Abstract
Background/Aims
We evaluated the performance, clinical role, and diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) in gastrointestinal intramural lesions.
Methods
Procedural and pathologic data were reviewed from consecutive patients undergoing EUS-FNA for intramural lesions. Final diagnoses were determined by surgical histopathologic conformation and the diagnosis of malignancy, including clinical follow-up with repeat imaging.
Results
Forty-six patients (mean age, 47 years; 24 males) underwent EUS-FNA. Lesions were located in the stomach (n=31), esophagus (n=5), and duodenum (n=10). The median lesion size was 2 cm (range, 1 to 20.6). Final diagnoses were obtained in 22 patients (48%). EUS-FNA was diagnostic in 40 patients (87%). The diagnostic accuracy of cytology for differentiating between benign and malignant lesions was 82%; diagnostic error occurred in three patients (6%). The cytologic results influenced clinical judgment in 78% cases. The primary reasons for negative or no clinical impact were false-negative results, misdirected patient management, and inconclusive cytology.
Conclusions
EUS-FNA exhibited an 87% diagnostic yield for gastrointestinal intramural lesions; the accuracy of cytology for differentiating malignancy was 82%. The limitations of EUS-FNA were primarily because of nondiagnostic sampling (9%) and probable diagnostic error (6%); these factors may influence the clinical role of EUS-FNA.
Keywords: Accuracy, Endoscopic ultrasound-guided fine needle aspiration, Extraintestinal mass, Intramural mass, Yield
INTRODUCTION
Endoscopic ultrasound (EUS) plays an important role in the characterization of subepithelial lesions by providing information regarding their layer of origin, echogenicity, size, and local invasion. However, sonographic characteristics alone may be insufficient to distinguish subepithelial and hypoechoic third or fourth layer lesions. Several studies estimate the accuracy of EUS for the diagnosis of subepithelial lesions to be 43% to 48%.1,2 There is currently no consensus regarding the best management strategy for diagnosing and treating small subepithelial lesions.3 Operative planning, the type of surgery, and the follow-up period vary dramatically with respect to the diagnosis.
EUS-guided fine needle aspiration (EUS-FNA) is a means of sampling for cytologic confirmation. In addition, EUS-FNA in combination with immunohistochemistry (IHC) assists in differentiating various subepithelial tumors. Previous studies have demonstrated that EUS-FNA accurately diagnoses subepithelial lesions with an accuracy of 80% to 98% compared with the final diagnosis, whereas inadequate cytologic sampling occurs in 10% to 18% of procedures.4-7 An accurate EUS-FNA diagnosis requires an adequate cytologic specimen for pathologic evaluation; it is also important to decrease the frequency of nondiagnostic results and diagnostic errors.8
The purpose of this study was to assess the performance characteristics, clinical role, and accuracy of EUS-FNA for the diagnosis of gastrointestinal (GI) intramural masses, and to estimate its clinical impact on patient management.
MATERIALS AND METHODS
Approval for this retrospective study was obtained from Institutional Review Boards of The Catholic University of Korea College of Medicine. Between June 2009 and November 2011, 46 consecutive patients who underwent EUS-FNA of upper GI intramural masses were identified from the medical records at Seoul St. Mary's Hospital. The following data were collected: patient demographics, location of the lesion, endoscopic and EUS characteristics of the lesion (size, echogenecity, and wall layer of origin), size of needle, number of needle passes, cytologic diagnosis from FNA, and final pathology based on a resected specimen, when available.
Following conventional esophagogastroduodenoscopy, EUS examinations were performed by using curvilinear array echoendoscopes (GFUCT-240; Olympus, Tokyo, Japan) connected to a US scanning system (SSD 5500; Aloka, Tokyo, Japan) by a single experienced endosonographer (Y.K. Cho). All samplings were achieved by using 22-gauge needles (Echotip; Wilson-Cook Inc., Winston-Salem, NC, USA; or Easyshot; Olymphus Medical System Corp., Ltd., Tokyo, Japan). Patients were followed up for 24 hours after the procedure to detect procedure-related complications. Because no on-site cytopathologist was available, at least three or four passes were conducted for each target lesion. Additional FNA passes were performed based on the endoscopist's gross assessment of sample adequacy. Ten to 20 repetitions of back and forth movements were performed per single needle pass. Cytologic samples were processed by the same experienced cytopathologist. Aspirates were placed on glass slides, and alcohol-fixed smears were prepared for cytologic examination. The remaining material was then flushed from the needle and collected in CytoLyt preservative (Cytyc Corp., Marlborough, MA, USA) for cell block preparation.
