Abstract
Competition among conspecific males for fertilizing the ova is one of the mechanisms of sexual selection, i.e. selection that operates on maximizing the number of successful mating events rather than on maximizing survival and viability 1. Sperm competition represents the competition between males after copulating with the same female 2, in which their sperm are coincidental in time and space. This phenomenon has been reported in multiple species of plants and animals 3. For example, wild-caught D. melanogaster females usually contain sperm from 2-3 males 4. The sperm are stored in specialized organs with limited storage capacity, which might lead to the direct competition of the sperm from different males 2,5.
Comparing sperm competitive ability of different males of interest (experimental male types) has been performed through controlled double-mating experiments in the laboratory 6,7. Briefly, a single female is exposed to two different males consecutively, one experimental male and one cross-mating reference male. The same mating scheme is then followed using other experimental male types thus facilitating the indirect comparison of the competitive ability of their sperm through a common reference. The fraction of individuals fathered by the experimental and reference males is identified using markers, which allows one to estimate sperm competitive ability using simple mathematical expressions 7,8. In addition, sperm competitive ability can be estimated in two different scenarios depending on whether the experimental male is second or first to mate (offense and defense assay, respectively) 9, which is assumed to be reflective of different competence attributes.
Here, we describe an approach that helps to interrogate the role of different genetic factors that putatively underlie the phenomenon of sperm competitive ability in D. melanogaster.
Keywords: Developmental Biology, Issue 78, Molecular Biology, Cellular Biology, Genetics, Biochemistry, Spermatozoa, Drosophila melanogaster, Biological Evolution, Phenotype, genetics (animal and plant), animal biology, double-mating experiment, sperm competitive ability, male fertility, Drosophila, fruit fly, animal model
Introduction
Since Geoff Parker noted the prevalence of sperm competition in insects and its evolutionary implications 2, a surge of studies in Drosophila and other species have tried to shed some light on this phenomenon at many different levels. Some examples of areas of interest have been the survey of its variation in natural populations 9,10, its genetic architecture and relevance of underlying genetic factors 11-14, and its role in driving coevolution between the sexes 15,16. In D. melanogaster females, the limited capacity of the specialized sperm-storage organs, a pair of spermatheca and the seminal receptacle 6,17, contributes to the competition of the sperm from different males. Approximately 1,500 sperm are transferred during mating to the female but only ~500 can be accommodated in the mentioned organs 18,19. In the laboratory, controlled double-mating experiments involving a reference male and one or more males of interest have been extensively used for evaluating sperm competitive ability 7,8.
Sperm competitive ability is estimated as the proportion of progeny sired by the experimental male in double-mating experiments over the total progeny, i.e. that from both the experimental and reference males. Sperm competitive ability comprises two components, each of them evaluated in a separate assay. In the offense assay, the ability of the experimental male sperm to displace the sperm from the first male, i.e. the reference male, is evaluated. Conversely, in the defense assay, the ability of the experimental male sperm to resist displacement or to reduce the fertilization success of the reference male sperm is evaluated. Depending on the type of assay, defense or offense, sperm competitive ability is estimated through the scores P1 or P2, respectively. P1 and P2 can only take values between 0 and 1. Intermediate values are usually interpreted as indirect evidence of sperm mixing, which suggests a physiological scenario involving direct sperm competition. Following the same rationale, extreme values can be interpreted as evidence for strong differential sperm competitive ability. Early studies showed that P2 in D. melanogaster is over 0.8 increasing as the time elapsed between the two matings lengthens 7. This same experimental design has been used in other Drosophila species, P2 being the commonly used statistic in studies to evaluate sperm competitive ability 20. For most species, the P2 values of the strains tested is higher than 0.6 21. Nevertheless, several other mechanisms unrelated to the direct competition between sperm of different males can yield identical scores (see Discussion).
Distinguishing progeny sired by the first or second males is possible through the use of easily identifiable markers. In early studies, one of the males was irradiated at sublethal doses of, for example, X-rays such that virtually all ova fertilized by irradiated sperm failed to hatch 7. Subsequently, mutations altering eye pigmentation or wing shape have been the most commonly used markers. Examples of the former are the mutations bw (brown) 9, cn (cinnabar)22 and w (white) 23, while the mutation Cy (Curly) 24 corresponds to the second type of phenotypes; some of these mutations have been combined in the same individual, e.g. cn bw. To a lesser extent, allozymes 25 and microsatellites 26,27 with known inheritance patterns have also been used.
