Abstract
Imaging mass spectrometry (IMS) is currently receiving large attention from the mass spectrometric community, although its use is not yet well known in the clinic. As matrix-assisted laser desorption/ionization time-of-flight (MALDI)-IMS can show the biomolecular changes in cells as well as tissues, it can be an ideal tool for biomedical diagnostics as well as the molecular diagnosis of clinical specimens, especially aimed at the prompt detection of premalignant lesions much earlier before overt mass formation, or for obtaining histologic clues from endoscopic biopsy. Besides its use for pathologic diagnosis, MALDI-IMS is also a powerful tool for the detection and localization of drugs, proteins, and lipids in tissue. Measurement of parameters that define and control the implications, challenges, and opportunities associated with the application of IMS to biomedical tissue studies might be feasible through a deep understanding of mass spectrometry. In this focused review series, new insights into the molecular processes relevant to IMS as well as other field applications are introduced.
Keywords: Spectrometry, mass, matrix-assisted laser desorption-ionization; Imaging mass spectrometer; Biological markers; Premalignant gastrointestinal lesions; Chemoprevention
INTRODUCTION
Although nucleic acid-based analysis of biological systems can begin to provide data on the nature of individual genes and on the systematic regulation of many genes, experimental evidences clearly show a disparity between the relative expression levels of mRNAs and their corresponding proteins.1 Therefore, the need to understand total protein expression is solved with the application of proteomics. Proteomics deals with the wide-ranging determination of gene and cellular function at the protein level, the principle of which is extended to include cell or tissue imaging by light and electron microscopy, high-throughput array and chip experiments, and genetic readout experiments as illustrated by the yeast two-hybrid assay and protein identification by liquid chromatography (LC) and mass spectrometry (MS). Derived from MS, matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) imaging mass spectrometry (IMS), which further advanced to MALDI imaging or MALDI-IMS, is a label-free bioanalytical technique used for spatially resolved chemical analysis of a sample. Usually, MALDI imaging is used for the analysis of a specially prepared tissue section thaw-mounted onto a glass slide (Fig. 1). For several decades, conventional histological analysis with hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) have been the main tools for visualizing and understanding tissue morphology and structure after endoscopic biopsy because IHC can visualize the spatial distribution of individual protein species directly in tissue based on pathological evaluation. However, a specific antibody is required for each protein, and the multiplexing capabilities of IHC are extremely limited, rarely visualizing more than two proteins simultaneously as well as providing nothing more than visual information. However, with the recent emergence of MALDI-IMS, it is becoming possible to study more complex proteomic patterns directly in tissue. Although confocal endomicroscopy, adopted from confocal laser scanning microscopy and white light microscopy, is unique in its ability to dynamically visualize cellular processes in their native environment free of artifacts and can also provide better information than just direct visualization for detecting endoscopic pathology, it provides very limited information and carries the risk of fluorescence dye and has a low level of representation.2,3 A huge development in the MALDI-IMS technique has been observed during the last decade, with it being considered one of the most promising innovative measurement techniques in biochemistry, and a powerful and versatile tool for spatially resolved chemical analysis of diverse sample types ranging from biological and plant tissues to biological and polymer thin films, geared toward future clinical applications, especially for gastrointestinal (GI) diseases.4 In this focused review series, accompanied by brief reviews of the developmental history of MS, we discuss the current challenges and perspectives of MALDI-IMS aimed for future clinical applications as a part of molecular pathology diagnosis as well as a fast track tool to detect premalignant GI lesions, including Barrett esophagus, chronic atrophic gastritis, and inflammatory bowel diseases.
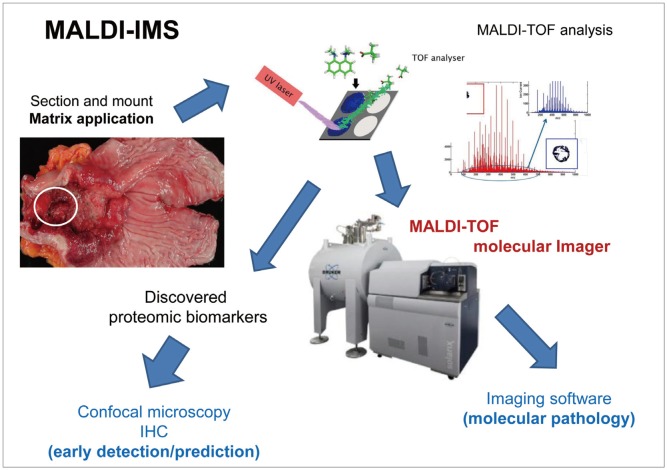
Fig. 1.
