Abstract
AIM: To describe the way stations of high-density lipoprotein (HDL) uptake and its lipid exchange in endothelial cells in vitro and in vivo.
METHODS: A combination of fluorescence microscopy using novel fluorescent cholesterol surrogates and electron microscopy was used to analyze HDL endocytosis in great detail in primary human endothelial cells. Further, HDL uptake was quantified using radio-labeled HDL particles. To validate the in vitro findings mice were injected with fluorescently labeled HDL and particle uptake in the liver was analyzed using fluorescence microscopy.
RESULTS: HDL uptake occurred via clathrin-coated pits, tubular endosomes and multivesicular bodies in human umbilical vein endothelial cells. During uptake and resecretion, HDL-derived cholesterol was exchanged at a faster rate than cholesteryl oleate, resembling the HDL particle pathway seen in hepatic cells. In addition, lysosomes were not involved in this process and thus HDL degradation was not detectable. In vivo, we found HDL mainly localized in mouse hepatic endothelial cells. HDL was not detected in parenchymal liver cells, indicating that lipid transfer from HDL to hepatocytes occurs primarily via scavenger receptor, class B, type I mediated selective uptake without concomitant HDL endocytosis.
CONCLUSION: HDL endocytosis occurs via clathrin-coated pits, tubular endosomes and multivesicular bodies in human endothelial cells. Mouse endothelial cells showed a similar HDL uptake pattern in vivo indicating that the endothelium is one major site of HDL endocytosis and transcytosis.
Keywords: High-density lipoprotein, Endocytosis, Endothelium, Human umbilical vein endothelial cells, Human coronary artery endothelial cells, Cholesterol
Core tip: The cardio-protective effect of high-density lipoprotein (HDL) is related to its ability to transfer lipids from the periphery, such as atherosclerotic plaques, back to the liver for excretion. Therefore, HDL has to cross the endothelial barrier. In the present work we analyzed the steps and way stations of HDL uptake and resecretion using novel fluorescent cholesterol surrogates in human endothelial cells as a model for the endothelial barrier. HDL uptake occurred via clathrin-coated pits, tubular endosomes and multivesicular bodies in human umbilical vein endothelial cells. Finally we compared key findings to the in vivo situation.
INTRODUCTION
Plasma concentrations of high-density lipoprotein (HDL) cholesterol exhibit an inverse association with the incidence of cardiovascular diseases. The cardio-protective effect of HDL is related to its ability to transfer lipids from the periphery back to the liver for excretion into the bile. This cholesterol clearance is called reverse cholesterol transport[1]. To achieve the removal of excess cholesterol deposited in the arterial intima, HDL must first cross the endothelial barrier to get into close proximity to macrophage foam cells found in atherosclerotic plaques. The mechanisms and way stations in this uptake and resecretion process of HDL seem to be redundant as several receptors mediate HDL uptake. Thus its details and the interplay of these receptors in the transport of HDL and its derived lipids across cells are not fully understood (for review see[2]).
Endocytosis and resecretion of HDL was first described by Bierman et al[3] and Stein et al[4] in rat aortic smooth muscle cells (for review see[5]). Bierman et al[3] suggested regurgitation of non-catabolized apolipoproteins by reverse endocytosis of HDL. Schmitz et al[6] described the interaction of HDL with cholesteryl ester-laden macrophages; subsequent to receptor-mediated binding, HDL internalization and transport into endosomes were demonstrated. These macrophages did not degrade HDL but rather resecreted internalized HDL particles on a path similar to the transferrin receptor[6]. Retroendocytosis of HDL particles was also demonstrated in a rat liver cell line[7]. During internalization, HDL is remodeled to larger apoE-containing HDL2-like particles[8]. Endocytosis and resecretion is not limited to HDL as it occurs for almost all lipoprotein classes: uptake and resecretion was described also for low density lipoprotein (LDL) or very LDL (VLDL)[3,9-13]. Additionally, apolipoprotein E (apoE) recycling has been reported to occur in hepatocytes and macrophages, where a part of the apoE associated with HDL escapes degradation[14-16] (for review see[17]).
In general, transport of molecules across barriers is determined by the water solubility, the size and charge of the corresponding molecule. Lipoproteins as well as apolipoprotein A-I(apoA-I) have been shown to be endocytosed/transcytosed in polarized hepatocytes and epithelial cells including endothelial cells[2,7,18-30]. Besides transendothelial transport, proteins can overcome the endothelial barrier by paracellular transport. The latter involves the modulation of interendothelial junctions in order to transport molecules larger than 6 nm (for review see[2]).
Scavenger receptor, class B, type I (SR-BI) has been shown to be involved in HDL particle uptake in polarized hepatocytes[22]. Ablation of SR-BI is associated with deregulation of cholesterol homeostasis in the arterial wall, thereby increasing the susceptibility to atherosclerosis[31]. Besides SR-BI, ATP binding cassette transporter A1 (ABCA1), ATP binding cassette transporter G1 (ABCG1), caveolin-1 and ecto-F1-ATPase are considered to be involved in HDL/apoA-I uptake or transcytosis[2,23,24,32-37]. Recently, transport of HDL back to the liver was demonstrated to occur via lymphatic vessels, with SR-BI being the main receptor mediating transcytosis of HDL across the lymphatic endothelium[38,39].
In this project we analyzed HDL uptake in endothelial cells. Therefore, we used light and electron microscopical methods enabling the visualization of HDL particles via crosslinking and their derived lipids using novel fluorescent cholesterol surrogates. Overall the process of HDL transfer must encompass: (1) binding of HDL to the apical side of the endothelial cells to receptors/proteins and its concomitant uptake; (2) transport of HDL particles and their cholesterol/cholesteryl esters to the basolateral side of the endothelial cells; and (3) excretion of HDL at the basolateral side of the endothelial cells. We demonstrate that HDL uptake via clathrin-coated pits leads to a rapid exchange of the cholesterol backbone visualized via a novel cholesterol surrogate. Furthermore, HDL was transported to multivesicular bodies without concomitant degradation, indicative of HDL resecretion.
