 7 (for women) or 14 (for men) drinks per week; history of tobacco dependency or use of tobacco or nicotine within 30 d; a first-degree relative with a history of schizoaffective disorder, depression, bipolar disorder, or suicide attempt; 2 or more first-degree relatives with a history of alcohol or substance dependence; or a first-degree family history of schizophrenia if the subject was less than 33 y of age (mean age of onset for schizophrenia plus 2 SDs). Criteria were assessed by the following: history, the Structured Clinical Interview for DSM Disorders (SCID), physical examination, blood tests, urine toxicology, and electrocardiography.
The protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute, and all subjects gave written informed consent after receiving an explanation of the study.
Study Medication
Two phase I clinical studies have been conducted using SEP-225289. In the first, 128 subjects were exposed to single doses of SEP-225289 ranging from 0.2 to 36 mg. In the second, 27 subjects were exposed to multiple doses of SEP-225289 ranging from 1 to 3 mg for 21 d. At the highest single dose administered (36 mg), 7 of the 9 subjects manifested symptoms consistent with stimulant-related exaggerated pharmacology, which resolved within 48 h of taking the drug without any intervention. This resolution was followed by a brief period of depressed mood in 2 subjects. In both studies, the most commonly observed adverse events (AEs) included headache, insomnia, dizziness, nausea, somnolence, diarrhea, and irritability (Sunovion Pharmaceuticals, Inc., unpublished data, 2011).
Selection of dose range for this PET occupancy study was based on the phase I clinical program. To produce drug plasma levels consistent with those observed in steady-state conditions in the clinical studies (i.e., clinically relevant doses), the single doses of SEP-225289 used in this PET study were higher than those that would be administered chronically.
Study Design
Subjects were assigned to 1 of 3 cohorts (A, B, or C), receiving a single dose of 8, 12, or 16 mg of SEP-225289, respectively. The final subject population had a mean age of 28.0 y (SD, 8.3 y) and consisted of cohorts A (2 men, 5 women), B (3 men, 2 women), and C (3 men, 4 women).
Dopamine and serotonin transporter occupancies were estimated in each subject with PET using the radioligands 11C-N-(3-iodoprop-2E-enyl)-2β-carbomethoxy-3β-(4-methylphenyl)nortropane (11C-PE2I) (24) and 11C-N,N-dimethyl-2-(2-amino-4-cyanophenylthio) benzylamine (11C-DASB) (25), respectively. The subjects received a baseline 11C-PE2I and 11C-DASB PET scan, with approximately a 1-h break between scans. The subjects returned 5–10 d after the baseline scans and were administered a single dose of SEP-225289, followed by occupancy scans with 11C-PE2I and 11C-DASB at approximately 24 and 27 h after dose administration, respectively. Because of scheduling constraints, 1 subject, S034, received the 11C-DASB occupancy scan first, followed by the 11C-PE2I occupancy scan, and 1 subject, S018, did not receive an 11C-DASB occupancy scan. The number of hours between study medication administration and PET was chosen to lie between the peak plasma concentration (median, 6–12 h, depending on dose) and the drug half-life (median, 46–64 h, depending on dose), as determined by previous clinical trials (Sunovion Pharmaceuticals, Inc., unpublished data, 2011). Because of the long half-life of SEP-225289, it is unlikely that the time between scans (or ligand order) had any effect on the measured occupancy.
Plasma concentrations of SEP-225289 were assayed from blood samples collected approximately 1 h before administration of a single dose of SEP-225289, immediately before injection of 11C-PE2I and 11C-DASB (~24 and 27 h after dose administration, respectively) and at the follow-up visit 7–14 d after dosing.
Image Acquisition and Analysis
MR images were acquired on a 3-T Signa Advantage system (GE Healthcare), as previously described (25). 11C-PE2I and 11C-DASB were prepared as previously described (25,26). PET was performed using an ECAT HR + scanner (Siemens/CTI) (24,25). Injected doses for each cohort are shown in Table 1.
TABLE 1.
