Abstract
Effective prevention and management of osteoporosis would require suitable methods for population screenings and early diagnosis. Current clinically-available diagnostic methods are mainly based on the use of either X-rays or ultrasound (US). All X-ray based methods provide a measure of bone mineral density (BMD), but it has been demonstrated that other structural aspects of the bone are important in determining fracture risk, such as mechanical features and elastic properties, which cannot be assessed using densitometric techniques. Among the most commonly used techniques, dual X-ray absorptiometry (DXA) is considered the current “gold standard” for osteoporosis diagnosis and fracture risk prediction. Unfortunately, as other X-ray based techniques, DXA has specific limitations (e.g., use of ionizing radiation, large size of the equipment, high costs, limited availability) that hinder its application for population screenings and primary care diagnosis. This has resulted in an increasing interest in developing reliable pre-screening tools for osteoporosis such as quantitative ultrasound (QUS) scanners, which do not involve ionizing radiation exposure and represent a cheaper solution exploiting portable and widely available devices. Furthermore, the usefulness of QUS techniques in fracture risk prediction has been proven and, with the last developments, they are also becoming a more and more reliable approach for assessing bone quality. However, the US assessment of osteoporosis is currently used only as a pre-screening tool, requiring a subsequent diagnosis confirmation by means of a DXA evaluation. Here we illustrate the state of art in the early diagnosis of this “silent disease” and show up recent advances for its prevention and improved management through early diagnosis.
Keywords: Diagnosis of osteoporosis, Screening techniques, X-ray based methods, Quantitative ultrasound, Peripheral sites, Bone mineral density
Core tip: Early diagnosis is the key for an appropriate osteoporosis management. To date, dual X-ray absorptiometry is the most commonly used and validated method for bone densitometry in clinical practice. Nevertheless, some important limitations like radiation dose and high costs do not allow it to be the true “gold standard technique” and make it unsuitable as a screening tool at the primary health care level for prevention purposes. As a consequence, interest in developing reliable pre-screening devices for osteoporosis assessment such as quantitative ultrasound scanners is growing up. Ultrasound-based techniques involve no radiation exposure, represent a cheap solution and they are also becoming more and more reliable for assessing bone quality.
INTRODUCTION
In recent years the prevalence and the awareness of osteoporosis are increasing and it has been estimated that 200 millions of individuals suffer from osteoporosis worldwide. Nevertheless, about 75% of these people represent undiagnosed cases and do not receive appropriate treatment.
According to the World Health Organization (WHO), osteoporosis is “a systemic skeletal disease characterized by low bone mass and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture”[1] .
Fractures resulting from osteoporosis lead to high rates of morbidity and mortality, reduce quality of life and are responsible for a sharp increase in healthcare costs[2-4]. As a consequence, with the gradual increase in life expectancy in developed countries, osteoporosis and consequent fragility fractures represent a major health problem in elderly women (older than 50 years) which will become a predominant portion in the next decades[2]. The socio-economic demand for the management of osteoporotic patients will also increase in the next years[5]: it would be both useful and necessary to adopt a preventive approach to the problem in postmenopausal women with the aim to stop or at least slow down the disease progression[2,6]. With this approach early diagnosis is essential for timely identification and treatment of patients who are at risk for osteoporotic fractures. In this context, diagnostic imaging of osteoporosis has two principal aims: (1) to identify the presence of osteoporosis, and (2) to derive prognostic information on the probability of future fractures by quantification of bone mass[7].
The diagnosis of osteoporosis relies on the quantitative assessment of BMD, which is currently considered the best predictor of osteoporotic fractures. The BMD value is the amount of bone mass per unit volume (volumetric density), or per unit area (areal density), and both can be measured in vivo by densitometric techniques[2]. Over the past 25 years, many non-invasive methods for osteoporosis diagnosis have been developed that rely on the attenuation of ionizing radiation to quantify BMD at different skeletal sites. Among the most commonly used X-ray based methods, quantitative computed tomography (QCT) and DXA allow quantification of bone loss while morphometry provides assessment of the presence of vertebral fractures.
Unfortunately, the application of the current “gold standard” method for bone densitometry, DXA, is not appropriate in primary healthcare or as a screening tool in order to improve the diagnostic outcomes because certain features, such as the use of radiation, the large size of the equipment, its relatively high costs and the limited availability of the measurements, prevent it from being a real benchmark for osteoporosis management. Moreover, all the X-ray based methods provide a measure of BMD but this parameter can explain only 60%-80% of the variability in bone strength, and it has been demonstrated that other mechanical aspects of the bone (microarchitectural parameters, bone geometry and elastic properties, which cannot be assessed by densitometric techniques[2,8] ) are important in determining fracture risk[2,9].
Quantitative ultrasound (QUS) methods for osteoporosis assessment, developed over the past 10 years, have showed some ability to determine bone quality and to provide information not only about bone density but also about its structure and elastic properties[10-12]. Their main limitation is represented by the fact that currently-available QUS devices can be applied only to peripheral sites of the skeleton: the calcaneus, the proximal phalanges of the hand, the tibial shaft and the radius. Nevertheless, QUS techniques are much faster, easier to use and more portable than DXA; they are also less expensive and do not employ ionizing radiation: these features suggest a future role for QUS as an effective screening tool for osteoporosis diagnosis.
This article gives an overview of the most widely used X-ray based techniques to perform osteoporosis diagnosis and of the most relevant developments in the field of QUS, underlining the corresponding advantages and limitations for their use in the clinical practice.
This review provides a complete framework for understanding and properly evaluates which tools or techniques can achieve the early diagnosis of osteoporosis.
X-RAY BASED METHODS
Before the advent of newer, highly accurate and precise quantitative techniques such as DXA and QCT, osteoporosis has been most commonly diagnosed by conventional Single Photon Absorptiometry (SPA), Single-energy-X-ray Absorptiometry (SXA) and Dual-Photon Absorptiometry (DPA)[7]. Thanks to the development of DPA and DXA it was possible to directly investigate the main anatomical reference sites: proximal femur and vertebral bodies[7].
Dual X-ray absorptiometry
Dual X-ray absorptiometry (DXA) method was introduced in 1987 as a successor of DPA. Among the different techniques that have been developed to assess osteoporosis disease in term of bone mass, bone mineral content, or other related aspects of skeletal mass or structure, the technique that has reached the more complete technical development and biological validation is DXA, which is currently regarded as the “gold standard” for osteoporosis diagnosis.
In DXA the production of photons, based on the use of an X-ray tube[13], leads to shorter imaging times (less than 5 min) with enhanced resolution and improved accuracy than in DPA using a radionuclide source. Like DPA, this technique determines BMD in two dimensions (from an anterior-posterior image).
A DXA scanner (Figure 1) consists of a mobile X-ray source, an examination table for the patient, and a detection system that detects radiation emerging from the bones being examined. The X-ray source is under the examination table and moves together with the detection system, which is located opposite the X-ray source and over the patient’s body.
Figure 1.
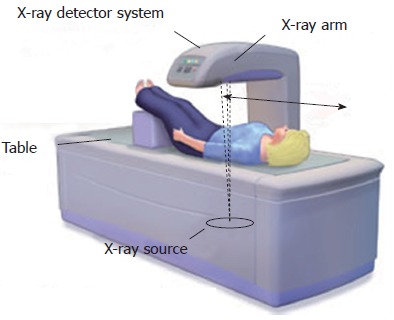
Bone densitometry scan (dual X-ray absorptiometry). Schematic representation of X-rays source and detector system in dual X-ray absorptiometry device. Adapted from Drugs.com[94].
