Abstract
Extracellular signal-regulated kinase (ERK) activation is important for both thymocyte development and T cell function. Classically, signal transduction from the T cell antigen receptor (TCR) to ERK is thought to be regulated by signaling from Ras guanine nucleotide exchange factors (GEFs), through the small G protein Ras, to the three-tiered Raf–MAPK/ERK kinase (MEK)–ERK kinase cascade. Developing and mature T cells express four members of two RasGEF families, RasGRP1, RasGRP4, son of sevenless 1 (Sos1), and Sos2, and several models describing combined signaling from these RasGEFs have been proposed. However, recent studies suggest that existing models need revision to include both distinct and overlapping roles of multiple RasGEFs during thymocyte development and novel, Ras-independent signals to ERK that have been identified in peripheral T cells.
Keywords: Ras, extracellular signal-regulated kinase, PAK, Bam32, Sos, phospholipase C-γ1
TCR signal transduction to Ras
Receptor-stimulated signal transduction through the small G protein Ras to the Raf–MEK–ERK kinase cascade is essential to multiple developmental and pathologic systems. As a result of this, defining and targeting the signaling proteins that underlie Ras and ERK activation has enormous therapeutic potential [1–4]. In T cells, signal transduction from two related receptors, the pre-TCR and TCR, to the small G protein Ras and the downstream Raf–MEK–ERK kinase cascade is absolutely required for normal intrathymic T cell development and mature T cell function [5–14]. Despite intense study for >20 years, the precise mechanisms that underlie TCR-stimulated Ras and ERK activation are not well understood. Although early studies in T cell lines have highlighted a protein kinase C (PKC)- and diacylglycerol (DAG)-dependent mechanism for Ras and ERK activation downstream of the RasGEF RasGRP1 [15–18], recent studies have shown that multiple Ras-dependent and Ras-independent mechanisms act in concert to stimulate ERK phosphorylation and downstream biological responsiveness. Furthermore, genetic studies predicted to downregulate Ras and ERK signaling have revealed pathological activation of these signaling pathways, highlighting the need for targeted genetic studies prior to therapeutic targeting of these important signaling pathways.
Although the initial mechanism of activation of the pre-TCR and TCR is different, the downstream signaling cascades that activate Ras are similar. Initiation of either pre-TCR signaling (by ligand-independent dimerization [19]) or TCR signaling (by peptide–MHC engagement [20]) brings the Src family kinases lymphocyte-specific protein tyrosine kinase (Lck) and feline yes-related protein (Fyn) to the TCR–CD3 complex where they phosphorylate immune receptor tyrosine-based activation motifs (ITAMs) on the TCRζ and CD3 chains. These ITAMs act as docking sites for the recruitment and activation of the tyrosine kinases spleen tyrosine kinase (SYK) and ζ chain-associated protein kinase of 70 kDa (ZAP70) [20]. SYK and/or ZAP70 then rapidly phosphorylate the membrane-bound adapter linker for activation of T cells (LAT) on multiple tyrosines on its cytoplasmic tail that are critical for its adapter function. The phosphorylated tyrosines on LAT act as binding sites for multiple proteins containing Src homology 2 (SH2)-domains, and based on these interactions LAT acts as a docking site for nucleation of a number of signaling complexes critical to T cell effector functions.
Phosphorylated LAT associates with two molecular complexes that regulate Ras activation: PLC-γ1–Grb2-related adaptor protein involved in T cell signaling (GADS)–SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76)–IL-2 inducible T cell kinase (ITK) and Grb2–Sos [21] (Figure 1). At LAT, activated PLC-γ1 cleaves phosphatidylinositol 4,5 bisphosphate (PIP2) generating inositol 1,4,5-trisphosphate (IP3), which stimulates release of calcium from intracellular stores and DAG. DAG binds the C1 domains of both novel PKCs and the RasGEF RasGRP1 [17], promoting their activation by recruiting these enzymes to the plasma membrane. Furthermore, novel PKCs directly phosphorylate RasGRP1 on T184 to increase its RasGEF activity [18].
Figure 1.
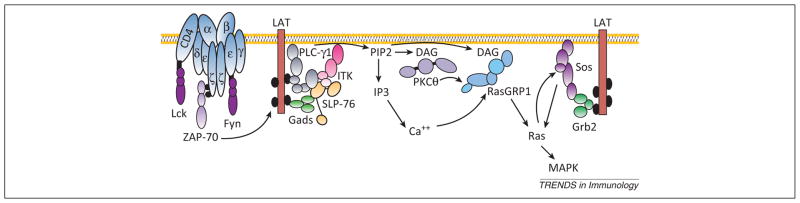
Canonical T cell receptor (TCR) signal transduction to Ras–mitogen-activated protein kinase (MAPK). After TCR binding and engagement of the upstream kinases lymphocyte-specific protein tyrosine kinase (Lck) and feline yes-related protein (Fyn), activated ζchain-associated protein kinase of 70 kDa (ZAP70) triggers phosphorylation of linker for activation of T cells (LAT). Phosphorylated LAT associates with two molecular complexes that regulate Ras activation: phospholipase C (PLC)-γ1–Grb2-related adaptor protein involved in T cell signaling (GADS)–SH2 domain-containing leukocyte phosphoprotein of 76 kDa (SLP-76) and growth factor receptor-bound protein 2 (Grb2)-son of sevenless (Sos). On LAT, activated PLC-γ1 cleaves phosphatidylinositol 4,5 bisphosphate (PIP2) generating inositol 1,4,5-trisphosphate (IP3), which stimulates release of calcium from intracellular stores and diacylglycerol (DAG). DAG then recruits protein kinase C (PKC)θ and RasGRP1 to the membrane, and the combined actions of Ca2+, DAG, and PKCθ activate Ras guanine nucleotide-releasing protein 1 (RasGRP1). The Ras guanine nucleotide exchange factors (GEFs) Sos1 and Sos2 are also recruited to LAT via the adapter Grb2. Sos proteins have basal RasGEF activity, which can be enhanced by the binding of activated Ras (Ras-GTP) to an allosteric binding pocket on Sos. Ras-GTP binding to Sos potentially allows for the engagement of a positive feedback loop, primed by either RasGRP1 or basal Sos activity, to produce high levels of Ras activation. Ras signals to multiple downstream effector pathways including the Raf–MAPK/ERK kinase (MEK)–extracellular signal-regulated kinase (ERK) kinase cascade.