MATERIALS AND METHODS
Cell culture
Human umbilical vein endothelial cells (HUVECs) and human coronary artery endothelial cells (HCAECs) (PromoCell, Germany) were cultured in flasks coated with 0.5% gelatin in Endothelial Cell Growth Medium (PromoCell) containing endothelial cell growth supplement, epidermal growth factor, basic fibroblast growth factor, heparin and hydrocortisone, supplemented with 5% fetal calf serum (PromoCell). Passages from 4 to 10 were used for the experiments. Prior to experiments, the medium was changed to serum-free Endothelial Cell Growth Medium containing the endothelial cell growth supplement mix (PromoCell).
Animal treatment
Animal experiments were conducted at the Swiss Federal Institute of Technology Zürich. All protocols for animal use and experiments were reviewed and approved by the Veterinary Office of Zurich (Switzerland). Male C57BL/6 mice (8 wk; 18-20 g) were obtained from the Jackson Laboratory (Bar Harbor, United States). Animals were kept on chow under standard conditions. For experiments, 200 μg of HDL-Alexa-568 (resembling 1/10 of total murine HDL) were injected into the tail vein. Mice had ad libitum access to water and were restrained from food afterwards. Mice were anesthetized by intraperitonal injection of Ketamin (40 mg/kg BW; Vetoquinol, Ittigen, Switzerland) and Rompun (2 mg/kg BW; Bayer HealthCare, Germany) after 1 h. Transcardial perfusion was performed with a 30 g needle at a rate of 3.2 mL/min with saline (1 min) and 4% formaldehyde/0.5% glutaraldeyde in PBS (14 min). Organs were collected and postfixed in 4% formaldehyde in PBS overnight.
Lipoprotein isolation and labeling with fluorescent dyes
HDL was prepared from plasma of healthy volunteers by sequential ultracentrifugation (d = 1.21 g/mL)[40]. The apolipoprotein part of HDL was covalently labeled with an Alexa Fluor 568 dye (Molecular Probes, United States) according to the manufacturer’s instructions. Additional loading of HDL with Bodipy-cholesteryl oleate (BP-CE) or Bodipy-cholesterol (BP-C) was done as described previously[41]. HUVECs and HCAECs were incubated with 50 μg/mL labeled HDL for up to 60 min. Cells were subsequently fixed in 4% para-formaldehyde at 4 °C for 30 min, washed twice with PBS, mounted with Fluoprep (Biomerieux, France) and imaged using a confocal microscope (LSM 5 Exciter, Zeiss, Germany). Alternatively, cells were incubated with labeled HDL for 60 min and subsequently lyzed using 1% cholate. Fluorescence intensities of Alexa 568 and the Bodipy label were measured using a fluorometer (Zenyth 3100, Anthos, Germany).
[3H-CE-, 125I-]-HDL labeling and uptake experiments
HDL was labeled with sodium [125] iodine (Hartmann Analytics, Germany) alone or additionally with [3H]-cholesteryl-oleate (Perkin Elmer, United States) as described previously[42]. For uptake experiments HUVECs were seeded in 12-well plates. To calculate unspecific binding, a 40-fold excess of unlabeled HDL was added to every fourth well. [3H-CE-, 125I]-HDL or [125I]-HDL was added to each well at a final concentration of 10 μg/mL. After up to 6 h, cell supernatants were collected for degradation measurements, which were performed as previously described[43]. Cells were washed twice with cold PBS + BSA (2 mg/mL) and twice with cold PBS. Cells were then lysed with 0.1 mol/L NaOH. [125I-] radioactivity in the lysates was counted using a gamma-counter (COBRAII Auto-gamma; Perkin Elmer). [3H]-radioactivity was counted using 15 mL Ready-Safe (Beckman Coulter; United States) and a beta-counter (Tri-Carb 2800TR; Perkin Elmer). Measurements were normalized to protein content, determined by the Bradford protein assay (Biorad, Germany).
Immunofluorescence microscopy on HUVECs
After incubation with HDL-Alexa 568 for 1 h, HUVECs were fixed in 4% para-formaldehyde at 4 °C for 30 min. For immunofluorescence staining, cells were permeabilized with ice-cold methanol for 20 s and blocked with 2.5% BSA in PBS for 30 min. Afterwards, samples were incubated with the primary antibody to LIMP II (Novus Biologicals, United States) diluted 1:200 in 1% BSA in PBS (buffer A) containing 1% horse serum for 1 h. Samples were then rinsed 3 times with buffer A containing 0.1% TWEEN-20. Next, cells were incubated with the secondary Alexa 488 conjugated antibody (Molecular Probes) diluted 1:250 in buffer A containing 1% horse serum. Finally cells were rinsed 3 times with buffer A containing 0.2% TWEEN-20, once with PBS and mounted with Fluoprep (Biomerieux). Cells were imaged using a fluorescence microscope (Axiovert 135, Zeiss).
Immunofluorescence microscopy on liver sections
Immunofluorescence was performed on free-floating 50 μm vibratome sections, because paraffin embedding quenched the Alexa signal. Sections were blocked with 4% BSA in PBS + 0.05% Tween-20 for 2 h and incubated with the following primary antibodies in PBS + 1% BSA + 0.05% Tween-20 over-night: anti-CD34 (Abcam ab8536; 1/100), anti-CD14 (Sigma HPA001887; 1/200) and anti-desmin (Sigma D1033; 1/50). After 5 washing steps with PBS, sections were incubated with secondary antibodies coupled to Alexa-488 (in PBS + 1% BSA + 0.05% Tween-20) for 2 h. After another 5 washing steps with PBS, samples were counterstained with TO-PRO-3 (Invitrogen), mounted and analyzed using a LSM 510 laser scanning confocal microscope (Zeiss, Germany).
Electron microscopy
Horseradish peroxidase (HRP) labeled HDL was prepared using the peroxidase labeling kit (Roche, Switzerland) according to the manufacturer’s instructions. HUVECs were incubated with 25 μg/mL HDL-HRP for 30 min. Cells were fixed in 0.1 mol/L cacodylate buffer (pH 7.4) containing 2.5% glutaraldehyde at 4 °C for 45 min. Cells were then washed twice with cacodylate buffer and twice with 0.05 mol/L Tris-HCl buffer (pH 7.6). HDL-HRP was visualized using the classical DAB-oxidation reaction[44]. Cells were postfixed in OsO4-ferrocyanide (15 min) and in 1% OsO4-veronalacetate (2 h). Samples were further processed for electron microscopy; after dehydration in a gradient of aqueous ethanol (70% overnight at 4 °C; 80%, 96% and 100% for 10 min at room temperature each). Afterwards they were embedded in Epon (Serva, Germany), 80-100 nm sections were cut with an UltraCut-UCT ultramicrotome (Leica Inc., Austria). Samples were transferred to copper grids, post-stained with uranyl acetate and lead citrate and examined with a Zeiss EM900 transmission electron microscope equipped with a wide-angle Dual speed CCD camera (Albert Tröndle Dünzelbach, Germany).