Definition
EUS-FNA cytologic results were classified into three categories: diagnostic, nondiagnostic, and diagnostic error. A nondiagnostic specimen was defined as an inadequate sampling or an inconclusive cytologic result by the cytopathologist from direct smears or a cell block. Diagnostic error was defined as sampling error or errors that occurred in tissue sampling or pathologic interpretation.
Cytologic EUS-FNA diagnoses were categorized as malignancy, atypical (favor reactive or favor neoplasia), negative for malignancy, or nondiagnostic. We considered all gastrointestinal stromal tumors (GISTs) to be potentially malignant, in accordance with the National Institutes of Health consensus statement.9 IHC analysis is necessary to confirm mesenchymal cell tumors; therefore, these lesions were analyzed further. Cytology diagnoses were categorized as accurate (IHC stains established a specific diagnosis) or suspicious (spindle cell identified, but quantity insufficient for specific-stain IHC analysis). An EUS-FNA diagnosis of GIST was based on the presence of spindle-type cells that stained positively for c-kit. Leiomyomas were defined by the presence of smooth muscle or spindle cells that stained positively for actin or desmin, and negatively for c-kit.
The reference standards for final diagnosis were the surgical histopathologic results, the diagnosis of malignancy including clinical history, or the response to clinical treatment and extended clinical follow-up (>12 months). A presumptive diagnosis based on EUS-FNA was reviewed and compared with the final confirmed diagnosis.
The impact of EUS-FNA on clinical management was subdivided into positive or negative effects. A positive impact was defined as provision of: 1) adequate cytology that influenced the decision to perform surgery; 2) adequate cytology and avoidance of surgery because of inoperable disease; or 3) a diagnosis that facilitated medical treatment. A negative impact or lack of impact was defined as provision of: 1) a false-negative result or the misdirection of patient management; 2) inconclusive cytology; or 3) an adequate cytologic diagnosis that did not influence patient management.7