DXA uses an X-ray beam composed of two different photon energies (constant and pulsed): the energy used is selected to compensate for the different attenuation coefficients of the mineralized bone and soft tissues of the skeletal site being analyzed[14]. In particular, the intensities of high-energy and low-energy photons are analyzed separately after the protons have passed through bones and soft tissue. The attenuation values of soft tissues are subtracted by an algorithm providing only the attenuation values of bone. These values are compared with standard values in phantoms of known density in order to obtain bone mineral content value (in grams). Dividing the bone mineral content by the projected area of the measured site (in square centimeters), it is possible to obtain the BMD value (in grams per square centimeter)[15]. BMD can be also expressed as a T-score and a Z-score, which represent the number of standard deviations (SDs) with respect to a reference average value. The T-score describes the difference between the BMD of the patient being examined and the mean BMD of a standard young adult population (20-30 years of age, when BMD typically reaches its peak value). The Z-score shows the difference between the patient’s BMD and the mean BMD of age- and gender-matched controls. DXA results are reported as numeric values for BMD, T-score and Z-score, and as a graphic curve in which patient’s parameter values are compared with those of a reference gender-matched population belonging to the same ethnic group. In 1994, the WHO defined the threshold levels for the diagnosis of osteopenia and osteoporosis with DXA. As a consequence, DXA measurements are currently the standard of reference for the clinical diagnosis of osteoporosis through bone densitometry. In particular, the WHO classifies BMD on the basis of the T-score (Figure 2) as normal (≥ -1.0), osteopenia (< -1.0 but > -2.5), osteoporosis (≤ -2.5), and severe osteoporosis (≤ -2.5 with a fragility fracture)[16,17].
Figure 2.
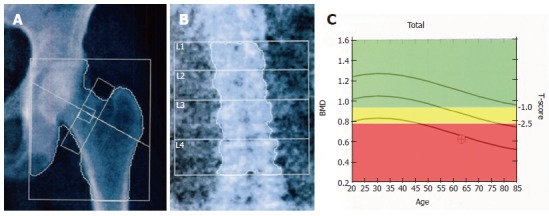
Dual X-ray absorptiometry examination on reference sites. A: Dual X-ray absorptiometry (DXA) scan of the femoral neck; B: DXA examination of the lumbar spine with analysis of the vertebral bodies (L1-L4); C: A graphic curve of T-score values normalized for age. The green area of the panel indicates normal T- score values (≥ -1.0), the yellow one indicates osteopenia (< -1.0 but > -2.5) and the red area indicates osteoporosis (≤ 2.5). BMD: Bone mineral density.
It has been shown that the reproducibility of BMD measurements, expressed as coefficient of variation (CV), is quite good: 1.12% for vertebrae, 2.21% for femoral neck and 1.32% for total hip[18].
The central skeleton (spine and femur) is the most relevant measurement site, since this is the site suffering the most severe fractures. In particular lumbar spine (from L1 to L4) and proximal femur (total hip, femoral neck, trochanter and WARD area) (Figure 2) are measured by axial DXA devices.
The DXA-method has also been applied for measurements of peripheral locations, such as the heel and distal radius. The choice to investigate the forearm can give an information on the possibility to have a wrist fracture and can be performed when evaluation of other sites is unfeasible; similarly, calcaneus measurements are particularly predictive of spine fractures[19,20] even if the WHO criterion for osteoporosis diagnosis (T score ≤ -2.5) is not applicable to the calcaneus. Anyway, since there is only a moderate correlation between the peripheral and axial BMD (r = 0.5-0.6), it has been estimated that over 40% of the patients investigated at peripheral bone sites would need an additional referral to the axial DXA measurement[21]. It has been shown that the most reliable prediction of future fractures is reached by measurements at the actual site of the future fracture. Thus, the risk of a hip fracture is best assessed by the proximal femur BMD, whereas vertebral fractures are best predicted by BMD measurement on the lumbar vertebrae[22].
Since DXA is a two-dimensional technique, it has some inherent limitations. It cannot help in distinguishing between cortical and trabecular bone neither in discriminating between changes due to bone geometry (e.g., variations in the third dimension) and those actually due to BMD variations (within a fixed volume of bone). Furthermore, the microstructural characteristics (e.g., trabecular shape, size, number, orientation, etc.) cannot be assessed. Moreover, other factors that can cause clinically relevant diagnostic errors should be taken into account: the presence of osteomalacia may result in an underestimation of bone mass; osteoarthritis at the spine or the hip may increase the measured bone density without improving the actual skeletal strength; soft tissue calcifications, previous fractures, severe scoliosis or vertebral deformities may be all sources of error in the diagnosis of osteoporosis by DXA[5].
The use of DXA requires well-trained personnel: incorrect patient positioning, scan analysis or mistakes in interpretation may lead to errors in diagnosis and consequent therapy[23]. Furthermore, it should not be forgotten that a DXA measurement always exposes the patient to a certain radiation dose: although the radiation dose in the modern DXA devices is small[13], it still interferes with the feasibility of the technique for large scale population screenings.
DXA is used also in pediatric populations to quantify the deficits in bone mineral associated with the various disorders causing osteopenia in children, and to detect the genetic susceptibility to osteoporosis[24]. However, DXA in children frequently leads to a misdiagnosis of osteoporosis and an underestimation of the amount of bone because growth and maturity significantly reduce the accuracy of DXA[24].
Quantitative computed tomography
In addition to DXA, quantitative computed tomography (QCT) has also been developed to quantify bone mineral content and to assess bone loss[25]. The main advantage of this technique is that cancellous bone can be examined separately from cortical bone[7]. In particular, in QCT a thin transverse slice through the body is imaged: the image can be segmented to give a quantitative measure of volumetric BMD (unlike DXA) of vertebrae, assessing the cancellous bone independently of surrounding cortical bone and possible aortic calcifications[26]. QCT can be performed to the spine (usually two to four vertebrae between T12 and L4 inclusive) on conventional whole body CT scanners, or to the appendicular skeleton at peripheral sites (radius, tibia) using smaller, less expensive, dedicated peripheral CT scanners (pQCT).
In QCT measurements, the X-ray absorption profiles are typically obtained when the source and the detectors rotate around the object. The absorption projections at different angles are then processed to reconstruct a three-dimensional illustration of the imaged object[27]. CT provides an image which is based on the linear X-ray absorption coefficients of the irradiated tissues. All clinical body CT scanners are similarly calibrated to the X-ray attenuation of water, resulting in CT numbers, measured in Hounsfield units (HU). In the quantitative determination of volumetric BMD, a calibration phantom is imaged simultaneously with the patient, to convert HU into bone mineral units. This phantom is made of different concentrations of calcium hydroxyapatite in water-equivalent plastic through which the average of the obtained vertebral density is calculated and compared with those of an healthy reference population. The density of each vertebral body is determined, and a mean value for all vertebral bodies is calculated. The results of the measurements are usually expressed as absolute values or as Z-score and T-score, as in all bone densitometry techniques[28].
In early CT scanners, QCT was applied to the lumbar spine using single two-dimensional (2D) 8-10 mm slices through the middle of each vertebral body. Over the last decade technical developments in CT, including complete and multiple rings of detectors and spiral rotation of the X-ray tube (spiral multi-detector computed tomography: MDCT), result in 3D images of tissue[29]. In this way analysis of the hip, a clinically important site of fracture, is more precise than in 2D single slices and scan times are below 10 s for the spine or femur[26]. Moreover, the next generation high-resolution pQCT (HR-pQCT) provide also an evaluation of trabecular bone structure (e.g., trabecular thickness, trabecular spacing and bone volume fraction). Patients with vertebral fractures were better identified by QCT than DXA measurements at lumbar spine or femoral neck[30]. With high resolution CT scans, also mild fractures, affecting the mechanical strength of bone but not bone mass, could be assessed by the determination of the structural parameters[31].
However, although this technique has important advantages over DXA, it has also disadvantages, some of which are common to DXA. First, it has been shown that BMD values depend on the bone marrow composition: this factor could provide an underestimation of bone mineral content values. Second, the radiation dose induced by a QCT scan of a hip is significantly higher than that of DXA (1 mSv vs 10 μSv), which limits the applicability of the technique not only for screening but also for standard diagnostics. In addition, this technique has difficulties with quality control, high cost compared with DXA, necessity of well-trained technicians for scan execution[28,32].
For the clinical use of QCT and pQCT in the management of osteoporosis, the International Society for Clinical Densitometry (ISCD) published its official position in 2008 stating that QCT of spine and pQCT of radius predict vertebral and hip fractures, respectively, in post-menopausal women[28].
Spinal QCT and pQCT can be used also in pediatric research in order to assess bone development in healthy children and in those at risk for poor bone health[26]. Central QCT is less widely used than pQCT because of its higher doses of ionizing radiation. The ISCD guidelines on the clinical application of pQCT in children definite advice to not use these techniques until more data has emerged[33].