Statistical analysis
Results were expressend as mean ± SD. Data were analyzed using a two-tailed Student’s t-test.
RESULTS
Uptake of HDL and its derived lipids in human endothelial cells
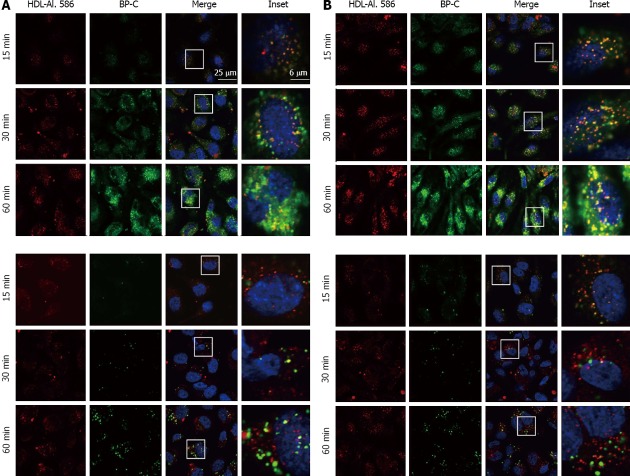
HDL uptake and transcytosis occur in endothelial cells[23] but the uptake path of HDL and the exchange behavior of its derived lipids during this process is elusive. To visualize this lipid transfer we directly labeled the apolipoprotein part of HDL covalently with Alexa 568 and the lipid part with a novel cholesterol surrogate. As HDL transports cholesterol in both its free and esterified form, we utilized Bodipy-cholesterol (BP-C) as well as Bodipy-cholesteryl oleate (BP-CE) as cholesterol surrogate markers. Size and shape of the reconstituted HDL particles containing either BP-C or BP-CE was assessed previously[41]. They were shown to be discoidal particles with a size comparable to native HDL. Both double-labeled HDL particles were applied to human umbilical vein endothelial cells (HUVECs) and human coronary artery endothelial cells (HCAECs) for the indicated time (Figure 1). HDL particle uptake occurred already after 5 min of incubation (not shown) and further proceeded and increased over a 3 h incubation period in HUVECs. HDL-Alexa 568 exhibited a vesicular transport pattern with some enrichment in the perinuclear area. Next, we determined the fate of both the apolipoprotein part and the lipid part of the HDL particle during endocytosis. Within 15 min both HDL-Alexa 568 and BP-C were clearly detected intracellularly in both HUVECs and HCAECs (Figure 1 upper panels). At this time point both labels still co-localized. With increasing time (up to 60 min) this co-localization decreased and BP-C accumulated mainly in the perinuclear area, indicating that cholesterol underwent transfer from the HDL particle to the cell. When BP-CE was employed as a marker for esterified cholesterol, the uptake of both the lipid and protein part occurred in parallel (Figure 1 lower panels), indicating that most of the esterified cholesterol during the 30 min uptake period is still contained within the HDL particle. After 60 min some of the BP-CE has been transferred to the perinuclear area. These data indicate that the transfer/exchange of free cholesterol between the HDL particle and the cells occurs faster than the transfer of esterified cholesterol.
Figure 1.
Detailed analysis of high density lipoprotein uptake and high density lipoprotein -derived lipid transfer in human umbilical vein endothelial cells and human coronary artery endothelial cells. A: HUVECs; B: HCAECs. HUVECs and HCAECs were incubated for the indicated time with HDL-Alexa 586, which was additionally labeled with either Bodipy-cholesterol (BP-C; upper panel) or Bodipy-cholesteryl oleate (BP-CE; lower panel). Samples were fixed and imaged using confocal microscopy. Note that brightness and contrast were increased at the 15 min time point of the BP-CE panel for better visibility (lower panel). HUVECs: Human umbilical vein endothelial cells; HCAECs: Human coronary artery endothelial cells; HDL: High density lipoprotein.
To analyze the way stations of HDL endocytosis we labeled the HDL particle covalently in the protein moiety with HRP, which can be visualized by transmission electron microscopy. HRP-HDL was clearly detected at the cell surface in clathrin-coated vesicles in HUVECs after 30 min of incubation. Furthermore, HDL was endocytosed and seen in tubular endosomes and multivesicular bodies (Figure 2). This HDL uptake pattern is similar to the one described earlier by our group in hepatic cells[41].
Figure 2.
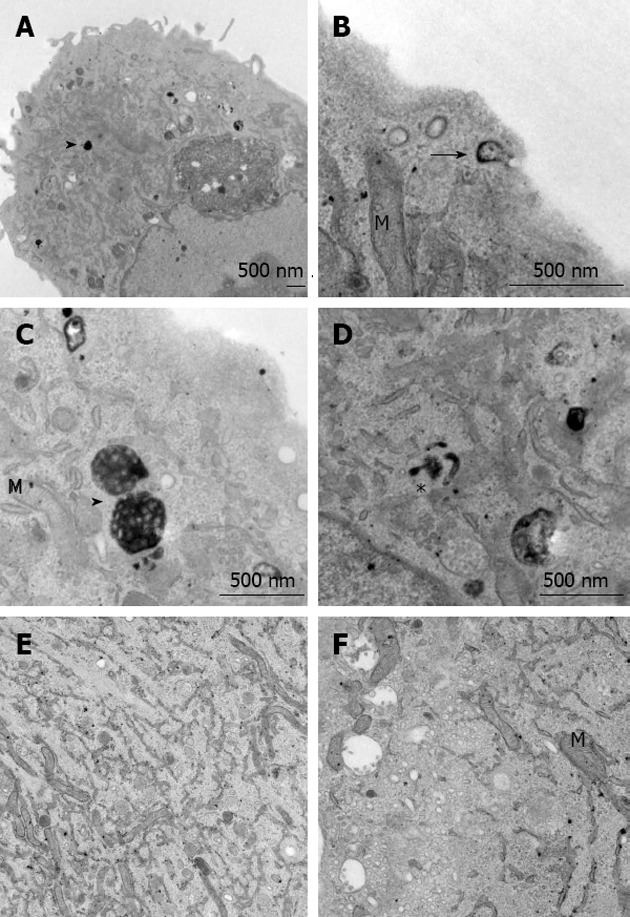
Ultrastructural analysis of high density lipoprotein uptake in human umbilical vein endothelial cells. Cells incubated with HRP-labeled HDL for 30 min (A-D) or untreated controls (E, F) were processed for electron microscopy. HRP-positive clathrin-coated vesicles were seen near the cell surface (arrow in B), in tubular endosomes (asterisk in D), and densely packed MVBs were found in the perinuclear area (arrow heads in A and C). No specific staining was seen in control samples (E, F). HRP: Horseradish peroxidase; HDL: High density lipoprotein; M: Mitochondria.