RESULTS
Characteristics of lesions
Forty-six patients (mean age, 47±16 years; 24 males) underwent EUS-FNA because of GI intramural mass lesions. The endoscopic and EUS characteristics of the lesions are summarized in Table 1. Lesions were located in the stomach in 31 patients (52%), esophagus in five patients (8%), and duodenum in 10 patients (17%). The median long axis of the lesions was 2 cm (range, 1 to 20.6).
Table 1.
Endoscopic and Endoscopic Ultrasound Characteristics of Intramural and Extraintestinal Masses (n=46)
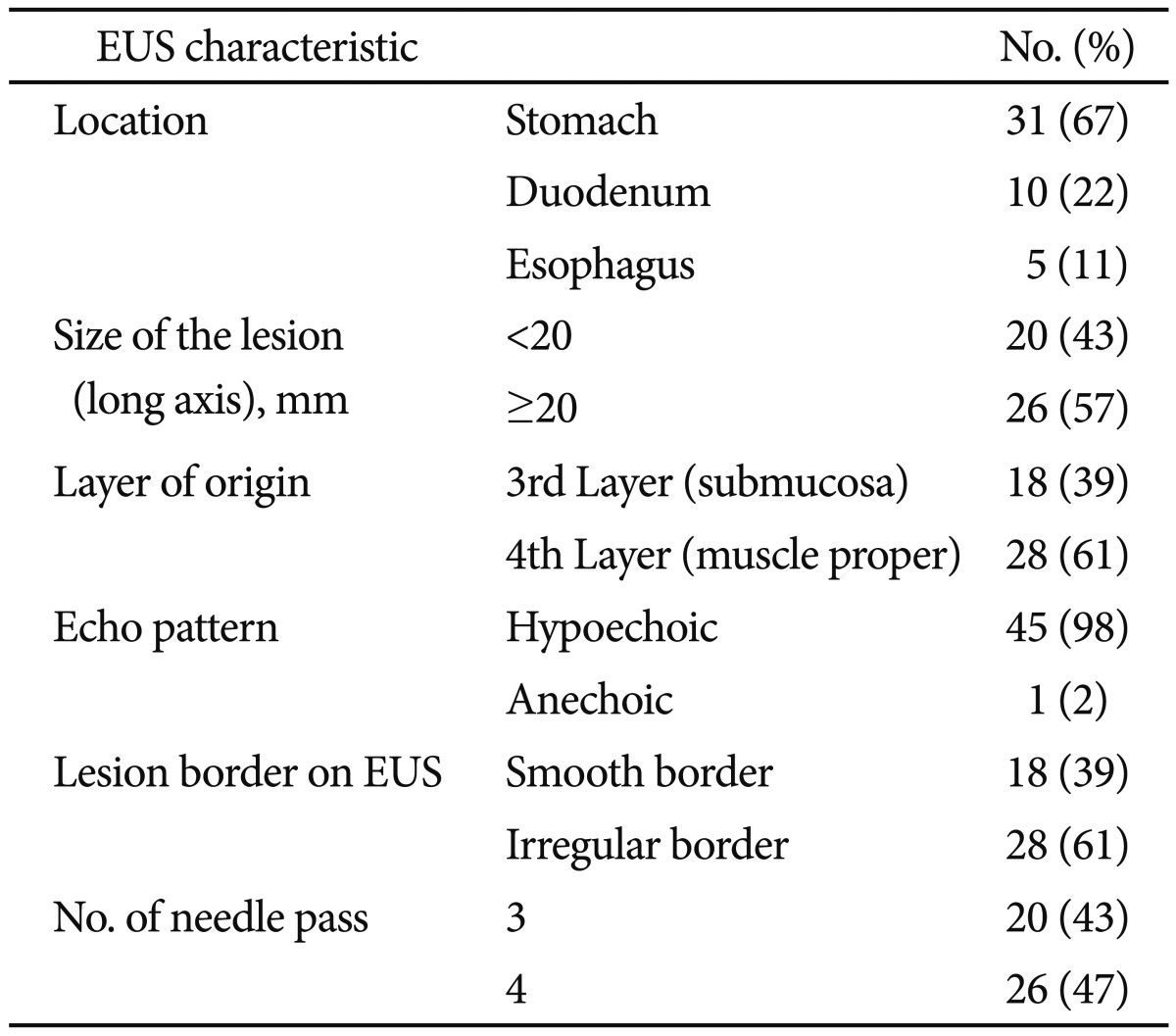
EUS, endoscopic ultrasound.
Diagnostic yield of EUS-FNA
FNA cytology was diagnostic in 39 lesions (85%). Cytologic samples from four lesions (9%) were nondiagnostic, and diagnostic error occurred for three lesions (6%). The cytologic yield of EUS-FNA was higher for gastric lesions (91%) compared with esophageal or duodenal lesions (60% and 89%, respectively). Ten lesions proved to be mesenchymal tumors that required IHC analysis for confirmatory diagnosis. Cytologic diagnosis was made for 70% of lesions (two lesions by using additive IHC stain and five lesions by using cytology alone). Samples from other lesions were insufficient for diagnosis.
Cytologic analysis was performed only for lesions that were sampled sufficiently for diagnosis. The diagnostic categories for cytology were malignancy, atypical, or negative for malignancy. Most mesenchymal tumors were classified as atypical (11 tumors) or malignant (two tumors). Two cases of mesenchymal tumors were diagnosed as negative for malignancy-these were assumed to be sampling errors. One adenocarcinoma was diagnosed cytologically as atypical spindle cells. One neuroendocrine tumor was diagnosed as negative for malignancy. All lesions of ectopic pancreas were diagnosed as negative for malignancy.