Injected Doses of 11C-PE2I and 11C-DASB for All Cohorts
| Cohort |
11C-PE2I injected dose
|
11C-DASB injected dose
|
||
|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |
| A | 543.9 ± 138.01 | 230.88–696.71 | 600.51 ± 78.44 | 399.23–672.29 |
| (14.70 ± 3.73) | (6.24–18.83) | (16.23 ± 2.12) | (10.79–18.17) | |
|
| ||||
| B | 356.31 ± 144.3 | 149.11–617.16 | 490.62 ± 193.51 | 167.24–671.92 |
| (9.63 ± 3.90) | (4.03–16.68) | (13.26 ± 5.23) | (4.52–18.16) | |
|
| ||||
| C | 542.79 ± 179.82 | 141.71–683.76 | 591.63 ± 88.06 | 441.04–682.28 |
| (14.67 ± 4.86) | (3.83–18.48) | (15.99 ± 2.38) | (11.92–18.44) | |
Data are in MBq, with mCi in parentheses.
All images were analyzed using MATLAB (The MathWorks). Subject motion during the scan was corrected as described previously (24). Automatic regions of interest were obtained using nonlinear registration techniques to warp 18 previously manually outlined MR images to the target image, as described elsewhere (26). The mean PET image was then coregistered to the segmented MR image to apply the regions of interest to the mean PET image, as well as individual PET frames (26,27). Time–activity curves were generated by plotting the average activity within a region over the time course of the PET acquisition.
Appropriate modeling methods for both 11C-PE2I and 11C-DASB have been previously determined (25,26). In this study, the outcome measure used was BPND = fNDBavail/KD, where fND is the fraction of free ligand in the reference region, Bavail is the density of available receptors, and KD is the radioligand equilibrium dissociation constant (28). BPND, the ratio of the concentration of specifically bound to nondisplaceable ligand at equilibrium, was used in this study because it does not require arterial sampling (28). Although BPND measurements are potentially sensitive to changes in the fraction of free ligand in the reference region, there is no evidence to suggest that SEP-225289 would affect these values.
11C-DASB BPND was calculated in the dorsal caudate, dorsal putamen, and midbrain using likelihood estimation in graphical analysis with the gray matter of the cerebellum as the reference tissue (25). 11C-PE2I BPND was calculated in the dorsal caudate and dorsal putamen using Logan graphical analysis with the cerebellum as the reference tissue (24).
Occupancy Measures
Occupancy, the percentage change in ligand binding due to SEP-225289 administration, was calculated as 100% × (baseline BPND – follow-up BPND)/baseline BPND. Because of variability in binding estimates, low or no transporter occupancy (which occurred only using 11C-DASB) occasionally resulted in negative values of estimated occupancy. Although negative occupancy estimates are not meaningful, to prevent bias, these negative values were used in the calculation of average occupancy.
Maximal occupancy was estimated by the equation occupancy = (plasma concentration of drug × OCCmax)/(plasma concentration of drug + IC50), where OCCmax is the maximum occupancy and IC50 is the plasma concentration of drug at 50% occupancy (29). Values for OCCmax and IC50 were fit using a nonlinear least-squares technique in MATLAB.
Safety Monitoring
Monitoring for AEs, defined as any reaction, side effect, or other undesirable event that occurred in conjunction with the study drug, whether or not the event was considered drug-related, took place from the time informed consent was signed through the end of the study. Subjects were also monitored for serious AEs occurring between the time of informed consent and 30 d after the final dose of SEP-225289.
RESULTS
Occupancy Results
Dopamine and serotonin transporter occupancies are plotted by oral SEP-225289 dose in Figure 1. In the dorsal putamen, average SEP-225289 occupancy was 9.8 (at 8 mg), 3.7 (at 12 mg), and 3.0 (at 16 mg) times higher at the dopamine transporter than the serotonin transporter. In the dorsal caudate, average SEP-225289 occupancy was 5.6 (at 8 mg), 5.1 (at 12 mg), and 5.7 (at 16 mg) times higher at the dopamine transporter than the serotonin transporter.
FIGURE 1.