The experimental design to test for differences in sperm competitive ability described here follows essentially that of Clark et al. 9. Results derived from these experiments give information solely about the differential paternity of the experimental male types under scrutiny. Assays that also make allowance for post-fertilization differences in fitness 14,28 and sperm visualization techniques 24 enable differences in P1 (or P2) scores to be interpreted as differences in sperm competence.
Figure 1 outlines the rationale of both the offense and the defense assays. To illustrate the logistics of the process, an offense experiment carried out in D. melanogaster 14 will be explained in detail. This particular offense assay was used to test for a measurable effect of the multigene family Sperm-specific dynein intermediate chain (Sdic) on sperm competitive ability. All the members of this multigene family reside in tandem on the X chromosome. Knockout males were generated by deleting the Sdic cluster. Because the deleted segment also included the essential gene short wing (sw) and the purpose of the study was to evaluate the relevance of Sdic, males carrying the Sdic-sw deletion were rescued by a transgenic copy of sw (symbolized as P{sw}, which also carried a mini-white reporter gene) on chromosome 2. Eye color was used as a visible marker for paternity identification. All the flies were in a white mutant background with the exception of those from the strain Oregon-R, which were used as reference males.
Protocol
Small-scale experiments should be performed to become familiar with the whole procedure.
1. Collecting Virgin Females and Naïve Males
The simplest version of the outlined experiment consists of four types of initial crosses, which involve the following combination of adults: a) w1118 individuals in order to collect virgin females; b) Oregon-R individuals in order to collect naïve reference males; c) P{sw} homozygous males and virgin females from a control line that carries the wild-type organization of Sdic in order to collect naïve experimental males (Type I); and d) P{sw} homozygous males and Sdic-sw-deletion-carrying virgin females in order to collect naïve experimental males which carry the Sdic-sw deficiency (Type II).
Set up multiple vials containing 8-10 females and 5 males each. Allow the females to lay eggs and transfer the adults to new vials every 3-5 days. Use bottles rather than vials if necessary and always use fresh food. The number of vials required will depend on the number of experimental male types under study and the number of individuals estimated to be necessary to detect differences (Table 1). More than 10 females per vial might result in over-crowding during larval growth, which may cause variation in the fertility of the progeny. Store vials at 25 °C in a temperature-controlled chamber.
Start collecting virgin females and naïve males on days 11th and 12th after setting up the initial crosses. The waiting period varies according to the temperature; lower temperatures result in longer developmental time delaying the collection of individuals. Another factor affecting the timing to eclosion is the type of medium. Nutritious food, such as cornmeal-yeast medium 29, assures proper development of the adult reproductive system, which facilitates mating. Collect unmated flies every 4-6 hr. When collecting, anesthetize flies by introducing CO2 into the vial, tap the flies down, and sex them under stereomicroscope (Figure 2). Place the desired flies into different vials by sex and phenotype and label the vials appropriately.
Note 1. Collection routine starts in the morning by discarding the adults that emerged during the previous night. Collect virgin females and naïve males once or twice during the day. Typically, D. melanogaster males become sexually mature 8 hr after eclosion at 25 °C 30. If flies are maintained under 12:12 hr light/dark cycles, two peaks of eclosion are expected: during the first 1-2 hr after the light is turned on, and during the 2 hr before the light is turned off. Eclosion occurs within 24 hr after the pupa darkens.
Note 2. No more than 10 females should be put into the same vial to prevent over-crowding. This also limits the loss of females in the case they have to be discarded because at least one of them is not virgin.
Note 3. In order to collect the appropriate number of virgin females and naïve males in a short time period, the following measures can be adopted. Set up 15-20 vials of w1118 for experiments involving two types of experimental males (Table 1). Lightly sprinkle the surface of the media and add a few dried active yeast pellets to facilitate oviposition. Transfer parents at least 4 times on consecutive days. To increase the available surface for pupation in each vial, insert multi-folded paper (7 x 5 cm) during the 4th or 5th day after the parents are transferred into another vial (Figure 3). If the number of emerged adults from the initial crosses is not enough, wait for a few more days and collect individuals from vials set up at different dates. Plan to carry out the experiments over several consecutive days to even out the workload.
2. Double-mating Experiments
Figure 4 shows the main steps involved in double-mating experiments performed in 14.
- On the morning of Day 1, set up the first mating. The Oregon-R males are the first to mate in the offense assay.