The final diagnosis was obtained for 22 lesions (48%) by performing surgical or endoscopic resection (n=13), cytologic diagnosis of malignancy (n=1), or clinical history and follow-up (n=8). The cytologic diagnoses for three lesions (6%) were discrepant with final pathology. In one case of a subepithelial mass that originated from the third layer of the duodenum, the EUS diagnosis was ectopic pancreas and the cytologic diagnosis was pancreatic ductal cells; IHC was not performed. Surgical pathology indicated a well-differentiated neuroendocrine tumor (Fig. 1). A subepithelial mass of fourth-layer origin in the antrum was diagnosed as having atypical spindle cells. Surgical pathology indicated adenocarcinoma. A cytologic result from a subepithelial mass in the ampulla demonstrated the presence of several inflammatory cell types. The lesion was diagnosed as a malignancy, such as a myofibroblastic tumor, based on imaging results and clinical course. Cytologic diagnoses and final diagnoses are summarized in Table 2.
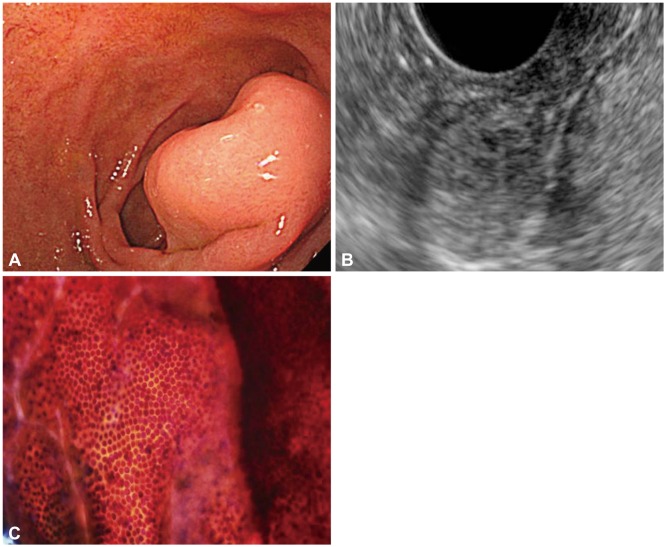
Fig. 1.
A subepithelial tumor exhibiting benign cytology that proved to be a neuroendocrine tumor. (A) A 2-cm subepithelial tumor of the duodenum. (B) Endoscopic ultrasound reveals a heterogenous hypoechoic mass containing multiple tiny cysts that originated from the submucosal layer. (C) Cytology was negative for malignancy. The final diagnosis after surgery was neuroendocrine tumor.
Table 2.
Concordance of Endoscopic Ultrasound and Cytologic Diagnosis with the Final Diagnosis (n=22)
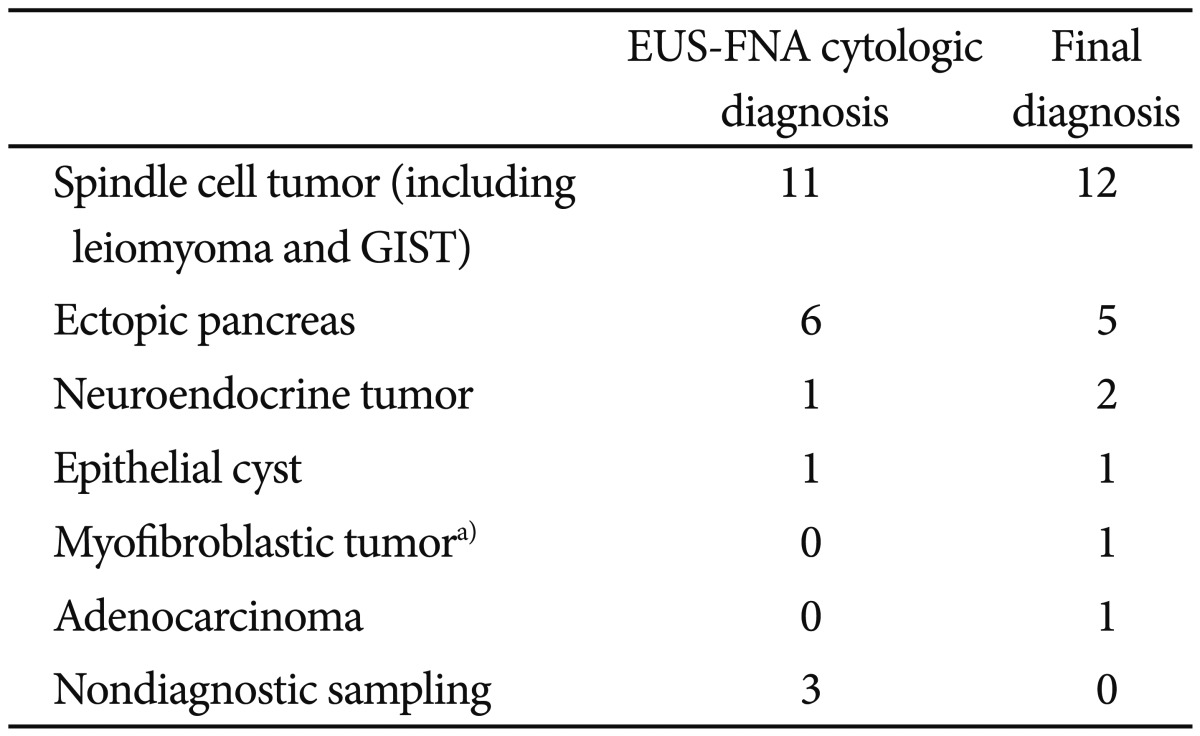
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration; GIST, gastrointestinal stromal tumor.
