Abstract
Purpose To review the current literature for the nonoperative and operative treatment for adult spinal deformity.
Recent Findings With more than 11 million baby boomers joining the population of over 60 years of age in the United States, the incidence of lumbar deformity is greatly increasing. Recent literature suggests that a lack of evidence exists to support the effectiveness of nonoperative treatment for adult scoliosis. In regards to operative treatment, current literature reports a varying range of improved clinical outcomes, curve correction, and complication rates. The extension of fusion to S1 compared with L5 and lower thoracic levels compared with L1 remains a highly controversial topic among literature.
Summary Most adult deformity patients never seek nonoperative or operative treatment. Of the few that seek treatment, many can benefit from nonoperative treatment. However, in selected patients who have failed nonoperative treatment and who are candidates for surgical intervention, the literature reflects positive outcomes related to surgical intervention as compared with nonoperative treatment despite varying associated ranges in morbidity and mortality rates. If nonoperative therapy fails in addressing a patient's complaints, then an appropriate surgical procedure that relieves neural compression, corrects excessive sagittal or coronal imbalance, and results in a solidly fused, pain-free spine is warranted.
Keywords: lumbar deformity, minimally invasive surgery, surgical treatment, sagittal balance, coronal deformity
Adult spinal deformity is one of the most challenging spinal disorders and by definition describes a complex spectrum of spinal diseases that present in adulthood including adult scoliosis, degenerative scoliosis, sagittal and coronal imbalance, and iatrogenic deformity, with or without spinal stenosis.1 With more than 11 million baby boomers in the United States joining the population of over 60 years of age, the number of patients with lumbar deformity including loss of lumbar lordosis, scoliosis, kyphosis, spondylolisthesis and lateral listhesis is greatly increasing.2 Fu et al3 retrospectively reported a high incidence of stenosis accompanying scoliosis among 36 adult patients, specifically 86% with central canal stenosis and 100% with foraminal stenosis. This article focuses on the current nonoperative and operative treatments for adult spinal deformity.
Etiology of Deformity
Presently, a scoliotic curve of >10 degrees exists in 1.4 to 12% of the population.4 Adult scoliosis can be divided into two main categories: (1) progression of childhood scoliosis or (2) degenerative scoliosis. The prevalence of both residual childhood scoliosis and degenerative scoliosis is ~6% in adults over the age of 50.5 Patients seeking treatment for back pain or radicular pain is typically an age-related degenerative phenomenon.
Degenerative, or de novo, scoliosis is usually seen in elderly adults over the age of 60. The scoliotic curve is caused by degeneration of the intervertebral discs and facet joints. The process of degeneration follows the predicted loss of disc hydration and disc space height, followed by increased loads on the facets leading to facet degeneration. The degeneration of these elements can cause instability in the spinal column leading to rotation, lateral listhesis, spondylolisthesis, kyphosis, or osteoporosis with vertebral body compression fractures. Axial, coronal, and sagittal deformity follows the asymmetric degenerative processes previously described. Unlike adult idiopathic scoliosis, with its array of curve patterns, the degenerative scoliosis curve typically occurs in the lumbar spine. As patients age and develop further degeneration of the vertebrae and surrounding structures, their curves may progress at a faster rate. Adult degenerative curves are typically of smaller magnitude than those seen in adult idiopathic scoliosis.6
Clinical presentation of adult spinal deformity varies greatly from minimal or no symptoms to severe pain with disability.7 A majority of patients remain asymptomatic with radiographic findings alone. However, when patients begin to complain of symptoms, these may vary from mild back pain without radiculopathy to severe back pain with neurogenic claudication, radiculopathy, and walking intolerance.8 A complete patient assessment requires not only appropriate imaging studies but a complete history and physical exam.
Diagnostic Evaluation and Imaging
During the physical examination of the patient, a three-dimensional assessment of the spine is appropriate to evaluate patient posture, neurological assessment, hip flexion contractures, leg length inequality, the presence of pelvic obliquity, evaluation of body habitus, and nutritional status.4 As such, proper imaging is technique-dependent and requires visualization of the entire spine in the coronal and sagittal plane, as well as the hip joints and femoral heads for accurate measurement of sagittal balance (sagittal vertical axis [SVA]) and spinopelvic parameters including pelvic incidence (PI), sacral slope (SS), and pelvic tilt (PT).
Imaging
Static standing full-length 36-inch anteroposterior and lateral radiographs of the whole spine, iliac crests, and hip joints should be obtained. All imaging should be taken with the patient standing with their knees fully extended. Specific lateral dynamic standing lumbar X-rays are also important to identify focal instability or spondylolisthesis. Bending films may help assess the flexibility of the scoliotic curve. Oblique X-rays can help identify the presence of pars defects. Once the appropriate radiographic studies have been obtained, the assessment of sagittal and coronal imbalance can then be determined.
Several radiographic measurements are required to determine the existing spinal deformity. Using the Cobb technique, the magnitude of the coronal deformity can be determined. The caudal and cranial extent of the deformity should be considered including the presence of fractional curves. Coronal malalignment measured by the center sacral ventral line should be evaluated and measured. Assessment of sagittal balance as measured by the C7 plumb line should also be considered and measured.8 The C7 plumb line is important in directing the surgeon as to the degree of global correction needed and assists in the identification of disc levels with asymmetric collapse.8
Magnetic resonance imaging (MRI) should be used to visualize any presence of central canal stenosis, facet hypertrophy, pedicular anomaly, foraminal encroachment, and degenerative disc disease. Computed tomography (CT) myelography can be used in place of an MRI to better observe the rotational deformity and bony anatomy. CT myelography allows detailed two-dimensional and three-dimensional views; MRI cannot assist surgeons in assessing anatomy in multiplanar views.9 In patients where osteoporosis may be a concern, it may be appropriate for such patients to have a dual-energy X-ray absorptiometry (DEXA) scan obtained to better assist in surgical planning.4
Spinopelvic Alignment
Normal lumbar lordosis increases in degree during development to skeletal maturity.10 Adult scoliotic patients demonstrate a significantly lower average lumbar lordosis and degree of thoracic kyphosis.10 If a patient's degree of curvature is significant enough to require surgery, the spinopelvic axis should be considered in regards to alignment. Literature has shown several correlations between pelvic and spinal parameters.10 11 12 PI serves as the main origin for proper spinal alignment and the structural basis of lumbar lordosis and thoracic kyphosis. Along with two positional parameters, SS and PT, the PI is critical in assessing the amount of correction for sagittal imbalance.10 11 Inadequate restoration of sagittal balance can lead to decreased surgical outcomes. Specific attention should be brought to patients who exhibit a high PI as they will require a greater increase in lumbar lordosis to restore sagittal balance.11 Rose et al13 were able to predict ideal sagittal balance using the formula, PI + lumbar lordosis + thoracic kyphosis ≤ 45 degrees, at 24 months. Lafage et al12 retrospectively reviewed the radiographs of 179 adult deformity patients to effectively predict the PT and global sagittal balance. Their study concluded that PI can be used to predict the PT and SVA as well as surgical parameters such as lumbar lordosis, thoracic kyphosis, and thoracolumbar kyphosis. The prediction of the postoperative SVA through PT served to increase the significant relationship between these two parameters.12 Smith et al14 conducted a comparative analysis of formulas for prediction of SVA. The study supported the use of the Lafage formulas as a better predictor of SVA due to its incorporation of PT and spinal compensatory changes.14 Spinopelvic measurements help shape the surgical approach to patients with adult spinal deformity who are being considered for surgical intervention. The sagittal parameters are proving to be more important in surgical considerations than the coronal deformity. As such, restoring sagittal balance should remain a priority from any surgical intervention.
Clinical Evaluation
Patients with adult deformity need comprehensive evaluations. Details of the patients' entire medical histories will be examined. Specifically, patients will be asked about their symptoms, treatment history, and if there is a family history of scoliosis. Patients who are being considered for operative intervention require a comprehensive review of systems. Consideration for coexisting medical comorbidities is paramount to the preoperative planning process. Symptomatic history should be reviewed with patients, specifically changes in body habitus, presence of gait disturbances, and any information regarding axial or radicular pain. Observed patient stance should be noted from an adequate distance to observe any trunk shift or presence of asymmetry in shoulder or pelvis and to evaluate the overall coronal and sagittal balance.2 Scoliotic patients with a sagittal imbalance will tend to extend hips, knees, and retrovert the pelvis to counterbalance the loss of lumbar lordosis.4 Evaluation should also involve flexion and side bending to evaluate curve rigidity. Any differences in leg length or pelvic obliquity should be noted along with palpation of the sacroiliac joints and trochanters to assess for any hip or knee contractures.2 Fu et al15 reported on the morbidity and mortality rates associated with spinal deformity surgeries from the Scoliosis Research Society (SRS) database. The study concluded that patients with higher American Society of Anesthesiologist grades were found to have increasingly higher rates of morbidity and related major complications. The study specified a total complication rate of 8.4% along with complication rates per grade ranging from 5.4 to 50%.15 Mok et al16 found that significantly increased rates of reoperation are related to comorbidities such as smoking, diabetes, age, alcohol usage, cardiovascular disease, and chronic medical conditions.