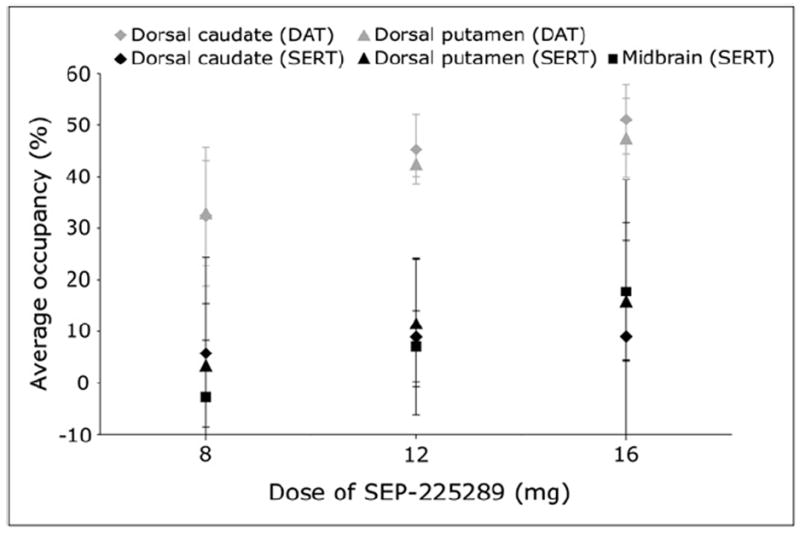
Average dopamine and serotonin transporter occupancy vs. oral dose of SEP-225289. Dopamine transporter occupancy was measured in dorsal caudate and dorsal putamen using PET with 11C-PE2I. Serotonin transporter occupancy was measured in dorsal caudate, dorsal putamen, and midbrain using PET with 11C-DASB. Each symbol represents mean value across all subjects in single cohort. Error bars indicate SD across cohort subjects. DAT = dopamine transporter; SERT = serotonin transporter.
The relationship between dopamine and serotonin occupancy is explored in Figure 2.
FIGURE 2.
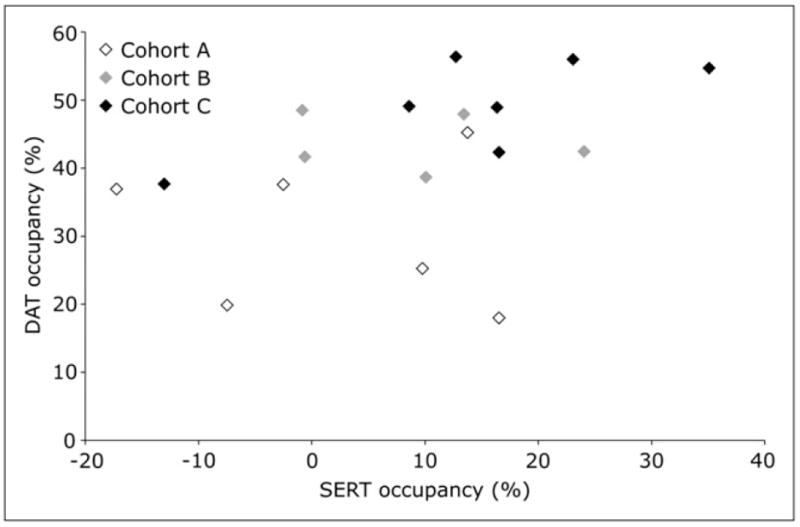
Average dopamine transporter occupancy versus average serotonin transporter occupancy. For each subject, mean dopamine transporter occupancy in dorsal caudate and dorsal putamen was calculated and plotted versus mean serotonin transporter occupancy in dorsal caudate, dorsal putamen, and midbrain. DAT = dopamine transporter; SERT = serotonin transporter.
Effects of SEP-225289 on Dopamine and Serotonin Transporter Binding
Figure 3 indicates tracer binding before and after administration of SEP-225289 in the subject with the highest measured serotonin transporter occupancy. Though this subject achieved high serotonin transporter occupancy, differences in 11C-DASB images before and after dosing of SEP-225289 were not as striking as 11C-PE2I binding differences. These results are representative in this study, because every subject achieved higher dopamine transporter occupancy than serotonin transporter occupancy.
FIGURE 3.