Schematic representation of the matrix-assisted laser desorption/ionization time-of-flight imaging mass spectrometry (MALDI-TOF-IMS) procedure. MALDI-IMS has developed as a promising tool for investigating the spatial distribution of biomolecules in intact tissue specimens. Ion densities of various molecules can be displayed as heat maps while preserving anatomical structures. For example, in gastric cancer, specially mounted tissues are subjected to matrix application and MALDI-TOF analysis. The discovered proteomes can be visualized in paraffin-embedded slides or visualized through confocal microscopy with a fluorescence-tagged biomarker antibody. MALDI-TOF molecular imaging can yield molecular images through an imaging software. IHC, immunohistochemistry.
PROGRESS OF MS DEVELOPMENT
General introduction to proteomics
Proteomics is defined as a large-scale study of proteins, particularly their functions and structures. MS has become a powerful tool in protein analysis and the key technology in the emerging field of proteomics, and it can also be used to measure the relative abundance of molecules.5 Quantitative proteomics is based on two-dimensional (2D) polyacrylamide gel electrophoresis and 2D difference gel electrophoresis fractionation coupled with peptide mass finger printing. In detail, 2D electrophoresis can separate a complex sample of proteins based on isoelectric point (pI), molecular mass (Mr), solubility, and relative abundance. 2D electrophoresis allows obtaining protein information such as protein modifications and/or changes in their expression levels. Proteomics has emerged from the laboratories of technologists and found widespread application in the clinical context.6 Recent advancements in proteomics technologies offer new opportunities for clinical applications in hospitals or specialized laboratories, including the identification of novel biomarkers, disease monitoring, detecting the adverse effects of drugs, and even evaluating the effects of environmental hazards. More advanced spectrometry technologies, including MALDI-IMS and the development of new protein array formats, have brought these analyses to a standard, which now has the potential to be used in clinical diagnostics.7
Introduction to clinical proteomics, including cancer proteomics
Clinical proteomics is a new field that has the potential to have many applications, including the identification of biomarkers, disease monitoring especially in the field of oncology, molecular diagnostics, prediction of treatment response, and evaluation of prognosis.8 In detail, expression proteomics or comparative proteomics evaluates the cellular production of proteins encoded by a particular gene and investigates the differential expression and posttranslational modifications of proteins according to healthy and diseased states. On the other hand, functional proteomics seeks to decipher protein-protein interactions and biochemical pathways involved in disease biology and targeted by newer molecular therapeutics. Combined with advanced spectrometry techniques (described below) and new protein array, molecular, and diagnostic imaging methods replacing current eye pathologies, further advances in proteomics technology could facilitate the accomplishment of the so called future 4P (personalized, predictive, precise, and preventive) medicine.9 Coupled with laser capture microdissection, high-density protein arrays, antibody arrays, and small molecular arrays, it could have a substantial impact on the proteomic profiling of human malignancies together with providing detailed real-time knowledge about the states of intracellular signaling circuitry and pathways in normal and malignant cells before and after therapy,10 including the potential of proteomics to identify proteins present in cancer tissue relevant for cancer screening.11
MALDI-TOF or surface-enhanced laser desorption/ionization-TOF-MS
MALDI ionizes samples out of a dry, transparent matrix by using high-energy laser pulses.4 MALDI is not a suitable method for large proteins as the resulting singly charged ion signals often result in near mass peaks, approximately several hundreds of daltons for a protein. Electrospray ionization (ESI) has recently emerged as a mild ionization method for producing intact ions in a vacuum from large and complex species in solution. This ionization method has more benefits for most biomacromolecular assemblies. The key features of a mass analyzer are its sensitivity, resolution, mass accuracy, and the ability to generate information-rich ion mass spectra from peptide fragments in proteomics analysis. The success of MS is driven by unique instrumentation designs, especially those operating on TOF and ion-trapping principles, which both use MS to detect the isolated large-scale protein mixtures. MALDI is commonly coupled to TOF-MS, which measures the mass of intact peptides, whereas ESI has usually been coupled to ion traps and triple quadruple instruments and used to generate fragment ion spectra of selected precursor ions. In most cases, MALDI-TOF-MS is more efficient for the analysis of relatively simple peptide mixtures, whereas integrated LC ESI-MS systems (LC-MS) are better options for the analysis of complex samples. For example, in the evaluation of patients with inflammatory bowel disease, although ulcerative colitis-associated colon cancer develops from dysplastic lesions caused by chronic inflammation, molecular throughput dissection to specifically link chronic inflammation and carcinogenesis in the colon has not been investigated, for which a label-free quantification analysis was performed (Fig. 2B). Furthermore, we had established an experimental animal model for colitic cancer and used proteomic analysis, based on 2D electrophoresis and MALDI-TOF-MS, to identify proteins involved in colitis-associated cancer.1 surface-enhanced laser desorption-TOF-MS technologies were applied in complex biological samples and/or for setting up new clinically relevant test systems adopting proteomic profiles of control and diseased states to find diagnostic biomarkers.