Osteoporosis is important when treating adult deformity patients. A complete review of osteoporosis and its medical management is beyond the scope of this article. However, it is a serious concern in patients being considered for surgical intervention. Medical management of osteoporosis can include the use of bisphosphonates that have been shown to increase bone density but require a period of lapse.17 18 Nonbisphosphonate antiresorptive agents can be taken during the period of lapse from bisphosphonates.18 A preoperative DEXA scan can assist in predicting the presence of osteoporosis, which is usually characterized by a femoral neck or spinal (L2–L4) T-score of less than −2.5.19 The presence of osteoporosis in patients who are candidates for spinal surgery can affect preoperative planning. These patients may need instrumentation for successful surgical results because of instability or deformity; however, certain principles should be observed. These include using multiple sites of fixation, accepting lesser degrees of deformity correction, and avoiding ending the instrumentation within kyphotic segments. Bone quality is an important consideration in evaluation the anterior or posterior approach to patient with thoracolumbar deformity. Anterior interbody grafts when placed in the presence of osteoporosis may indeed lead to iatrogenic end plate fracture, which may in turn cause implant subsidence. Great care must be taken to preserve such end plates during surgical preparation to avoid these complications. Advances in perioperative medical management, as well as improved instrumentation systems, may also contribute to improving patient outcomes in patients with osteoporosis (Fig. 1).
Figure 1.
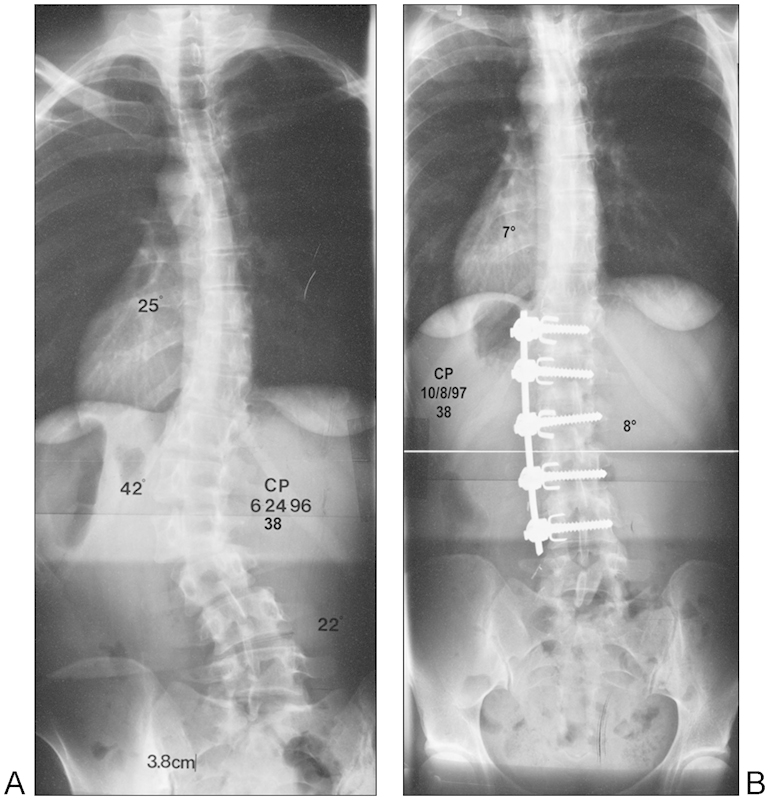
A 38-year-old woman with scoliosis as demonstrated by anteroposterior (A) X-ray who underwent anterior instrumentation and fusion (B).
Treatment of Deformity
Nonoperative Treatment
Nonsurgical management is offered as the first line of conservative care but its efficacy is not well supported in the literature. In the absence of neurological deficit or significant instability, nonoperative care should be initiated with all patients. In the absence of cardiovascular contraindications, physical therapy, stretching, and aerobic conditioning are encouraged in such patients.8 Other treatments for deformity include core strengthening, specifically aqua therapy, walking, cycling, and weight lifting.20 Nonsteroidal anti-inflammatory drugs (NSAIDs) can often alleviate the arthritic type of symptoms. However, it is critical to counsel the patients about the specific side effects such as gastrointestinal irritation, elevation of blood pressure, thrombocytopenia, and renal toxicity. Other nonnarcotic medicines such as antidepressants and anticonvulsants could also be considered. If patients suffer from night pain and difficulty sleeping, tricyclic antidepressants can offer assistance with these problems. Gabapentin and pregabalin may decrease neurogenic pain and assist with sleep. However, the major side effect of such medications is sedation, and it is not well tolerated by some patients. If a patient cannot tolerate the side effects during the day, they often take it only at night for sleep and nerve pain relief.
In an acute exacerbation of back pain and radiculopathy, there may be some role for narcotic pain medicine. However, the chronic use of these medicines is not recommended. The long-term side effects and addiction potential should be strongly considered when prescribing these medicines. Vestergaard et al21 reviewed the risk of fractures associated with the treatment of morphine and opiate therapy. Using a nationwide register of 10,015 (8%) case subjects and 12,108 (3.2%) control subjects who used morphine or opiates, the study reported an increased fracture risk associated with morphine, fentanyl, methadone, oxycodone, nicomorphine, ketobemidone, tramadol, and codeine.21 In a separate study, Vestergaard et al22 also reviewed the risk of fractures associated with the use of NSAIDs. The study reported an increase in fracture risk associated with low doses of common pain relievers such as ibuprofen, diclofenac, and acetaminophen; however, they attributed this increase to falls as opposed to weakened bone structure.22
Only a few patients can benefit from temporary relief with bracing in combination with exercise as it has been shown to be ineffective in significantly preventing curve progression in adult spinal deformity.2 8 20 Despite the possibility for pain relief, brace discomfort and trunk muscle balancing should be weighed in the decision making to use as a form of nonoperative treatment. It is quite reasonable to consider the use of alternative treatments in the nonoperative management. These include acupuncture, chiropractic care, yoga, and Pilates.
Injection therapy is another alternative nonoperative option. Although the evidence for injection therapy as a tool to decrease or eliminate pain is not founded in the literature, patients often experience extended pain relief with injection therapy, thus reducing the need for medication in such patients.23 Injection therapy can include epidural steroids, facet blocks, nerve root blocks, and trigger-point injections. Nonoperative treatments may be used alone or in any combination. The goal of such nonoperative modalities is to enable the patient to manage the pain and to maintain functional abilities.
Several studies have assessed the outcomes of adult deformity patients following nonoperative treatments as compared with surgical treatment.9 24 25 26 27 28 Smith et al25 reported on a total of 317 patients who experienced back pain in adults with scoliosis. From the 317 patients involved in the retrospective review, 147 patients underwent surgery for adult deformity and 170 were treated nonoperatively. At the 2-year follow-up evaluation, patients receiving operative treatment demonstrated significant improvement in patient outcomes reporting lower Numerical Rating Scale and Oswestry Disability Index (ODI) scores as compared with patients receiving nonoperative treatment. The study concluded that surgical treatment can result in significantly improved back pain in those patients who are symptomatic.25 Li et al26 reported on 83 patients, 34 of whom were treated operatively and 49 who were treated nonoperatively. Compared with the nonoperative group at 2-year follow-up, patients in the operative group demonstrated significant improvement in pain, self-image, mental health, health-related quality of life, and overall satisfaction with their treatment.26 As reflected in literature, a lack of evidence exists to support the effectiveness of nonoperative treatment.8 20 To strengthen the evidence, Glassman et al29 assessed the documented costs of nonoperative treatment for adult deformity and concluded that despite the substantial mean treatment over a 2-year period at a mean cost of $10,815 per patient for the nonoperative care, there was no improvement in health status of these patients.29
Operative Treatment
After conservative treatments have been exhausted, surgical correction can be considered as an option. The discussion between the patient and the surgeon must include a fulfillment of nonoperative efforts and a disclosure of the potential risks of surgical intervention. The main goals of surgery involve thorough decompression of involved neural elements and reestablishment of both coronal and sagittal balance.1 20 Surgical indications include symptomatic low back pain and/or leg pain greater than 6 months in duration that has been treated with nonoperative efforts with worsening cardiopulmonary function, documented progression of the curve, presentation of neurological symptoms, declining sagittal or coronal balance, and/or decompensation.2 8 Contraindications for surgery include cardiopulmonary conditions or associated comorbidities, later stages of osteopenia, and physical or mental condition that would impair patients for surgery conditioning and preparation and appropriate rehabilitation.4
Development of surgical strategies includes a thorough preoperative assessment, sagittal and coronal balance, and degenerative lumbar strategies. As mentioned, surgical correction of spinal deformities relies upon the restoration of spinal balance and alignment. The chosen surgical approach to be used should be assessed during preoperative planning. Recent studies have attempted to provide a surgical guideline based on preoperative imaging studies and patient symptoms. Gupta6 and other surgical management assessments1 2 20 specify that radiographic findings are indicative of chosen surgical intervention. Bess et al30 reported that increased coronal plane deformity in radiographic findings was evidence for operative management in younger patients. However, the study suggested that pain and disability measurements were indicative for operative management in older patients instead of radiographic measurements.30 Glassman et al31 reported that adult scoliotic patients receiving operative treatment were more likely to have higher degrees of thoracic and thoracolumbar/lumbar curves, more persistent leg pain, and higher patient-reported daily back pain as opposed to patients receiving nonoperative treatment.31 Lowe et al32 and Ames et al33 emphasize the importance of classifying curve patterns from preoperative radiographs to formulate appropriate surgical treatment algorithms. The goals for appropriate surgical treatment can vary from patient to patient. Depending on severity of symptoms, preoperative imaging studies, and patient comorbidities, surgical intervention can vary from decompressive procedures alone to combined anterior and posterior fusions in conjunction with decompression of the neural elements.