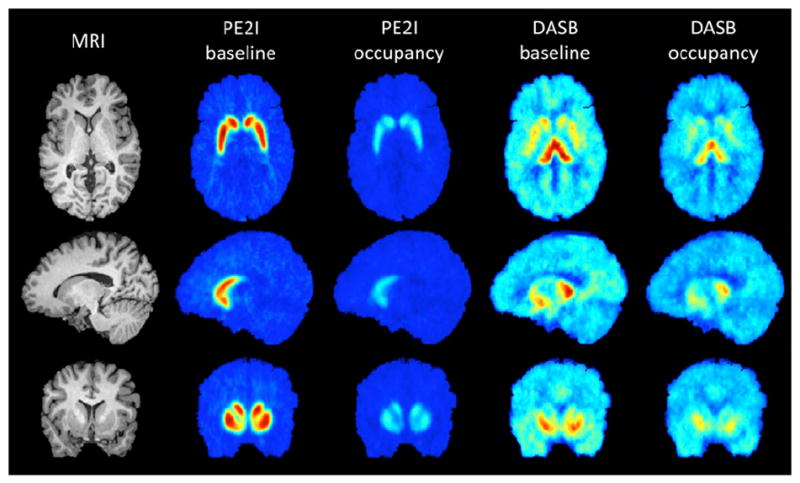
MR and mean PET images, before and after administration of SEP-225289 for the subject achieving highest serotonin transporter occupancy. Axial, sagittal, and coronal slices are shown in top, middle, and bottom rows, respectively. Left column shows MR images of subject, for anatomic reference. PET images are means of the last 6 frames of each scan (corresponding to last 60 min of scanning). Baseline scanning occurred 5–10 d before administration of SEP-225289. Difference in binding between baseline and occupancy scans is due to SEP-225289 transporter occupancy. Dopamine transporter occupancy in this subject (55%, as indicated by PE2I PET scans) was markedly higher than serotonin transporter occupancy (35%, as indicated by the DASB PET scans).
As expected, the 24- and 27-h plasma drug levels increased as a function of oral SEP-225289 dose, as shown in Figure 4. The R2 values for the regression lines for the 24-and 27-h plasma levels were 0.98 and greater than 0.99, respectively.
FIGURE 4.
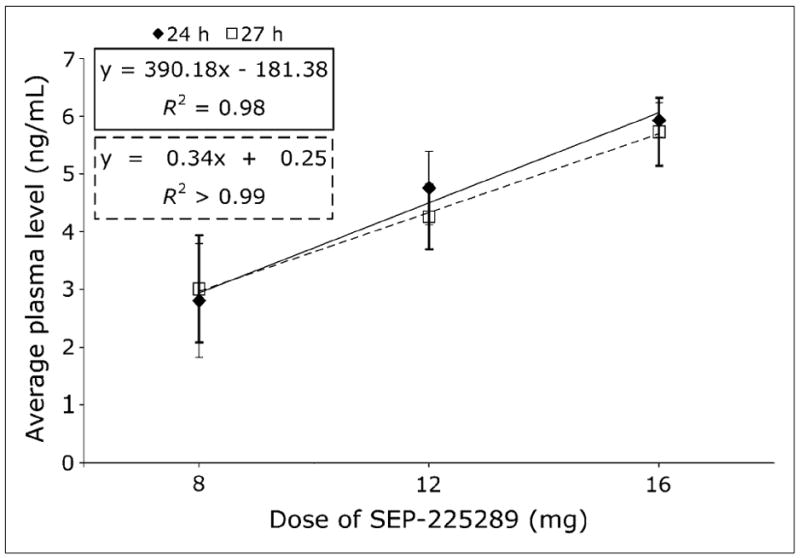
SEP-225289 plasma levels vs. dose. Plasma level was averaged over all subjects in each cohort. Error bars indicate SD across cohort subjects.
SEP-225289 plasma levels at 24 h (except for subject S034, for whom the 27-h plasma value was used) versus average occupancy (mean of dorsal caudate and dorsal putamen occupancy), as determined from the 11C-PE2I scans, are shown in Figure 5. The parameters of the best-fit curve to the data were a maximum occupancy of 84.9% and a 4.5 ng/mL drug plasma concentration at 50% occupancy (IC50).
FIGURE 5.