The RasGEFs Sos1 and Sos2 are also recruited to LAT via the adapter Grb2 [21]. Membrane-recruited Sos is initially in an autoinhibited conformation [22] and has low catalytic activity. This autoinhibited structure must be disrupted for full Sos activation. Ras-GTP (activated Ras) can bind an allosteric pocket on Sos distinct from its catalytic RasGEF domain [23]. This binding relieves auto-inhibition of Sos and increases its catalytic activity up to 80-fold, suggesting that activated Ras is required for efficient Sos-dependent signaling. Biochemical and modeling studies assessing Sos1- and RasGRP1-dependent Ras activation have proposed that RasGRP1 (and not Sos) is the first RasGEF engaged after TCR ligation, and is sufficient for Ras activation following weak TCR stimulation [24]. However, strong TCR ligation could potentially trigger a RasGRP1–Ras–Sos1–Ras positive feedback loop and enhance Ras activation several fold [24,25]. Once activated, Ras signals to multiple downstream effector pathways that are important for both T cell development and effector function (Figure 1).
In this review, we highlight recent advances in understanding the relative contributions of multiple signaling inputs to the activation of Ras and ERK in both developing and mature T cells. During intrathymic T cell development, regulated expression of the RasGEFs Sos1 and RasGRP family members at distinct developmental stages dictates their relative contribution to Ras and ERK activation [16,26,27]. In peripheral T cells, Sos- and RasGRP1-dependent signaling cannot completely explain TCR-dependent ERK activation [24,28]. Here, a recently identified B cell lymphocyte adaptor molecule of 32 kDa (Bam32)–PLC-γ1–p21-activated kinase 1 (PAK1) complex stimulates ERK independently of Ras, and cooperates with Ras-dependent signals to activate fully the Raf–MEK–ERK kinase cascade [29].
RAS activation during thymocyte development
Genetic studies of thymocyte development have shown that signal transduction from the pre-TCR and TCR, through the adapters LAT and SLP76, to the small G protein Ras and downstream mitogen activated protein kinase (MAPK) cascades is required during several stages [7,11,13,14,30,31]. For a brief review of thymocyte development, see Box 1. However, a mechanistic understanding of Ras activation during thymocyte development has been elusive. Recently, two models have been proposed to explain how Ras is activated to drive thymocyte development.
Box 1. Thymocyte development.
αβ T cell development is initiated when immature progenitor cells migrate from the fetal liver or adult bone marrow to the thymus, where they undergo receptor-driven differentiation programs that test proper rearrangement and function of the TCRβand TCRα chains. TCRβ gene rearrangement and expression is assessed at the CD4−CD8− (DN)3 stage, where surface TCRβ pairs with a pre-TCRα chain to form a pre-TCR, which dimerizes and signals in a ligand-independent manner to drive proliferation and differentiation to the DP stage (βselection) [19]. At the CD4+CD8+ (DP) stage, TCRα gene rearrangement and the quality of signaling through the paired, mature αβ TCR are assessed. In the thymic cortex, cells that fail to signal through their mature TCR die by neglect, whereas cells expressing a TCR with a weak affinity for self-antigen and self-MHC selectively survive and differentiate into CD4 or CD8 single positive (SP) thymocytes (positive selection). Positively selected cells then migrate into the medulla, where cells expressing a TCR with too strong an affinity for self-antigen and self-MHC generate strong TCR signals and die via TCR-driven apoptotic pathways (negative selection) [6].
Model 1: differential activation of RasGRP1 and Sos controls positive and negative selection
The first model addresses only the TCR checkpoint (positive and negative selection), and is based upon the signaling properties of each RasGEF. Studies using fetal thymic organ cultures from OT-I TCR-transgenic thymocytes have shown that positively selecting ligands promote weak but sustained Ras and ERK activation, whereas negatively selecting ligands induce strong signaling to Ras and ERK [8,32]. Microscopic analysis of RasGRP1, Sos1, and Grb2 localization has revealed that positively selecting (low-affinity) ligands induce colocalization of RasGRP1 (but not Sos1) with Ras at the Golgi, whereas negatively selecting (high-affinity) ligands induce colocalization of RasGRP1, Sos1, and Grb2 with Ras at the plasma membrane [32]. Correlation of these observations with the biochemical and modeling data describing the engagement of a RasGRP1–Ras–Sos–Ras positive feedback loop following strong, but not weak, TCR stimulation [25] has led to a hypothesis whereby weak TCR stimulation by low-potency ligands signals to Ras via RasGRP1 alone to support positive selection, whereas stronger ligands engage a RasGRP1–Ras-Sos–Ras feedback loop to promote negative selection [33].
Model 2: expression profile dictates RasGEF usage during thymocyte development
The second model describing how Ras is activated during thymocyte selection is based both on genetic studies and the expression profile of the RasGEFs Sos1 and RasGRP1 at distinct developmental checkpoints. A summary of the genetic studies relevant to this review is given in Table 1. Studies examining RasGRP1-deficient mice have shown that RasGRP1 is required for positive selection; however, development to the CD4+CD8+ (DP) stage (β selection) and negative selection have been reported to be intact in RasGRP1-deficent mice [16,34]. By contrast, deletion of Sos1 in developing thymocytes [Sos1(T)−/− mice] has shown that Sos1 plays a role in pre-TCR-driven proliferation and gene expression during β selection, but positive and negative selection remain intact in Sos1(T)−/− mice [26]. Furthermore, deletion of Sos1 (but not RasGRP1) markedly reduces anti-CD3ε induced proliferation and DP thymocyte development in Rag2−/− mice, suggesting that Sos1 is likely the dominant RasGEF required for proliferation at the CD4+CD8+ (DN)3 stage [26].