Decompression Alone
Decompression alone can be considered in small degenerative curves without instability, with complaints of radiculopathy more than back pain. This procedure can decrease the neurological compression and alleviate claudication or radicular symptoms but can possibly result in further postoperative deformity or the development of iatrogenic postsurgical spinal instability.4 34 Matsumura et al34 reported the clinical outcomes of 25 patients with degenerative scoliosis who underwent microscopic bilateral decompression via a unilateral (MDBU) approach. The study reported a recovery rate of 58.7% at 2-year follow-up and concluded that the MDBU approach preservation of the posterior elements minimizes the development of postoperative instability.34 Transfeldt et al35 retrospectively evaluated the functional outcomes of 85 patients who underwent decompression alone, a limited decompression, or a decompression and full curve fusion. Complications were lower (10%) among the patients who underwent decompression alone whereas the decompression and fusion group had a higher rate (56%) of complications. In regards to lumbar lordosis and curve correction, only the decompression and fusion group demonstrated improvements, and the decompression alone and limited decompression groups remained the same. Patient-reported outcomes also clearly indicated that the decompression and fusion patients were more satisfied with their outcomes than the patients who underwent decompression alone.35 Kelleher et al36 retrospectively reported on the effectiveness of minimally invasive decompression alone in treating 28 patients with stenosis combined with or without spondylolisthesis and scoliosis. The study concluded that scoliotic patients, specifically with lateral listhesis, demonstrated a significantly higher revision rate.36 Liu et al37 retrospectively reported improved patient reported outcomes for 112 patients following decompression with or without fusion at a mean 5.7 years of follow-up, thereby demonstrating that decompression alone or with fusion is sufficient in treating pain associated with deformity.37
Anterior Alone
Traditional anterior alone approaches for treating adult deformities is anterior lumbar interbody fusion (ALIF) with anterior instrumentation and most recently lateral interbody fusion, which has been popularized with limited indications. Anterior spinal fusion with instrumentation has been shown to have excellent deformity correction and high patient satisfaction in well-selected patients. Despite improvements in instrumentation and technique, literature on rigid anterior instrumented fusion for adult scoliosis is sparse.38 39 40 41 42 43 44 45 46 47 48
The decision to use an anterior approach may provide benefits such as preservation of additional motion segments. However, several variables must be taken into account when making this decision. Adult scoliosis can be associated with marked degeneration, so it is paramount to consider the condition of the segments above and below the planned fusion and adjust fusion levels accordingly to attain a balanced stable fusion. Deviren et al49 has shown that flexibility of the major and lumbosacral curve decreases with patient age and that the curve magnitude and patient age are the main predictors of structural flexibility. Understanding of major and fractional curve flexibility is useful information in estimating how surgical options for deformity correction may change on adult deformity patients. When addressing the correctability of a curve, the sagittal, coronal, and axial alignments must be considered. Essential to the correction are balance in the coronal and sagittal planes, factors that are more important than the absolute degree of correction. Understanding curve rigidity is useful in surgical planning in adults who may be less tolerant of residual end vertebral tilt and obliquity of the subjacent disc.
In selected patients, anterior alone approaches may restore sagittal and coronal balance, thereby restoring disc height and foraminal height while restoring lumbar lordosis. There are several advantages of anterior alone surgery in addition to curve correction, including the ability to enhance fusion by the use of a larger interbody graft or cage or via a larger fusion surface area, indirect decompression of the neural elements, global curve correction, and preservation of the posterior spinal musculature.50 An anterior approach also allows for complete visualization of the disc space. Despite the advantages of an anterior approach, there are several potential complications associated with this procedure, which include vascular damage, ileus and left iliac artery thrombosis, pseudarthrosis, ilioinguinal and iliohypogastric nerve injuries, subsidence, graft displacement, ureteral and/or bladder damage, abdominal hernia, and retrograde ejaculation in male patients, most of which are very low.50 51 52 53 54 Crandall and Revella55 compared ALIF to posterior lumbar interbody fusion (PLIF) and reported no significant difference in clinical outcomes or complication rates. Radiographic outcomes among both ALIF and PLIF patients were similar as well as the patient-reported Visual Analog Scale (VAS) and ODI outcomes.55 Kim et al56 retrospectively reported on the associated complications and patient satisfaction rates of 62 patients who underwent anterior thoracolumbar approach for treatment of deformity. The study reported an 82.2% satisfaction rate following surgery, but many patients reported dissatisfaction in regards to their anterior incision, specifically related to moderate to severe pain over the thoracolumbar scar area (32.3%) and a bulging appearance (43.5%). Patients (24.2%) also reported functional disturbance with their daily activities as a result of the anterior incision.
In 2004, Bergey et al57 described the lateral transpsoas approach for 21 patients who were diagnosed with discogenic pain, adjacent-level instability, and degenerative scoliosis. At a mean follow-up of 3.1 years, 30% of patients reported groin/thigh paresthesia and 27% reported groin/thigh pain. Despite the risk of postoperative numbness and/or pain, patients reported an average 5.9 decrease in VAS scores. The study reported that unlike traditional anterior approaches, the lateral approach greatly facilitated access to the L1–L4 level lumbar spine and lacked mobilization of the greater vessels; however, access to the L5–S1 level, occasionally the L4–L5 level, can be complicated by the iliac crest.57 Mundis et al53 conducted a literature review of the lateral approach, suggesting significant improvements in VAS and ODI scores and degrees of coronal and sagittal correction and decreased blood loss and shortened hospital stays in comparison with open anterior procedures. The literature did reflect differing complications rate; however, major complications were low and most studies cited a transient motor deficit but reported resolution at subsequent follow-up visits.53 Isaacs et al58 reported a 12.1% major complication rate, similar to that reported in the literature, among 107 patients who underwent lateral interbody fusion for the treatment of adult scoliosis.
The use of lateral interbody fusion combined with posterior pedicle instrumentation may be more beneficial when compared with an traditional approach as it minimizes vessel injury, bowel injury, and sexual dysfunction and may provide better access to the interbody space.58 However, the lateral approach has its own potential complications mostly related to manipulation of the lumbar plexus. Tormenti et al59 cited a higher curve correction of 70.2% for eight patients who underwent a combined lateral transpsoas and posterior approach compared with 44.7% for four patients who underwent posterior approach only. However, six combined approach patients reported postoperative thigh paresthesias or dysesthesias and two demonstrated sustained motor radiculopathies. Paresthesia resolved in all but one patient by 10 months postoperatively, and motor radiculopathy had resolved in the two patients by 3 months. Patient-reported VAS scores were similar for both groups.59 Wang and Mummaneni60 retrospectively demonstrated a 97% fusion rate at treated levels and 20-degree coronal balance correction in 23 patients who underwent lateral interbody fusion with posterior pedicle instrumentation. Dakwar et al61 reported on a total of 25 patients, eight of whom received lateral interbody fusion with posterior pedicle screw instrumentation and 15 received lateral plates. At 1-year follow-up, the study reported improved VAS scores by an average of 5.7 points preoperatively to postoperatively, and ODI scores showed an improvement of 23.7%. Postoperatively, only three patients (12%) were found to have sensory deficits that resolved by the 3-month follow-up. In regards to correction, one-third of the patients in the study did not demonstrate a correction of sagittal balance; however, 20 (80%) patients at 6 months postoperatively had demonstrated solid fusion. Their study determined that lateral interbody fusion with or without posterior instrumentation is a reasonable alternative to other anterior and posterior approaches to correct adult degenerative deformities.61 We do believe that stand-alone lateral interbody fusion is indicated in a select group of patients: those with good bone stock so that the cage subsidence is not a significant concern, advanced facet arthropathy in which there is no significant instability, and smaller magnitude of deformity that the coronal and sagittal balance is not required to be addressed (Figs. 1 and 2).