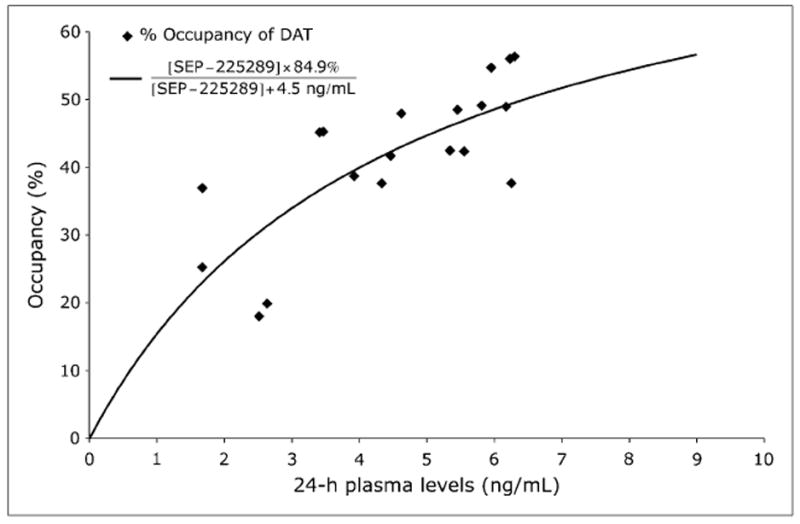
Dopamine transporter occupancy vs. plasma level. Percentage occupancy of dopamine transporter as measured with 11C-PE2I PET images is plotted. Curve was fit to data using a nonlinear least-squares method. Parameters for best-fit curve were maximum occupancy of 84.9% and 4.5 ng/mL drug plasma concentration at 50% occupancy. These parameters are indicated in equation of fitted curve shown on top left corner. For 1 subject (S034), 27-h plasma level was used, because this subject received 11C-PE2I scan at approximately 27 h after dose administration. DAT = dopamine transporter.
SEP-225289 plasma levels at 27 h (except for subject S034, for whom the 24-h plasma value was used) versus average occupancy (mean of dorsal caudate, dorsal putamen, and midbrain occupancy), as determined from the 11C-DASB scans are shown in Figure 6. The serotonin transporter occupancy values were too low to permit the data to be fit to a curve that allows the calculation of IC50 or estimation of maximal occupancy.
FIGURE 6.
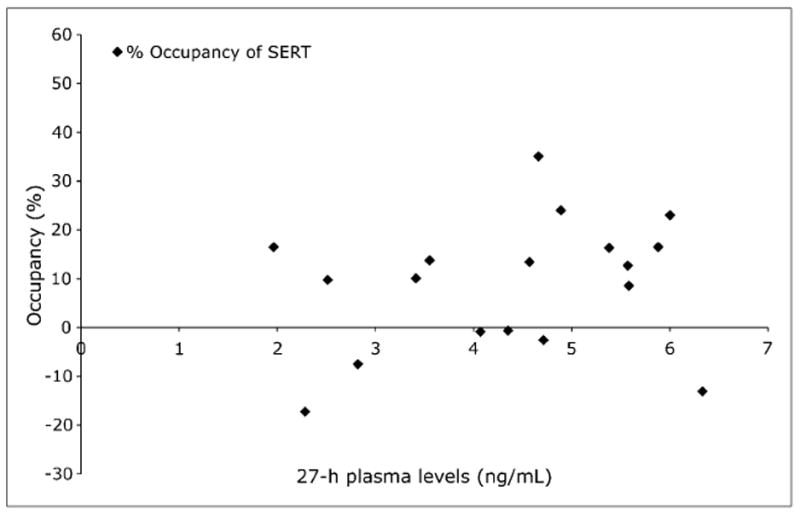
Serotonin transporter occupancy vs. plasma level. Percentage occupancy of serotonin transporter as measured with 11C-DASB PET images is plotted. Negative values occur because of measurement noise. For 1 subject (S034), 24-h plasma level was used, because this subject received 11C-DASB scan at approximately 24 h after dose administration. SERT = serotonin transporter.