HDL particles exchange lipids during the transfer process
To quantify HDL uptake in HUVECs the protein part of the HDL particle was radioactively labeled with iodine and the lipid moiety was loaded with [3H]-cholesteryl oleate. HUVECs were incubated for 1 h with the double-labeled HDL particles and specific HDL cell association was analyzed. Unspecific [3H-CE-, 125I-]-HDL cell association was measured in the presence of a 40-fold excess of unlabeled HDL, which competed for labeled HDL cell association. Within this hour HDL cell association was 128 ± 51 ng HDL/mg cell protein (n = 2). A time course study of HDL cell association (0-6 h) showed the expected saturation curve (Figure 3A). HDL degradation was below the detection limit (not shown). Furthermore, Alexa-HDL did not co-localize with the lysosomal marker LIMPII in HUVECs (Figure 3B) indicating again that the uptake route of HDL does not involve lysosomes. Next, [3H]-cholesteryl oleate transfer was quantified. During a time period of 1 h an equivalent of 3787 ± 918 ng HDL/mg cell protein for [3H]-cholesteryl oleate, showing that lipid transfer was detected (n = 3). To follow cholesterol and cholesteryl oleate delivery to HUVECs in detail, we again used the fluorescent cholesterol surrogates. After HDL uptake for 1 h the ratio of the apolipoprotein label Alexa 568 to BP-C changed (Figure 3C); after labeling HDL particles had an Alexa 568/BP-C ratio of 2.6, and after 1 h this ratio inverted to 0.9 in the cell lysate, indicating transfer of BP-C from the HDL particle to the cells. A similar behavior was observed for BP-CE. The initial ratio of Alexa 568/BP-CE was 7.1, and this ratio was altered even to a higher extent to 1.2, again providing evidence for remodeling of the HDL particles during the endocytosis process. We found no uptake of HDL-Alexa 568 at 4 °C, indicating that holo-HDL particle uptake was inhibited. BP-C uptake from the double labeled particles was largely decreased at 4 °C and BP-CE uptake was completely blocked, indicating that lipid transfer from HDL was also temperature dependent (data not shown).
Figure 3.
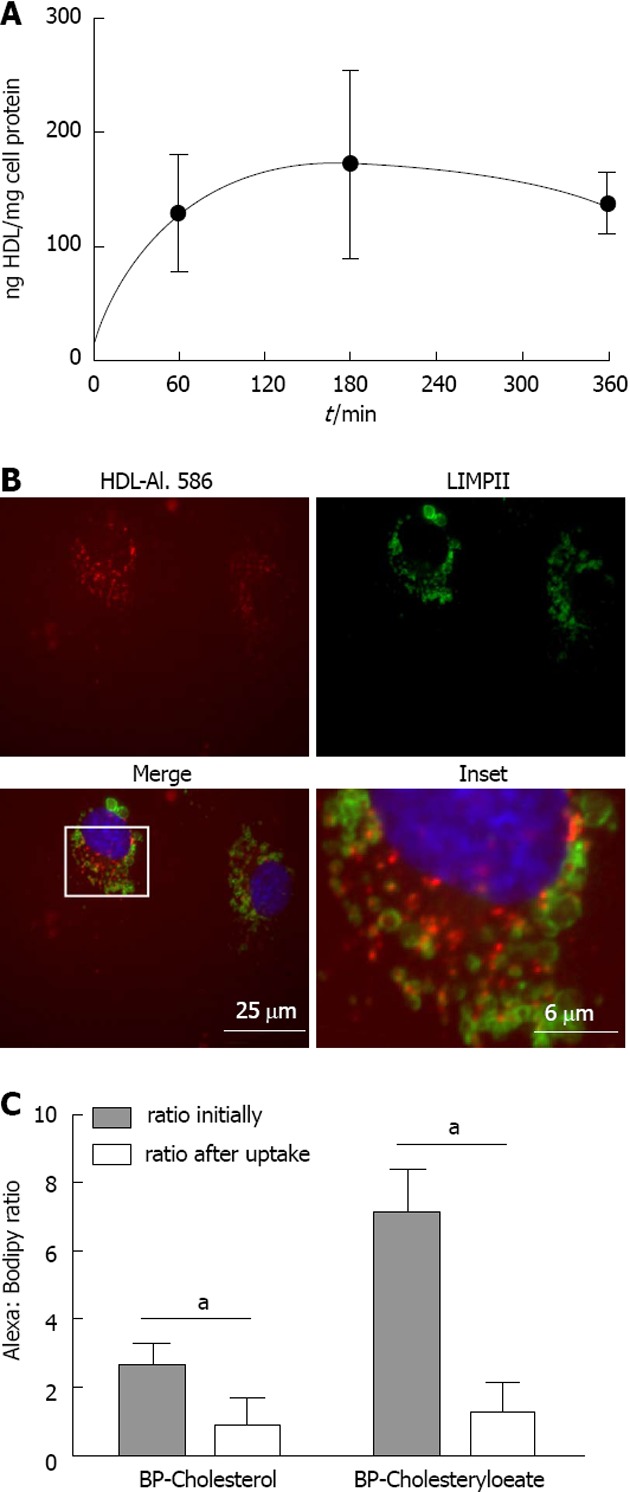
Quantification of high density lipoprotein uptake. A: Time course of HDL uptake; HUVECs were incubated with iodinated HDL for up to 6 h and specific cell association was measured using a gamma counter. Measurements were normalized to protein content, determined by the Bradford protein assay; B: HUVECs were incubated with Alexa 568 labeled HDL for 1 h. Cells were fixed and immunofluorescene was performed using an antibody against LIMPII and visualized using fluorescence microscopy. No colocalization of LIMPII, a marker for the lysosomal compartment, and HDL-Alexa 568 was seen; C: HUVECs were incubated with HDL-Alexa-BP-C or -BP-CE for 60 min. Cells were then lyzed and the fluorescence intensity for each label was measured using a fluorometer. Results are expressed as the Alexa: Bodipy ratio initially and after the 60 min incubation period (n = 3). aP < 0.05 between groups. HDL: High density lipoprotein; BP-C: Bodipy-cholesterol; BP-CE: Bodipy-cholesteryl oleate; HUVECs: Human umbilical vein endothelial cells.