- Using the aspirator, set up vials containing 10 4-5-day-old white-eyed w1118 virgin females and 10 red-eyed Oregon-R naïve males each. Two to three vials are set up daily for 5 consecutive days. The number of vials to be set up might vary depending on the number of available individuals. Allow the flies to mate for 2 hr.
- Discard Oregon-R males and place each female into a new vial using an aspirator (Figure 5). Sex identification can be achieved by visual inspection of a few morphological differences (Figure 2). Label each vial appropriately and leave the female in the vial for 2 days (hereafter "v1").
- On Day 3, set up the second mating. Selection of males and cross set up should be performed at random in order to minimize any potential bias towards any of the experimental male types.
- Two hours before the light is turned off, transfer again the female into a new vial with an aspirator. Label the new vial appropriately (hereafter "v2").
- Introduce three 5-6-day-old experimental males of the same genotype into v2.
- Repeat 2.2.1 and 2.2.2 for each female.
- On Day 4, two hours right before the light is turned on (i.e. no more than 12 hr after 2.2.1), discard males by using the aspirator.
On Day 6, transfer each female again into another new vial. Label the new vials appropriately (hereafter "v3").
On Day 10, discard the w1118 females.
- Examine the progenies in v2 and v3. The first inspection is performed after 13-15 days after the female is introduced into v2 (or v3), e.g. on Day 17 after the female first started ovipositing in v2. The second inspection is performed exactly after 17 days. This time frame poses a safe upper temporal boundary that guarantees no second generation in the same vial. Two inspections prevent over-crowding while facilitating progeny counting.
- On Day 17, inspect v1 and ensure that there are red-eyed progeny present; if not mark the vial accordingly. Presence of white-eyed progeny indicates that the female was not virgin at the time of initial mating while no progeny indicates that mating with the reference or the experimental males did not occur.
- On Day 17, perform the first inspection of v2. Anesthetize emerged progeny in v2 with CO2 and sort them by eye color and sex. Record progeny numbers fathered by the first and second males. Figure 6 shows the expected phenotypes in the progeny of each mating scheme. In this particular experiment 14, only female progeny can be unambiguously assigned as sired by the reference or the experimental males and therefore only the information from daughters can be used to calculate P2. If with other markers the paternity of male offspring can be unambiguously assigned, progeny counts of both sexes can be used in downstream calculations. Discard the progeny but keep v2 for second inspection.
- On Day 20, proceed as in 2.5.2 with the newly emerged progeny in v2 and then discard the vial.
- On Day 20, inspect the emerged progeny in v3 for the first time and follow step 2.5.2.
- On Day 23, proceed as in 2.5.3 with the newly emerged progeny in v3.
Note 1: Due to the potential inflating effect on P scores that multiple mating might have (2.2.2 and 2.2.3), mating can be limited to a particular time frame. The time frame will depend on the remating frequency associated with the genotype of the female and males used. The probability of multiple mating is specially reduced during night time 11.
Note 2: Do not add live yeast pellets because this might cause problems due to potential overgrowth of the yeast when the number of adult flies is low.
3. Data Analysis
Recorded progeny counts should be organized appropriately for easy visual inspection and efficient analysis with a suitable statistical package (e.g. JMP from SAS Institute) or free web-based tools (e.g. http://vassarstats.net/).
Sperm competitive ability. Add counts from v2 and v3. Female flies that have given rise to no or very limited progeny (e.g. <10), died during the procedure, or were only successfully inseminated by one of the two males are considered as non-informative and are excluded from any downstream statistical analysis (2.5.1 and Table 2). Calculate the P2 score for each informative female.
Test whether there are statistically significant differences in P2 values among the experimental males compared. This can be done using parametric (e.g. Tukey's HSD) or non-parametric (e.g. Steel-Dwass) tests depending on several factors including the skewness of the distribution of P2 values and the dependency between the variance and the mean. The angular transformation is commonly applied to proportions such as P2 prior to the use of parametric tests 31.
Mating rate differences (optional). For each experimental male type, calculate the number of doubly mated females and those that only mated with the first male (the reference male in the offense assay and the experimental male in the defense assay). Using a two-tailed Fisher's exact test (available at http://www.langsrud.com/fisher.htm), determine whether there are statistically significant differences between the two types of experimental males.
Sex ratio (optional). For each experimental male, use the chi-square test to determine whether the sex ratio in the progeny from each female deviates from the expected 1:1 ratio.