Safety Monitoring
Eight AEs occurred in cohort A (8 mg), including nausea and emesis, elevated mood, anxiety, and insomnia. Four AEs occurred in cohort B (12 mg), including 2 subjects who reported having a headache. Twenty-three AEs occurred in the highest dose cohort, cohort C (16 mg), including headache, insomnia, nausea, diarrhea, palpitations, and dizziness. In all cohorts, all AEs believed to be related to the study medication resolved within 32 h, except for insomnia experienced by 1 subject in cohort C that lasted 8 nights. No serious AEs were reported.
DISCUSSION
In this study, PET was performed before and after administration of a single dose of SEP-225289, a potential triple monoamine reuptake inhibitor. Drug occupancy at the dopamine and serotonin transporters was assessed (norepinephrine transporter occupancy was not evaluated) within the same individual, and this occupancy was examined relative to the administered dose and plasma levels of SEP-225289. In addition, subjects were monitored for AEs and serious AEs throughout the study duration. No serious AEs occurred, and most of the AEs reported in this study were of mild or moderate severity and self-limiting.
In vitro data suggested that the functional potencies of SEP-225289 for the dopamine and serotonin transporters were similar. However, in this study, in vivo dopamine transporter occupancy was, on average, at least 3 times greater than that of serotonin transporter, and dopamine transporter occupancy exceeded the serotonin transporter occupancy for every subject for whom both were measured. In addition, Figure 2 indicates little correlation between average dopamine and serotonin transporter occupancies. Although in vitro assays are an important first step in determining whether a compound engages its target, because of disparities in temperature, pH, concentration, and other conditions, in vitro affinity may not predict in vivo occupancy. Also, of critical importance is determining the relatively narrow therapeutic dose range for these compounds, which is based on tolerability and therefore not easily predicted from in vitro models. For these reasons, many consider in vivo occupancy studies more useful in predicting a drug’s therapeutic potential than in vitro measures.
PET occupancy studies can also provide estimations of drug IC50 and extrapolation of maximal occupancy, based on fitted occupancy versus plasma curves (29). Mean serotonin transporter occupancy across all cohorts based on 11C-DASB PET scans was 8.8%, and 6 subjects (3 receiving 8 mg, 2 receiving 12 mg, and 1 receiving 16 mg of SEP-225289) attained less than 1% serotonin transporter occupancy, on average. These values were therefore too low for meaningful curve fits. However, the dopamine transporter occupancy as measured by 11C-PE2I was significant. Dopamine transporter occupancies were as high as 56%, allowing the calculation of SEP-225289 plasma concentration at 50% dopamine transporter occupancy (4.5 ng/mL) and an extrapolation of maximal occupancy (85%). Because maximal occupancy is an extrapolation based on current data, this value will need to be verified by in vivo measurements of SEP-225289 occupancy and plasma concentrations at higher doses.
Although SEP-225289 was originally developed as a triple–monoamine reuptake inhibitor based on promising in vitro data, this PET occupancy study suggests that the serotonin transporter occupancy of SEP-225289 is too low to produce an antidepressant effect at these exposures. Even though 3 subjects achieved serotonin transporter occupancies greater than 20%, all serotonin transporter occupancies were well below the 80% threshold established for clinical effectiveness of selective serotonin reuptake inhibitors (14). Because of its high dopamine transporter occupancy, however, SEP-225289 is a potentially clinically effective dopamine reuptake inhibitor. Although the therapeutic threshold for dopamine transporter occupancy remains unclear, the dopamine transporter occupancy of SEP-225289 in this study is well above occupancy of bupropion (<22%) (18). Additionally, depending on its norepinephrine transporter occupancy, SEP-225289 may be an effective norepinephrine–dopamine reuptake inhibitor.
CONCLUSION
The in vivo target occupancy of novel therapeutics is best assessed early in a development program to provide clinical direction. PET allows the calculation of this target occupancy directly in vivo and augments in vitro, ex vivo, and other preclinical data.
This PET study used a within-subject design to investigate the differential binding of a new drug, SEP-225289, to dopamine and serotonin transporters. Despite similar in vitro potencies, the in vivo occupancies of SEP-225289 at serotonin and dopamine transporters were significantly different, as has been previously observed with other drugs. This study demonstrates the advantages of in vivo occupancy evaluations and the potential contributions of PET to this and similar development programs.