HDL uptake in mouse endothelium
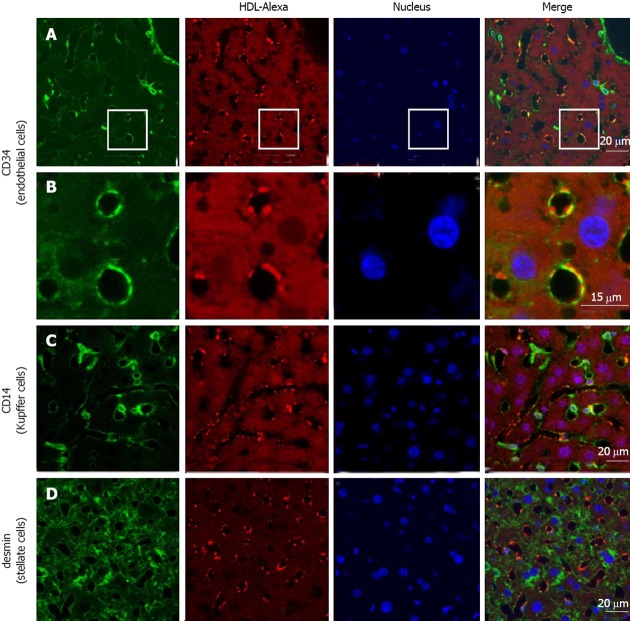
To confirm the in vitro data, we administered HDL-Alexa 568 via tail vein injection into C57BL/6 mice. Mice were sacrificed after 1 h, and liver sections were processed for immunohistochemistry. We used CD34 as marker for endothelial cells. Figure 4 shows co-localization of Alexa 568, the marker for the HDL particle, and CD34. Both CD34 and HDL-Alexa 568 showed intense staining in cells lining the sinusoid vessels. In addition, liver sections were also stained with CD14 and desmin, which are markers for Kupffer and stellate cells, respectively (Figure 4). Almost no co-localization of HDL Alexa 568 with CD14 or desmin was found, indicating that these cells did not bind and take up HDL to a significant amount within 1 h. The intensity of HDL staining did not differ between periportal and pericentral regions. HDL staining was predominantly found in sinusoidal endothelial cells and not in the endothelial cells of larger vessels (i.e., central vein and portal vein). Taken together, these data demonstrate that the Alexa-labeled HDL particles can be used to follow HDL uptake in vivo and that transfer routes and possibly lipid exchange can be monitored in the in vivo setting.
Figure 4.
High density lipoprotein uptake in non-parenchymal liver cells. Fluorescently labeled high density lipoprotein (HDL)-Alexa 568 was intravenously injected into C57BL/6 mice. After 60 min, tissues were fixed by transcardial perfusion. Liver sections were stained for cellular markers by immunofluorescence and analyzed by confocal microscopy. HDL is localized in endothelial cells [A and B (inset)] and to a limited amount in Kupffer cells (C), whereas stellate cells show no detectable HDL staining (D).
DISCUSSION
Transendothelial transport of proteins occurs via para- or trans-cellular pathways, with transport of HDL being described to proceed via transcytosis[23,37-39]. For this, HDL must be endocytosed in the first step, and then the particle is transported intracellularly from the apical to the basolateral side of the cell, and finally the particle itself is excreted. In the present work we performed a detailed morphological analysis of HDL endocytosis and the transfer of its lipids using novel cholesterol surrogates in endothelial cells. HDL uptake occurred via clathrin-coated pits, leading to a rapid exchange of cholesterol cargo (Figures 1 and 3). Intracellularly, HDL was transported via endosomes to multivesicular bodies (Figure 2) to await its resecretion. This endocytosis path was similar to earlier observations in hepatic cell lines[41]. In vivo mouse endothelial cells in the liver exhibited a comparable HDL uptake pattern.
Here we report that HDL was detected at the cell surface in clathrin-coated invaginations using DAB staining and electron microscopy (Figure 2), indicative clathrin-mediated endocytosis. Indeed, using HepG2 cells, we saw a similar binding and uptake behavior of HDL, with the involvement of endosomal vesicles and multivesicular bodies in the uptake process[41]. This uptake path is similar to that reported here for endothelial cells. In addition, in both cases lysosomes were not involved in this process and thus HDL degradation was not detected. HDL catabolism is limited to certain tissues such as the kidney (for review see[45]). Hepatic HDL catabolism is disturbed in cases of imbalanced metabolism like obesity, with leptin being one regulator, as in ob/ob mice HDL degradation in the liver is decreased[46]. We did not find an involvement of caveolae in this uptake process; however, SR-BI, which seems to participate in endothelial HDL uptake at least in part, was reported to co-precipitate with caveolin[47]. As the caveolar density differs considerably between different cell types, the involvement of caveolae in HDL endocytosis might be cell type specific. For instance, we found strong HDL staining in caveolae in CHO cells using HDL-HRP staining (data not shown). Taken together, the data indicate that HDL and its cargo are taken up by endothelial cells via a vesicular path involving clathrin-coated vesicles and multivesicular bodies, with HDL catabolism being low.