Representative Results
Table 2 summarizes some salient features of two offense experiments (Assays 1 and 2) in which D. melanogaster experimental males with and without (Type I and II, respectively) a functional Sdic cluster are compared 14. After taking into account different incidences encountered with some replicates, 58-83% of the females were found to be informative and therefore the paternity counts of their progeny could be used for calculating P2. Results of non-parametric tests were consistent with a lower sperm competitive ability in males without the Sdic cluster (Type II) as compared to males with the intact cluster (Type I) 14. This pattern was reproducible across the offense assays performed. A similar but not statistically significant trend was found in the defense assays performed in parallel (not shown). Importantly, absence of mating rate differences (step 3.4) ruled out the possibility that the male genotype could affect female remating behavior 14.
| Offense Assay | Defense Assay | |||||
| Experimental ♂ Type | w1118 ♀ | OR-R ♂ | Experimental ♂ | w1118 ♀ | Experimental ♂ | OR-R ♂ |
| I | 60-70 | 60-70 | 180-210 | 60-70 | 60-70 | 180-210 |
| II | 60-70 | 60-70 | 180-210 | 60-70 | 60-70 | 180-210 |
Table 1. Number of required individuals in double-mating experiments for comparing two experimental male types. OR-R, Oregon-R.
| ♀ | ||||||
| Experimental ♂ Type | Initial Count | No successful fertilization with reference ♂ | No successful fertilization with experimental ♂ | Problematic 1 | Informative (%) | P2 2 |
| Assay 1 | ||||||
| B+ (Type I) | 60 | 5 | 6 | 2 | 47 (78%) | 1.000 (1.000-0.987) |
| A- (Type II) | 55 | 1 | 7 | 5 | 42 (76%) | 0.986 (1.000-0.936) |
| Assay 2 | ||||||
| I+ (Type I) | 74 | 9 | 7 | 15 | 43 (58%) | 1.000 (1.000-0.979) |
| E- (Type II) | 75 | 3 | 8 | 2 | 62 (83%) | 0.983 (1.000-0.927) |
Table 2. Summary of the impact of different events on the number of females used in offense assays performed to test differences among experimental male types. 1 Died, escaped during the assay, or giving rise to a number of offspring below a threshold value. 2 Median (interquartile range).
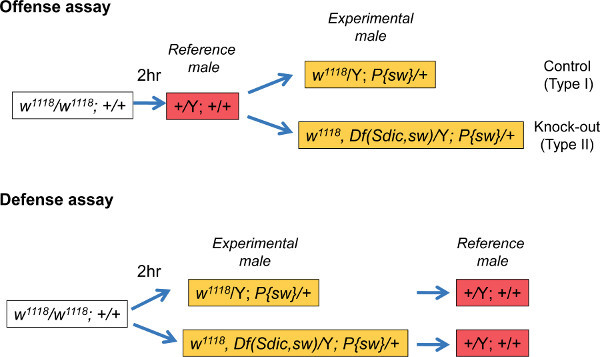 Figure 1. Experimental design for the offense and defense assays. The color code used for each genotype denotes the eye color of the adult fly. Oregon-R males are used as a reference for comparing indirectly the sperm competitive ability of two experimental males: one carrying the wild-type form of the genetic factor under study (Type I) and one in which the functionality of the genetic factor has been perturbed (Type II). The Sdic cluster, the genetic factor in this case, is located on the X chromosome while the sw transgene is located on chromosome 2. The experiments depicted are part of those performed in 14. Df(Sdic, sw), deficiency including the Sdic multigene family and the adjacent gene sw. Y, Y chromosome; P{sw}, transgene; w1118, a mutant allele of the white gene.
Figure 1. Experimental design for the offense and defense assays. The color code used for each genotype denotes the eye color of the adult fly. Oregon-R males are used as a reference for comparing indirectly the sperm competitive ability of two experimental males: one carrying the wild-type form of the genetic factor under study (Type I) and one in which the functionality of the genetic factor has been perturbed (Type II). The Sdic cluster, the genetic factor in this case, is located on the X chromosome while the sw transgene is located on chromosome 2. The experiments depicted are part of those performed in 14. Df(Sdic, sw), deficiency including the Sdic multigene family and the adjacent gene sw. Y, Y chromosome; P{sw}, transgene; w1118, a mutant allele of the white gene.