Table 1.
Animal models of Ras/ERK signaling in T cell development and function
| Mouse model | Developmental effect | Refs |
|---|---|---|
| Grb2+/− |
|
[46] |
| Grb2(T)−/− |
|
[51] |
| Rasgrp1−/− |
|
[16,26,27,34,38] |
| Rasgrp4−/− |
|
[38] |
| Rasgrp1/Rasgrp4 DKO |
|
[38] |
| Sos1(T)−/− |
|
[26,27] |
| Sos2−/− |
|
[27] |
| Sos1(T)/Rasgrp1 DKO |
|
[27] |
| Rasgrf2−/− |
|
[37] |
| Rasa1−/− (p120RasGAP deficient) |
|
[71] |
| Nras−/− |
|
[72,73] |
| Hras−/− |
|
[72] |
| Mapk3−/− (ERK1 deficient) |
|
[7,9] |
| Mapk1−/− (ERK2 deficient) |
|
[7,74,75] |
| Mapk3/Mapk1 DKO (ERK1 and ERK2 deficient) |
|
[7,47] |
| Lat-Y136F |
|
[11,12,54,55] |
INF, interferon.
These genetic results can be viewed in the context of RasGEF protein expression changes during T cell development. Sos1 expression decreases fivefold between the DN3 and DP stages, with a reciprocal fivefold increase in RasGRP1 expression [16,26,33]. These results suggest that the RasGEFs Sos1 and RasGRP1 are developmentally regulated in thymocytes, which is a major determinant for Ras activation at the pre-TCR and TCR checkpoints.
Regulation of RasGEF expression as a key determinant controlling thymocyte development is reminiscent of previous reports on the relation between genetic findings and regulated protein expression for the SYK kinases SYK and ZAP70. SYK expression is elevated in DN3 thymocytes and is important during β selection but rapidly decreases as cells move past the DN3 stage, whereas ZAP70 expression increases only after β selection and is required for positive and negative selection [35,36]. These data suggest that the regulated expression of multiple enzyme families may be an integral part of T cell development.
To summarize, two models have been proposed relating RasGEF signaling to T cell developmental changes. In the first model, the biochemical properties of individual Ras-GEFs and their subsequent activation mechanisms primarily determine the relative importance of individual RasGEFs during thymocyte selection. In the second model, regulated changes in RasGEF expression determine the primary mechanism of Ras activation at different developmental stages.
Toward an integrated model of RasGEF signaling during thymocyte development
Neither of the described models sufficiently explains Ras activation at both the pre-TCR and TCR developmental checkpoints. Mice that are deficient for Sos1 specifically in the T cell lineage [Sos1(T)−/−] contain some cells that develop beyond the DN3 stage [26], suggesting a role for other RasGEFs in β selection. Rasgrp1−/− mice, which in theory are unable to initiate RasGRP1–Ras–Sos–Ras signaling, show intact negative selection [34]. Furthermore, transgenic expression of a dominant-negative H-Ras construct is unable to block negative selection induced by strong ligands [13,30]. These studies suggest that Ras-dependent signaling may not be involved in negative selection. Two recent studies have provided insight into these questions.
Recently, the combined roles of Sos1, Sos2, and RasGRP1 during thymocyte development were assessed. Sos1(T)/Rasgrp1 double knockout (DKO) mice showed a marked defect in development beyond the DN3 stage, suggesting a combined role for Sos1 and RasGRP1 during β selection [27]. Furthermore, the extent to which β selection was blocked in individual Sos1(T)/Rasgrp1 DKO mice correlated with the efficiency of Lck–Cre-mediated deletion of the floxed Sos1 allele, suggesting that residual Sos1 expression plays a role in allowing thymocytes to escape the β selection checkpoint in the absence of RasGRP. At the TCR checkpoint, RasGRP1 was both necessary and sufficient for positive selection. However, although negative selection was intact in either Rasgrp1−/− or Sos1(T)/Sos2 DKO mice, combined deletion of Sos1 and RasGRP1 efficiently blocked negative selection, suggesting that negative selection requires signaling either by Sos1 or RasGRP1. Sos2 deletion did not alter thymocyte development either alone or in combination with Sos1 and/or RasGRP1 deletion, suggesting it is not required during T cell development. Similar to Sos2, the RasGEF RasGRF2 is expressed in thymocytes but is not required for normal T cell development [37].
A study assessing early T cell development in Rasgrp1−/−, Rasgrp4−/−, and Rasgrp1/Rasgrp4 DKO mice showed a previously unappreciated role for RasGRP proteins in early T cell development [38]. Although deletion of RasGRP1 alone partially blocked development beyond the DN3 stage and reduced the total number of DP thymocytes, this block was markedly enhanced by RasGRP4 deletion, suggesting that RasGRP4 can partially compensate for the loss of RasGRP1 early in thymocyte development. Intriguingly, the reduction in thymic cellularity was as or more severe in Rasgrp1/Rasgrp4 DKO mice [38] than in Sos1(T)/Rasgrp1 DKO mice [27], whereas the representative histograms (and assessment of thymocyte subsets) showed a more significant DN-to-DP block in the case of combined Sos1 and RasGRP1 deletion. Although these differences might simply have been due to animal-to-animal variability in these two studies [27,38], these data may provide important insight into the potential biological functions of Sos1 and RasGRP4 in early T cell development. RasGRP1 deletion was common to both studies, therefore, any differences observed between Rasgrp1/ Rasgrp4 DKO mice and Sos1(T)/Rasgrp1 DKO mice can be attributed to the different biological functions of Sos1 and RasGRP4. Sos1 is required for maximal pre-TCR-dependent proliferation beyond the DN3 stage [26], which is integral for differentiation to the DP stage [39], but Sos1 does not play a role in thymocyte survival signaling [26]. By contrast, the extreme reduction in thymic cellularity observed in Rasgrp1/Rasgrp4 DKO mice suggests that, in addition to its reported importance in β selection [38], RasGRP4 might play a critical role in either precursor proliferation prior to β selection or in thymocyte survival. Teasing apart the potentially complementary roles of Sos1 and RasGRP4 in early T cell development will require side-by-side genetic studies assessing independent and combined deletion of Sos1, RasGRP4, and RasGRP1. At the DP stage, Rasgrp1−/− mice show a significant defect in positive selection and subsequently reduced numbers of peripheral CD4+ and CD8+ lymphocytes [16], and RasGRP1/RasGRP4 DKO mice have almost no peripheral T cells, suggesting that RasGRP4 may play a role in positive selection as well.