In general, HDL metabolism has to be very versatile in order to adapt to the different environments such as peripheral tissues or the central cholesterol distributor, the liver. Thus HDL/lipid transport is complex, with the existing cholesterol gradient being one driving force at least for free cholesterol exchange. In addition, selective cholesteryl ester uptake from HDL particles occurs mainly in liver and adrenal, with HDL degradation being low in these tissues[48]. During HDL endocytosis, the lipid moiety of the HDL particle is altered massively. Using the novel fluorescent cholesterol surrogates Bodipy-cholesterol and Bodipy-cholesteryl oleate (Figure 1) or double-radiolabeled HDL particles cholesterol and cholesteryl ester, which were contained within the HDL particle before endocytosis, were localized intracellularly after uptake. These data are in agreement with our previous work on HepG2 cells[41]. Some studies have reported different transport routes for HDL and its associated free sterol in polarized hepatocytes[20,22,30], with the endoplasmic recycling compartment and multivesicular bodies being the main transfer sites of HDL cargo.
Until now, HDL uptake was visualized in in vitro systems, but in the present work we extended these studies to mice. Alexa-labeled HDL was delivered to mice by tail-vein injection and after 1 h the label was detected in endothelial cells of the liver. Out et al[49] reported that SR-BI was not involved in the transfer of HDL-derived lipids to endothelial cells of mouse liver, as SR-BI ablation did not lead to an alteration in this uptake. This suggests an involvement of clathrin-coated pits rather than caveolae in the uptake process. In mice, we observed strong staining of HDL-Alexa 568 in endothelial cells but not in Kupffer or stellate cells (Figure 4). As there are still some discrepancies between in vitro and in vivo data, more work is needed to assess HDL endocytosis in animal models. A recent study showed that HDL transcytosis occurs with the involvement of lymphatic vessels in the removal of cholesterol from peripheral tissues on its way back to the liver for disposal[39].
In summary, we visualized HDL uptake and its lipid exchange in endothelial cells in vitro and in vivo. The endothelium is one major site of HDL endocytosis and transcytosis, while other liver cell types seem to play a minor role in HDL endocytosis.
ACKNOWLEDGMENTS
We thank Jelena Brankovic and Regina Wegscheider for excellent technical assistance.
COMMENTS
Background
High-density lipoprotein (HDL) particles transfer lipids from the periphery to the liver for excretion into the bile. This so-called reverse cholesterol transport is one mechanism thought to contribute to the anti-atherosclerotic effects of HDL. To remove cholesterol from macrophages residing in the arterial intima and thus preventing atherosclerotic plaque progression, HDL has to cross the endothelial barrier. However, the route by which HDL crosses the endothelial barrier and many details concerning its travel back to the liver via lymphatic vessels are still enigmatic.
Research frontiers
In order to excert its main anti-atherosclerotic function, HDL has to cross the vascular endothelium to reach atherosclerotic lesions. This transport can either involve transcytosis of the particles with endocytosis in a first step or paracellular transport of HDL. The authors show that HDL endocytosis occurs via clathrin-coated pits, tubular endosomes and multivesicular bodies in primary human endothelial cells. In the present study, the authors provide evidence for the important role of HDL endocytosis in endothelial cells also in vivo.
Innovations and breakthroughs
In this project, the authors characterized HDL endocytosis in endothelial cells in vitro and in vivo in great detail. Therefore, the authors used light and electron microscopical methods enabling the visualization of HDL particles and their derived lipids using novel fluorescent cholesterol surrogates. Moreover the authors injected fluorescently labeled HDL into mice and analyzed HDL uptake in hepatocytes in vivo. Taken together the authors found that endothelial cells play an important role in HDL endocytosis in vitro and in vivo.
Applications
The role of endothelial HDL endocytosis and transcytosis during atherogenesis and in atherosclerotic lesions is not clear. Our study indicates that the endothelium is a major site of HDL endocytosis and therefore there is need for future studies.
Terminology
Atherosclerosis is a thickening of the arteries, a stepwise process which starts with the accumulation of excess lipids in the arterial wall. The so called atherosclerotic plaque can subsequently rupture and block arteries leading for example to stroke. Atherogenesis is the development of atherosclerosis and influenced by many factors, such as lipid and lipoprotein levels.
Peer review
This is a convincing study elucidating the distribution of cholesterol and cholesteryl esters during HDL uptake. The combination of new fluorescent marker techniques, classical combined radio-labeling approaches, electron microscopy and fluorescence microscopy is a strength of this carefully designed study.
Footnotes
References
- 1.Rader DJ, Daugherty A. Translating molecular discoveries into new therapies for atherosclerosis. Nature. 2008;451:904–913. doi: 10.1038/nature06796. [DOI] [PubMed] [Google Scholar]
- 2.von Eckardstein A, Rohrer L. Transendothelial lipoprotein transport and regulation of endothelial permeability and integrity by lipoproteins. Curr Opin Lipidol. 2009;20:197–205. doi: 10.1097/MOL.0b013e32832afd63. [DOI] [PubMed] [Google Scholar]
- 3.Bierman EL, Stein O, Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974;35:136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- 4.Stein O, Stein Y. Comparative uptake of rat and human serum low-density and high-density lipoproteins by rat aortic smooth muscle cells in culture. Circ Res. 1975;36:436–443. doi: 10.1161/01.res.36.3.436. [DOI] [PubMed] [Google Scholar]