Morphometry
In order to improve the correct diagnosis of osteoporosis other imaging techniques have been developed, and in an effort to identify vertebral fractures, morphometric methods were introduced. Vertebral morphometry is a quantitative method based on the measurement of distinct vertebral dimensions, calculating relative changes (or differences) in vertebral height as indicators of fracture. These measurements may be obtained from conventional spine radiographs (morphometric X-ray radiography) or absorptiometric images (morphometric X-ray absorptiometry). Before measuring vertebral heights, the reader must identify the vertebral levels. The vertebral bodies should be marked so that they can be identified more easily on follow-up radiographs[34].
After the radiographs are digitized, an operator manually selects the four corners of the vertebra of interest. The software automatically determines the midpoints between the anterior and posterior corner points of the upper and lower endplates and calculates the posterior, middle, and anterior heights of each vertebra and specific indexes derived from height measurements for defining vertebral deformities. Morphometric X-ray absorptiometry is currently the most widely used digital technique for assessing vertebral height although it is unable to distinguish between true vertebral fractures and vertebral deformities due to degenerative spine and disk disease.
A combination of semiquantitative visual (conventional radiography) and quantitative morphometric methods may be the best approach for defining fractures but not for prevention aims, as suggested by Kanis et al[35], the National Osteoporosis Foundation[36], and the International Osteoporosis Foundation[37].
ULTRASOUND METHODS
In order to measure quantitative parameters and assess tissue properties, QUS techniques are also used. Interest in US methods can be attributed primarily to the fact that they do not involve radiation exposure. In addition, US devices offer the advantages of small size, portability, quick and simple measurements, low costs compared with both DXA and QCT, shorter investigation times with respect to DXA.
QUS of bone has been introduced approximately two decades ago as a method for investigating bone structural features and elastic properties of bone tissues, which could not be assessed using densitometric techniques[38], and has been applied particularly in post-menopausal osteoporosis[39,40]. The recent technical innovations of some of the commercially-available devices have made it possible to apply QUS to different skeletal areas of study, using this non-invasive method in order to complete the clinical picture of the patient (state of bones and information of fracture risk).
Physical principles of US methods
US is a mechanical wave characterized by a frequency exceeding the threshold of audibility of the human ear (> 20 kHz)[40]. The typical QUS methods, unlike the usual US techniques that are based on the reflection of US waves, involve the transmission (transversely or longitudinally) of US pulses through the investigated bone tissue and the detection of the transmitted pulses once they have passed through the medium. The bone to be investigated is placed between the two probes, broadcaster and receiver (Figure 3).
Figure 3.
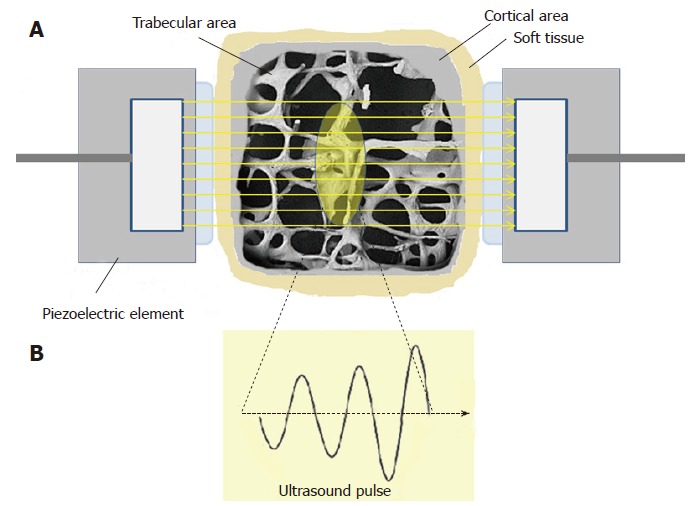
Quantitative ultrasound method. A: Ultrasound beam through a bone tissue of phalanx (transmission way) in a typical quantitative ultrasound measurement (section view); B: Ultrasound pulse.
Particularly, the US pulse is transmitted to the cortex and after propagation along the cortical bone layer parallel to its long axis is received by another ultrasound transducer at a known distance. Today, most of the devices use several transducers and the bi-directional transmission techniques in order to increase the repeatability of the measurements and to correct the soft tissue related errors[41].
The bone tissue has a high coefficient of acoustic attenuation that increases exponentially with the increase of wave frequency: then, when investigating bones, it is necessary to use lower frequencies than those used in the common ecographic scan of soft tissues.
The first US parameters employed for characterizing bone tissues are: Speed of Sound (SoS) and Broadband Ultrasound Attenuation (BUA). More complex parameters have been developed from combination of SoS and BUA: amplitude dependent speed of sound (AD-SoS), stiffness, quantitative ultrasound index (QUI)[40]. In the diagnosis of osteoporosis, these latter have proved to be more useful in identifying subjects with low BMD and therefore at high risk of fracture[42].
Performance of US systems
Several studies have been focused on evaluating the performance of US systems in terms of stability, accuracy, and ability in the discrimination of patients with osteoporotic fractures. Most of these studies involve comparison of the QUS method with X-ray methods such as DXA or QCT[42-47].
It has been shown that ultrasonography allows to obtain useful information, such as the distribution of the mineralized matrix within the bone (the connectivity and the thickness of the trabeculae) and the different resistance to loading of the bone tissue according to the trabecular orientation[48,49].
In the last decades, a large number of studies have confirmed the usefulness of QUS in predicting osteoporotic fractures of the calcaneus (particularly in elderly women aged 65-70 years or older), the distal metaphysis of the phalanx, the radius and the tibia[50].
In particular, the calcaneus was chosen as a site for measurement since it is easily accessible, with the medial and lateral aspects being relatively flat and parallel, therefore resulting well-suited for optimizing the geometry of transmission of the US wave through it. It contains approximately 90% trabecular bone, which has a high metabolic turnover rate and a pattern of bone loss similar to the spine[9,50]. Numerous important prospective studies have been carried out to assess fracture risk by QUS at the calcaneus[51]. The EPIC-Norfolk prospective population study[50], conducted in an English male and female population, proved the effectiveness of QUS at the calcaneus in predicting fracture risk, for both males and females. The same result was confirmed by a prospective study (SEMOF study) performed on more than 7000 Swiss women[52].
The versatility of QUS has suggested that its potential might be evaluated in fields other than those related to osteoporosis. In particular, pediatricians are interested in using this technique for studying skeletal maturation, thanks to its absence of ionizing radiation. Some normative data have been collected for calcaneus, phalanx, radius and tibia in subjects ranging from ages of 3 to 18 years in seven countries[53-58]. However, QUS devices are manufactured principally for application in adults and adaption for the smaller anatomical structures of children may not be available.
Among the other skeletal sites in which QUS has been used, phalanges offer some advantages for studying bone status, since they may reflect more systemic bone loss and, for example, the metaphysis has both cortical and cancellous bone. When osteopenia is developing, the cortical thickness of these small tubular bones decreases while the medullary cavity enlarges due to endosteal resorption of bone[59]. The metaphysis of the phalanges of the last four fingers of the hand have been investigated by QUS, DXA and QCT to evaluate the association among phalangeal morphometric parameters, AD-SoS, Ultrasound Bone Profile Index (UBPI), and spinal fracture status. The results of analysis showed that the phalanx is sensitive to bone tissue changes occurring with aging and in presence of osteoporosis. The sensitivity of UBPI, AD-SoS, and morphometric variables for spinal fracture discrimination were similar to that of the lumbar spine as measured by X-ray techniques[60]. Several other studies about the relationships between ultrasonic parameters and phalanx bones show that ultrasound SOS in the compact bone is strongly related to the cortical thickness and cortical area, while the attenuation seems to be more closely linked to the area of the medullary canal. Many studies have been performed on both samples of animal bone tissue[43] and phantoms[61], either through mathematical simulations were carried out, and then confirmed by in vivo assessments in humans[49].