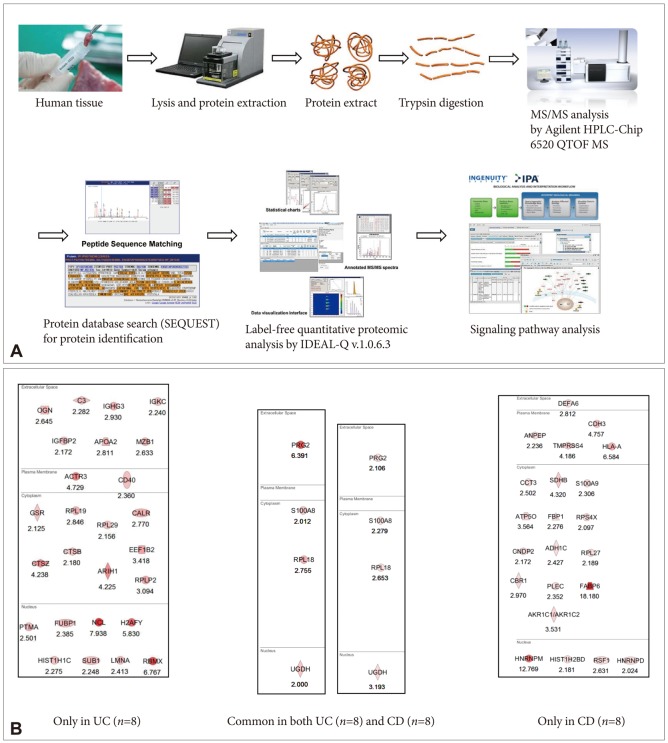
Fig. 2.
(A) Schematic representation of label-free quantification. (B) Proteome discovery for biomarkers predicting the risk of colitic cancer in patients with ulcerative colitis (UC) and Crohn disease (CD). The proteomes specifically identified as significantly increased in both UC and CD are considered potential markers in high-risk patients such as those with long-standing disease and extensive involvement of active diseases.
Stable isotope labeling in MS-based quantification
High-throughput proteomic analysis allows performing shotgun methods involving isotopic tagging of peptides. Methods of labeling samples with stable isotopes can be performed chemically or metabolically. The commonly used isotope tagging/labeling technologies include isotope-coded affinity tagging (ICAT), stable isotope labeling with amino acids in cell culture (SILAC), and isobaric tag for relative and absolute quantitation (iTRAQ) (Fig. 2A). ICAT is one of the most used chemical isotope labeling methods, and it depends on the sensitivity of MS to quantify relative protein abundance in a mixture of two different protein samples with the cysteine residues labeled. For the MS analysis, protein abundance is quantified using MS followed by selective identification of only the differentially expressed ICAT pairs using tandem MS (MS/MS).10 SILAC is a simple and reliable method for impartial comparative proteomic experiments and works in cellular proteomes through normal metabolic processes, incorporating nonradioactive, stable isotope-containing amino acids in newly synthesized proteins. In the growth medium for SILAC labeling, natural light amino acids are replaced by heavy SILAC amino acids. SILAC provides precise relative quantitative analysis free of chemical manipulation. iTRAQ is a powerful technology for the simultaneous identification and quantitation of protein abundance in four to eight different samples using tandem MS. Each label is composed of a peptide reactive group (N-hydroxysuccinimide ester) and an isobaric tag of 145 Da that consists of a balancer group (carbonyl) and a reporter group (based on N-methylpiperazine).