Figure 2.
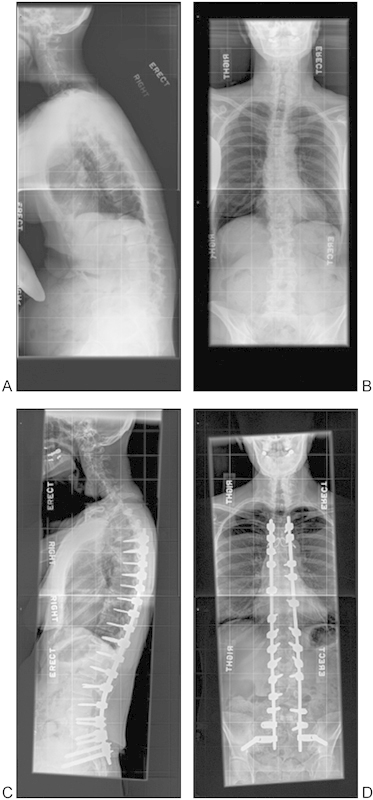
Patient with sagittal plane deformity (A, B) who had posterior only surgery with Ponte osteotomies (C, D) at 5-year follow-up.
Posterior Approaches
The posterior approaches in treating adult lumbar spine deformity have been the mainstay of surgical treatment for quite some time. These include PLIF and transforaminal lumbar interbody fusion (TLIF) in conjunction with posterior instrumentation. Li et al62 retrospectively reported on 46 patients who underwent TLIF for treatment of degenerative scoliosis. The study reported postoperative improvements in mean Cobb angles, mean lordosis angles, and mean segmental lordosis angles. The correction rates were reported as 67.8% for Cobb angles, 44.4% for mean lordosis angles, and 80% for mean segmental lordosis angles. The study also noted improved patient reported outcomes and an 81% satisfaction rate with the surgery.62 Burneikiene et al63 suggested that TLIF was an effective treatment for scoliosis but can be accompanied by a high complication rate. Similar to Li et al,52 their study reported similar improvements in patient-reported outcomes and radiographic improvements among 29 patients. However, the study reported a high complication rate of 31% for systemic complications, 49% for hardware or surgery-related complications, and a revision rate of 28%.63 Charosky et al64 reported an overall complication rate of 39% with a revision rate of 26% due to mechanical or neurological complications for 306 patients following anterior surgery, an anteroposterior approach, or posterior surgery only for treatment of adult deformity. The study noted that risk factors to take into consideration when discussing surgical management included the number of levels requiring instrumentation, fusion to S1, whether or not the patient required a pedicle subtraction osteotomy (PSO), and preoperative pelvic parameters, specifically if the PT was greater than 26 degrees.64 Wu et al65 reported significantly improved ODI scores and a 76.9% satisfaction rate for 26 patients following instrumented PLIF for degenerative lumbar scoliosis. Radiographic results demonstrated significant improvements in scoliosis and lumbar lordosis angles.65 Pateder et al66 retrospectively reported on the radiographic outcomes and complication rates for 45 posterior only patients compared with 35 patients who underwent combined anterior-posterior surgery for treatment of adult lumbar scoliosis. The study demonstrated no significant difference between curve or balance correction between the posterior only or combined groups. The study reported similar major complications rates between outpatient posterior only (24%) and combined (23%) procedures versus a 45% complication rate among inpatient procedures.66
Combined Anterior-Posterior Fusion
A combined approach is necessary for a larger degree of curvature along with coronal and sagittal imbalance.6 8 However, combined procedures can lead to increased operative time and can result in an increase patient medical stress leading to increased complication and morbidity rate.6 53 Despite the disadvantages of a combined approach, literature demonstrates higher fusion rates, higher degree of deformity correction, and better overall patient-reported outcomes.6 59 67 68 With proper patient selection, the TLIF/PLIF approach has the advantage of a high fusion rate and optimal neural decompression.69 70 If more than one level is involved, the PLIF approach has its disadvantages including the limitation of the degree correction at any single level, the increased operative time associated with each additional level, the increased amount of blood loss and risk to neural elements associated with each additional level, and the increased expense resulting from the combined use of cages and screws needed to complete the case.69 Zimmerman et al71 reported on a total of 35 patients; 16 patients received posterior spinal fusion. A complication rate of 49% was reported at the 2-year follow-up, 26% of which were major complications. The study reported a statistically significant improvement in ODI scores with an average of 13.3 points, and the Short Form Survey-36 physical and mental outcomes showed an improvement of 13.7% and 16.2% preoperatively to postoperatively.71 Zeng et al72 reported on 43 patients divided into two group; 21 patients were treated with posterior long segment fusion. At the 2-year follow-up, the study found radiographic evidence of grade 1 fusion in 65% of the patients, and 9.5% failed to fuse. Patients also reported an overall satisfaction rate of 86% (Fig. 2).72
Good et al68 retrospectively compared the clinical outcomes of 24 patients who underwent a combined fusion with 24 patients who underwent a posterior only approach at a minimum 2-year follow-up. The study concluded that both groups demonstrated similar postoperative radiographic correction and alignment along with no significant difference in postoperative complications. Despite the higher rates of pseudarthrosis in the combined group, the patient-reported SRS and ODI scores were similar and demonstrated significant improvement from baseline.68 Tsai et al73 retrospectively evaluated the clinical outcomes of 58 patients who underwent instrumented PLIF. VAS and ODI scores improved 5 and 15.9 points, respectively, at the minimum 2-year follow-up. Patients also reported a 72% satisfaction rate.73 Ploumis et al74 reported improved functional outcomes and decreased pain levels for 28 patients who underwent posterolateral fusion for degenerative lumbar scoliosis. As discussed earlier, lateral interbody fusion minimizes the morbidity related to traditional anterior thoracolumbar approach, which becomes a good alternative procedure for patients who require combined anterior-posterior fusion (Fig. 3). A “hybrid” technique such as using lateral interbody fusion with open pedicle screws or extreme lateral interbody fusion (XLIF) with percutaneous pedicle screws can be used as a less disruptive technique, resulting in a potential avoidance of complications associated with traditional open anterior or posterior approaches.60 61 67
Figure 3.
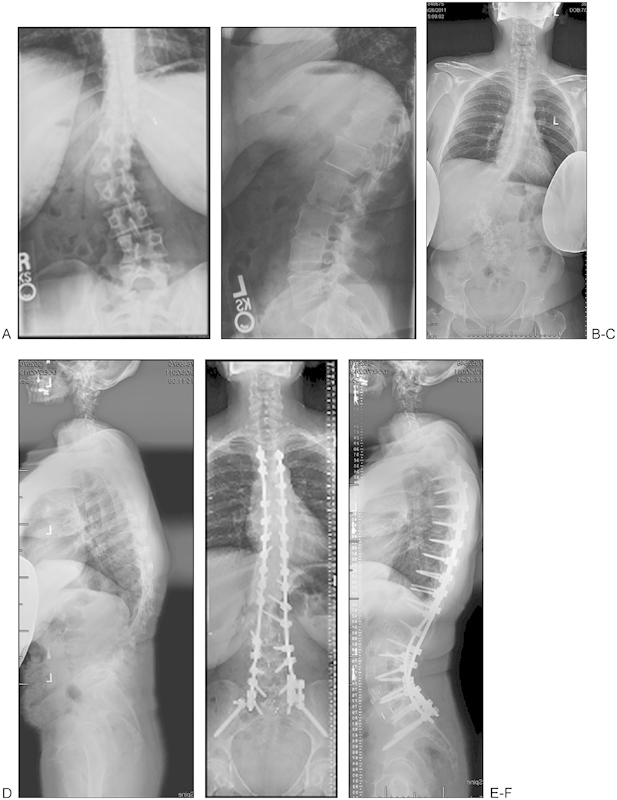
Patient with progressive adult scoliosis. Anteroposterior (A) and lateral (B) X-rays obtained in 2004 demonstrate the progression compared with anteroposterior (C) and lateral (D) X-rays obtained in 2009. Postoperative anteroposterior (E) and lateral (F) X-rays demonstrate that the patient underwent a two-staged extreme lateral interbody fusion (XLIF) with posterior spinal fusion (PSF).