A clinical validation of QUS at the phalanx was provided by the European multi-centre study (PhOS) performed on over 10000 women: precision in identifying osteoporotic subjects was very good[44]. In addition, in the BOS study, Hartl et al[42] showed that the diagnostic performance of QUS at the calcaneus and the phalanx was comparable with central DXA. The percentages of correct classification of osteoporotic subjects with or without vertebral fractures depended on the different QUS instruments used and were the following: 66.5% for Achilles BUA device; 64.8% for Achilles SOS device; 63.9% for Achilles STIFFNESS device; 65.2% for Sahara BUA device; 61.1% for Sahara SOS device; 71.1% for Bone Profiler AD-SoS and 59.1% for Bone Profiler UBPI. On the other hand, results of the same discriminant analysis obtained by DXA device were the following: 60.4% for DXA lumbar spine; 47.8% for DXA neck; 59.1% for DXA Ward’s triangle; 66.3% for DXA trochanter and 62% for DXA total hip[42]. Also in the European study, OPUS (analogous to the BOS study), all US parameters on phalanx and calcaneus showed a significant association with osteopenic vertebral deformities acquired[47].
Interestingly, an Italian study proved that QUS at the phalanx is more sensitive in discriminating subjects with and without vertebral fractures immediately post-menopause, prior to the age of 70 years, while the effectiveness of the method in predicting hip fractures in the elderly population was inadequate; on the contrary, QUS at the calcaneus resulted more sensitive in the subsequent period, at the age of 70 years and older[52,62,63]. Recently, the study of bone tissue by US has been extended to the male population both in Italy and in Germany, obtaining good results also in this case[64-67]. In addition, NORA, the largest study of post-menopausal osteoporosis conducted in the United States (performed on 200000 women), showed a high degree of predictability for phalanx fracture risk [odds ratio (OR) = 4.86], forearm (OR = 2.86) and heel (OR = 1)[68].
Lately, to study the bone tissue in newborns and preterm, other anatomical peripheral sites, such as the metacarpal bone and the humerus, were also chosen as investigation sites for the following reasons: (1) they lend themselves well to the analysis of bone maturation in perinatal period as they have larger dimensions than the phalanges of the hands; (2) are easily accessible; and (3) do not exhibit the newborn to any risk of shock during the measurement.
QUS results can be expressed as absolute values or as T-score and Z-score, thanks to well-established and validated normative reference curves, allowing bone loss to be followed up over time[69]. Recently, Kanis et al[70] published tables for calculating 10-year fracture risk from the results of QUS of the phalanx and other clinical risk factors. Afterwards the ISCD has provided recommendations and possible algorithms for the diagnosis and management of osteoporosis[71].
The QUS parameters have shown good precision, stability over time and independence from the presence of soft tissue, enabling effective follow-up of osteoporosis therapies. The role of US-based techniques might be to identify patients at risk for osteoporosis as a first-line pre-screening tool for large population, but there are no consensus criteria yet.
New advances in US diagnosis of osteoporosis
Recently, new QUS techniques to assess the femur and the spine have been developed. In vitro studies have shown a high correlation between QUS measurements and BMD in human femur cadaver specimens[72,73]. Since the accuracy achieved in vitro was very good, the new methodology could be promising and applicable, in fact it would be more appropriate to perform QUS at central sites, which are more commonly involved by osteoporotic insufficiency fractures, rather than the usual peripheral ones.
In recent years, Barkmann et al[74] developed the first QUS device (Femur Ultrasound Scanner, FemUS) for measurements at the proximal femur. This prototype utilizes ultrasonic waves in transmission by using two US transducers which are able to transmit and receive ultrasonic waves. For better ultrasound coupling, the transducers are submerged in a temperature-controlled water bath with a stable temperature of 34 ± 0.5 °C (a potential limiting step for the good performance of the scan). Inflatable water-filled membranes were used to establish contact between the water bath that contains the transducers and the patient’s skin. Patients were positioned on a scan table (lying between the transducers) and the proximal femur region was scanned by ultrasonic beams perpendicular to the surface of the table. According to the authors, significant correlations with total hip BMD measured by DXA were found with a correlation coefficient R2 up to 0.72. The main limitation of this study is its restricted sample size. In addition, based on this study, Grimal et al[75] implemented a novel QUS technique that probes the femoral neck cortical compartment. The sensitivity and the feasibility of the method to variations of femoral neck cortical properties and strength was tested in an in vivo study. Nine femurs were subjected to QUS, QCT and DXA measurements, and mechanical tests (to assess strength). This study has shown the ability of QUS to measure critical determinants of bone strength in the cortical compartment of the femoral neck. The correlations between QUS and DXA with strength were found to be very similar: R2 = 79% for QUS, R2 = 78% for DXA (total proximal femur).
On the other hand, to date, only one in vivo QUS-based measurement at lumbar spine was published recently using a commercial sonography scanner to measure backscattered signals from the lumbar vertebrae. However, the number of subjects was small (nine), and correlations with BMD were moderate[76].
Currently, a new-method for osteoporosis diagnosis on reference sites was developed at the Italian Institute of Clinical Physiology of the National Council of Research in Lecce (Echolight project, partially supported by FESR P.O. Apulia Region 2007-2013-Action 1.2.4 (grant number 3Q5AX31)). A new fully automatic algorithm was implemented to calculate the same diagnostic parameters provided by a DXA examination (BMD, T-score and Z-score) starting from an US scan of both lumbar spine and femoral neck and exploiting advanced spectral and statistical analyses on both the echographic images and the “raw” US signals (Figure 4). The method seems to be very promising. Preliminary results showed that US diagnosis coincided with the corresponding DXA ones for 86.1% of spines and for 81.0% of femurs[77-89].
Figure 4.
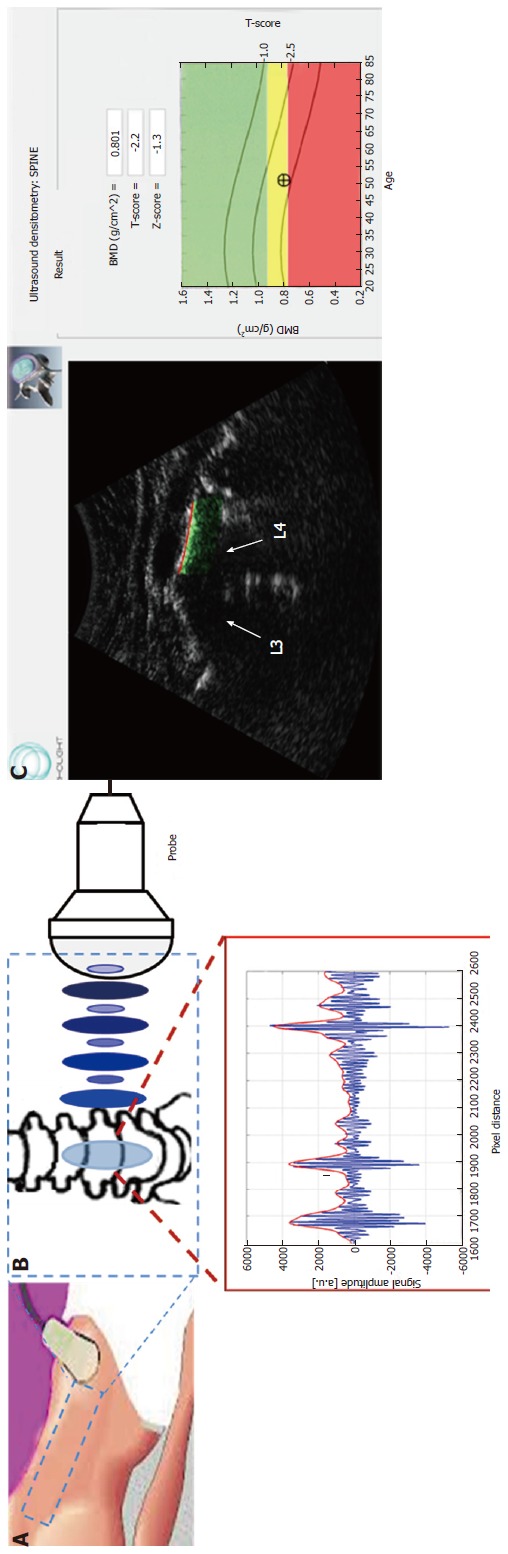
A new ultrasound-based methodology. A: Ultrasound (US) scan of lumbar spine; B: Physical principles of the ultrasound technique based on the reflection of US waves; C: Selection of the target bone interface and automatic detection of the region of interest for diagnostic evaluations and medical report provided with BMD, T-score and Z-score values. BMD: Bone mineral density.