Label-free quantification
To study the differential protein expression in complex biological samples, strategies for rapid, highly reproducible, and accurate quantification are needed. Although isotope labeling and fluorescent labeling techniques have been widely used in quantitative proteomics research, researchers are increasingly turning to label-free shotgun proteomics techniques for faster, cleaner, and simpler results. MS-based label-free quantitative proteomics falls into two general categories.11
MALDI imaging mass spectrometry
MALDI-IMS has become a powerful tool for the detection and localization of drugs, proteins, and lipids in tissue, the principle of which is schematically shown in Fig. 1. Nevertheless, this approach can only identify low mass molecules such as lipids, pharmaceuticals, and peptides. Tryptic enzymatic digestion protocols for different kinds of tissues-formalin-fixed paraffin-embedded (FFPE) and frozen tissues-are combined with MALDI-ion mobility MS (MALDI-IM-MS). This combination enables localization and identification of proteins on the basis of their related digested peptides. IM separates isobaric ions that cannot be identified by conventional MALDI-TOF-MS. The amount of detected peaks per measurement is higher than that by conventional MALDI-TOF, which enables mass and time selected ion images and the identification of separated ions. These experiments demonstrate the feasibility of direct protein identification by IM-TOF-IMS from tissue. Therefore, the approach of tissue digestion combined with MALDI-IM-TOF-IMS allows a proteomics bottom-up strategy for different kinds of tissue samples, especially FFPE tissues conserved for a long time in hospital sample banks. The combination of IM with IMS marks the development of IMS approaches as real proteomic tools, which brings new perspectives to biological studies.12 IMS provides clear images with regard to the distribution of hundreds of biomolecules in a single measurement, as well as determines the cellular profile of the biological system. IMS is especially useful for characterizing the distribution of several kinds of lipids in various tissues, which is of utmost importance in cancer metabolism.13
FUNDAMENTALS OF IMS
MALDI-IMS is an MS technique applied to the chemical analysis of surfaces; it is a new technology for assessing spatial molecular arrangements in tissue sections, going far beyond the current microscopy techniques in providing hundreds of different molecular images from a single scan without the need for target-specific reagents. Ion beams for secondary ion MS and lasers for MALDI-MS are commonly used surface probes for IMS that typically provide nanometer and micrometer lateral resolution. The intrinsic label-free capabilities of IMS allow the spatial distribution of each molecule on the surface to be measured simultaneously by the detection of its specific molecular weight. The chemical and localization information obtained by IMS can be compared using the results of immunohistochemical staining (IHS). IMS integrates naturally into proteomics workflows for peptides and proteins,12 whereas IHS only shows pathology based on antibody binding in situ, for which a high-affinity antibody is needed. As the main principle of IMS is to use the analytical power of MS to create chemical images showing the distribution of both known and unknown molecules in a sample, IMS uses a different set of molecular properties for analyte detection and characterization compared with approaches based on fluorescence or radioactivity; thereby, IMS is able to observe molecules that are inaccessible to more traditional IHS techniques.13 Therefore, unknown or novel targets can be identified and validated with chemical imaging through a retrospective analysis of the data obtained from an experiment. Therefore, IMS differs from many conventional imaging methods because IMS analyzes the number of atoms and molecules in creating images. In conclusion, IMS is a true discovery-oriented tool for proteomics that integrates conventional proteomics workflows and can be perceived as either an alternative or a complementary proteomics technique.14
KINDS OF MALDI-IMS FOR THE MOLECULAR IMAGING OF PREMALIGNANT GI LESIONS
Histology-guided IMS
One of the advantages of IMS is that spatial localization of analytes within a tissue is preserved in comparison to homogenization. It allows for the differentiation of tissue regions based on molecular features. Therefore, IMS can be integrated with histology to directly target diseased regions and correlate the peaks that are upregulated/downregulated in specific regions with the reference information provided by histology. To date, histology-guided imaging has become the standard for applying IMS to clinical diagnostics. H&E staining is commonly performed in clinical tissue samples. This technique allows visualization of cells with bright field microscopy by labeling the nucleus and cytoplasm of the cells. A trained pathologist can then use the H&E-stained tissue to establish a diagnosis. Methods of histology-guided IMS have been developed that allow the observation of both the histological features and the MS image of the tissue. In such methods, the histological stain is applied to a serial section of the tissue that is to be analyzed by IMS, which dissipates the concern about MS-friendly stains. One drawback of this method is that it can be difficult to obtain serial sections reproducibly without tearing or folding the tissue in the cryostat. This can cause a misalignment of the sections, causing a loss of the correlation of small features. Another method is to apply the histological stain directly to the tissue that is to be analyzed by IMS. This method requires using MS-compatible stains such as cresyl violet or methylene blue, or performing imaging first and washing off the matrix before applying the histological stain. The drawback of this method is that H&E staining is not compatible with IMS. Therefore, the standard workflow would have to be changed to include nonstandard histological stains and a nonroutine evaluation of the results. The future of IMS in clinical diagnostics relies on incorporating this method into the current diagnostic workflow, and its results must be compared and correlated with histological results.15
Single-cell IMS
Single-cell IMS is another powerful technique used to map the distributions of endogenous biomolecules with subcellular resolution.16 Currently, secondary ion MS is the predominant technique for single-cell IMS, owing to its submicron lateral resolution and surface sensitivity. However, recent methodological and technological developments aimed at improving the spatial resolution of MALDI have made this technique a potential platform for single-cell IMS. MALDI opens the field of single-cell IMS to new possibilities, including singlecell proteomic imaging and atmospheric pressure analyses; however, sensitivity is a challenge. In this report, we estimate the availability of proteins and lipids in a single cell and discuss strategies used to improve sensitivity at the single-cell level.