Integration of these studies with existing ideas suggests a new, more comprehensive model for RasGEF signaling during thymocyte development (Figure 2). In this model, functional redundancy exists between Sos1, RasGRP1, and RasGRP4 downstream of both the pre-TCR and TCR, although the relative expression levels of these RasGEFs dictate their importance at each developmental checkpoint. During β selection, while Ras is preferentially activated by Sos1 (rather than RasGRP1), the lower expression of RasGRP1 is compensated for by RasGRP4, so that these two families of RasGEFs (Sos and RasGRP) act in concert to promote pre-TCR-mediated proliferation and differentiation at the β selection checkpoint. Furthermore, the partial functional redundancy observed between Sos1, RasGRP1, and RasGRP4 during β selection acts as a failsafe mechanism to ensure the production of a sufficient immune repertoire in the event that signaling via one or more RasGEFs is perturbed early in thymocyte development.
Figure 2.
Integrated model of Ras activation during thymocyte development. At the CD4−CD8− (DN)3 stage, ligand-independent pre-T cell receptor (TCR) signals are transmitted to Ras and extracellular signal-regulated kinase (ERK) by the combined actions of son of sevenless 1 (Sos1), Ras guanine nucleotide-releasing protein 1 (RasGRP1), and RasGRP4 to stimulate proliferation and differentiation to the CD4+CD8+ (DP) stage. In the thymic cortex, positively selecting signals are transmitted from the TCR to Ras via RasGRP1. Downstream of Ras, ERK activation is required for efficient positive selection. In the medulla, higher-potency ligands trigger Ras activation via either RasGRP1 or Sos1 to promote negative selection. Here, activation of ERK seems to be dispensable, and activation of other mitogen-activated protein kinases [MAPKs; c-jun N-terminal kinase (JNK), p38, and ERK5] are likely more important. Ras guanine nucleotide exchange factors (GEFs) whose individual deletion blocks thymocyte development for a given signal are shown in red, whereas RasGEFs that require combined deletion with a second RasGEF to cause a developmental block are shown in blue. Proteins whose role at a given developmental checkpoint have not been demonstrated by developmental studies are shown in green.
At the TCR checkpoint, there is differential activation of RasGEFs depending upon ligand potency. In the cortex, positively selecting ligands signal to Ras via RasGRP1 (and possibly RasGRP4), but not Sos1. The lack of Sos1 signaling is likely due to both the low expression of Sos1 and the requirement for Ras-GTP-mediated Sos1 activation. In the medulla, negatively selecting ligands generate sufficient upstream signaling through LAT to induce high amounts of Ras activation by either RasGRP1 (in the absence of Sos1), or to engage basal Sos1 activity on its own (in the absence of RasGRP1) to induce a Sos1–RasGTP–Sos1–Ras positive feedback loop capable of generating high amounts of Ras activation and promoting negative selection. The role of RasGRP4 in negative selection has not been assessed. This functional redundancy by Sos1 and RasGRP1 during negative selection may act to ensure appropriate central tolerance.
So why is negative selection impaired after combined Sos1/RasGRP1 deletion [27], while it is intact in mice expressing a dominant negative H-Ras(N17) transgene [13,30]? One interpretation is that Sos1 and RasGRP1 might have Ras-independent, yet overlapping, signaling functions that are required for negative selection. Sos proteins are reported to have RasGEF-independent functions including acting as either a RacGEF [40] or as scaffold proteins to facilitate LAT oligomerization [41]. Intriguingly, both Rac and LAT signaling are important for negative selection [11,42]. However, whether RasGRP1 plays an overlapping role with Sos in either of these signaling pathways is unknown.
Alternatively, expression of dominant negative H-Ras(N17) may be insufficient to block TCR-dependent Ras activation. Although H-Ras(N17) is commonly used as a broad inhibitor of Ras activation, it does not effectively block DAG- or PKC-dependent Ras activation in certain biological settings [43,44]. Furthermore, although overexpression of dominant negative H-Ras(N17) can inhibit TCR-stimulated ERK phosphorylation in Jurkat T cells [15], it does not efficiently inhibit TCR-stimulated ERK phosphorylation in primary T lymphocytes [45]. In thymocytes isolated from H-Ras(N17) transgenic mice, TCR-stimulated ERK phosphorylation is reduced, but not eliminated, whereas c-Jun N-terminal kinase (JNK) phosphorylation remains intact [46]. These data indicate that transgenic H-Ras(N17) expression provides sufficient Ras inhibition to block positive selection induced by low-potency ligands, but may not achieve the level of Ras inhibition necessary to block negative selection. However, a conclusive answer as to whether RasGEF-dependent Ras activation is required for negative selection will require genetic studies with targeted mutation of the RasGEF domains of Sos1 and/or RasGRP1.
