Since their first discovery nearly 50 years ago1, anthracyclines, including doxorubicin (Adriamycin), daunorubicin (Cerubidine), epirubicin (Ellence) and idarubicin (Idamycin PFS) have been successfully developed as potent anti-cancer therapeutics with significant efficacy in lymphomas and many solid tumors. Particularly in patients with breast cancer, they are the primary choices of therapy. However, cardiotoxicityhas been a central limiting complication in treating patients since the agents acutely producearrhythmias, LV dysfunction, and pericarditis, and chronically lead to LV dysfunction and heart failure2. The toxicity is clearly dose-related with sharp rises in LV dysfunction with cumulative doses above 400-450 mg/m2 for doxorubicin.3 When cardiac imaging was employed, the incidence of HF was 5%, 26%, and 48% in patients receiving 400, 550, and 700 mg/m2 of doxorubicin. As a result, most oncologists typically limit the dose to 450-500 mg/m2. Children are especially vulnerable with rates of significant LV dysfunction of 5% at 15 yrs of follow-up, increasing to 10% for cumulative doses of >550 mg/m24. Heart failure may present many years after treatment. Mediastinal irradiation is an additional risk factor that may also be particularly problematic in children5. To date, our only proven protective measure is adherence to “stopping guidelines” for total dose.Unfortunately, thistypically limits the total dose an individual patient could receive, and for particularly problematic cancers, oncologists would like to use higher doses.
This issue may be particularly problematic in patients with breast cancer who are positive for human epidermal growth factor receptor 2 (Her2). This receptor is amplified in ~30% of breast cancers, leading to activation of signaling pathways that promote proliferation of the tumor cells and block tumor cell death6. Initial strategies for treatment included concurrent anthracycline and the anti-Her2 monoclonal antibody, trastuzumab (Herceptin). However this led to a high incidence of cardiotoxicity. Trastuzumab is now administered after completion of anthracycline dosing, and that has reduced the incidence of heart failure7, 8. Despite all of the above machinations to reduce cardiotoxicity of anthracyclines and trastuzumab, this still remains a thorn in the side of the oncologist and cardiologist. Clearly, strategies to limit cardiotoxicity while maintaining anti-cancer efficacy are desperately needed.
Earlier studies have uncovered a number of mechanisms involved in anthracycline-induced cardiac injury9, 10. The most extensively characterized mechanism is anthracycline-induced reactive oxygen species (ROS) and subsequent oxidative stress-induced DNA damage, mitochondrial dysfunction, sarcomere damage and loss of pro-survival signaling. Unfortunately, numerous approaches focusing on the use of antioxidants were generally ineffective, due at least in part to the fact that ROS-induced cytotoxicity is a shared mechanism for both cardiotoxicity and tumoricidal activity. Consequently, our history of identifying novel strategies to prevent cardiotoxicity while preserving anti-cancer efficacy has a very checkered past with few (or no) true successes11.
However, recent findings from the Yeh laboratory12 and the Lee laboratory reported in this issue of Circulation 13 offered two novel approaches to treat or prevent doxorubicin-induced cardiomypathy (Figure 1). Although starting from very different vantage points, both laboratories exploited the subtle but important molecular differences between cardiomyocytes and cancer cells. In both cases, these differences were used as the mechanistic basis to develop strategies to protect cardiomyocytes against Dox treatment without affecting its anti-cancer efficacy. It is indeed remarkable and exciting that novel, and possibly game-changing approaches to an age-old conundrum (anthracycline induced cardiotoxicity) were identified within a few months of one another.
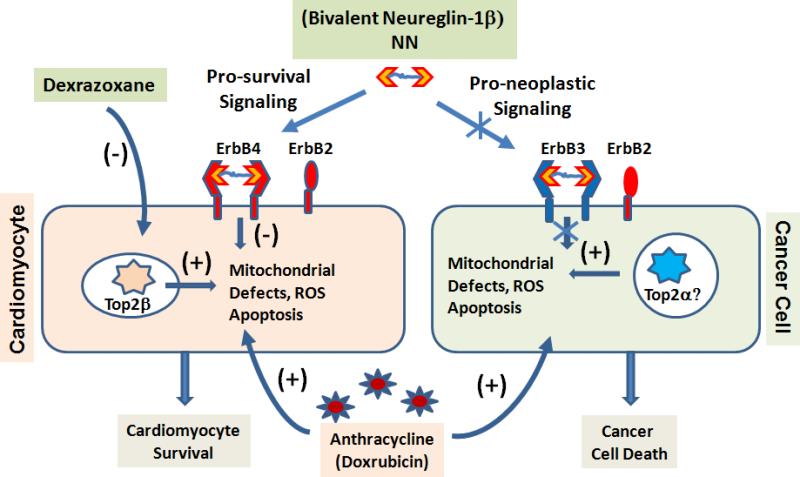
Figure 1.