- 5.Röhrl C, Stangl H. HDL endocytosis and resecretion. Biochim Biophys Acta. 2013;1831:1626–1633. doi: 10.1016/j.bbalip.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz G, Robenek H, Lohmann U, Assmann G. Interaction of high density lipoproteins with cholesteryl ester-laden macrophages: biochemical and morphological characterization of cell surface receptor binding, endocytosis and resecretion of high density lipoproteins by macrophages. EMBO J. 1985;4:613–622. doi: 10.1002/j.1460-2075.1985.tb03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeLamatre JG, Sarphie TG, Archibold RC, Hornick CA. Metabolism of apoE-free high density lipoproteins in rat hepatoma cells: evidence for a retroendocytic pathway. J Lipid Res. 1990;31:191–202. [PubMed] [Google Scholar]
- 8.Alam R, Yatsu FM, Tsui L, Alam S. Receptor-mediated uptake and ‘retroendocytosis’ of high-density lipoproteins by cholesterol-loaded human monocyte-derived macrophages: possible role in enhancing reverse cholesterol transport. Biochim Biophys Acta. 1989;1004:292–299. doi: 10.1016/0005-2760(89)90076-3. [DOI] [PubMed] [Google Scholar]
- 9.Aulinskas TH, Coetzee GA, Gevers W, van der Westhuyzen DR. Evidence that recycling of low density lipoprotein receptors does not depend on delivery of receptors to lysosomes. Biochem Biophys Res Commun. 1982;107:1551–1558. doi: 10.1016/s0006-291x(82)80176-9. [DOI] [PubMed] [Google Scholar]
- 10.Aulinskas TH, Oram JF, Bierman EL, Coetzee GA, Gevers W, van der Westhuyzen DR. Retro-endocytosis of low density lipoprotein by cultured human skin fibroblasts. Arteriosclerosis. 1985;5:45–54. doi: 10.1161/01.atv.5.1.45. [DOI] [PubMed] [Google Scholar]
- 11.Aulinskas TH, van der Westhuyzen DR, Bierman EL, Gevers W, Coetzee GA. Retro-endocytosis of low density lipoprotein by cultured bovine aortic smooth muscle cells. Biochim Biophys Acta. 1981;664:255–265. doi: 10.1016/0005-2760(81)90048-5. [DOI] [PubMed] [Google Scholar]
- 12.Greenspan P, St Clair RW. Retroendocytosis of low density lipoprotein. Effect of lysosomal inhibitors on the release of undegraded 125I-low density lipoprotein of altered composition from skin fibroblasts in culture. J Biol Chem. 1984;259:1703–1713. [PubMed] [Google Scholar]
- 13.Snyder ML, Polacek D, Scanu AM, Fless GM. Comparative binding and degradation of lipoprotein(a) and low density lipoprotein by human monocyte-derived macrophages. J Biol Chem. 1992;267:339–346. [PubMed] [Google Scholar]
- 14.Heeren J, Grewal T, Laatsch A, Rottke D, Rinninger F, Enrich C, Beisiegel U. Recycling of apoprotein E is associated with cholesterol efflux and high density lipoprotein internalization. J Biol Chem. 2003;278:14370–14378. doi: 10.1074/jbc.M209006200. [DOI] [PubMed] [Google Scholar]
- 15.Rellin L, Heeren J, Beisiegel U. Recycling of apolipoprotein E is not associated with cholesterol efflux in neuronal cells. Biochim Biophys Acta. 2008;1781:232–238. doi: 10.1016/j.bbalip.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Rensen PC, Jong MC, van Vark LC, van der Boom H, Hendriks WL, van Berkel TJ, Biessen EA, Havekes LM. Apolipoprotein E is resistant to intracellular degradation in vitro and in vivo. Evidence for retroendocytosis. J Biol Chem. 2000;275:8564–8571. doi: 10.1074/jbc.275.12.8564. [DOI] [PubMed] [Google Scholar]
- 17.Heeren J, Beisiegel U, Grewal T. Apolipoprotein E recycling: implications for dyslipidemia and atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:442–448. doi: 10.1161/01.ATV.0000201282.64751.47. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Ahmed AM, Tran TL, Lin J, McFarlane N, Boreham DR, Igdoura SA, Truant R, Trigatti BL. The inhibition of endocytosis affects HDL-lipid uptake mediated by the human scavenger receptor class B type I. Mol Membr Biol. 2007;24:442–454. doi: 10.1080/09687680701300410. [DOI] [PubMed] [Google Scholar]
- 19.Xiao C, Watanabe T, Zhang Y, Trigatti B, Szeto L, Connelly PW, Marcovina S, Vaisar T, Heinecke JW, Lewis GF. Enhanced cellular uptake of remnant high-density lipoprotein particles: a mechanism for high-density lipoprotein lowering in insulin resistance and hypertriglyceridemia. Circ Res. 2008;103:159–166. doi: 10.1161/CIRCRESAHA.108.178756. [DOI] [PubMed] [Google Scholar]
- 20.Wüstner D, Mondal M, Huang A, Maxfield FR. Different transport routes for high density lipoprotein and its associated free sterol in polarized hepatic cells. J Lipid Res. 2004;45:427–437. doi: 10.1194/jlr.M300440-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Strauss JG, Zimmermann R, Hrzenjak A, Zhou Y, Kratky D, Levak-Frank S, Kostner GM, Zechner R, Frank S. Endothelial cell-derived lipase mediates uptake and binding of high-density lipoprotein (HDL) particles and the selective uptake of HDL-associated cholesterol esters independent of its enzymic activity. Biochem J. 2002;368:69–79. doi: 10.1042/BJ20020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver DL, Wang N, Xiao X, Tall AR. High density lipoprotein (HDL) particle uptake mediated by scavenger receptor class B type 1 results in selective sorting of HDL cholesterol from protein and polarized cholesterol secretion. J Biol Chem. 2001;276:25287–25293. doi: 10.1074/jbc.M101726200. [DOI] [PubMed] [Google Scholar]
- 23.Rohrer L, Ohnsorg PM, Lehner M, Landolt F, Rinninger F, von Eckardstein A. High-density lipoprotein transport through aortic endothelial cells involves scavenger receptor BI and ATP-binding cassette transporter G1. Circ Res. 2009;104:1142–1150. doi: 10.1161/CIRCRESAHA.108.190587. [DOI] [PubMed] [Google Scholar]
- 24.Rohrer L, Cavelier C, Fuchs S, Schlüter MA, Völker W, von Eckardstein A. Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim Biophys Acta. 2006;1761:186–194. doi: 10.1016/j.bbalip.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 25.Rhode S, Breuer A, Hesse J, Sonnleitner M, Pagler TA, Doringer M, Schütz GJ, Stangl H. Visualization of the uptake of individual HDL particles in living cells via the scavenger receptor class B type I. Cell Biochem Biophys. 2004;41:343–356. doi: 10.1385/CBB:41:3:343. [DOI] [PubMed] [Google Scholar]