Imaging cells and tissues
A broad range of chemical species has been investigated using IMS within biological specimens. Low-mass ions such as Na+, K+, Ca++, Cl-, and Br- derived from biological samples have been investigated using secondary ion mass (SIMS) imaging and can be used to diagnose the physiological state of cells and serve as markers of cellular origin. As an increased signal for K+ ions corresponding to a higher K+ concentration was observed in the interior of a cell, whereas the opposite was found for Na+ and Ca++ ions, a change in the intensity of the MS signal from inorganic ions might indicate cell damage or the development of pathology, including tumor growth. As another example, proliferative diabetic retinopathy demonstrates an increase in the relative intensity of Fe, Ca, Al, Zn, and Cu, as well as vitamin A fragment-1 ion signals compared with nondiabetic tissue.
MALDI-IMS for preneoplastic pancreatic cancer and premalignant GI lesions
To show the potential applications of MALDI-IMS, Grüner et al.17 identified novel biomarkers predicting the development of preneoplastic pancreatic lesions, including intraepithelial neoplasia (PanIN), intraductal papillary mucinous neoplasm (IPMN), and early pancreatic ductal adenocarcinoma (PDAC)-critical pancreatic diseases showing high mortality rates upon late detection-using MALDI-IMS. Their results showed that PanIN and IPMN could be distinguished from normal pancreatic tissue and PDAC by 26 significant m/z species. Besides the molecular imaging diagnostic power, they found that albumin and thymosin-β4 can be detected by LC-MS/MS. Precancerous conditions such as gastric cancer or Barrett esophagus can also be visualized through MALDI-IMS.
APPLICATIONS OF MALDI FOR PREMALIGNANT GI LESIONS BEYOND IMS: BIOMARKER DETECTION, DRUG DISCOVERY, ETC.
Molecular imaging for early detection of premalignant lesions: far beyond conventional biomarkers
Because IMS is a powerful tool with the ability to investigate a broad mass range of molecules, from small molecules to large proteins, through creating detailed distribution maps of selected compounds, its usefulness in biomarker discovery toward clinical applications has obtained success by correlating the molecular expression of tissues acquired from IMS with well-established histology. Ye et al.15 has demonstrated the versatility of IMS in terms of clinical applications such as biomarker diagnostics of different diseases, prognostics of disease severity, and metabolic response to drug treatment, and concluded that the ever growing applications with the continuous development of this powerful analytical tool will lead to a better understanding of the biology of diseases and improvements in clinical diagnostics. Changes in protein levels in a tissue can correlate with the disease state. The protein levels can be monitored to reveal not only the type but also the severity of the disease, especially in patients with premalignant lesions before progressing into overt malignancy. Through the excellent endoscopic treatment technology, these biomarkers might aid in decision making in cancer prevention and surveillance. Six common types of cancer, including Barrett esophagus associated with esophageal cancer, breast cancer, colon cancer, hepatocellular carcinoma, gastric cancer, and thyroid carcinoma, have been probed with MALDI-IMS by Meding and Walch.18 Patient diagnosis begins with the identification of tumor origin and tumor classification; when a primary tumor cannot be identified, cancer of unknown primary site is diagnosed. Researchers use MALDI-IMS to establish distinct protein biomarkers for each type of known cancer. Cancer cell-specific spectra are extracted and classified based on their proteomic differences with high confidence. For example, we performed label-free quantification (Fig. 2A) with TOF/TOF analysis using biopsied samples from either eight patients with ulcerative colitis or eight patients with Crohn disease, and determined significant proteomic biomarkers signifying cancer-prone proteomes after a Gene-Go analysis (Fig. 2B). These results can be easily validated with IHC staining or confocal microscopy with fluorescence-tagged antibody, after which high-risk group patients can be screened for colitic cancer.
Molecular imaging for drug discovery
One of the most important steps in drug discovery is the identification of appropriate pharmacological targets, considering that many diseases have multiple manifestations that include significant changes in biochemical processes and physiological activities, as well as the plethora of different and interconnected mechanisms involved in normal and pathological functioning of an organism. One method to address this question is to screen and compare the biochemistry, physiology, and morphology of healthy, diseased, and pharmacologically manipulated organs and biological systems using high-throughput methods to attempt to link the measurable changes in chemical abundance to the possible cause of disease and response to drug treatment. MALDI-IMS has been used broadly in such biochemical characterization of cells, and normal and diseased tissues, as well as in the study of fundamental cellular biology. The current drug discovery process is heavily dependent on the synthesis of combinatorial libraries on arrays of polymer resin particles. Because of its specificity, sensitivity, speed, robustness, and low sample consumption, IMS is becoming an important analytical tool for screening combinatorial libraries to identify compounds and determine the purity of the synthesis. TOF-SIMS imaging strategies for the analysis of peptide and nonpeptide combinatorial libraries have been developed. To optimize MS library screening, several important parameters were evaluated, such as linker systems, sample conditioning procedures, linker cleavage, and the extraction of unbound analytes. The detection limit of this approach was determined to be 480 peptide molecules. The microfabrication of smaller desorption/ionization on silicon (DIOS) sample arrays makes DIOS-IMS a probable candidate for the high-throughput screening of combinatorial libraries in the future.