Although the methodologies for assessing bone status using US pulses are presently much less developed than those employing X-rays, the potential of ultrasound extends far beyond the currently available QUS techniques and is still largely unexploited.
CONCLUSION
Because of the increasing aging of the world population, the number of people affected by osteoporosis is also augmenting and the complications related to this disease create significant social and economic burdens. Accurate and early diagnosis of osteoporosis would result in better clinical management, in terms of prevention and adequate pharmacologic or surgical treatment.
The currently available methods for bone densitometry are mainly based on the use of either X-rays, considered as the “gold standard” reference, or ultrasound. These techniques interact differently with bone tissues because of the different physical phenomena on which they are based. The X-ray absorption is mainly controlled by the amount of mineral in the bone tissue and so it does not provide information about organic composition or microstructure, which significantly contribute to the mechanical properties of bone that actually influences fracture risk assessment. In contrast, the propagation of ultrasound in bone tissue is affected by the tissue structure, the organic and inorganic composition of the calcified matrix and by the properties of the bone marrow, which have a significant effect on US propagation[90,91]. X-ray diagnostic methods, because of their high costs, radiation dose (more higher in QCT than in DXA), large size of the equipment and limited availability of the dedicated infrastructures, are best suited for a second level diagnosis of osteoporosis rather than for screening purposes of primary health care. These limits of X-ray techniques have resulted in an increasing interest in developing reliable pre-screening tools for osteoporosis, such as QUS scanners[92,93], especially in those countries where the availability of DXA is very limited (Table 1).
Table 1.
Advantages and disadvantages of the most commonly used diagnostic methods currently available for the treatment of osteoporosis
| Dual- X-ray absorptiometry | Quantitative computed tomography | Quantitative ultrasound | |||
|
PROS |
CONS |
PROS |
CONS |
PROS |
CONS |
| High accuracy | X-rays | True density values | Higher radiation dose than DXA | Quick, cheap and radiation-free devices | Different guidelines for definition of osteoporosis |
| Assessment on reference sites (spine and hip) | High cost | Discriminates completely trabecular from cortical bone | Limited accessibility | Portable systems | Only peripheral anatomical sites |
| Several validation studies available in literature | Does not distinguish between cortical and trabecular components | High resolution | Shorter investigation times than DXA | Not directly comparable with the gold standard DXA | |
| Does not discriminate bone microarchitecture | Useful to investigate bone structural features and elastic properties of bone tissue | ||||
| Not useful as a screening tool | Not useful as a screening tool | Useful as a screening tool | |||
DXA: Dual X-ray absorptiometry.
Nowadays there is no procedure for standardizing the QUS devices currently in use and they are all applied to peripheral anatomical sites, instead of referring to lumbar spine and to femur that are the most critical and at the same time the most valuable diagnostic sites (Figure 5). In addition, while a large number of QUS devices are currently available on the market, particularly for measurement at the calcaneus, only for a very small minority of these the scientific validity has been confirmed by literature-available clinical studies.
Figure 5.
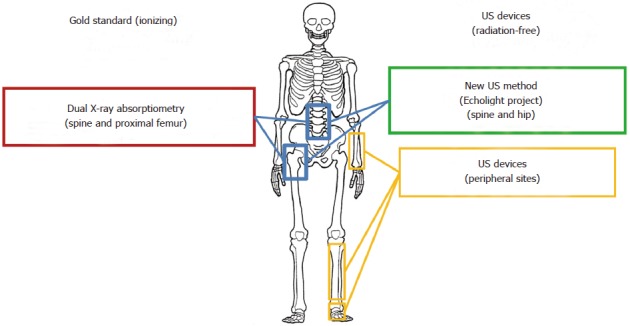
Available systems for osteoporosis diagnosis on main anatomical reference sites. US: Ultrasound.
It would be necessary to validate the US-based method for clinical and epidemiological screening at central skeleton sites, such as femur or femoral neck and vertebrae, as well as it has been done for DXA scan in order to encourage the widespread use of US techniques for the diagnosis of osteoporosis. The results of US diagnosis of osteoporosis on spine and hip could open up new perspectives for early and reliable diagnosis of osteoporosis.
The possibility of having a rapid, reliable, portable, non-ionizing and space-saving device allows to perform osteoporosis screening, reducing waiting lists and leaving the use of X-ray techniques only for a high level investigation for specific pathologic definitions and for some other therapeutic pathways. Through the new US-based techniques, benefits of the diagnostic DXA could be guaranteed without the contraindications due to X-ray use. In fact, future and reliable QUS methods could be applied to different populations including women, men, children, newborn and preterm infants.
Footnotes
Supported by Partially funded by FESR P.O. Apulia Region 2007-2013-Action 1.2.4, No. 3Q5AX31
P- Reviewer: La Montagna, G S- Editor: Song XX L- Editor: A E- Editor: Liu XM
References
- 1.Kanis JA, Burlet N, Cooper C, Delmas PD, Reginster JY, Borgstrom F, Rizzoli R; European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19:399–428. doi: 10.1007/s00198-008-0560-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albanese CV, De Terlizzi F, Passariello R. Quantitative ultrasound of the phalanges and DXA of the lumbar spine and proximal femur in evaluating the risk of osteoporotic vertebral fracture in postmenopausal women. Radiol Med. 2011;116:92–101. doi: 10.1007/s11547-010-0577-1. [DOI] [PubMed] [Google Scholar]
- 3.Ensrud KE, Thompson DE, Cauley JA, Nevitt MC, Kado DM, Hochberg MC, Santora AC, Black DM. Prevalent vertebral deformities predict mortality and hospitalization in older women with low bone mass. Fracture Intervention Trial Research Group. J Am Geriatr Soc. 2000;48:241–249. doi: 10.1111/j.1532-5415.2000.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper C. The crippling consequences of fractures and their impact on quality of life. Am J Med. 1997;103:12S–17S; discussion 17S-19S. doi: 10.1016/s0002-9343(97)90022-x. [DOI] [PubMed] [Google Scholar]
- 5.Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359:1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338:736–746. doi: 10.1056/NEJM199803123381107. [DOI] [PubMed] [Google Scholar]
- 7.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 8.Glüer CC, Wu CY, Jergas M, Goldstein SA, Genant HK. Three quantitative ultrasound parameters reflect bone structure. Calcif Tissue Int. 1994;55:46–52. doi: 10.1007/BF00310168. [DOI] [PubMed] [Google Scholar]
- 9.Hayes WC, Piazza SJ, Zysset PK. Biomechanics of fracture risk prediction of the hip and spine by quantitative computed tomography. Radiol Clin North Am. 1991;29:1–18. [PubMed] [Google Scholar]
- 10.Kaufman JJ, Einhorn TA. Ultrasound assessment of bone. J Bone Miner Res. 1993;8:517–525. doi: 10.1002/jbmr.5650080502. [DOI] [PubMed] [Google Scholar]
- 11.Njeh CF, Boivin CM, Langton CM. The role of ultrasound in the assessment of osteoporosis: a review. Osteoporos Int. 1997;7:7–22. doi: 10.1007/BF01623454. [DOI] [PubMed] [Google Scholar]
- 12.Fuerst T, Glüer CC, Genant HK. Quantitative ultrasound. Eur J Radiol. 1995;20:188–192. doi: 10.1016/0720-048x(95)00650-f. [DOI] [PubMed] [Google Scholar]