Osteotomy
Surgical correction of sagittal imbalance can be accomplished using a variety of posterior osteotomies that have varying degrees of correction, complication rates, and challenges in execution. Smith-Peterson osteotomy (SPO), PSO, and vertebral column resection (VCR) have been the traditional procedures of choice. The SPO, originally described in 194575 for correction of a fixed kyphotic deformity in the rheumatoid spine, involves resection of the lamina, bilateral facets, and ligaments at the selected level. After ensuring that the exiting nerve roots are free, the osteotomy is closed. The SPO is a relatively safe technique that can be performed at multiple levels and can yield up to 10 degrees of lordosis per level. Each millimeter of bone resected provides ~1 degree of correction, with a maximal correction of 10 to 15 degrees. Chang et al76 reported an 81.9 degree mean lordosis correction with 17.1cm mean correction of sagittal balance in 83 patients that underwent SPO. Of the patients included in the study, 72 patients were satisfied with their treatment.76
In 1985, Thomasen77 described a PSO for treating ankylosing spondylitis. The PSO is a challenging procedure involving all three columns and may be associated with significant blood loss. When comparing SPO to PSO, pedicle subtraction demonstrates a larger degree of correction and proves to be more effective despite the demanding technicality of the procedure. In a PSO, the posterior elements are resected (lamina, spinous process, facets) in addition to the pedicles and a wedge-shaped portion of the posterior vertebral body. The anterior vertebral body is then fractured during closing the osteotomy, and ~30 degrees of lordotic correction is achieved. LaFage et al78 reported the degrees of correction per level following PSO of 70 patients. Procedures at L1 and L2 showed 24 degrees of correction in 6 and 15 patients, respectively. L3 showed 25 degrees of correction in 29 patients, and L4 showed 22 degrees of correction in 20 patients. Overall, PSO resulted in a postoperative increase of 29 degrees of lordosis and 8 degrees of kyphosis. Cho et al79 found a 34% complication rate associated with PSO in 141 patients. Rose et al13 reported the clinical outcomes of 40 patients who underwent pedicle subtraction for the treatment of adult scoliosis. At 2-year follow-up, ODI and SRS scores improved from preoperative values and 55% of patients maintained sagittal balance. However, these procedures usually resulted in significantly increased operative times, blood loss, and complication rates including but not limited to aortic rupture, cauda equina syndrome, paraplegia, and superior mesenteric artery syndrome.
The VCR was first described by Bradford80 in the late 1980s, involves the complete resection of the vertebral body and posterior elements of the affected level, and is reserved for advanced coronal/sagittal deformities that cannot be corrected with a PSO or SPO alone. The complications from this procedure are potentially devastating, and it generally requires long operative times. Suk et al81 reported 16 patients who underwent a posterior VCR. Their indication for this procedure was scoliosis of more than 80 degrees with flexibility less than 25%. There was a 59% deformity correction reported. However, complications were encountered in four patients, including one with complete permanent paralysis.
Fusion to L5 versus S1
Extending fusion to S1 compared with L5 is a highly controversial topic within the literature. By extending the fusion to S1, the stability increases but the procedure also runs the risk of increasing chances of pseudarthrosis, operative time, revision rate, and the rate of sacral insufficiency fractures.6 8 To reduce complications associated with the extension of fusion, anterior instrumentation such as fixed angle plates and vertebral body compression screws along with iliac screws or bolts is warranted.1 Cho et al82 reported significantly improved coronal imbalance and lateral listhesis among 22 patients who underwent fusion to S1 as compared with 28 patients who underwent fusion to L5. Schwab et al7 found that the perioperative complications of patients who had fixation at the sacrum or below were higher than those patients whose fixation ended above S1. Harimaya et al83 retrospectively reviewed clinical and radiographic results of 33 patients who had lumbosacral fixation failure. The results demonstrated that sole use of bilateral S1 screws can lead to loosening or pullout in conjunction with L5–S1 cage or graft collapse or subsidence. Using distal fixation to the S1 screws strengthens the sacral screws from collapsing.83 Cho et al84 reviewed 250 adult patients who underwent primary or revision surgery for treatment of scoliosis. More osteotomy procedures and fusions to the sacrum were performed on the revision patients as compared with the primary group. In regards to complication rates, the revision group demonstrated significantly similar complication rates in relation to the extended fusion. The revision group, specifically patients aged 40 to 60, demonstrated better improvement from the surgery despite the reported high complication rate of 58.9%.84 Cho et al85 retrospectively reported on the clinical outcomes following fusion to the sacrum. The study found that 42% of patients demonstrated sagittal decompensation following posterior instrumentation at a mean follow-up of 3.5 years. Other reported outcomes in this group included a significant loss of sagittal C7 plumb line and a declining improvement in lumbar lordosis.85 Kebaish86 reviewed current techniques and indications for the use of sacropelvic fixation; including long fusions that extend instrumentation into the pelvis. Due to the dynamic biomechanical forces and physiology of the sacrum, complications occur such as implant prominence, implant loosening, and instrument-related issues. Common techniques for sacropelvic fixation include the use of S2 alar iliac screws; this technique is associated with reduced implant prominence and allows the use of a single rod reducing the number or required connections. Kebaish86 reported decreased complication rates when compared with available techniques; at the 2-year follow-up, 1.9% required additional surgery. Considering the management of the fractional curve (L5 obliquity) and the rigid fractional curve (oblique takeoff at lumbosacral junction), if the fractional curve is not addressed, the patient will never obtain coronal balance. In adult scoliosis, rigid fractional curves need to be included in the fusion and managed with ALIF or TLIF to prevent potential coronal decompensation.
Complications
Complications are associated with all procedures. Surgical treatment for adult deformity, regardless of corrective procedure, is associated with high complication rates.6 Literature-reported complications include pseudarthrosis, infection, neurological deficits, cerebrospinal fluid leaks, failure of implants, catastrophic injury, adjacent segment disease, systemic complications, and pulmonary embolism.6 16 Sansur et al87 reported an overall complication rate of 13.4% for treatment of adult scoliosis. The study concluded that osteotomies, revisions, and combined approaches resulted in significantly higher complication rates.87 Smith et al88 retrospectively reviewed the rate of complications associated with surgery for scoliosis in relation to patient age. The study concluded that older patients in comparison with younger patients had a significantly greater complication rate at 2-year follow-up. However, despite the greater risk of complications, elderly patients, in comparison to younger patients, demonstrated a greater extent of improvement in standardized measures of disability, pain, and health-related quality of life.77 Smith et al89 reported a total infection (superficial and deep) rate of 3.7% from 5801 adult scoliosis patients following surgery. The rate of infection also increased when surgery included a fusion.89 Mok et al16 reported a reoperation rate of 26% at 2-year follow-up among 89 patients who underwent surgery to treat adult deformity as compared with 65% of patients who did not require a revision procedure. Scheufler et al90 retrospectively reviewed the clinical outcomes and complications of 30 adult scoliotic patients. The study reported a major complication rate of 59.9% and a minor complication rate of 23.4%. Despite the high major complication rate, 83% of patients were satisfied with the treatment at the 1-year follow-up.90