Novel strategies in cardioprotection against anthracycline induced cardiotoxicity. Mechanism based engineering of biased NRG ligand and topoisomerase 2β inhibition prevents Dox induced cardiomyocyte death.
In the report by Zhang et, a critical role of topoisomerase-2β (Top2β) in anthracycline cardiotoxicity was discovered based on studies in both cultured cells and intact animals. They demonstrated that Dox- mediated DNA damage and subsequent mitochondrial defects were Top2β dependent processes in cardiomyocytes12. Dox-induced genomic DNA strand breaks, mitochondrial loss, cardiomyocyte death, and eventual LV dysfunction were markedly reduced in Top2β KO mice. Since Top2β is selectively expressed in heart, but Top2α is absent, a β isoform-specific inhibition of Top2 may offer a novel strategy to prevent Dox induced cardiotoxicity without affecting its anti-cancer activities if Top2α remains functional in cancer cells. This finding also breathes interesting new life into an age-old debate over whether, and/or how, dexrazoxane, an iron-chelator and topoisomerase inhibitor, is cardioprotective against Dox treatment14, 15. Indeed, after so many years of clinical observation, revealing the functional role of topoisomerase 2β in Dox-mediated cardiac injury might offer a bona fide mechanism for the beneficial effect of dexrazoxane. Largely because of that, another trial in the clinic with dexrazoxane or similar compounds is likely.
The report in this issue of Circulation from the Lee lab offered yet, another novel approach to achieve biased protection in cardiomyocytes against Dox treatment 13. Neuregulin-1β (NRG1B) functions through its receptors, ErbB2 and ErbB4, to exert potent cardioprotection16 against Dox-induced injury17. However, it can also induce oncogenic activity through receptor-mediated signaling in cancer cells. This poses an especially critical dilemma for breast cancer patients, even with staged Dox/trastuzumab therapy. Interestingly, ErbB4 is enriched in cardiomyocytes and can transduce protective signaling through homodimer interaction, while cancer cells express ErbB3 which requires ErbB3/ErbB2 heterodimer interaction to transduce downstream oncogenic effect. Lee and his colleagues exploited this property using a modified NGR1B ligand 18. By tethering two molecules with a linker, this bivalent NRG1B ligand (NN) has a strong preference for homodimer signaling over heterodimer signaling in the target cells18. In both in vitro and in vivo, the newly engineered NN showed a potent cardioprotective effect against Dox treatment (where ErbB4 homodimers signal downstream to pro-survival pathways) and also demonstrated significantly reduced pro-growth signaling and pro-neoplastic potential in cancer cells (where ErbB4 homodimers fail to signal to downstream pathways) 13.
Despite these exciting advancements, clinical translation of these newly established cardioprotective strategies need to be further validated. Both studies showed potent protective effects to attenuate Dox-induced cardiomyopahty in mice, but did not provide in vivo evidence of preserving anti-cancer potency in an animal model. Since Dox-induced cardiomyopathy can develop many years after treatment, longer term observation will also be critical. Nevertheless, these two reports represent significant advances in our current therapeutic approaches to anthracycline-induced cardiomyopathy. They also highlight the importance of better understanding of the disease processes and the therapeutic agents at a mechanistic and fundamental level in order to achieve rational design of therapies. These two successes demonstrate again that mechanism based engineering (genetic or protein) holds great promise to tackle complex and challenging diseases in addition to cardiotoxicity of cancer therapies.
Acknowledgements
The authors thank Ms. Wendy Buck for her kind assistance in manuscript preparation.
Funding Sources: This work was supported in part by National Institutes of Health grants HL070079, HL103205, HL108186, HL098954 (YW) and HL61688, HL114124, HL091799 (TF).