ERK activation during thymocyte development
Combined deletion of ERK1 and ERK2 shows that Raf–MEK–ERK signaling is required for β selection downstream of the pre-TCR and for positive selection downstream of the TCR [7,9]. Furthermore, the intensity, duration, and location of ERK activation change between positive and negative selection [8,32], which has led to the hypothesis that ERK activation is involved in this decision. Using Sos1(T)−/− and Rasgrp1−/− mice, the RasGEFs that are responsible for Ras-dependent ERK activation in developing thymocytes have been defined. Sos1 is essential for ERK activation downstream of the pre-TCR at the DN3 stage, but becomes progressively less important as T cells mature [26]. In DP thymocytes, Sos1 deletion reduces TCR-stimulated ERK phosphorylation by 30–40%, whereas RasGRP1 deletion almost completely eliminates TCR-stimulated ERK activation [27]. Negative selection can still occur in both RasGRP1-deficient [34] and ERK1 and ERK2 double-deficient mice [47], thus, other Ras-dependent MAPK pathways may be more important for negative selection. Transgenic studies have shown a role for JNK, p38, and ERK5 during negative selection [48–50]. Furthermore, pharmacological inhibition of p38, but not ERK1 and ERK2, blocks negative selection [50]. Grb2+/− and Grb2(T)−/− mice, which have impaired negative selection, have normal TCR-stimulated ERK activation but defective JNK and p38 activation [46,51]. These data suggest that differential activation of MAPK cascades by the combined actions of Sos1 and RasGRP1 versus RasGRP1 alone may regulate positive versus negative selection (Figure 2). However, this idea remains to be tested.
Ras and ERK signaling in peripheral lymphocytes
Although the requirements for Ras-dependent ERK activation are well defined during thymocyte development, RasGEF-dependent signaling does not seem to fully explain TCR-dependent ERK activation in peripheral T cells. Knockout, knockdown, and mutational studies in murine CD8+ T cells [52], Jurkat T cells [18,24], or human T cells [24,28] have shown that signaling via RasGRP1 accounts for ~50% of TCR-stimulated ERK activation in peripheral T cells. However, whether Sos1 and Sos2 play a role in TCR-stimulated ERK activation in this setting is controversial. In agreement with a previous study assessing knockdown of Sos1 in Jurkat T cells and human CD4+ T cells [24], deletion of Sos1 in murine CD4+ T cells showed a modest (10–15%) reduction in TCR-dependent ERK phosphorylation [26]. By contrast, a recent study has reported that combined Sos1/2 knockdown in human peripheral T cells did not alter TCR-stimulated ERK phosphorylation [28], and instead Sos1 knockdown antagonized the effects of RasGRP1 depletion so that TCR-stimulated ERK phosphorylation was normal after combined Sos1/RasGRP1 knockdown [28]. This study, however, did not explore TCR-dependent ERK activation at submaximal doses of TCR stimulation more within the physiological range of stimulation, or provide a kinetic analysis of TCR-dependent ERK activation. RasGRP1 and Sos1 have been shown to have additive effects on ERK phosphorylation in other studies [24,25,27], therefore, careful experimentation will be required to resolve this controversy. Furthermore, because thymic development is severely impaired in both Sos1(T)/Rasgrp1 DKO and Rasgrp1/Rasgrp4 DKO mice, floxed alleles that allow for the peripheral deletion of RasGEFs after thymocyte development is complete will need to be developed to answer these questions. However, it is clear that canonical Ras-dependent pathways do not completely describe TCR-stimulated ERK activation, and that alternative modes of activating ERK must be considered. Studies characterizing TCR-dependent ERK activation in a murine model of lymphoproliferative disease provide insights into alternative pathways to ERK.
LAT-Y136F knock-in mice have enhanced ERK phosphorylation
We [12] and another laboratory [5] have independently generated and characterized mice with a germline mutation in the PLC-γ1 binding site of LAT (LAT-Y136F mice). LAT-Y136F mice show an early (DN3) block in thymocyte development; however, with age, these mice develop an overwhelming T helper (TH)2 CD4+ T cell lymphoproliferation characterized by lymphadenopathy, splenomegaly, and multiorgan lymphocyte infiltration. Consistent with mutation of the PLC-γ1 binding site on LAT, isolated CD4+ T cells from LAT-Y136F mice showed defective TCR-dependent PLC-γ1 phosphorylation and Ca2+ flux [12]. RasGRP1-dependent ERK activation normally requires LAT-dependent PLC-γ1 activation [17,53], thus, TCR-dependent ERK activation should be similarly decreased in LAT-Y136F CD4+ T cells. Unexpectedly, LAT-Y136F CD4+ T cells have relatively normal TCR-dependent ERK activation after resting to reduce basal signaling [12], and paradoxical hyperactivation of ERK in freshly isolated cells [54].
The enhanced ERK phosphorylation observed in the absence of appreciable LAT–PLC-γ1 signaling suggests that LAT-independent ERK activation could contribute to the disease phenotype of LAT-Y136F mice. Biochemical studies have been used together with a candidate genetic approach to assess either novel signaling proteins whose connection to ERK have been reported but poorly defined (e.g., Bam32 [54]) or alternative LAT–PLC -γ1-independent means of activating known signaling intermediates (e.g., RasGRP1 and Sos1/2 [55]).
The adapter Bam32 [also known as dual adaptor for phosphotyrosine and 3-phosphoinositides 1 (Dapp1)] is required in mouse CD4+ T cells for optimal ERK activation, cytokine production [interleukin (IL)-2 and IL-4], and cell proliferation [56]. Bam32 deletion in LAT-Y136F mice reduces ERK activation and overall disease burden [54]. Furthermore, introduction of a hyperactivatable ERK2 allele [10] partially restores ERK phosphorylation and disease progression in Bam32-deficient LAT-Y136F mice [54], suggesting that Bam32-dependent ERK activation is partially responsible for the LAT-Y136F disease phenotype. Mechanistic studies have identified a novel trimolecular complex, comprising Bam32, the serine/threonine kinase PAK1 (Box 2), and PLC-γ1, which is important for ERK activation in both Jurkat T cells and human CD4+ T cells [29].