Conclusion
Most adult deformity patients never seek nonoperative or operative treatment. Of the few who seek treatment, many can benefit from nonoperative treatment. However, in selected patients who have failed nonoperative treatment and who are candidates for surgical intervention, the literature reflects positive outcomes related to surgical intervention as compared with nonoperative treatment despite varying associated ranges in morbidity and mortality rates.9 24 26 27 28 If nonoperative therapy fails in addressing a patient's complaints, then an appropriate surgical procedure that relieves neural compression, corrects excessive sagittal or coronal imbalance, and results in a solidly fused, pain-free spine is warranted.1 20 The chosen surgical approach to be used should be assessed during preoperative planning to best determine the approach to be used. Regardless of corrective procedure, surgical treatment for adult deformity can be associated with high complication rates.6 However, despite the increased complication risk, the literature demonstrates that elderly patients experience a greater improvement in standardized measures of disability, pain, and health-related quality of life in comparison with younger patients.88 When assessing the correct surgical procedure, extension of fusion to S1 compared with L5 should be considered as it is a highly controversial topic among literature. By extending the fusion to S1, the stability increases but the procedure also runs the risk of increasing chances of pseudarthrosis, operative time, revision rate, and the rate of sacral insufficiency fractures.6 20 Surgical goals should focus on restoring sagittal balance, as this is predictive of improved patient outcomes.7
Footnotes
Disclosures C. A. Patty, None M. A. Scott, None H. L. Price, None L. F. Hamlin, None T. L. Williams, None V. Deviren, Consultant: NuVasive, Stryker, Medtronic, Guidepoint; Royalties: NuVasive; Fellowship Support: OREF, Omega, AOSpine J. S. Uribe, Consultant: NuVasive, Orthofix; Research Grant: NuVasive D. O. Orndorff, Paid Consultant: Integra, Stryker J. A. Youssef, Consultant: NuVasive, Integra; Royalties: NuVasive (potential conflict for an interbody device), Integra, Aesculap (potential conflict for an interbody device), Osprey Biomedical, Amedica (potential conflict for an interbody device); Research Support: DePuy, NuVasive, BioSurface Engineering Technologies, Globus Medical, Advanced Technologies in Regenerative Medicine, Axial Biotech; Ownership: ASC Durango; Stock/Options: Amedica, Pioneer, Vertiflex, Benvenue, Paradigm, Promethean Surgical Devices, ISD, Spinicity
References
- 1.Birknes J K, White A P, Albert T J, Shaffrey C I, Harrop J S. Adult degenerative scoliosis: a review. Neurosurgery. 2008;63(3, Suppl):94–103. doi: 10.1227/01.NEU.0000325485.49323.B2. [DOI] [PubMed] [Google Scholar]
- 2.Silva F E, Lenke L G. Adult degenerative scoliosis: evaluation and management. Neurosurg Focus. 2010;28:E1–E10. doi: 10.3171/2010.1.FOCUS09271. [DOI] [PubMed] [Google Scholar]
- 3.Fu K M, Rhagavan P, Shaffrey C I, Chernavvsky D R, Smith J S. Prevalence, severity, and impact of foraminal and canal stenosis among adults with degenerative scoliosis. Neurosurgery. 2011;69:1181–1187. doi: 10.1227/NEU.0b013e31822a9aeb. [DOI] [PubMed] [Google Scholar]
- 4.Youssef J A, Hamlin L F. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2009. Adult spinal deformity; pp. 721–726. [Google Scholar]
- 5.Vanderpool D W, James J I, Wynne-Davies R. Scoliosis in the elderly. J Bone Joint Surg Am. 1969;51:446–455. [PubMed] [Google Scholar]
- 6.Gupta M C. Degenerative scoliosis. Options for surgical management. Orthop Clin North Am. 2003;34:269–279. doi: 10.1016/s0030-5898(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 7.Schwab F, Lafage V, Farcy J P. et al. Surgical rates and operative outcome analysis in thoracolumbar and lumbar major adult scoliosis: application of the new adult deformity classification. Spine. 2007;32:2723–2730. doi: 10.1097/BRS.0b013e31815a58f2. [DOI] [PubMed] [Google Scholar]
- 8.Sengupta K. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2012. Adult spinal deformity; pp. 349–367. [Google Scholar]
- 9.Crawford C H, Glassman S D. Fusing adult degenerative deformities of the lumbar spine. Semin Spine Surg. 2011;23:222–226. [Google Scholar]
- 10.Mendoza-Lattes S, Ries Z, Gao Y, Weinstein S L. Natural history of spinopelvic alignment differs from symptomatic deformity of the spine. Spine. 2010;35:E792–E798. doi: 10.1097/BRS.0b013e3181d35ca9. [DOI] [PubMed] [Google Scholar]
- 11.Patel A A, Daubs M. Spinal-pelvic relationships: implications for spine surgery. SpineLine. 2010;11(2):18–21. [Google Scholar]
- 12.Lafage V, Schwab F, Vira S, Patel A, Ungar B, Farcy J P. Spino-pelvic parameters after surgery can be predicted: a preliminary formula and validation of standing alignment. Spine. 2011;36:1037–1045. doi: 10.1097/BRS.0b013e3181eb9469. [DOI] [PubMed] [Google Scholar]
- 13.Rose P S, Bridwell K H, Lenke L G. et al. Role of pelvic incidence, thoracic kyphosis, and patient factors on sagittal plane correction following pedicle subtraction osteotomy. Spine. 2009;34:785–791. doi: 10.1097/BRS.0b013e31819d0c86. [DOI] [PubMed] [Google Scholar]
- 14.Smith J S, Bess S, Shaffrey C I. et al. International Spine Study Group . Dynamic changes of the pelvis and spine are key to predicting postoperative sagittal alignment after pedicle subtraction osteotomy: a critical analysis of preoperative planning techniques. Spine. 2012;37:845–853. doi: 10.1097/BRS.0b013e31823b0892. [DOI] [PubMed] [Google Scholar]
- 15.Fu K M, Smith J S, Polly D W Jr. et al. Scoliosis Research Society Morbidity and Mortality Committee . Correlation of higher preoperative American Society of Anesthesiology grade and increased morbidity and mortality rates in patients undergoing spine surgery. J Neurosurg Spine. 2011;14:470–474. doi: 10.3171/2010.12.SPINE10486. [DOI] [PubMed] [Google Scholar]
- 16.Mok J M, Cloyd J M, Bradford D S. et al. Reoperation after primary fusion for adult spinal deformity: rate, reason, and timing. Spine. 2009;34:832–839. doi: 10.1097/BRS.0b013e31819f2080. [DOI] [PubMed] [Google Scholar]
- 17.Harris K B, Nealy K L, Jackson D J, Thornton P L. The clinical use of denosumab for the management of low bone mineral density in postmenopausal women. J Pharm Pract. 2012;25:310–318. doi: 10.1177/0897190012442061. [DOI] [PubMed] [Google Scholar]
- 18.Watts N B, Diab D L. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95:1555–1565. doi: 10.1210/jc.2009-1947. [DOI] [PubMed] [Google Scholar]
- 19.Sirola J, Rikkonen T, Tuppurainen M, Honkanen R, Kröger H. Should risk of bone fragility restrict weight control for other health reasons in postmenopausal women?—A ten year prospective study. Maturitas. 2012;71:162–168. doi: 10.1016/j.maturitas.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Russo A, Bransford R, Wagner T, Lee M J, Chapman J R. Adult degenerative scoliosis insights, challenges, and treatment outlook. Curr Orthop Pract. 2008;19:357–365. [Google Scholar]
- 21.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with the use of morphine and opiates. J Intern Med. 2006;260:76–87. doi: 10.1111/j.1365-2796.2006.01667.x. [DOI] [PubMed] [Google Scholar]
- 22.Vestergaard P, Rejnmark L, Mosekilde L. Fracture risk associated with use of nonsteroidal anti-inflammatory drugs, acetylsalicylic acid, and acetaminophen and the effects of rheumatoid arthritis and osteoarthritis. Calcif Tissue Int. 2006;79:84–94. doi: 10.1007/s00223-006-0020-8. [DOI] [PubMed] [Google Scholar]