- 26.Pagler TA, Rhode S, Neuhofer A, Laggner H, Strobl W, Hinterndorfer C, Volf I, Pavelka M, Eckhardt ER, van der Westhuyzen DR, et al. SR-BI-mediated high density lipoprotein (HDL) endocytosis leads to HDL resecretion facilitating cholesterol efflux. J Biol Chem. 2006;281:11193–11204. doi: 10.1074/jbc.M510261200. [DOI] [PubMed] [Google Scholar]
- 27.Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezón E, Champagne E, Pineau T, Georgeaud V, Walker JE, Tercé F, et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature. 2003;421:75–79. doi: 10.1038/nature01250. [DOI] [PubMed] [Google Scholar]
- 28.Klinger A, Reimann FM, Klinger MH, Stange EF. Clathrin-mediated endocytosis of high density lipoprotein3 in human intestinal Caco-2 cells. A post-embedding immunocytochemical study. Biochim Biophys Acta. 1997;1345:65–70. doi: 10.1016/s0005-2760(96)00164-6. [DOI] [PubMed] [Google Scholar]
- 29.Eckhardt ER, Cai L, Sun B, Webb NR, van der Westhuyzen DR. High density lipoprotein uptake by scavenger receptor SR-BII. J Biol Chem. 2004;279:14372–14381. doi: 10.1074/jbc.M313793200. [DOI] [PubMed] [Google Scholar]
- 30.Bravo E, Botham KM, Mindham MA, Mayes PA, Marinelli T, Cantafora A. Evaluation in vivo of the differential uptake and processing of high-density lipoprotein unesterified cholesterol and cholesteryl ester in the rat. Biochim Biophys Acta. 1994;1215:93–102. doi: 10.1016/0005-2760(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 31.Van Eck M, Twisk J, Hoekstra M, Van Rij BT, Van der Lans CA, Bos IS, Kruijt JK, Kuipers F, Van Berkel TJ. Differential effects of scavenger receptor BI deficiency on lipid metabolism in cells of the arterial wall and in the liver. J Biol Chem. 2003;278:23699–23705. doi: 10.1074/jbc.M211233200. [DOI] [PubMed] [Google Scholar]
- 32.Cavelier C, Lorenzi I, Rohrer L, von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim Biophys Acta. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Chao WT, Fan SS, Chen JK, Yang VC. Visualizing caveolin-1 and HDL in cholesterol-loaded aortic endothelial cells. J Lipid Res. 2003;44:1094–1099. doi: 10.1194/jlr.M300033-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Chao WT, Fan SS, Yang VC. Visualization of the uptake of high-density lipoprotein by rat aortic endothelial cells and smooth muscle cells in vitro. Histochem J. 2002;34:233–239. doi: 10.1023/a:1021789429893. [DOI] [PubMed] [Google Scholar]
- 35.Chao WT, Tsai SH, Lin YC, Lin WW, Yang VC. Cellular localization and interaction of ABCA1 and caveolin-1 in aortic endothelial cells after HDL incubation. Biochem Biophys Res Commun. 2005;332:743–749. doi: 10.1016/j.bbrc.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 36.Lin YC, Lin CH, Kuo CY, Yang VC. ABCA1 modulates the oligomerization and Golgi exit of caveolin-1 during HDL-mediated cholesterol efflux in aortic endothelial cells. Biochem Biophys Res Commun. 2009;382:189–195. doi: 10.1016/j.bbrc.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Cavelier C, Ohnsorg PM, Rohrer L, von Eckardstein A. The β-chain of cell surface F(0)F(1) ATPase modulates apoA-I and HDL transcytosis through aortic endothelial cells. Arterioscler Thromb Vasc Biol. 2012;32:131–139. doi: 10.1161/ATVBAHA.111.238063. [DOI] [PubMed] [Google Scholar]
- 38.Kahn ML, Rader DJ. Lymphatics as a new active player in reverse cholesterol transport. Cell Metab. 2013;17:627–628. doi: 10.1016/j.cmet.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 39.Lim HY, Thiam CH, Yeo KP, Bisoendial R, Hii CS, McGrath KC, Tan KW, Heather A, Alexander JS, Angeli V. Lymphatic vessels are essential for the removal of cholesterol from peripheral tissues by SR-BI-mediated transport of HDL. Cell Metab. 2013;17:671–684. doi: 10.1016/j.cmet.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Schumaker VN, Puppione DL. Sequential flotation ultracentrifugation. Methods Enzymol. 1986;128:155–170. doi: 10.1016/0076-6879(86)28066-0. [DOI] [PubMed] [Google Scholar]
- 41.Röhrl C, Meisslitzer-Ruppitsch C, Bittman R, Li Z, Pabst G, Prassl R, Strobl W, Neumüller J, Ellinger A, Pavelka M, et al. Combined light and electron microscopy using diaminobenzidine photooxidation to monitor trafficking of lipids derived from lipoprotein particles. Curr Pharm Biotechnol. 2012;13:331–340. doi: 10.2174/138920112799095338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stangl H, Hyatt M, Hobbs HH. Transport of lipids from high and low density lipoproteins via scavenger receptor-BI. J Biol Chem. 1999;274:32692–32698. doi: 10.1074/jbc.274.46.32692. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983;98:241–260. doi: 10.1016/0076-6879(83)98152-1. [DOI] [PubMed] [Google Scholar]
- 44.Graham RC, Karnovsky MJ. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966;14:291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- 45.Moestrup SK, Nielsen LB. The role of the kidney in lipid metabolism. Curr Opin Lipidol. 2005;16:301–306. doi: 10.1097/01.mol.0000169350.45944.d4. [DOI] [PubMed] [Google Scholar]
- 46.Silver DL, Wang N, Tall AR. Defective HDL particle uptake in ob/ob hepatocytes causes decreased recycling, degradation, and selective lipid uptake. J Clin Invest. 2000;105:151–159. doi: 10.1172/JCI8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babitt J, Trigatti B, Rigotti A, Smart EJ, Anderson RG, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. doi: 10.1074/jbc.272.20.13242. [DOI] [PubMed] [Google Scholar]
- 48.Glass C, Pittman RC, Weinstein DB, Steinberg D. Dissociation of tissue uptake of cholesterol ester from that of apoprotein A-I of rat plasma high density lipoprotein: selective delivery of cholesterol ester to liver, adrenal, and gonad. Proc Natl Acad Sci USA. 1983;80:5435–5439. doi: 10.1073/pnas.80.17.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Out R, Hoekstra M, Spijkers JA, Kruijt JK, van Eck M, Bos IS, Twisk J, Van Berkel TJ. Scavenger receptor class B type I is solely responsible for the selective uptake of cholesteryl esters from HDL by the liver and the adrenals in mice. J Lipid Res. 2004;45:2088–2095. doi: 10.1194/jlr.M400191-JLR200. [DOI] [PubMed] [Google Scholar]