Box 2. PAK kinases.
PAKs are serine/threonine kinases that belong to the Ste20 family of kinases first identified in yeast [76]. There are six PAK isoforms that can be divided into two groups, group I (PAKs 1–3) and group II (PAKs 4–6). PAK kinases are involved in multiple cellular processes including ERK signaling, cytoskeletal reorganization, apoptotic signaling, cell migration, oncogenic transformation, and neurite outgrowth [76]. All PAKs have an N-terminal regulatory domain and a highly conserved C-terminal kinase domain. The regulatory domains of all PAKs consist of a GTPase-binding domain (PBD) and several proline-rich regions that serve as docking sites for SH3 domain-containing proteins; additionally, group I PAKs possess an autoinhibitory domain (PID) overlapping with the PBD. In resting cells PAK1 exists as a trans-inhibited homodimer, in which the N-terminal PID of one PAK1 molecule binds to and inhibits the kinase domain of another PAK1 molecule [77]. Binding of activated Rac or Cdc42 to the PBD dissociates PAK1 homodimers and activates PAK1 by releasing the PID-mediated inhibition, leading to autophosphor-ylation of T423 in the activation loop [77]. In T cells, Vav and PAK interacting exchange factor (PIX), two Rac1/Cdc42 GEFs, have been implicated in PAK1 activation and shown to be important for cytoskeletal rearrangement [78–80].
Bam32–PLC-γ1–PAK1 complexes contribute to normal TCR-stimulated, Ras-independent ERK activation
In T cells, the Bam32–PLC-γ1–PAK1 complex works in a cooperative manner to activate the Raf–MEK–ERK kinase cascade independently of Ras. In Bam32-deficient CD4+ T cells, it has been shown that TCR-stimulated ERK phosphorylation depends both on Ras-dependent and Bam32-dependent (but Ras-independent) signaling pathways [29], because overexpression of dominant-negative Ras (dnRas) or Bam32 deletion leads to similar reductions in TCR-stimulated ERK activation. Furthermore, the effects of Bam32 deletion and dnRas are additive and nonoverlapping, such that ERK phosphorylation is completely inhibited in the absence of both signaling pathways. Bam32-dependent activation of ERK depends upon PAK1 directly phosphorylating Raf-1 on S338 and MEK1 on S298 [57,58].
Mapping the interactions between Bam32, PLC-γ1, and PAK1 has revealed that the phospholipase function of PLC-γ1 is not required for Bam32-mediated ERK activation; instead, PLC-γ1 is not tyrosine phosphorylated and has a scaffolding role in this complex. Binding of the PLC-γ1 C-SH2 domain to Bam32 requires S141 of Bam32, whereas the SH3 domain of PLC-γ1 (in conjunction with Bam32) binds a proline-rich region of PAK1. PLC-γ1–PAK1 interaction leads to the dissociation of trans-inhibited PAK1 dimers into active PAK1 monomers and downstream signaling to Raf and MEK. Bam32–PLC-γ1-dependent PAK1 activation is independent of molecules previously shown to activate PAK1 in T cells, including LAT, SLP-76, Ras, and Rac1/Cdc42.
When assessing the interplay between different PLC-γ1 complexes, Bam32 overexpression has been shown to limit LAT–PLC-γ1 interaction, and reciprocally, LAT overexpression disrupts Bam32–PLC-γ1 interaction, suggesting potential competition between these two complexes [29]. Such competition might manifest in several physiological and pathological settings. LAT hypophosphorylation and decreased LAT–PLC-γ1 interaction have been observed in anergy [59], lupus [60], human T-lymphotropic virus (HTLV)-1 infection [61], and treatment with the polyunsaturated fatty acids (PUFAs) [62]. In these cases, reduced LAT–PLC-γ1 interactions may result in more Bam32–PLC-γ1–PAK1 complexes. In the setting of intact LAT phosphorylation, Bam32 upregulation could also result in increased Bam32–PLC-γ1–PAK1 complex formation. In B cells, CD40 ligation results in a 5–10-fold increase in Bam32 protein levels [63]. It would be interesting to know whether Bam32 expression is also regulated upon T cell activation.
PLC-γ1-independent, Ras-dependent ERK activation
Bam32-dependent, Ras-independent signaling [29] does not completely account for ERK hyperactivation in LAT-Y136F mice [54]. Furthermore, biochemical assessment of isolated LAT-Y136F CD4+ T cells shows basal Ras hyper-activation [55], suggesting that signaling by one or more RasGEFs is dysregulated in these cells. RasGRP1 activation normally depends on PLC-γ1-dependent DAG formation and should be defective in LAT-Y136F mice, therefore, Sos1/2 signaling, perhaps via alternative LAT-independent activation [64], might be important in this setting. However, genetic studies have shown that RasGRP1, and not Sos1/2, is the major RasGEF responsible for ERK hyperactivation in LAT-Y136F CD4+ T cells [55] via activation of a noncanonical Lck–PKCθ–RasGRP1 pathway. Aspects of this signaling pathway, and specifically Lck-dependent PKCθ activation, have previously been reported in the setting of CD28 co-stimulation [65]. Genetic assessment of altered ERK signaling in LAT-Y136F mice [54,55] highlight how normal or minor physiological signaling pathways can become pathologically activated when appropriate feedback controls are lost.