Unique applications of MALDI-IMS for clinical diagnostics
Besides drug distribution/development and biomarker discovery for the early detection of premalignant lesions, as well as molecular imaging replacing pathological examination, IMS has been used for several other clinical applications. For instance, although routine clinical fracture risk assessments do not consider the quality of the bone mineral matrix, and osteoporosis is a well-known disease for which biomarkers have been extensively studied, little attention has been devoted to bone material quality. Zoehrer et al.19 used TOF-SIMS-IMS to investigate the spatial distribution and the relation of phosphorus and calcium in bone, and SIMS-IMS clearly showed a greater frequency of areas with high Ca++ intensity in the tissue samples with fragility fractures than the control group. As another potential of application, biodegradable polymers are of high interest in the medical field because of their potential applications in tissue engineering and as drug delivery carriers. Supramolecular polymers have gained much interest as potential drug delivery carriers because of their compatibility with weak water-soluble compounds and their potential for controlled drug release. Klerk et al.20 used SIMS-IMS to elucidate the molecular distributions in and around such polymers. In addition, Tanaka et al.21 used MALDI-IMS to study the lipid profile of arteriovenous fistula tissues.
FUTURE PERSPECTIVES OF MALDI-IMS FOR PREMALIGNANT GI LESIONS
Three-dimensional MALDI-IMS
One of the more exciting advances in the field of clinical IMS application is the development of three-dimensional (3D)-MALDI-IMS.22 After registration of scanned images and histologically stained images, the anatomical information from histology is fused with the mass spectra from MALDI-IMS. Three-dimensional-MALDI-IMS allows studying a broad mass range of molecular species by creating a lateral and vertical distribution map of selected compounds. This technique serves as a powerful discovery tool for pathologists and to the pharmaceutical industry by allowing a more complete visualization of tissue samples, which improves the ability to identify distinct molecular signatures and drug distribution throughout the entire tissue.23 Advances involving imaging acquisition speed, image resolution, and data processing are always ongoing in the biological sciences. By adding the third dimension to the traditional 2D-IMS method, there is a greater need for improvements in sample preparation, data acquisition, and data file transfer. Advances in computational tools will need to be made to improve the efficiency of data transfer and processing that will be much more time consuming when collecting a third dimension of imaging data. Three-dimensional imaging shows great promise for advancing clinical diagnostics, but there are still many areas for potential improvement before it becomes a widely used technique. Approximately 1,000 peaks can be observed in each dataset. Conclusively, the IMS sections are collected at an equal distance, 200 µm, instead of 400 to 500 mum used previously, thus enabling the use of virtual z-stacks and 3D volume renderings to investigate differential localization patterns. However, this 3D-IMS technology pipeline has been studied mostly in solid organs such as the brain or kidney rather than in hollow viscous organs such as the stomach or colon.
Alternative to FFPE tissues
Formalin fixation and paraffin embedding is the standard procedure for preserving biological tissue samples for histological analysis and for long-term storage at room temperature. It preserves the cellular and morphological details of the tissue, and is a well-established processing method used by pathologists.24 As the analysis of human tissue specimen is key to the identification of novel biomarkers that can be used to create more specific therapies and treatments, recent progress has also been made in the analysis of FFPE tissues in MALDI-IMS. With the advancement of tissue microarray (TMA) technology, multiple FFPE core biopsies can also be assembled in a single block.25 However, proteomic analysis of these tissues is still not easy, and further development of this technology is needed in the near future. Therefore, direct analysis of FFPE-TMA tissue with IMS might allow a detailed diagnostic evaluation of multiple tissue samples in a single experiment without the laborious extraction and purification of proteins. Especially, the advantages of high speed and throughput, easy sample handling, and excellent reproducibility make this technology a favorable approach for the proteomic analysis of clinical research cohorts with large sample numbers.
Quantitative imaging
Quantitative imaging is of great interest in the field of MS and in the application of IMS in the clinical setting. Advances are slowly being made in the development of a reproducible, quantitative IMS method. For instance, Takai et al.26,27 have developed a quantitative MALDI-IMS approach to analyze the concentration of a drug in mouse tissue after specific time points, a method based on generating a calibration curve for the drug by spiking different concentrations of the drug of interest directly onto a tissue section and analyzing it with IMS. On the other hand, Terp et al.28 used comparative, quantitative LC-MS/MS proteome analysis of a unique metastasis model comprising three isogenic human breast cancer cell lines that are equally tumorigenic in mice to identify proteins associated with cancer cell aggressiveness, and found LRRC59, CD59, and CSPG4 as candidates. Until now, there is no universal technique for obtaining high-resolution images and robust quantitative information, but progress is being made and there is potential for future improvements that would greatly influence the field of clinical diagnostics as well as many other scientific fields. In conclusion, MALDI-IMS enables the quantification of an administered therapeutic peptide on biological tissue sections or visualizes the in vivo distribution of the peptide.