- 23.DePalma M J, Slipman C W. Evidence-informed management of chronic low back pain with epidural steroid injections. Spine J. 2008;8:45–55. doi: 10.1016/j.spinee.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Everett C R, Patel R K. A systematic literature review of nonsurgical treatment in adult scoliosis. Spine. 2007;32(19, Suppl):S130–S134. doi: 10.1097/BRS.0b013e318134ea88. [DOI] [PubMed] [Google Scholar]
- 25.Smith J S Shaffrey C I Berven S et al. Spinal Deformity Study Group Improvement of back pain with operative and nonoperative treatment in adults with scoliosis Neurosurgery 20096586–93.; discussion 93–94 [DOI] [PubMed] [Google Scholar]
- 26.Li G, Passias P, Kozanek M. et al. Adult scoliosis in patients over sixty-five years of age: outcomes of operative versus nonoperative treatment at a minimum two-year follow-up. Spine. 2009;34:2165–2170. doi: 10.1097/BRS.0b013e3181b3ff0c. [DOI] [PubMed] [Google Scholar]
- 27.Smith J S, Shaffrey C I, Berven S. et al. Spinal Deformity Study Group . Operative versus nonoperative treatment of leg pain in adults with scoliosis: a retrospective review of a prospective multicenter database with two-year follow-up. Spine. 2009;34:1693–1698. doi: 10.1097/BRS.0b013e3181ac5fcd. [DOI] [PubMed] [Google Scholar]
- 28.Bridwell K H, Glassman S, Horton W. et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine. 2009;34:2171–2178. doi: 10.1097/BRS.0b013e3181a8fdc8. [DOI] [PubMed] [Google Scholar]
- 29.Glassman S D, Carreon L Y, Shaffrey C I. et al. The costs and benefits of nonoperative management for adult scoliosis. Spine. 2010;35:578–582. doi: 10.1097/BRS.0b013e3181b0f2f8. [DOI] [PubMed] [Google Scholar]
- 30.Bess S, Boachie-Adjei O, Burton D. et al. International Spine Study Group . Pain and disability determine treatment modality for older patients with adult scoliosis, while deformity guides treatment for younger patients. Spine. 2009;34:2186–2190. doi: 10.1097/BRS.0b013e3181b05146. [DOI] [PubMed] [Google Scholar]
- 31.Glassman S D, Schwab F J, Bridwell K H, Ondra S L, Berven S, Lenke L G. The selection of operative versus nonoperative treatment in patients with adult scoliosis. Spine. 2007;32:93–97. doi: 10.1097/01.brs.0000251022.18847.77. [DOI] [PubMed] [Google Scholar]
- 32.Lowe T, Berven S H, Schwab F J, Bridwell K H. The SRS classification for adult spinal deformity: building on the King/Moe and Lenke classification systems. Spine. 2006;31(19, Suppl):S119–S125. doi: 10.1097/01.brs.0000232709.48446.be. [DOI] [PubMed] [Google Scholar]
- 33.Ames C P, Smith J S, Scheer J K. et al. Impact of spinopelvic alignment on decision making in deformity surgery in adults. J Neurosurg Spine. 2012;16:547–564. doi: 10.3171/2012.2.SPINE11320. [DOI] [PubMed] [Google Scholar]
- 34.Matsumura A, Namikawa T, Terai H. et al. The influence of approach side on facet preservation in microscopic bilateral decompression via a unilateral approach for degenerative lumbar scoliosis. Clinical article. J Neurosurg Spine. 2010;13:758–765. doi: 10.3171/2010.5.SPINE091001. [DOI] [PubMed] [Google Scholar]
- 35.Transfeldt E E, Topp R, Mehbod A A, Winter R B. Surgical outcomes of decompression, decompression with limited fusion, and decompression with full curve fusion for degenerative scoliosis with radiculopathy. Spine. 2010;35:1872–1875. doi: 10.1097/BRS.0b013e3181ce63a2. [DOI] [PubMed] [Google Scholar]
- 36.Kelleher M O, Timlin M, Persaud O, Rampersaud Y R. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine. 2010;35:E981–E987. doi: 10.1097/BRS.0b013e3181c46fb4. [DOI] [PubMed] [Google Scholar]
- 37.Liu W, Chen X S, Jia L S, Song D W. The clinical features and surgical treatment of degenerative lumbar scoliosis: a review of 112 patients. Orthop Surg. 2009;1:176–183. doi: 10.1111/j.1757-7861.2009.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn H K. Anterior stabilization of thoracolumbar injuries. Clin Orthop Relat Res. 1984;189:116–124. [PubMed] [Google Scholar]
- 39.Hopf C G Eysel P Dubousset J Operative treatment of scoliosis with Cotrel-Dubousset-Hopf instrumentation. New anterior spinal device Spine 199722618–627.; discussion 627–628 [DOI] [PubMed] [Google Scholar]
- 40.Kaneda K Shono Y Satoh S Abumi K New anterior instrumentation for the management of thoracolumbar and lumbar scoliosis. Application of the Kaneda two-rod system Spine 1996211250–1261.; discussion 1261–1262 [DOI] [PubMed] [Google Scholar]
- 41.Turi M, Johnston C E II, Richards B S. Anterior correction of idiopathic scoliosis using TSRH instrumentation. Spine. 1993;18:417–422. [PubMed] [Google Scholar]
- 42.Majd M E, Castro F P Jr, Holt R T. Anterior fusion for idiopathic scoliosis. Spine. 2000;25:696–702. doi: 10.1097/00007632-200003150-00008. [DOI] [PubMed] [Google Scholar]
- 43.Sweet F A, Lenke L G, Bridwell K H, Blanke K M, Whorton J. Prospective radiographic and clinical outcomes and complications of single solid rod instrumented anterior spinal fusion in adolescent idiopathic scoliosis. Spine. 2001;26:1956–1965. doi: 10.1097/00007632-200109150-00005. [DOI] [PubMed] [Google Scholar]
- 44.Dwyer A F. Experience of anterior correction of scoliosis. Clin Orthop Relat Res. 1973;93:191–214. doi: 10.1097/00003086-197306000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Goel V K, Gilbertson L G. Basic science of spinal instrumentation. Clin Orthop Relat Res. 1997;335:10–31. [PubMed] [Google Scholar]
- 46.Spiegel D A, Flynn J M, Drummond D S. Anterior instrumentation in the treatment of scoliosis. Univ of PA Orthopaedic Journal. 1998;11:19–26. [Google Scholar]
- 47.Smith J A, Deviren V, Berven S, Bradford D S. Does instrumented anterior scoliosis surgery lead to kyphosis, pseudarthrosis, or inadequate correction in adults? Spine. 2002;27:529–534. doi: 10.1097/00007632-200203010-00014. [DOI] [PubMed] [Google Scholar]
- 48.Simmons E D. Surgical treatment of patients with lumbar spinal stenosis with associated scoliosis. Clin Orthop Relat Res. 2001;384:45–53. doi: 10.1097/00003086-200103000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Deviren V, Berven S, Kleinstueck F, Antinnes J, Smith J A, Hu S S. Predictors of flexibility and pain patterns in thoracolumbar and lumbar idiopathic scoliosis. Spine. 2002;27:2346–2349. doi: 10.1097/00007632-200211010-00007. [DOI] [PubMed] [Google Scholar]
- 50.Than K D, Wang A C, Rahman S U. et al. Complication avoidance and management in anterior lumbar interbody fusion. Neurosurg Focus. 2011;31:E6. doi: 10.3171/2011.7.FOCUS11141. [DOI] [PubMed] [Google Scholar]
- 51.Guyer R D, Fulp T. St. Louis, MO: Quality Medical Publishing, Inc.; 1999. Perirectus retroperitoneal approach for anterior lumbar interbody fusion; pp. 203–216. [Google Scholar]
- 52.Zdeblick T A. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. Mini-ALIF with cages; pp. 321–333. [Google Scholar]
- 53.Mundis G M, Akbarnia B A, Phillips F M. Adult deformity correction through minimally invasive lateral approach techniques. Spine. 2010;35(26, Suppl):S312–S321. doi: 10.1097/BRS.0b013e318202495f. [DOI] [PubMed] [Google Scholar]
- 54.Jarrett C D, Heller J G, Tsai L. Anterior exposure of the lumbar spine with and without an “access surgeon”: morbidity analysis of 265 consecutive cases. J Spinal Disord Tech. 2009;22:559–564. doi: 10.1097/BSD.0b013e318192e326. [DOI] [PubMed] [Google Scholar]
- 55.Crandall D G, Revella J. Transforaminal lumbar interbody fusion versus anterior lumbar interbody fusion as an adjunct to posterior instrumented correction of degenerative lumbar scoliosis: three year clinical and radiographic outcomes. Spine. 2009;34:2126–2133. doi: 10.1097/BRS.0b013e3181b612db. [DOI] [PubMed] [Google Scholar]
- 56.Kim Y B, Lenke L G, Kim Y J. et al. The morbidity of an anterior thoracolumbar approach: adult spinal deformity patients with greater than five-year follow-up. Spine. 2009;34:822–826. doi: 10.1097/BRS.0b013e31818e3157. [DOI] [PubMed] [Google Scholar]
- 57.Bergey D L, Villavicencio A T, Goldstein T, Regan J J. Endoscopic lateral transpsoas approach to the lumbar spine. Spine. 2004;29:1681–1688. doi: 10.1097/01.brs.0000133643.75795.ef. [DOI] [PubMed] [Google Scholar]