 Figure 2. Sex identification in D. melanogaster.(A) Dorsal, (B) lateral, and (C) ventral view of 1 hr old females (left) and males (right) anesthetized with CO2 under the stereomicroscope. The male genitalia is substantially more pigmented than the vaginal plate giving rise to an apparent dark spot; this is the most reliable character to use for sexing. Also, the tarsus of the male forelegs possess a fringe of dark bristles (sex comb) absent in the female. (D) Reliable and rapid gender identification of older flies by naked eye is highly recommended when transferring individuals into vials using aspirators. In older adults, male abdomen is dorsally much darker than that of the female. Females are usually larger and have a paler abdomen than males. The ovipositor makes the female abdomen pointed.
Figure 2. Sex identification in D. melanogaster.(A) Dorsal, (B) lateral, and (C) ventral view of 1 hr old females (left) and males (right) anesthetized with CO2 under the stereomicroscope. The male genitalia is substantially more pigmented than the vaginal plate giving rise to an apparent dark spot; this is the most reliable character to use for sexing. Also, the tarsus of the male forelegs possess a fringe of dark bristles (sex comb) absent in the female. (D) Reliable and rapid gender identification of older flies by naked eye is highly recommended when transferring individuals into vials using aspirators. In older adults, male abdomen is dorsally much darker than that of the female. Females are usually larger and have a paler abdomen than males. The ovipositor makes the female abdomen pointed.
 Figure 3. Multi-folded paper used to increase the available surface for wandering larvae.
Figure 3. Multi-folded paper used to increase the available surface for wandering larvae.
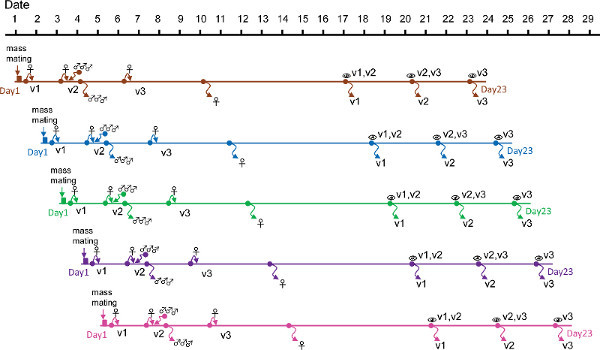 Figure 4. Outline of the experiment to assay differences in sperm competitive ability in D. melanogaster. Mass mating for 2 hr using virgin w1118 females and Oregon-R males are initiated every day for 5 days. In each mass mating, once terminated, 12-14 females must be aspirated separately into individual vials (v1). Two days later, each female is transferred into a new vial (v2) together with three experimental males of the same genotype. These individuals are allowed to mate overnight. Males are discarded after ~12 hr while females are allowed to oviposit for 2 days. Subsequently, females are transferred into new vials (v3), allowed to oviposit again, and finally discarded after 3 days. Thirteen to fifteen and 17 days after the females started to oviposit in both v2 and v3, progeny fathered by the experimental and reference males are identified using appropriate markers and recorded. After the progenies in v2 and v3 are inspected twice, the vials are discarded. Downward arrow, discarded individuals; eye symbol, vial inspection. Adapted from 9. Click here to view larger figure.
Figure 4. Outline of the experiment to assay differences in sperm competitive ability in D. melanogaster. Mass mating for 2 hr using virgin w1118 females and Oregon-R males are initiated every day for 5 days. In each mass mating, once terminated, 12-14 females must be aspirated separately into individual vials (v1). Two days later, each female is transferred into a new vial (v2) together with three experimental males of the same genotype. These individuals are allowed to mate overnight. Males are discarded after ~12 hr while females are allowed to oviposit for 2 days. Subsequently, females are transferred into new vials (v3), allowed to oviposit again, and finally discarded after 3 days. Thirteen to fifteen and 17 days after the females started to oviposit in both v2 and v3, progeny fathered by the experimental and reference males are identified using appropriate markers and recorded. After the progenies in v2 and v3 are inspected twice, the vials are discarded. Downward arrow, discarded individuals; eye symbol, vial inspection. Adapted from 9. Click here to view larger figure.