Acknowledgments
This study was funded by Sunovion Pharmaceuticals Inc. (formerly Sepracor Inc.).
Footnotes
No other potential conflict of interest relevant to this article was reported.
References
- 1.Leuchter AF, Lesser IM, Trivedi MH, et al. An open pilot study of the combination of escitalopram and bupropion-SR for outpatients with major depressive disorder. J Psychiatr Pract. 2008;14:271–280. doi: 10.1097/01.pra.0000336754.19566.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenox-Smith AJ, Jiang Q. Venlafaxine extended release versus citalopram in patients with depression unresponsive to a selective serotonin reuptake inhibitor. Int Clin Psychopharmacol. 2008;23:113–119. doi: 10.1097/YIC.0b013e3282f424c2. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Fava M, Wisniewski SR, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med. 2006;354:1243–1252. doi: 10.1056/NEJMoa052964. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Skolnick P. Triple uptake inhibitors: therapeutic potential in depression and beyond. Expert Opin Investig Drugs. 2007;16:1365–1377. doi: 10.1517/13543784.16.9.1365. [DOI] [PubMed] [Google Scholar]
- 5.McMillen BA, Shank JE, Jordan KB, Williams HL, Basile AS. Effect of DOV 102,677 on the volitional consumption of ethanol by Myers’ high ethanol-preferring rat. Alcohol Clin Exp Res. 2007;31:1866–1871. doi: 10.1111/j.1530-0277.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- 6.Ross S, Peselow E. Pharmacotherapy of addictive disorders. Clin Neuropharmacol. 2009;32:277–289. doi: 10.1097/wnf.0b013e3181a91655. [DOI] [PubMed] [Google Scholar]
- 7.Castells X, Casas M, Perez-Mana C, Roncero C, Vidal X, Capella D. Efficacy of psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2010;2 doi: 10.1002/14651858.CD007380.pub3. CD007380. [DOI] [PubMed] [Google Scholar]
- 8.Mease PJ. Further strategies for treating fibromyalgia: the role of serotonin and norepinephrine reuptake inhibitors. Am J Med. 2009;122:S44–S55. doi: 10.1016/j.amjmed.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Basile AS, Janowsky A, Golembiowska K, et al. Characterization of the anti-nociceptive actions of bicifadine in models of acute, persistent, and chronic pain. J Pharmacol Exp Ther. 2007;321:1208–1225. doi: 10.1124/jpet.106.116483. [DOI] [PubMed] [Google Scholar]
- 10.Tizzano JP, Stribling DS, Perez-Tilve D, et al. The triple uptake inhibitor (1R,5S)-(1)-1-(3,4-dichlorophenyl)-3-azabicyclo[3.1.0] hexane hydrochloride (DOV 21947) reduces body weight and plasma triglycerides in rodent models of diet-induced obesity. J Pharmacol Exp Ther. 2008;324:1111–1126. doi: 10.1124/jpet.107.133132. [DOI] [PubMed] [Google Scholar]
- 11.Steffen KJ, Roerig JL, Mitchell JE, Uppala S. Emerging drugs for eating disorder treatment. Expert Opin Emerg Drugs. 2006;11:315–336. doi: 10.1517/14728214.11.2.315. [DOI] [PubMed] [Google Scholar]
- 12.Schreiber R, Lew R, Hardy L, Cremers T, Fang QK, Campbell U. Pharmacological characterization of the triple monoamine transporter inhibitor SEP-225289. Paper presented at: Meeting of Society for Neuroscience; October 20, 2009; Chicago, IL. 2009. [Google Scholar]
- 13.Rabiner EA, Gunn RN, Wilkins MR, Sedman E, Grasby PM. Evaluation of EMD 128 130 occupancy of the 5-HT1A and the D2 receptor: a human PET study with [11C]WAY-100635 and [11C]raclopride. J Psychopharmacol. 2002;16:195–199. doi: 10.1177/026988110201600301. [DOI] [PubMed] [Google Scholar]
- 14.Meyer JH, Wilson AA, Sagrati S, et al. Serotonin transporter occupancy of five selective serotonin reuptake inhibitors at different doses: an [11C]DASB positron emission tomography study. Am J Psychiatry. 2004;161:826–835. doi: 10.1176/appi.ajp.161.5.826. [DOI] [PubMed] [Google Scholar]