Interestingly, another study has also shown that RasGRP1 deletion could limit disease in LAT-Y136F mice [66], although in this study the effect of RasGRP1 deletion was primarily due to worsening the early β selection defect observed in LAT-Y136F mice. Although there are certainly confounding developmental aspects of many of the studies assessing disease burden in LAT-Y136F mice, combining disease assessment with careful biochemical assessment of isolated LAT-Y136F CD4+ T cells can lead to keen insights regarding the tremendous plasticity of TCR-dependent signaling. In LAT-Y136F mice, perturbation of canonical signaling leads to the pathological rewiring of TCR-dependent pathways to ERK [54,55]. Furthermore, perturbations in LAT signaling can also lead to changes in gene expression that may further alter signaling within immune cells [67]. Modulation of LAT signaling is just one of several examples of genetic manipulations that, although predicted to blunt immune development and signaling, instead resulted in pathological immune dysregulation [68]. Understanding how these genetic manipulations alter the overall signaling environment leading to disease is paramount, because these studies will help inform future targeted therapeutic choices by separating promising candidate targets from molecules whose targeting might have unforeseen adverse consequences.
Concluding remarks
It is well established that TCR-dependent Ras and ERK activation are crucial for both normal intrathymic T cell development and mature T cell function. Developmental studies in mice lacking multiple RasGEFs have shown us that Ras- and ERK-dependent signals are important to drive pre-TCR-dependent proliferation at the β selection checkpoint and TCR-dependent thymocyte selection. However, the relative importance of different RasGEFs differs depending on the developmental stage. Downstream of the pre-TCR Sos1, RasGRP1 and RasGRP4 cooperate to drive proliferation beyond the β selection checkpoint. At the TCR checkpoint, RasGRP1 is required for positive selection, whereas either RasGRP1 or Sos1 is required for negative selection [27]. Although these data highlight the importance of Ras-dependent signaling at multiple stages of thymocyte development, the role of ERK1/2-dependent signaling (one of several downstream effectors of Ras) during TCR-dependent thymocyte development has been more controversial.
ERK1 and ERK2 double-deficient mice show defective pre-TCR-driven proliferation and TCR-mediated positive selection [7]. Furthermore, the overall profile of MAPK activation changes when DP thymocytes are activated by positively versus negatively selecting ligands, suggesting that the coordinated efforts of multiple MAPKs may regulate this complex decision [32]. However, the majority (>95%) of TCR-dependent ERK1 and ERK2 activation in DP thymocytes is RasGRP1-dependent, and negative selection can still occur in both Rasgrp1−/− [27,34] and ERK1/2 DKO [47] mice. By contrast, transgenic studies have shown a role for other MAPKs including JNK, p38, and ERK5 during negative selection [48,49]. Further experimentation using multiple models of negative selection will be required to determine whether ERK1/2 activation plays a role in negative selection, or whether other Ras-dependent MAPK pathways are activated to control this process.
Unlike developing T cells where mouse knockout studies have been instrumental in our understanding of Ras-dependent signaling, the severe developmental block observed in Rasgrp1−/− mice has limited their usefulness in the study of peripheral T cells. Instead, understanding of TCR-dependent ERK activation in peripheral cells is based primarily on overexpression and knockdown studies. These studies have revealed that in peripheral T cells, there are multiple complementary pathways that can lead to ERK activation (Figure 3). TCR-dependent phosphorylation of LAT activates Ras via RasGRP1 and Sos1. RasGRP1 can also be activated independently of LAT via Lck–PKCθ interactions. Furthermore, TCR stimulation induces the formation of Bam32–PLC-γ1–PAK1 complexes that can normally synergize with Ras-dependent pathways to enhance ERK activation.
Figure 3.
Ras-dependent versus Ras-independent extracellular signal-regulated kinase (ERK) signaling. In peripheral T lymphocytes, two molecular complexes, both of which are capable of signaling to the Raf–MAPK/ERK kinase (MEK)–ERK kinase cascade, compete for phospholipase C (PLC)-γ1 binding. Association of PLC-γ1 with linker for activation of T cells (LAT) promotes canonical T cell receptor (TCR) signaling (Figure 1) and activation of the Raf–MEK–ERK kinase cascade via Ras. This pathway requires PLC-γ1 catalytic activity (red). By contrast, association of PLC-γ1 with the adapter B cell lymphocyte adaptor molecule of 32 kDa (Bam32) and the kinase p21-activated kinase 1 (PAK1) causes dissociation of PAK1 dimers promoting PAK1 activation. PAK1 can then signal to the Raf–MEK–ERK kinase cascade by phosphorylating both Raf and MEK. In this alternative pathway, PLC-γ1 is catalytically inactive (blue) and instead acts as a scaffold to dissociate pre-existing PAK1 dimers. Normally, both pathways act together to promote ERK activation, as MEK1 phosphorylation on S298 (by PAK1) potently enhances its phosphorylation on S217/S221 by Raf. Changes in the balance between expression of the adapters LAT and Bam32 can sequester PLC-γ1 and alter the relative activities of these two pathways. These two PLC-γ1-dependent pathways are likely to be responsible for the majority of TCR-dependent ERK activation, although LAT–son of sevenless (Sos) and lymphocyte-specific protein tyrosine kinase (Lck)–protein kinase C (PKC)θ–Ras guanine nucleotide-releasing protein 1 (RasGRP1) pathways also contribute to TCR-dependent ERK signaling.
How might these multiple, signaling inputs be used in concert to regulate signaling through the Raf–MEK–ERK kinase cascade? Both LAT and Sos1 are subject to inhibitory feedback phosphorylation by ERK [69,70], suggesting that PAK1 complexes might be more important for prolonged ERK activation and/or activation of distinct pools of ERK. Furthermore, there exists a potential competition between the LAT–PLC-γ1–RasGRP1–Ras and the Bam32–PLC-γ1–PAK1 pathways, dependent on PLC-γ1 usage, which may act to fine tune ERK activation in certain physiological settings.