- 58.Isaacs R E, Hyde J, Goodrich J A, Rodgers W B, Phillips F M. A prospective, nonrandomized, multicenter evaluation of extreme lateral interbody fusion for the treatment of adult degenerative scoliosis: perioperative outcomes and complications. Spine. 2010;35(26, Suppl):S322–S330. doi: 10.1097/BRS.0b013e3182022e04. [DOI] [PubMed] [Google Scholar]
- 59.Tormenti M J, Maserati M B, Bonfield C M, Okonkwo D O, Kanter A S. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7–E13. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 60.Wang M Y, Mummaneni P V. Minimally invasive surgery for thoracolumbar spinal deformity: initial clinical experience with clinical and radiographic outcomes. Neurosurg Focus. 2010;28:E9. doi: 10.3171/2010.1.FOCUS09286. [DOI] [PubMed] [Google Scholar]
- 61.Dakwar E, Cardona R F, Smith D A, Uribe J S. Early outcomes and safety of the minimally invasive, lateral retroperitoneal transpsoas approach for adult degenerative scoliosis. Neurosurg Focus. 2010;28:E8–E14. doi: 10.3171/2010.1.FOCUS09282. [DOI] [PubMed] [Google Scholar]
- 62.Li F, Chen Q, Chen W, Xu K, Wu Q. Posterior-only approach with selective segmental TLIF for degenerative lumbar scoliosis. J Spinal Disord Tech. 2011;24:308–312. doi: 10.1097/BSD.0b013e3181f9a7d5. [DOI] [PubMed] [Google Scholar]
- 63.Burneikiene S, Nelson E L, Mason A, Rajpal S, Serxner B, Villavicencio A T. Complications in patients undergoing combined transforaminal lumbar interbody fusion and posterior instrumentation with deformity correction for degenerative scoliosis and spinal stenosis. Surg Neurol Int. 2012;3:25. doi: 10.4103/2152-7806.92933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charosky S Guigui P Blamoutier A Roussouly P Chopin D; Study Group on Scoliosis. Complications and risk factors of primary adult scoliosis surgery: a multicenter study of 306 patients Spine 201237693–700. [DOI] [PubMed] [Google Scholar]
- 65.Wu C H, Wong C B, Chen L H, Niu C C, Tsai T T, Chen W J. Instrumented posterior lumbar interbody fusion for patients with degenerative lumbar scoliosis. J Spinal Disord Tech. 2008;21:310–315. doi: 10.1097/BSD.0b013e318148b256. [DOI] [PubMed] [Google Scholar]
- 66.Pateder D B, Kebaish K M, Cascio B M, Neubaeur P, Matusz D M, Kostuik J P. Posterior only versus combined anterior and posterior approaches to lumbar scoliosis in adults: a radiographic analysis. Spine. 2007;32:1551–1554. doi: 10.1097/BRS.0b013e318067dc0e. [DOI] [PubMed] [Google Scholar]
- 67.Anand N, Baron E M, Thaiyananthan G, Khalsa K, Goldstein T B. Minimally invasive multilevel percutaneous correction and fusion for adult lumbar degenerative scoliosis: a technique and feasibility study. J Spinal Disord Tech. 2008;21:459–467. doi: 10.1097/BSD.0b013e318167b06b. [DOI] [PubMed] [Google Scholar]
- 68.Good C R, Lenke L G, Bridwell K H. et al. Can posterior-only surgery provide similar radiographic and clinical results as combined anterior (thoracotomy/thoracoabdominal)/posterior approaches for adult scoliosis? Spine. 2010;35:210–218. doi: 10.1097/BRS.0b013e3181c91163. [DOI] [PubMed] [Google Scholar]
- 69.Wang M Y. PLIF for the treatment of adult spinal deformity. Acta Neurochir (Wien) 2011;153:557. doi: 10.1007/s00701-010-0910-4. [DOI] [PubMed] [Google Scholar]
- 70.Resnick D K. Lumbar interbody fusion: current status. Contemporary Spine Surgery. 2009;10:1–6. [Google Scholar]
- 71.Zimmerman R M, Mohamed A S, Skolasky R L, Robinson M D, Kebaish K M. Functional outcomes and complications after primary spinal surgery for scoliosis in adults aged forty years or older: a prospective study with minimum two-year follow-up. Spine. 2010;35:1861–1866. doi: 10.1097/BRS.0b013e3181e57827. [DOI] [PubMed] [Google Scholar]
- 72.Zeng Y, White A P, Albert T J. et al. Surgical strategy in adult lumbar scoliosis: the utility of categorization into two groups based on primary symptom, each with two year minimum follow-up. Spine. 2012;37:E556–E561. doi: 10.1097/BRS.0b013e31824af5c6. [DOI] [PubMed] [Google Scholar]
- 73.Tsai T H, Huang T Y, Lieu A S. et al. Functional outcome analysis: instrumented posterior lumbar interbody fusion for degenerative lumbar scoliosis. Acta Neurochir (Wien) 2011;153:547–555. doi: 10.1007/s00701-010-0909-x. [DOI] [PubMed] [Google Scholar]
- 74.Ploumis A, Albert T J, Brown Z, Mehbod A A, Transfeldt E E. Healos graft carrier with bone marrow aspirate instead of allograft as adjunct to local autograft for posterolateral fusion in degenerative lumbar scoliosis: a minimum 2-year follow-up study. J Neurosurg Spine. 2010;13:211–215. doi: 10.3171/2010.3.SPINE09603. [DOI] [PubMed] [Google Scholar]
- 75.Smith-Petersen M N, Larson C B, Aufranc O E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. J Bone Joint Surg Am. 1945;27:1–11. [PubMed] [Google Scholar]
- 76.Chang K W, Cheng C W, Chen H C, Chang K I, Chen T C. Closing-opening wedge osteotomy for the treatment of sagittal imbalance. Spine. 2008;33:1470–1477. doi: 10.1097/BRS.0b013e3181753bcd. [DOI] [PubMed] [Google Scholar]
- 77.Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142–152. [PubMed] [Google Scholar]
- 78.Lafage V, Schwab F, Vira S. et al. Does vertebral level of pedicle subtraction osteotomy correlate with degree of spinopelvic parameter correction? J Neurosurg Spine. 2011;14:184–191. doi: 10.3171/2010.9.SPINE10129. [DOI] [PubMed] [Google Scholar]
- 79.Cho S K, Bridwell K H, Lenke L G. et al. Major complications in revision adult deformity surgery: risk factors and clinical outcomes with two to seven year follow up. Spine. 2011;37:489–500. doi: 10.1097/BRS.0b013e3182217ab5. [DOI] [PubMed] [Google Scholar]
- 80.Bradford D S. Vertebral column resection. Orthop Trans. 1987;11:502. [Google Scholar]
- 81.Suk S I, Chung E R, Kim J H, Kim S S, Lee J S, Choi W K. Posterior vertebral column resection for severe rigid scoliosis. Spine. 2005;30:1682–1687. doi: 10.1097/01.brs.0000170590.21071.c1. [DOI] [PubMed] [Google Scholar]
- 82.Cho K J, Suk S I, Park S R. et al. Short fusion versus long fusion for degenerative lumbar scoliosis. Eur Spine J. 2008;17:650–656. doi: 10.1007/s00586-008-0615-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Harimaya K, Mishiro T, Lenke L G, Bridwell K H, Koester L A, Sides B A. Etiology and revision surgical strategies in failed lumbosacral fixation of adult spinal deformity constructs. Spine. 2011;36:1701–1710. doi: 10.1097/BRS.0b013e3182257eaf. [DOI] [PubMed] [Google Scholar]
- 84.Cho S K, Bridwell K H, Lenke L G. et al. Comparative analysis of clinical outcome and complications in primary vs. revision adult scoliosis surgery. Spine. 2011;37:393–401. doi: 10.1097/BRS.0b013e31821f0126. [DOI] [PubMed] [Google Scholar]
- 85.Cho K J, Suk S I, Park S R. et al. Risk factors of sagittal decompensation after long posterior instrumentation and fusion for degenerative lumbar scoliosis. Spine. 2010;35:1595–1601. doi: 10.1097/BRS.0b013e3181bdad89. [DOI] [PubMed] [Google Scholar]
- 86.Kebaish K M. Sacropelvic fixation: techniques and complications. Spine. 2010;35:2245–2251. doi: 10.1097/BRS.0b013e3181f5cfae. [DOI] [PubMed] [Google Scholar]
- 87.Sansur C A, Smith J S, Coe J D. et al. Scoliosis research society morbidity and mortality of adult scoliosis surgery. Spine. 2011;36:E593–E597. doi: 10.1097/BRS.0b013e3182059bfd. [DOI] [PubMed] [Google Scholar]
- 88.Smith J S, Shaffrey C I, Glassman S D. et al. Spinal Deformity Study Group . Risk-benefit assessment of surgery for adult scoliosis: an analysis based on patient age. Spine. 2011;36:817–824. doi: 10.1097/BRS.0b013e3181e21783. [DOI] [PubMed] [Google Scholar]
- 89.Smith J S, Shaffrey C I, Sansur C A. et al. Scoliosis Research Society Morbidity and Mortality Committee . Rates of infection after spine surgery based on 108,419 procedures: a report from the Scoliosis Research Society Morbidity and Mortality Committee. Spine. 2011;36:556–563. doi: 10.1097/BRS.0b013e3181eadd41. [DOI] [PubMed] [Google Scholar]
- 90.Scheufler K M Cyron D Dohmen H Eckardt A Less invasive surgical correction of adult degenerative scoliosis. Part II: Complications and clinical outcome Neurosurgery 2010671609–1621.; discussion 1621 [DOI] [PubMed] [Google Scholar]