- 13.Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK. Radiation exposure in bone mineral density assessment. Appl Radiat Isot. 1999;50:215–236. doi: 10.1016/s0969-8043(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 14.Williams JE, Wells JC, Wilson CM, Haroun D, Lucas A, Fewtrell MS. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am J Clin Nutr. 2006;83:1047–1054. doi: 10.1093/ajcn/83.5.1047. [DOI] [PubMed] [Google Scholar]
- 15.Schousboe JT, Debold CR. Reliability and accuracy of vertebral fracture assessment with densitometry compared to radiography in clinical practice. Osteoporos Int. 2006;17:281–289. doi: 10.1007/s00198-005-2010-5. [DOI] [PubMed] [Google Scholar]
- 16.Garg MK, Kharb S. Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J Endocrinol Metab. 2013;17:203–210. doi: 10.4103/2230-8210.109659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, Licata A, Benhamou L, Geusens P, Flowers K, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- 18.Patel R, Blake GM, Rymer J, Fogelman I. Long-term precision of DXA scanning assessed over seven years in forty postmenopausal women. Osteoporos Int. 2000;11:68–75. doi: 10.1007/s001980050008. [DOI] [PubMed] [Google Scholar]
- 19.Johnell O, Gullberg B, Allander E, Kanis JA. The apparent incidence of hip fracture in Europe: a study of national register sources. MEDOS Study Group. Osteoporos Int. 1992;2:298–302. doi: 10.1007/BF01623186. [DOI] [PubMed] [Google Scholar]
- 20.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health. 2002;56:466–470. doi: 10.1136/jech.56.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blake GM, Chinn DJ, Steel SA, Patel R, Panayiotou E, Thorpe J, Fordham JN. A list of device-specific thresholds for the clinical interpretation of peripheral x-ray absorptiometry examinations. Osteoporos Int. 2005;16:2149–2156. doi: 10.1007/s00198-005-2018-x. [DOI] [PubMed] [Google Scholar]
- 22.Bouxsein ML, Palermo L, Yeung C, Black DM. Digital X-ray radiogrammetry predicts hip, wrist and vertebral fracture risk in elderly women: a prospective analysis from the study of osteoporotic fractures. Osteoporos Int. 2002;13:358–365. doi: 10.1007/s001980200040. [DOI] [PubMed] [Google Scholar]
- 23.Watts NB. Fundamentals and pitfalls of bone densitometry using dual-energy X-ray absorptiometry (DXA) Osteoporos Int. 2004;15:847–854. doi: 10.1007/s00198-004-1681-7. [DOI] [PubMed] [Google Scholar]
- 24.Gilsanz V, Wren T. Assessment of bone acquisition in childhood and adolescence. Pediatrics. 2007;119 Suppl 2:S145–S149. doi: 10.1542/peds.2006-2023G. [DOI] [PubMed] [Google Scholar]
- 25.Adams JE. Radiogrammetry and radiographic absorptiometry. Radiol Clin North Am. 2010;48:531–540. doi: 10.1016/j.rcl.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 26.Adams JE. Quantitative computed tomography. Eur J Radiol. 2009;71:415–424. doi: 10.1016/j.ejrad.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 27.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011;26:50–62. doi: 10.1002/jbmr.171. [DOI] [PubMed] [Google Scholar]
- 28.Engelke K, Adams JE, Armbrecht G, Augat P, Bogado CE, Bouxsein ML, Felsenberg D, Ito M, Prevrhal S, Hans DB, et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:123–162. doi: 10.1016/j.jocd.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Kalender WA. CT: the unexpected evolution of an imaging modality. Eur Radiol. 2005;15 Suppl 4:D21–D24. doi: 10.1007/s10406-005-0128-3. [DOI] [PubMed] [Google Scholar]
- 30.Wu SY, Jia HH, Hans D, Lan J, Wang LY, Li JX, Cai YZ. Assessment of volumetric bone mineral density of the femoral neck in postmenopausal women with and without vertebral fractures using quantitative multi-slice CT. J Zhejiang Univ Sci B. 2009;10:499–504. doi: 10.1631/jzus.B0820409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wegrzyn J, Roux JP, Arlot ME, Boutroy S, Vilayphiou N, Guyen O, Delmas PD, Chapurlat R, Bouxsein ML. Determinants of the mechanical behavior of human lumbar vertebrae after simulated mild fracture. J Bone Miner Res. 2011;26:739–746. doi: 10.1002/jbmr.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prevrhal S, Engelke K, Genant HK. pQCT: peripheral quantititive computed tomography. In: Grampp S, editor. Radiology of osteoporosis. 2nd ed. Berlin, Heidelberg: Springer-Verlag; 2008. pp. 143–162. [Google Scholar]
- 33.Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, Moyer-Mileur L, Shepherd J, Specker B, Ward K, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J Clin Densitom. 2008;11:59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim YM, Demissie S, Genant HK, Cheng X, Yu W, Samelson EJ, Kiel DP, Bouxsein ML. Identification of prevalent vertebral fractures using CT lateral scout views: a comparison of semi-automated quantitative vertebral morphometry and radiologist semi-quantitative grading. Osteoporos Int. 2012;23:1007–1016. doi: 10.1007/s00198-011-1774-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanis JA, Black D, Cooper C, Dargent P, Dawson-Hughes B, De Laet C, Delmas P, Eisman J, Johnell O, Jonsson B, et al. A new approach to the development of assessment guidelines for osteoporosis. Osteoporos Int. 2002;13:527–536. doi: 10.1007/s001980200069. [DOI] [PubMed] [Google Scholar]
- 36.Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Osteoporos Int. 1998;8 Suppl 4:S3–S80. [PubMed] [Google Scholar]
- 37.International Osteoporosis Foundation, European Society of Musculoskeletal Radiology. Vertebral Fracture Initiative Resource Document 2003. Available from: http://www iofbonehealth org/vfi/assets /resources/Resource-Document pdf 2009.
- 38.Njeh CF, Nicholson PHF, Langton CM. The physics of ultrasound applied to bone. In: Njeh CF, Hans D, Fuerst T, Gluer CC, Genant HK, et al., editors. Quantitative ultrasound: assessment of osteoporosis and bone status. London, UK: Martin Dunitz Ltd; 1999. pp. 67–75. [Google Scholar]
- 39.Hans D, Njeh CF, Genant HK, Meunier PJ. Quantitative ultrasound in bone status assessment. Rev Rhum Engl Ed. 1998;65:489–498. [PubMed] [Google Scholar]
- 40.Glüer CC. Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. The International Quantitative Ultrasound Consensus Group. J Bone Miner Res. 1997;12:1280–1288. doi: 10.1359/jbmr.1997.12.8.1280. [DOI] [PubMed] [Google Scholar]
- 41.Bossy E, Talmant M, Defontaine M, Patat F, Laugier P. Bidirectional axial transmission can improve accuracy and precision of ultrasonic velocity measurement in cortical bone: a validation on test materials. IEEE Trans Ultrason Ferroelectr Freq Control. 2004;51:71–79. doi: 10.1109/tuffc.2004.1268469. [DOI] [PubMed] [Google Scholar]
- 42.Hartl F, Tyndall A, Kraenzlin M, Bachmeier C, Gückel C, Senn U, Hans D, Theiler R. Discriminatory ability of quantitative ultrasound parameters and bone mineral density in a population-based sample of postmenopausal women with vertebral fractures: results of the Basel Osteoporosis Study. J Bone Miner Res. 2002;17:321–330. doi: 10.1359/jbmr.2002.17.2.321. [DOI] [PubMed] [Google Scholar]
- 43.Cadossi R, Canè V. Pathways of transmission of ultrasound energy through the distal metaphysis of the second phalanx of pigs: an in vitro study. Osteoporos Int. 1996;6:196–206. doi: 10.1007/BF01622735. [DOI] [PubMed] [Google Scholar]