- 15.Reimold M, Solbach C, Noda S, et al. Occupancy of dopamine D1, D2 and serotonin2A receptors in schizophrenic patients treated with flupentixol in comparison with risperidone and haloperidol. Psychopharmacology (Berl) 2007;190:241–249. doi: 10.1007/s00213-006-0611-0. [DOI] [PubMed] [Google Scholar]
- 16.Uppoor RS, Mummaneni P, Cooper E, et al. The use of imaging in the early development of neuropharmacological drugs: a survey of approved NDAs. Clin Pharmacol Ther. 2008;84:69–74. doi: 10.1038/sj.clpt.6100422. [DOI] [PubMed] [Google Scholar]
- 17.Grimwood S, Hartig PR. Target site occupancy: emerging generalizations from clinical and preclinical studies. Pharmacol Ther. 2009;122:281–301. doi: 10.1016/j.pharmthera.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 18.Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology (Berl) 2002;163:102–105. doi: 10.1007/s00213-002-1166-3. [DOI] [PubMed] [Google Scholar]
- 19.Mamo D, Graff A, Mizrahi R, Shammi CM, Romeyer F, Kapur S. Differential effects of aripiprazole on D2, 5-HT2, and 5-HT1A receptor occupancy in patients with schizophrenia: a triple tracer PET study. Am J Psychiatry. 2007;164:1411–1417. doi: 10.1176/appi.ajp.2007.06091479. [DOI] [PubMed] [Google Scholar]
- 20.Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G. D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry. 1995;152:1444–1449. doi: 10.1176/ajp.152.10.1444. [DOI] [PubMed] [Google Scholar]
- 21.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 22.Hagberg G, Gefvert O, Bergstrom M, et al. N-[11C]methylspiperone PET, in contrast to [11C]raclopride, fails to detect D2 receptor occupancy by an atypical neuroleptic. Psychiatry Res. 1998;82:147–160. doi: 10.1016/s0925-4927(98)00020-1. [DOI] [PubMed] [Google Scholar]
- 23.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 24.DeLorenzo C, Kumar JSD, Zanderigo F, Mann JJ, Parsey RV. Modeling considerations for in vivo quantification of the dopamine transporter using [C-11]PE2I and positron emission tomography. J Cereb Blood Flow Metab. 2009;29:1332–1345. doi: 10.1038/jcbfm.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogden RT, Ojha A, Erlandsson K, Oquendo MA, Mann JJ, Parsey RV. In vivo quantification of serotonin transporters using [11C]DASB and positron emission tomography in humans: modeling considerations. J Cereb Blood Flow Metab. 2007;27:205–217. doi: 10.1038/sj.jcbfm.9600329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milak MS, DeLorenzo C, Zanderigo F, et al. In vivo quantification of human serotonin 1A receptor using 11C-CUMI-101, an agonist PET radiotracer. J Nucl Med. 2010;51:1892–1900. doi: 10.2967/jnumed.110.076257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLorenzo C, Klein A, Mikhno A, et al. SPIE Medical Imaging. Bellingham, WA: SPIE; 2009. A new method for assessing PET-MRI coregistration; pp. 72592W–72598. [Google Scholar]
- 28.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 29.Rabiner EA, Wilkins MR, Turkheimer F, et al. 5-Hydroxytryptamine1A receptor occupancy by novel full antagonist 2-[4-[4-(7-chloro-2,3-dihydro-1,4-benzdioxyn-5-yl)-1-piperazinyl]butyl]-1, 2-benzisothiazol-3-(2H)-one-1,1-dioxide: a[11C][O-methyl-3H]-N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-py ridinyl)cyclohexanecarboxamide trihydrochloride (WAY-100635) positron emission tomography study in humans. J Pharmacol Exp Ther. 2002;301:1144–1150. doi: 10.1124/jpet.301.3.1144. [DOI] [PubMed] [Google Scholar]