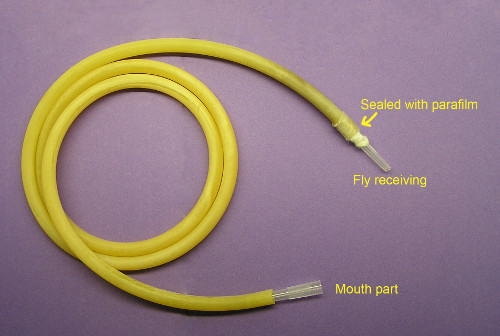 Figure 5. Flexible aspirator. Assembled from amber latex tubing, one 1 ml graduated tip for receiving flies and another functioning as disposable mouthpart. To prevent flies being sucked into the tube, fine mesh fabric is used to shield the fly-receiving tip at the junction to the tube.
Figure 5. Flexible aspirator. Assembled from amber latex tubing, one 1 ml graduated tip for receiving flies and another functioning as disposable mouthpart. To prevent flies being sucked into the tube, fine mesh fabric is used to shield the fly-receiving tip at the junction to the tube.
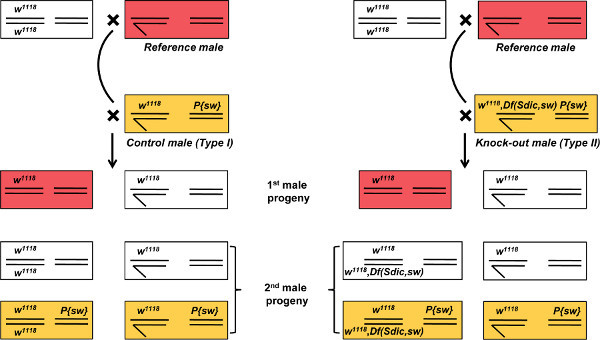 Figure 6. Expected phenotypes in the progeny of the offense assay. Left and right, crosses to evaluate the sperm competitive ability of control and knock-out males respectively. Genotype and eye color for parentals and progeny of both fathers are shown. Because of the particular genetic markers used in this assay, only the female progeny can be used for calculating P1 (or P2) scores (see text for details); part of the male progeny sired by the experimental male is white-eyed and therefore phenotypically indistinguishable from the male progeny of the reference male. Df, deficiency; P{sw}, transgene; w1118 mutant allele of the white gene. Click here to view larger figure.
Figure 6. Expected phenotypes in the progeny of the offense assay. Left and right, crosses to evaluate the sperm competitive ability of control and knock-out males respectively. Genotype and eye color for parentals and progeny of both fathers are shown. Because of the particular genetic markers used in this assay, only the female progeny can be used for calculating P1 (or P2) scores (see text for details); part of the male progeny sired by the experimental male is white-eyed and therefore phenotypically indistinguishable from the male progeny of the reference male. Df, deficiency; P{sw}, transgene; w1118 mutant allele of the white gene. Click here to view larger figure.
Discussion
We have described the experimental design to assess differences in the relative contribution of genetically distinct D. melanogaster males to the progeny in controlled double-mating experiments 7,8. This has been done in the context of a genetic factor hypothesized to influence sperm competitive ability and it has been illustrated within the offense assay although a similar procedure applies to the defense assay (Figure 1). This experimental design can be modified to test other aspects of paternity success such as the influence of the female genotype 32. An example of the modifications that can be incorporated is the direct monitoring of copulation between the female and the second male. Further, it is also important to note that the relative performance of the experimental male types is dependent on the genotype of the tester male and female used and therefore cannot be extrapolated to other scenarios involving different tester males and females 10,32,33.
Since the collection of the required individuals (virgin females and naïve males) and scoring of the progeny are labor intensive, the number of distinct experimental male types to be evaluated should be adjusted according to the number of well-trained personnel available. The experiments outlined here can be easily handled by one person over six weeks. In fact, up to five experimental male types could be evaluated in parallel. The only additional measure to be adopted is to prolong the number of days setting up initial mass matings and collecting the required individuals for them.
In our experience, the starting number of females used (60-70) is enough to accommodate reduction in sample size without compromising our statistical power to detect differences among the experimental male types (Table 2). The decrease in number of informative females can result from different factors, for example lack of evidence of double mating or premature death. Nevertheless, the number of females needed for each experimental male type in order to achieve adequate statistical power will vary depending on the magnitude of the effect of the genetic factor under study. A pilot experiment is highly advisable in order to estimate the suitable level of replication.