CONCLUSIONS
The field of clinical proteomics offers opportunities to identify new disease biomarkers in body fluids, cells, and tissues, as well as predictors for premalignant lesions that can be missed or underevaluated in routine endoscopy examination. These biomarkers suggestive of premalignant lesions can be used in clinical applications for specific treatment or therapy monitoring, including endoscopic submucosal dissection, heater probe ablation, or others. New protein array formats and improved spectrometry technologies have conferred the potential for these analyses to be used in clinical diagnostics.29 Like biopsied tissue in endoscopy, fluid proteomics can provide additional opportunities for treatment, especially for premalignant lesions, beyond the current methods based on cytology or tumor markers. IMS has developed into a technique that can solve actual problems in current biomedical research. Although it still has its limitations, developments in IMS instrumentation are continuously eliminating the technical barriers and limitations, resulting in an increase in its analytical value. Furthermore, all MS imaging implementations need to be combined with other molecular imaging techniques and standard analytical protocols applied to tissue homogenates to provide a positive identification of the molecules associated with a spatial structure. Although the applications of IMS need further development, studies in this field will facilitate the clinical applications together with contributing to increasing the knowledge of doctors, especially GI endoscopists.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Yeo M, Kim DK, Park HJ, et al. Loss of transgelin in repeated bouts of ulcerative colitis-induced colon carcinogenesis. Proteomics. 2006;6:1158–1165. doi: 10.1002/pmic.200500390. [DOI] [PubMed] [Google Scholar]
- 2.Goetz M, Malek NP, Kiesslich R. Microscopic imaging in endoscopy: endomicroscopy and endocytoscopy. Nat Rev Gastroenterol Hepatol. 2013 Jul 30; doi: 10.1038/nrgastro.2013.134. Epub. DOI: 10.1038/nrgastro.2013.134. [DOI] [PubMed] [Google Scholar]
- 3.Goetz M, Watson A, Kiesslich R. Confocal laser endomicroscopy in gastrointestinal diseases. J Biophotonics. 2011;4:498–508. doi: 10.1002/jbio.201100022. [DOI] [PubMed] [Google Scholar]
- 4.Alexandrov T. MALDI imaging mass spectrometry: statistical data analysis and current computational challenges. BMC Bioinformatics. 2012;13(Suppl 16):S11. doi: 10.1186/1471-2105-13-S16-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghafourian S, Sekawi Z, Raftari M, Ali MS. Application of proteomics in lab diagnosis. Clin Lab. 2013;59:465–474. [PubMed] [Google Scholar]
- 6.Tabb DL. Quality assessment for clinical proteomics. Clin Biochem. 2013;46:411–420. doi: 10.1016/j.clinbiochem.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Apweiler R, Aslanidis C, Deufel T, et al. Approaching clinical proteomics: current state and future fields of application in cellular proteomics. Cytometry A. 2009;75:816–832. doi: 10.1002/cyto.a.20779. [DOI] [PubMed] [Google Scholar]
- 8.Azad NS, Rasool N, Annunziata CM, Minasian L, Whiteley G, Kohn EC. Proteomics in clinical trials and practice: present uses and future promise. Mol Cell Proteomics. 2006;5:1819–1829. doi: 10.1074/mcp.R600008-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Wulfkuhle JD, Paweletz CP, Steeg PS, Petricoin EF, 3rd, Liotta L. Proteomic approaches to the diagnosis, treatment, and monitoring of cancer. Adv Exp Med Biol. 2003;532:59–68. doi: 10.1007/978-1-4615-0081-0_7. [DOI] [PubMed] [Google Scholar]
- 10.Bichsel VE, Liotta LA, Petricoin EF., 3rd Cancer proteomics: from biomarker discovery to signal pathway profiling. Cancer J. 2001;7:69–78. [PubMed] [Google Scholar]
- 11.Verma M, Wright GL, Jr, Hanash SM, Gopal-Srivastava R, Srivastava S. Proteomic approaches within the NCI early detection research network for the discovery and identification of cancer biomarkers. Ann N Y Acad Sci. 2001;945:103–115. doi: 10.1111/j.1749-6632.2001.tb03870.x. [DOI] [PubMed] [Google Scholar]