a)The diagnosis was based on clinical and radiological evidence.
Performance characteristics of EUS-FNA
Cytologic diagnoses made by using EUS-FNA were in accordance with the final diagnoses in 17 of 22 lesions. With respect to the differentiation between benign and potentially malignant lesions, six lesions were true positives, 12 were true negatives, four lesions for which the specimens were inadequate for cytology were false negatives, and no lesions were false positives. EUS-FNA had a sensitivity of 60%, a specificity of 100%, a positive predictive value of 100%, a negative predictive value of 75%, and an accuracy of 82% for differentiating benign lesions from potentially malignant lesions (Table 3).
Table 3.
Performance Characteristics of Endoscopic Ultrasound-Guided Fine Needle Aspiration for Differentiating Benign from Malignant (or Potentially Malignant) Lesions
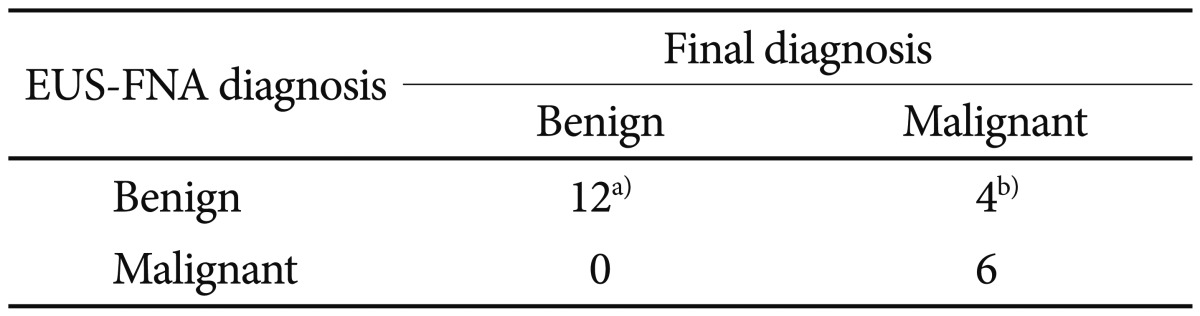
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration.
a)One lesions sampled inadequately were regarded as true negative cases; b)Three lesions proved to be diagnostic errors were regarded as false negative cases: one lesion with cytology of spindle cell tumor proved to be adenocarcinoma of stomach, one lesion with cytology of several inflammatory cell proved to be myofibroblstic tumor of duodenum, one lesion with cytology of pancreatic ductal cell proved to be carcinoid tumor of duodenum.
Clinical impact of EUS-FNA
EUS-FNA had a positive effect on clinical management in 78% of cases by influencing the decision to perform surgery (22%), avoidance of surgery because of inoperable disease (2%), or the exclusion of malignancy and facilitation of medical treatment (54%).
Cytologic results had no effect on clinical management in 22% of cases for the following reasons: nondiagnostic specimens (12%), a false-negative result, the misdirection of patient management (9%), or adequate cytology but the result had no influence on patient management (7%) (Table 4).
Table 4.
How Endoscopic Ultrasound-Guided Fine Needle Aspiration Diagnosis Influenced on Clinical Management?