- 44.Wüster C, Albanese C, De Aloysio D, Duboeuf F, Gambacciani M, Gonnelli S, Glüer CC, Hans D, Joly J, Reginster JY, et al. Phalangeal osteosonogrammetry study: age-related changes, diagnostic sensitivity, and discrimination power. The Phalangeal Osteosonogrammetry Study Group. J Bone Miner Res. 2000;15:1603–1614. doi: 10.1359/jbmr.2000.15.8.1603. [DOI] [PubMed] [Google Scholar]
- 45.Louis O, Moreels X, Osteaux M. Reproducibility of phalanx osteosonography and relation with forearm peripheral quantitative computed tomography: single finger versus average measurement on the last four fingers. Eur J Radiol. 1998;28:270–275. doi: 10.1016/s0720-048x(97)00180-0. [DOI] [PubMed] [Google Scholar]
- 46.Feltrin GP, Nardin M, Marangon A, Khadivi Y, Calderone M, De Conti G. Quantitative ultrasound at the hand phalanges: comparison with quantitative computed tomography of the lumbar spine in postmenopausal women. Eur Radiol. 2000;10:826–831. doi: 10.1007/s003300051013. [DOI] [PubMed] [Google Scholar]
- 47.Glüer CC, Eastell R, Reid DM, Felsenberg D, Roux C, Barkmann R, Timm W, Blenk T, Armbrecht G, Stewart A, et al. Association of five quantitative ultrasound devices and bone densitometry with osteoporotic vertebral fractures in a population-based sample: the OPUS Study. J Bone Miner Res. 2004;19:782. doi: 10.1359/JBMR.040304. [DOI] [PubMed] [Google Scholar]
- 48.De Terlizzi F, Battista S, Cavani F, Canè V, Cadossi R. Influence of bone tissue density and elasticity on ultrasound propagation: an in vitro study. J Bone Miner Res. 2000;15:2458–2466. doi: 10.1359/jbmr.2000.15.12.2458. [DOI] [PubMed] [Google Scholar]
- 49.Barkmann R, Lüsse S, Stampa B, Sakata S, Heller M, Glüer CC. Assessment of the geometry of human finger phalanges using quantitative ultrasound in vivo. Osteoporos Int. 2000;11:745–755. doi: 10.1007/s001980070053. [DOI] [PubMed] [Google Scholar]
- 50.Khaw KT, Reeve J, Luben R, Bingham S, Welch A, Wareham N, Oakes S, Day N. Prediction of total and hip fracture risk in men and women by quantitative ultrasound of the calcaneus: EPIC-Norfolk prospective population study. Lancet. 2004;363:197–202. doi: 10.1016/S0140-6736(03)15325-1. [DOI] [PubMed] [Google Scholar]
- 51.Bauer DC, Glüer CC, Cauley JA, Vogt TM, Ensrud KE, Genant HK, Black DM. Broadband ultrasound attenuation predicts fractures strongly and independently of densitometry in older women. A prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1997;157:629–634. [PubMed] [Google Scholar]
- 52.Krieg MA, Cornuz J, Ruffieux C, Van Melle G, Büche D, Dambacher MA, Hans D, Hartl F, Häuselmann HJ, Kraenzlin M, et al. Prediction of hip fracture risk by quantitative ultrasound in more than 7000 Swiss women > or = 70 years of age: comparison of three technologically different bone ultrasound devices in the SEMOF study. J Bone Miner Res. 2006;21:1457–1463. doi: 10.1359/jbmr.060615. [DOI] [PubMed] [Google Scholar]
- 53.Baroncelli GI, Federico G, Bertelloni S, de Terlizzi F, Cadossi R, Saggese G. Bone quality assessment by quantitative ultrasound of proximal phalanxes of the hand in healthy subjects aged 3--21 years. Pediatr Res. 2001;49:713–718. doi: 10.1203/00006450-200105000-00017. [DOI] [PubMed] [Google Scholar]
- 54.Gimeno Ballester J, Azcona San Julián C, Sierrasesúmaga Ariznabarreta L. [Bone mineral density determination by osteosonography in healthy children and adolescents: normal values] An Esp Pediatr. 2001;54:540–546. [PubMed] [Google Scholar]
- 55.Sawyer A, Moore S, Fielding KT, Nix DA, Kiratli J, Bachrach LK. Calcaneus ultrasound measurements in a convenience sample of healthy youth. J Clin Densitom. 2001;4:111–120. doi: 10.1385/jcd:4:2:111. [DOI] [PubMed] [Google Scholar]
- 56.Barkmann R, Rohrschneider W, Vierling M, Tröger J, de TF, Cadossi R, Heller M, Glüer CC. German pediatric reference data for quantitative transverse transmission ultrasound of finger phalanges. Osteoporos Int. 2002;13:55–61. doi: 10.1007/s198-002-8338-8. [DOI] [PubMed] [Google Scholar]
- 57.Zadik Z, Price D, Diamond G. Pediatric reference curves for multi-site quantitative ultrasound and its modulators. Osteoporos Int. 2003;14:857–862. doi: 10.1007/s00198-003-1456-6. [DOI] [PubMed] [Google Scholar]
- 58.Baroncelli GI, Federico G, Vignolo M, Valerio G, del Puente A, Maghnie M, Baserga M, Farello G, Saggese G. Cross-sectional reference data for phalangeal quantitative ultrasound from early childhood to young-adulthood according to gender, age, skeletal growth, and pubertal development. Bone. 2006;39:159–173. doi: 10.1016/j.bone.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 59.Aguado F, Revilla M, Villa LF, Rico H. Cortical bone resorption in osteoporosis. Calcif Tissue Int. 1997;60:323–326. doi: 10.1007/s002239900236. [DOI] [PubMed] [Google Scholar]
- 60.Guglielmi G, Njeh CF, de Terlizzi F, De Serio DA, Scillitani A, Cammisa M, Fan B, Lu Y, Genant HK. Palangeal quantitative ultrasound, phalangeal morphometric variables, and vertebral fracture discrimination. Calcif Tissue Int. 2003;72:469–477. doi: 10.1007/s00223-001-1092-0. [DOI] [PubMed] [Google Scholar]
- 61.Njeh CF, Richards A, Boivin CM, Hans D, Fuerst T, Genant HV. Factors influencing the speed of sound through the proximal phalanges. J Clin Densitom. 1999;2:241–249. doi: 10.1385/jcd:2:3:241. [DOI] [PubMed] [Google Scholar]
- 62.Pluskiewicz W. Quantitative ultrasound and hip fractures. J Bone Miner Res. 2007;22:1311; author reply 1312. doi: 10.1359/jbmr.070331. [DOI] [PubMed] [Google Scholar]
- 63.Camozzi V, De Terlizzi F, Zangari M, Luisetto G. Quantitative bone ultrasound at phalanges and calcaneus in osteoporotic postmenopausal women: influence of age and measurement site. Ultrasound Med Biol. 2007;33:1039–1045. doi: 10.1016/j.ultrasmedbio.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Soballa T, Schlegel J, Cadossi R, Isani R, Heilmann P, Ziegler R, Wüster C. Osteosonography of the phalanges of men. Med Klin (Munich) 1998;93:131–136. doi: 10.1007/BF03044830. [DOI] [PubMed] [Google Scholar]
- 65.Montagnani A, Gonnelli S, Cepollaro C, Mangeri M, Monaco R, Bruni D, Gennari C. Quantitative ultrasound at the phalanges in healthy Italian men. Osteoporos Int. 2000;11:499–504. doi: 10.1007/s001980070092. [DOI] [PubMed] [Google Scholar]
- 66.Montagnani A, Gonnelli S, Cepollaro C, Mangeri M, Monaco R, Gennari L, Gennari C. Usefulness of bone quantitative ultrasound in management of osteoporosis in men. J Clin Densitom. 2001;4:231–237. doi: 10.1385/jcd:4:3:231. [DOI] [PubMed] [Google Scholar]
- 67.Magkos F, Manios Y, Babaroutsi E, Sidossis LS. Contralateral differences in quantitative ultrasound of the heel: the importance of side in clinical practice. Osteoporos Int. 2005;16:879–886. doi: 10.1007/s00198-004-1761-8. [DOI] [PubMed] [Google Scholar]
- 68.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women: results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 69.Ekman A, Michaëlsson K, Petrén-Mallmin M, Ljunghall S, Mallmin H. Dual X-ray absorptiometry of hip, heel ultrasound, and densitometry of fingers can discriminate male patients with hip fracture from control subjects: a comparison of four different methods. J Clin Densitom. 2002;5:79–85. doi: 10.1385/jcd:5:1:079. [DOI] [PubMed] [Google Scholar]
- 70.Kanis JA, Johnell O, Oden A, De Laet C, de Terlizzi F. Ten-year probabilities of clinical vertebral fractures according to phalangeal quantitative ultrasonography. Osteoporos Int. 2005;16:1065–1070. doi: 10.1007/s00198-004-1805-0. [DOI] [PubMed] [Google Scholar]