- 12.Stauber J, MacAleese L, Franck J, et al. On-tissue protein identification and imaging by MALDI-ion mobility mass spectrometry. J Am Soc Mass Spectrom. 2010;21:338–347. doi: 10.1016/j.jasms.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Schwamborn K. Imaging mass spectrometry in biomarker discovery and validation. J Proteomics. 2012;75:4990–4998. doi: 10.1016/j.jprot.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 14.MacAleese L, Stauber J, Heeren RM. Perspectives for imaging mass spectrometry in the proteomics landscape. Proteomics. 2009;9:819–834. doi: 10.1002/pmic.200800363. [DOI] [PubMed] [Google Scholar]
- 15.Ye H, Gemperline E, Li L. A vision for better health: mass spectrometry imaging for clinical diagnostics. Clin Chim Acta. 2013;420:11–22. doi: 10.1016/j.cca.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Passarelli MK, Ewing AG. Single-cell imaging mass spectrometry. Curr Opin Chem Biol. doi: 10.1016/j.cbpa.2013.07.017. Epub 2013 Aug 12. DOI: 10.1016/j.cbpa.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grüner BM, Hahne H, Mazur PK, et al. MALDI imaging mass spectrometry for in situ proteomic analysis of preneoplastic lesions in pancreatic cancer. PLoS One. 2012;7:e39424. doi: 10.1371/journal.pone.0039424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meding S, Walch A. MALDI imaging mass spectrometry for direct tissue analysis. Methods Mol Biol. 2013;931:537–546. doi: 10.1007/978-1-62703-056-4_29. [DOI] [PubMed] [Google Scholar]
- 19.Zoehrer R, Perilli E, Kuliwaba JS, Shapter JG, Fazzalari NL, Voelcker NH. Human bone material characterization: integrated imaging surface investigation of male fragility fractures. Osteoporos Int. 2012;23:1297–1309. doi: 10.1007/s00198-011-1688-9. [DOI] [PubMed] [Google Scholar]
- 20.Klerk LA, Dankers PY, Popa ER, et al. TOF-secondary ion mass spectrometry imaging of polymeric scaffolds with surrounding tissue after in vivo implantation. Anal Chem. 2010;82:4337–4343. doi: 10.1021/ac100837n. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Zaima N, Yamamoto N, et al. Distribution of phospholipid molecular species in autogenous access grafts for hemodialysis analyzed using imaging mass spectrometry. Anal Bioanal Chem. 2011;400:1873–1880. doi: 10.1007/s00216-011-4850-5. [DOI] [PubMed] [Google Scholar]
- 22.Thiele H, Heldmann S, Trede D, et al. 2D and 3D MALDI-imaging: conceptual strategies for visualization and data mining. Biochim Biophys Acta. 2013 Mar 04; doi: 10.1016/j.bbapap.2013.01.040. Epub. DOI: 10.1016/j.bbapap.2013.01.040. [DOI] [PubMed] [Google Scholar]
- 23.Andersson M, Groseclose MR, Deutch AY, Caprioli RM. Imaging mass spectrometry of proteins and peptides: 3D volume reconstruction. Nat Methods. 2008;5:101–108. doi: 10.1038/nmeth1145. [DOI] [PubMed] [Google Scholar]
- 24.Lemaire R, Desmons A, Tabet JC, Day R, Salzet M, Fournier I. Direct analysis and MALDI imaging of formalin-fixed, paraffin-embedded tissue sections. J Proteome Res. 2007;6:1295–1305. doi: 10.1021/pr060549i. [DOI] [PubMed] [Google Scholar]
- 25.Casadonte R, Caprioli RM. Proteomic analysis of formalin-fixed paraffin-embedded tissue by MALDI imaging mass spectrometry. Nat Protoc. 2011;6:1695–1709. doi: 10.1038/nprot.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai N, Tanaka Y, Inazawa K, Saji H. Quantitative analysis of pharmaceutical drug distribution in multiple organs by imaging mass spectrometry. Rapid Commun Mass Spectrom. 2012;26:1549–1556. doi: 10.1002/rcm.6256. [DOI] [PubMed] [Google Scholar]
- 27.Takai N, Tanaka Y, Watanabe A, Saji H. Quantitative imaging of a therapeutic peptide in biological tissue sections by MALDI MS. Bioanalysis. 2013;5:603–612. doi: 10.4155/bio.13.13. [DOI] [PubMed] [Google Scholar]
- 28.Terp MG, Lund RR, Jensen ON, Leth-Larsen R, Ditzel HJ. Identification of markers associated with highly aggressive metastatic phenotypes using quantitative comparative proteomics. Cancer Genomics Proteomics. 2012;9:265–273. [PubMed] [Google Scholar]
- 29.Apweiler R, Aslanidis C, Deufel T, et al. Approaching clinical proteomics: current state and future fields of application in fluid proteomics. Clin Chem Lab Med. 2009;47:724–744. doi: 10.1515/CCLM.2009.167. [DOI] [PubMed] [Google Scholar]