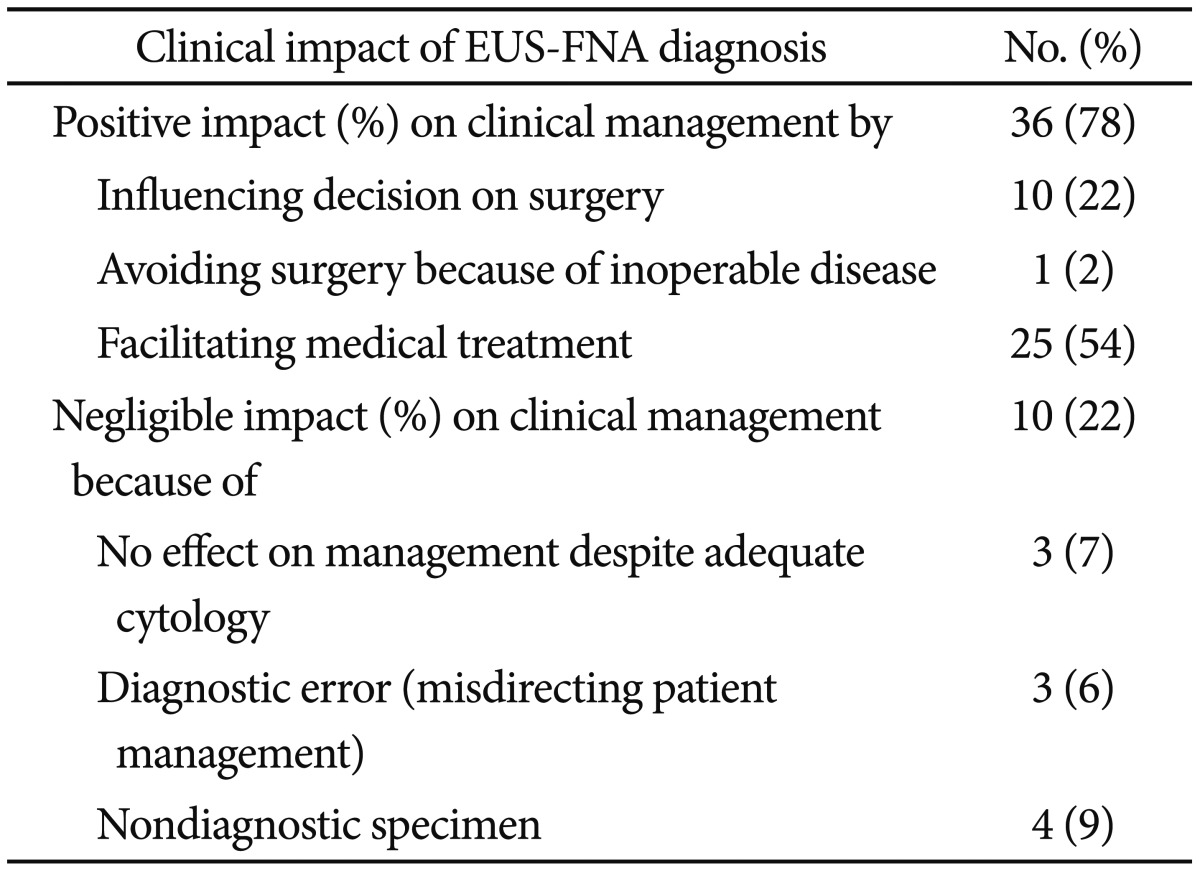
EUS-FNA, endoscopic ultrasound-guided fine needle aspiration.
DISCUSSION
We assessed the performance characteristics, clinical role, and accuracy of EUS-FNA in evaluating intramural masses. We report a yield of 87% for EUS-FNA sampling of submucosal lesions in the upper GI tract. Although our overall yield was similar to that in other recent studies, the proportion of diagnoses that were based on cytology and IHC was lower. In our study, spindle cell tumors comprised 33% of all EUS-FNA diagnoses (15 of 46 lesions). Twenty percent of spindle cell lesions were adequately stained for these antibodies. There are many reports of mesenchymal cell tumors in the literature. For example, a study from Japan reported a sample adequacy rate of 83% for gastric submucosal tumors.10 However, this included 39% of samples that met suggestive cytologic criteria without IHC staining. We suspect that, in most cases, this is related to the absence of on-site cytopathology and small size of lesions. The actual number of passes required for diagnosis may be less relevant when an on-site cytopathologist confirms specimen adequacy.