Footnotes
Conflict of Interest Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dimarco A, Gaetani M, Orezzi P, Scarpinato BM, Silvestrini R, Soldati M, Dasdia T, Valentini L. ‘Daunomycin’, a new antibiotic of the rhodomycin group. Nature. 1964;201:706–707. doi: 10.1038/201706a0. [DOI] [PubMed] [Google Scholar]
- 2.Von Hoff DD, Layard MW, Basa P, Davis HL, Jr., Von Hoff AL, Rozencweig M, Muggia FM. Risk factors for doxorubicin-induced congestive heart failure. Ann Intern Med. 1979;91:710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 3.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer. 2003;97:2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 4.Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. doi: 10.1056/NEJM199103213241205. [DOI] [PubMed] [Google Scholar]
- 5.Chen MH, Colan SD, Diller L. Cardiovascular disease: Cause of morbidity and mortality in adult survivors of childhood cancers. Circ Res. 2011;108:619–628. doi: 10.1161/CIRCRESAHA.110.224519. [DOI] [PubMed] [Google Scholar]
- 6.Jacot W, Fiche M, Zaman K, Wolfer A, Lamy PJ. The her2 amplicon in breast cancer: Topoisomerase iia and beyond. Biochim Biophys Acta. 2013;1836:146–157. doi: 10.1016/j.bbcan.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: What the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 8.Christodoulou C, Kostopoulos I, Kalofonos HP, Lianos E, Bobos M, Briasoulis E, Gogas H, Razis E, Skarlos DV, Fountzilas G. Trastuzumab combined with pegylated liposomal doxorubicin in patients with metastatic breast cancer. Phase ii study of the hellenic cooperative oncology group (hecog) with biomarker evaluation. Oncology. 2009;76:275–285. doi: 10.1159/000207504. [DOI] [PubMed] [Google Scholar]
- 9.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin- induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Geisberg C, Pentassuglia L, Sawyer DB. Cardiac side effects of anticancer treatments: New mechanistic insights. Curr Heart Fail Rep. 2012;9:211–218. doi: 10.1007/s11897-012-0098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladas EJ, Jacobson JS, Kennedy DD, Teel K, Fleischauer A, Kelly KM. Antioxidants and cancer therapy: A systematic review. J Clin Oncol. 2004;22:517–528. doi: 10.1200/JCO.2004.03.086. [DOI] [PubMed] [Google Scholar]
- 12.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 13.Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced pro-neoplastic potential. Circulation. 2013;128:XX–XXX. doi: 10.1161/CIRCULATIONAHA.113.002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasinoff BB, Herman EH. Dexrazoxane: How it works in cardiac and tumor cells. Is it a prodrug or is it a drug? Cardiovasc Toxicol. 2007;7:140–144. doi: 10.1007/s12012-007-0023-3. [DOI] [PubMed] [Google Scholar]
- 15.Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, Liu LF. Topoisomerase iibeta mediated DNA double-strand breaks: Implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67:8839–8846. doi: 10.1158/0008-5472.CAN-07-1649. [DOI] [PubMed] [Google Scholar]
- 16.Yan X, Morgan JP. Neuregulin1 as novel therapy for heart failure. Curr Pharm Des. 2011;17:1808–1817. doi: 10.2174/138161211796391010. [DOI] [PubMed] [Google Scholar]
- 17.Sawyer DB, Zuppinger C, Miller TA, Eppenberger HM, Suter TM. Modulation of anthracycline-induced myofibrillar disarray in rat ventricular myocytes by neuregulin-1beta and anti-erbb2: Potential mechanism for trastuzumab-induced cardiotoxicity. Circulation. 2002;105:1551–1554. doi: 10.1161/01.cir.0000013839.41224.1c. [DOI] [PubMed] [Google Scholar]
- 18.Jay SM, Kurtagic E, Alvarez LM, de Picciotto S, Sanchez E, Hawkins JF, Prince RN, Guerrero Y, Treasure CL, Lee RT, Griffith LG. Engineered bivalent ligands to bias erbb receptor-mediated signaling and phenotypes. J Biol Chem. 2011;286:27729–27740. doi: 10.1074/jbc.M111.221093. [DOI] [PMC free article] [PubMed] [Google Scholar]