Statistical differences in P1 (or P2) scores indicate variation in male fertilization efficiency although they do not inform about the underlying mechanisms contributing to such differences. This is due to multiple variables that could impact sperm competitive ability (extensively reviewed in 20 and 34). Sperm attributes like size, number and motility can impact on the competition of the sperm directly 35. In addition, other mechanisms not related to direct sperm competition can also contribute to differences in P1 or P2 values. For example, first male sperm can be preferentially removed, repositioned, or flushed out before or during the mating with the second male 19,20. This can occur by mechanical stimulation, for example, during copulation and via molecules present in the seminal fluid of the second male, which will affect first male sperm negatively 19,36. Therefore, complementary assays that evaluate the impact of those additional indirect mechanisms must be performed, ideally prior to the double-mating experiment described here. Example of these assays are those testing for differences in zygote viability (e.g. larval survival) and egg hatchability (a proxy for successful egg fertilization) 28,37, which ultimately result in differences in progeny number.
Sperm imaging techniques can also be highly informative 19,24,28. Essentially, one of the males is modified genetically such that the sperm is labeled with a fluorescent fusion protein (e.g. that of the reference male with a green fluorescent protein) helping monitoring sperm dynamics in the female reproductive tract. This approach combined with further staining using 4',6-diamidino-2-phenyllindole (DAPI) was used to directly identify sperm counts from the first and second males in dissected seminal receptacles of D. melanogaster females 24,28. In one study 24, differences in sperm counts were in good agreement with differences in P2 values supporting the notion that those differences in male paternity were reflective of true differences in sperm competitive ability and not of indirect mechanisms.
Subsequent refinements have been achieved by generating transgenic strains that expressed green and red fluorescent tags fused to sperm-specific proteins 19. These improvements have allowed researchers to monitor the sperm of the two directly competing male sperm with an unprecedented degree of resolution. In conclusion, the execution of double-mating experiments and additional assays like the ones suggested help to narrow down the nature of any differences in P1 or P2 scores found among experimental male types, which opens the possibility to dissect the genetics basis of the naturally occurring variation in sperm competitive ability.
Disclosures
No conflicts of interest declared.
Acknowledgments
The authors thank NSF (MCB-1157876) for funding. We also thank Alberto Civetta, John Roote, and two anonymous referees for their comments.
References
- Darwin C. The descent of man and selection in relation to sex. John Murray; 1871. [Google Scholar]
- Parker GA. Sperm competition and its evolutionary consequences in the insects. Biol. Rev. 1970;45:525–567. [Google Scholar]
- Birkhead TR, Møller AP. Sperm competition and sexual selection. Academic Press; 1998. [Google Scholar]
- Jones B, Clark AG. Bayesian sperm competition estimates. Genetics. 2003;163:1193–1199. doi: 10.1093/genetics/163.3.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RC, McKechnie SW, McKenzie JA. Multiple mating and sperm displacement in natural populations of Drosophila melanogaster. Theor. Appl. Genet. 1982;62:89–96. doi: 10.1007/BF00276292. [DOI] [PubMed] [Google Scholar]
- Jonsson UB. Sperm transfer, storage, displacement, and utilization in Drosophila melanogaster. Genetics. 1962;47:1719–1736. doi: 10.1093/genetics/47.12.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman E, Parker GA. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1976;1:145–155. [Google Scholar]
- Gromko MH, Gilbert DG, Richmond RC. In: Sperm Competition and the Evolution of Animal Mating Systems. Smith RL, editor. Academic Press; 1984. pp. 372–427. [Google Scholar]
- Clark AG, Aguade M, Prout T, Harshman LG, Langley CH. Variation in sperm displacement and its association with accessory gland protein loci in Drosophila melanogaster. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Begun DJ, Prout T. Female x male interactions in Drosophila sperm competition. Science. 1999;283:217–220. doi: 10.1126/science.283.5399.217. [DOI] [PubMed] [Google Scholar]
- Civetta A, Clark AG. Chromosomal effects on male and female components of sperm precedence in Drosophila. Genet. Res. 2000;75:143–151. doi: 10.1017/s0016672399004292. [DOI] [PubMed] [Google Scholar]