The level of experience of the endoscopist, the needle gauge, the number of needle passes attempted, the location of the lesion, and the on-site presence of a cytotechnologist or pathologist may affect cytologic adequacy.11-13 In this study, neither the number of needle passes nor the size of the lesion was related to diagnostic yield. The median size of lesions that were insufficiently sampled or subject to diagnostic error was 2×1.5 cm. Most lesions in this study were approximately 2 cm (range, 1 to 20.6) and therefore insufficient for Trucut biopsy. Many lesions were located in the stomach, duodenum, and angulated portion, which also restricted the use of 19-gauge needles and Trucut biopsy because of potential handling difficulties. All procedures were performed with a 22-gauge needle, which facilitated adequate sampling irrespective of lesion size and location. However, this size may be insufficient to obtain a cell block, which is useful for diagnosing the subtypes of submucosal tumors. The reason for comparatively lower yields of the esophageal lesions is unknown; the esophagus is straighter and therefore allows easier needle handling compared with other organs such as the stomach. Because failed procedures were performed during the early stages of the learning curve, this may have affected cytologic outcomes.2
The limited value of EUS-FNA for the differential diagnosis of subepithelial masses was primarily the result of the rate of nondiagnosis and diagnostic error. The former is determined by technical failures, unsatisfactory specimens, and atypical and suspicious diagnoses; the latter is determined by false-positive and false-negative diagnoses, and errors in interpretation.8 Discordance between cytologic results and the final pathologic diagnosis may critically influence and misdirect patient management. In this study, the diagnostic accuracy of EUS-FNA for differentiating benign lesions from potentially malignant lesions and malignant lesions was 82%. There were three cases exhibiting discordance with the final pathology. Of these, two cases in which the cytologic diagnosis was benign were confirmed clinically to be a duodenal neuroendocrine tumor and ampulla myoblastic tumor, respectively, and one patient with a presumed GIST in the gastric antrum with atypical spindle cells was diagnosed with differentiated adenocarcinoma. Pathologic review suggested that atypical spindle cells were rarely obtained from the stromal portion of adenocarcinomas. This was possibly because of difficulties in communication with pathologists and insufficient specimens for IHC. In contrast, high-grade GIST should be distinguished from poorly differentiated carcinoma based on spindle cell features. The use of immunocytochemistry may be helpful in making this differential diagnosis. Tumor cells from GIST are positive for c-kit, whereas smooth muscle cells from the bowel wall and from spindle cell carcinoma are negative for this marker. Spindle cell carcinoma is positive for cytokeratin expression, whereas GIST is not. A duodenal submucosal tumor was initially misdiagnosed as ectopic pancreas tissue by using EUS. Subsequent surgical resection revealed a well-differentiated neuroendocrine tumor. Retrospective review revealed that the aspirate contained pancreatic ductal epithelium. To reduce the false-negative rate, it is critical to obtain a sufficient sample for cytology. In addition, the provision of sufficient information to the pathologist is also important, in particular when no on-site pathologist is available.
The decision to resect an intramural lesion also depends on the findings from EUS. Considering the morphologic EUS features of a tumor in combination with its cytohistologic FNA assessment and location enhances the power and accuracy of the preoperative diagnosis of subepithelial masses. However, EUS imaging alone is unable to differentiate hypoechoic third layer masses. If EUS-FNA cytology is able to accurately differentiate potentially malignant lesions, unnecessary procedures would be avoided, particularly for lesions of borderline size (i.e., 1 to 2 cm). National Comprehensive Cancer Network guidelines regarding GIST recommend resection of masses of >2 cm, and clinical follow-up for lesions of <1 cm. The lack of guidelines for lesions of medium size may lead to confusion regarding patient management.3,14 To date, there is no consensus regarding the indications for use of EUS-guided tissue sampling. In our study, 73% of sampling results influenced clinical judgment. In particular, unnecessary surgery was avoided in the majority of patients. The role of EUS-FNA in clinical management depends on the appropriateness of the indication. To avoid unnecessary procedures, EUS-FNA should be performed in accordance with the appropriate indication to positively influence the clinical outcome.