- 71.Krieg MA, Barkmann R, Gonnelli S, Stewart A, Bauer DC, Del Rio Barquero L, Kaufman JJ, Lorenc R, Miller PD, Olszynski WP, et al. Quantitative ultrasound in the management of osteoporosis: the 2007 ISCD Official Positions. J Clin Densitom. 2008;11:163–187. doi: 10.1016/j.jocd.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 72.Barkmann R, Laugier P, Moser U, Dencks S, Padilla F, Haiat G, Heller M, Glüer CC. A method for the estimation of femoral bone mineral density from variables of ultrasound transmission through the human femur. Bone. 2007;40:37–44. doi: 10.1016/j.bone.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 73.Dencks S, Barkmann R, Padilla F, Haïat G, Laugier P, Glüer CC. Wavelet-based signal processing of in vitro ultrasonic measurements at the proximal femur. Ultrasound Med Biol. 2007;33:970–980. doi: 10.1016/j.ultrasmedbio.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 74.Barkmann R, Dencks S, Laugier P, Padilla F, Brixen K, Ryg J, Seekamp A, Mahlke L, Bremer A, Heller M, et al. Femur ultrasound (FemUS)--first clinical results on hip fracture discrimination and estimation of femoral BMD. Osteoporos Int. 2010;21:969–976. doi: 10.1007/s00198-009-1037-4. [DOI] [PubMed] [Google Scholar]
- 75.Grimal Q, Grondin J, Guérard S, Barkmann R, Engelke K, Glüer CC, Laugier P. Quantitative ultrasound of cortical bone in the femoral neck predicts femur strength: results of a pilot study. J Bone Miner Res. 2013;28:302–312. doi: 10.1002/jbmr.1742. [DOI] [PubMed] [Google Scholar]
- 76.Garra BS, Locher M, Felker S, Wear KA. Measurements of ultrasonic backscattered spectral centroid shift from spine in vivo: methodology and preliminary results. Ultrasound Med Biol. 2009;35:165–168. doi: 10.1016/j.ultrasmedbio.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muratore M, Conversano F, Casciaro E, Soloperto G, Franchini R, Greco A, Quarta E, Grimaldi A, Casciaro S. High Correlation between a New Ultrasonic Method for Spinal Densitometry and Dual X-ray Absorptiometry. Proceedings of EULAR 2013-Annual European Congress of Rheumatology. Annals of the Rheumatic Diseases. EULAR. 2013;72(Suppl 3):AB0624. [Google Scholar]
- 78.Conversano F, Casciaro E, Greco A, Pisani P, Franchini R, Quarta E, Muratore M, Casciaro S. Evaluation of Bone Mineral Density on Femoral Neck: Preliminary Clinical Validation of a New Ultrasonic Method. Proceedings of EULAR 2013-Annual European Congress of Rheumatology. Annals of the Rheumatic Diseases. EULAR. 2013;72(Suppl 3):AB0612. [Google Scholar]
- 79.Casciaro S, Conversano F, Casciaro E, Franchini R, Renna MD, Greco A, Quarta E, Quarta L, Muratore M. A New Ultrasonic Method for Diagnosis of Osteoporosis on Hip and Spine. ECTS 2013-Annual Congress of the European Calcified Tissue Society; 2013 May 18-21; Lisbon, Portugal. Bone Abstracts. 2013;1:323. [Google Scholar]
- 80.Muratore M, Conversano F, Casciaro E, Soloperto G, Franchini R, Greco A, Quarta E, Casciaro S. Strong Diagnostic Agreement between a Novel Ultrasound-Based Method for Lumbar Densitometry and Dual-Energy X-ray Absorptiometry. ECTS 2013-Annual Congress of the European Calcified Tissue Society; 2013 May 18-21; Lisbon, Portugal. Bone Abstracts. 2013;1:324. [Google Scholar]
- 81.Conversano F, Casciaro E, Greco A, Pisani P, Franchini R, Grimaldi A, Quarta E, Muratore M, Casciaro S. Comparative Assessment of Bone Mineral Density of the Femoral Neck between Dual-Energy X-ray Absorptiometry and a New Ultrasonic Method. ECTS 2013-Annual Congress of the European Calcified Tissue Society; 2013 May 18-21; Lisbon, Portugal. Bone Abstracts. 2013;1:322. [Google Scholar]
- 82.Casciaro S, Conversano F, Renna MD, Franchini R, Greco A, Casciaro E, Quarta E, Muratore M. A New Ultrasound Method for Osteoporosis Diagnosis on Main Anatomical Reference Sites. Osteopor Int. 2013;24(Suppl 1):S208. [Google Scholar]
- 83.Conversano F, Casciaro E, Soloperto G, Greco A, Franchini R, Grimaldi A, Muratore M, Casciaro S. High Correlation between a New Ultrasound-Based Methodology for Spinal Densitometry and Dual X-ray Absorptiometry. Osteopor Int. 2013;24(Suppl 1):S153 [. [Google Scholar]
- 84.Casciaro S, Conversano F, Casciaro E, Pisani P, Greco A, Franchini R, Quarta L, Muratore M. Comparative Assessment of a New Ultrasound-Based Methodology for Femoral Neck Densitometry and Dual X-Ray Absorptiometry. Osteopor Int. 2013;24(Suppl 1):S216. [Google Scholar]
- 85.Casciaro S, Conversano F, Casciaro E, Greco A, Franchini R, Raho L, Quarta L, Calcagnile F, Quarta E, Muratore M Validazione Clinica Preliminare di una Nuova Metodica Ultrasonica per la Densitometria Ossea del Collo del Femore. Atti del VII Congresso "OrtoMed"-Società Italiana di Ortopedia e Medicina, 2012 December 13-15; Firenze, 106 . [Google Scholar]
- 86.Casciaro S, Conversano F, Franchini R, Greco A, Casciaro E, Costanza D, Quarta L, Grimaldi A, Quarta E, Muratore M Nuova Metodica Ultrasonica per la Densitometria Ossea sui Principali Siti di Riferimento per la Diagnosi dell'Osteoporosi. Atti del VII Congresso "OrtoMed"-Società Italiana di Ortopedia e Medicina, 2012 December 13-15; Firenze, 105 . [Google Scholar]
- 87.Conversano F, Casciaro E, Soloperto G, Greco A, Raho L, Quarta L, Calcagnile F, Quarta E, Muratore M, Casciaro S Prima Validazione Clinica Di Una Nuova Metodica Ultrasonica per La Valutazione della Densità di Massa Ossea Sulla Colonna Vertebrale. Atti del XII Congresso Nazionale SIOMMMS, 2012 November 15-17; Bologna. Numero3-4-2012, 121 . [Google Scholar]
- 88.Casciaro S, Conversano F, Franchini R, Greco A, Casciaro E, Frisenda S, Calcagnile F, Quarta L, Grimaldi A, Quarta E, et al. Nuova Metodica Ultrasonica per la Valutazione della Densità di Massa Ossea su Colonna Vertebrale e Femore Prossimale. Atti del XII Congresso Nazionale SIOMMMS, 2012 November 15-17; Bologna Numero3-4-2012, 117 . [Google Scholar]
- 89.Casciaro S, Conversano F, Casciaro E, Greco A, Franchini R, Costanza D, Quarta L, Grimaldi A, Quarta E, Muratore M Primi Risultati della Validazione Clinica di una Nuova Metodica Ultrasonica per la Valutazione della Densità di Massa Ossea sul Collo del Femore. Atti del XII Congresso Nazionale SIOMMMS, 2012 November 15-17; Bologna, 80 . [Google Scholar]
- 90.Aula AS, Töyräs J, Hakulinen MA, Jurvelin JS. Effect of bone marrow on acoustic properties of trabecular bone--3D finite difference modeling study. Ultrasound Med Biol. 2009;35:308–318. doi: 10.1016/j.ultrasmedbio.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 91.Nicholson PH, Bouxsein ML. Bone marrow influences quantitative ultrasound measurements in human cancellous bone. Ultrasound Med Biol. 2002;28:369–375. doi: 10.1016/s0301-5629(01)00508-7. [DOI] [PubMed] [Google Scholar]
- 92.Pais R, Campean R, Simon S, Bolosiu CR, Muntean L, Bolosiu HD. Accuracy of Quantitative Ultrasound Parameters in the Diagnosis of Osteoporosis. Cent Eur J Med. 2010;5:478–485. [Google Scholar]
- 93.Holi MS, Radhakrishnan S, Swaranamani S, Ayavelan NA. Quantitative ultrasound technique for the assessment of osteoporosis and prediction of fracture risk. J Pure Appl Ultrason. 2005;27:55–60. [Google Scholar]
- 94.Bone Densitometry. Drugs.com. Available from: http://www.drugs.com/cg/bone-densitometry.html.