- Greenspan L, Clark AG. Associations between variation in X chromosome male reproductive genes and sperm competitive ability in Drosophila melanogaster. Int. J. Evol. Biol. 2011;2011:214280. doi: 10.4061/2011/214280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T, Neubaum DM, Wolfner MF, Partridge L. The role of male accessory gland protein Acp36DE in sperm competition in Drosophila melanogaster. Proc. Biol. Sci. 2000;267:1097–1105. doi: 10.1098/rspb.2000.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh SD, et al. Functional evidence that a recently evolved Drosophila sperm-specific gene boosts sperm competition. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2043–2048. doi: 10.1073/pnas.1121327109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitnick S, Markow TA, Spicer GS. Evolution of multiple kinds of female sperm-storage organs in Drosophila. Evolution. 1999;53:1804–1822. doi: 10.1111/j.1558-5646.1999.tb04564.x. [DOI] [PubMed] [Google Scholar]
- Pitnick S, Miller GT, Schneider K, Markow TA. Ejaculate-female coevolution in Drosophila mojavensis. Proc. Biol. Sci. 2003;270:1507–1512. doi: 10.1098/rspb.2003.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonidez JF. The internal phenomenon of reproduction in Drosophila. Biol. Bull. 1920;39:207–230. [Google Scholar]
- Miller GT, Pitnick S. Sperm-female coevolution in Drosophila. Science. 2002;298:1230–1233. doi: 10.1126/science.1076968. [DOI] [PubMed] [Google Scholar]
- Manier MK, et al. Resolving mechanisms of competitive fertilization success in Drosophila melanogaster. Science. 2010;328:354–357. doi: 10.1126/science.1187096. [DOI] [PubMed] [Google Scholar]
- Simmons L, Siva-Jothy M. In: Sperm competition and sexual selection. Birkhead TR, Møller AP, editors. Academic Press; 1998. p. 826. [Google Scholar]
- Singh SR, Singh BN, Hoenigsberg HF. Female remating, sperm competition and sexual selection in Drosophila. Genetics and Molecular Research. 2002;1:178–215. [PubMed] [Google Scholar]
- Markow TA. A comparative investigation of the mating system of Drosophila hydei. Animal Behaviour. 1985;33:775–781. [Google Scholar]
- Barbadilla A, Quezada-Díaz JE, Ruiz A, Santos M, Fontdevila A. The evolutionary history of Drosophila buzzatii. XVII. Double mating and sperm predominance. Genet. Sel. Evol. 1991;23:133–140. [Google Scholar]
- Civetta A. Direct visualization of sperm competition and sperm storage in Drosophila. Curr. Biol. 1999;9:841–844. doi: 10.1016/s0960-9822(99)80370-4. [DOI] [PubMed] [Google Scholar]
- Turner ME, Anderson WW. Sperm predominance among Drosophila pseudoobscura karyotypes. Evolution. 1984;38:983–995. doi: 10.1111/j.1558-5646.1984.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Harshman LG, Clark AG. Inference of sperm competition from broods of field-caught Drosophila. Evolution. 1998. pp. 1334–1341. [DOI] [PubMed]
- Imhof M, Harr B, Brem G, Schlotterer C. Multiple mating in wild Drosophila melanogaster revisited by microsatellite analysis. Mol. Ecol. 1998;7:915–917. doi: 10.1046/j.1365-294x.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- Price CS, Dyer KA, Coyne JA. Sperm competition between Drosophila males involves both displacement and incapacitation. Nature. 1999;400:449–452. doi: 10.1038/22755. [DOI] [PubMed] [Google Scholar]
- Ashburner M. Drosophila: A Laboratory Manual. Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Greenspan RJ. CSHL; 1997. Fly Pushing: The Theory and Practice of Drosophila Genetics. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry : the principles and practice of statistics in biological research. 3d. Freeman, W.H; 1994. [Google Scholar]
- Clark AG, Begun DJ. Female genotypes affect sperm displacement in Drosophila. Genetics. 1998;149:1487–1493. doi: 10.1093/genetics/149.3.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AG, Dermitzakis ET, Civetta A. Nontransitivity of sperm precedence in Drosophila. Evolution. 2000;54:1030–1035. doi: 10.1111/j.0014-3820.2000.tb00102.x. [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sperm competition. Curr. Biol. 2004;14:100–102. [PubMed] [Google Scholar]
- Pizzari T, Parker GA. In: Sperm biology: an evolutionary perspective. Birkhead TR, Hosken DJ, Pitnick S, editors. Academic Press; 2008. p. 674. [Google Scholar]
- Ram KR, Wolfner MF. Seminar influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integr. Comp. Biol. 2007;47:427–445. doi: 10.1093/icb/icm046. [DOI] [PubMed] [Google Scholar]
- Civetta A, Rosing KR, Fisher JH. Differences in sperm competition and sperm competition avoidance in Drosophila melanogaster. Animal Behaviour. 2008;75:1739–1746. [Google Scholar]