In conclusion, EUS-FNA exhibited a high diagnostic yield for most small intramural and extraintestinal lesions, and a diagnostic accuracy of 82% for differentiating malignant lesions. The results of most procedures influenced clinical judgment. However, EUS-FNA has some limitations for the differential diagnosis of intramural masses, primarily because of nondiagnostic sampling (9%), and more importantly, the likelihood of diagnostic error (6%); these factors may influence the clinical utility of EUS-FNA. Better tissue acquisition techniques and more accurate pathologic interpretation are necessary for improved differential diagnosis.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Hwang JH, Saunders MD, Rulyak SJ, Shaw S, Nietsch H, Kimmey MB. A prospective study comparing endoscopy and EUS in the evaluation of GI subepithelial masses. Gastrointest Endosc. 2005;62:202–208. doi: 10.1016/s0016-5107(05)01567-1. [DOI] [PubMed] [Google Scholar]
- 2.Karaca C, Turner BG, Cizginer S, Forcione D, Brugge W. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–727. doi: 10.1016/j.gie.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Ha CY, Shah R, Chen J, Azar RR, Edmundowicz SA, Early DS. Diagnosis and management of GI stromal tumors by EUS-FNA: a survey of opinions and practices of endosonographers. Gastrointest Endosc. 2009;69:1039–1044. doi: 10.1016/j.gie.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 4.Wiersema MJ, Hawes RH, Tao LC, et al. Endoscopic ultrasonography as an adjunct to fine needle aspiration cytology of the upper and lower gastrointestinal tract. Gastrointest Endosc. 1992;38:35–39. doi: 10.1016/s0016-5107(92)70327-7. [DOI] [PubMed] [Google Scholar]
- 5.Matsui M, Goto H, Niwa Y, Arisawa T, Hirooka Y, Hayakawa T. Preliminary results of fine needle aspiration biopsy histology in upper gastrointestinal submucosal tumors. Endoscopy. 1998;30:750–755. doi: 10.1055/s-2007-1001416. [DOI] [PubMed] [Google Scholar]
- 6.Ando N, Goto H, Niwa Y, et al. The diagnosis of GI stromal tumors with EUS-guided fine needle aspiration with immunohistochemical analysis. Gastrointest Endosc. 2002;55:37–43. doi: 10.1067/mge.2002.120323. [DOI] [PubMed] [Google Scholar]
- 7.Chen VK, Eloubeidi MA. Endoscopic ultrasound-guided fine-needle aspiration of intramural and extraintestinal mass lesions: diagnostic accuracy, complication assessment, and impact on management. Endoscopy. 2005;37:984–989. doi: 10.1055/s-2005-870272. [DOI] [PubMed] [Google Scholar]
- 8.Eltoum IA, Chhieng DC, Jhala D, et al. Cumulative sum procedure in evaluation of EUS-guided FNA cytology: the learning curve and diagnostic performance beyond sensitivity and specificity. Cytopathology. 2007;18:143–150. doi: 10.1111/j.1365-2303.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. doi: 10.1053/hupa.2002.123545. [DOI] [PubMed] [Google Scholar]
- 10.Mekky MA, Yamao K, Sawaki A, et al. Diagnostic utility of EUS-guided FNA in patients with gastric submucosal tumors. Gastrointest Endosc. 2010;71:913–919. doi: 10.1016/j.gie.2009.11.044. [DOI] [PubMed] [Google Scholar]
- 11.Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci. 2011;56:1757–1762. doi: 10.1007/s10620-011-1646-6. [DOI] [PubMed] [Google Scholar]
- 12.Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218–1223. doi: 10.1016/j.gie.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 13.Philipper M, Hollerbach S, Gabbert HE, et al. Prospective comparison of endoscopic ultrasound-guided fine-needle aspiration and surgical histology in upper gastrointestinal submucosal tumors. Endoscopy. 2010;42:300–305. doi: 10.1055/s-0029-1244006. [DOI] [PubMed] [Google Scholar]
- 14.Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST): update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5(Suppl 2):S1–S29. [PubMed] [Google Scholar]