Although overexpression and knockdown studies have been fundamental in revealing how ERK1 and ERK2 can be activated in peripheral T cells, they cannot reveal the physiological consequences of activating each of these pathways. Here, new mouse models that allow deletion of RasGEFs after thymocyte development is completed are required. Combiningthese modelswith Bam32 and/orPAK1knockout mice may lead to a better understanding of the physiological mechanisms that regulate Ras-dependent versus Ras-independent ERK activation. Such studies will also lead to a better understanding of how different PLC-γ1 complexes are manipulated to activate differentially ERK alone versus ERK and Ca2+ to control peripheral cell function.
Why might defining Ras-dependent and Ras-independent pathways to ERK be important? Signal transduction through Ras and ERK is critical not only to normal homeostasis and proliferation, but is also hyperactivated in several disease states. Understanding the interplay of the multiple pathways leading to ERK activation in T cells, and how ablation of specific signals to ERK can modify T cell responsiveness, will undoubtedly lead to new therapeutics targeting ERK activation to modulate immune function.
Acknowledgments
We would like to thank Connie Sommers and Lakshmi Balagopalan for helpful suggestions and careful reading of the manuscript. This research was supported by the Intramural Research Program of the NIH, CCR, NCI.
References
- 1.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 2.Gysin S, et al. Therapeutic strategies for targeting ras proteins. Genes Cancer. 2011;2:359–372. doi: 10.1177/1947601911412376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logue JS, Morrison DK. Complexity in the signaling network: insights from the use of targeted inhibitors in cancer therapy. Genes Dev. 2012;26:641–650. doi: 10.1101/gad.186965.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 5.Aguado E, et al. Induction of T helper type 2 immunity by a point mutation in the LAT adaptor. Science. 2002;296:2036–2040. doi: 10.1126/science.1069057. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11:666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer AM, et al. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 8.McNeil LK, et al. A requirement for sustained ERK signaling during thymocyte positive selection in vivo. Proc Natl Acad Sci USA. 2005;102:13574–13579. doi: 10.1073/pnas.0505110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pages G, et al. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 10.Sharp LL, et al. The influence of the MAPK pathway on T cell lineage commitment. Immunity. 1997;7:609–618. doi: 10.1016/s1074-7613(00)80382-9. [DOI] [PubMed] [Google Scholar]
- 11.Sommers CL, et al. Mutation of the phospholipase C-gamma1-binding site of LAT affects both positive and negative thymocyte selection. J Exp Med. 2005;201:1125–1134. doi: 10.1084/jem.20041869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sommers CL, et al. A LAT mutation that inhibits T cell development yet induces lymphoproliferation. Science. 2002;296:2040–2043. doi: 10.1126/science.1069066. [DOI] [PubMed] [Google Scholar]
- 13.Swan KA, et al. Involvement of p21ras distinguishes positive and negative selection in thymocytes. EMBO J. 1995;14:276–285. doi: 10.1002/j.1460-2075.1995.tb07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, et al. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 15.Izquierdo M, et al. p21ras couples the T cell antigen receptor to extracellular signal-regulated kinase 2 in T lymphocytes. J Exp Med. 1993;178:1199–1208. doi: 10.1084/jem.178.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dower NA, et al. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 17.Carrasco S, Merida I. Diacylglycerol-dependent binding recruits PKCtheta and RasGRP1 C1 domains to specific subcellular localizations in living T lymphocytes. Mol Biol Cell. 2004;15:2932–2942. doi: 10.1091/mbc.E03-11-0844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roose JP, et al. A diacylglycerol-protein kinase C-RasGRP1 pathway directs Ras activation upon antigen receptor stimulation of T cells. Mol Cell Biol. 2005;25:4426–4441. doi: 10.1128/MCB.25.11.4426-4441.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pang SS, et al. The structural basis for autonomous dimerization of the pre-T-cell antigen receptor. Nature. 2010;467:844–848. doi: 10.1038/nature09448. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Garvin JE, et al. T cell activation. Annu Rev Immunol. 2009;27:591–619. doi: 10.1146/annurev.immunol.021908.132706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balagopalan L, et al. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sondermann H, et al. Structural analysis of autoinhibition in the Ras activator Son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Margarit SM, et al. Structural evidence for feedback activation by Ras. GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- 24.Roose JP, et al. Unusual interplay of two types of Ras activators, RasGRP and SOS, establishes sensitive and robust Ras activation in lymphocytes. Mol Cell Biol. 2007;27:2732–2745. doi: 10.1128/MCB.01882-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das J, et al. Digital signaling and hysteresis characterize ras activation in lymphoid cells. Cell. 2009;136:337–351. doi: 10.1016/j.cell.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kortum RL, et al. Targeted Sos1 deletion reveals its critical role in early T-cell development. Proc Natl Acad Sci USA. 2011;108:12407–12412. doi: 10.1073/pnas.1104295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kortum RL, et al. Deconstructing Ras signaling in the thymus. Mol Cell Biol. 2012;32:2748–2759. doi: 10.1128/MCB.00317-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warnecke N, et al. TCR-mediated Erk activation does not depend on Sos and Grb2 in peripheral human T cells. EMBO Rep. 2012;13:386–391. doi: 10.1038/embor.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouquette-Jazdanian AK, et al. LAT-independent Erk activation via Bam32-PLC-gamma1-Pak1 complexes: GTPase-independent Pak1 activation. Mol Cell. 2012;48:298–312. doi: 10.1016/j.molcel.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberola-Ila J, et al. Positive and negative selection invoke distinct signaling pathways. J Exp Med. 1996;184:9–18. doi: 10.1084/jem.184.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maltzman JS, et al. Conditional deletion reveals a cell-autonomous requirement of SLP-76 for thymocyte selection. J Exp Med. 2005;202:893–900. doi: 10.1084/jem.20051128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daniels MA, et al. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 33.Prasad A, et al. Origin of the sharp boundary that discriminates positive and negative selection of thymocytes. Proc Natl Acad Sci USA. 2009;106:528–533. doi: 10.1073/pnas.0805981105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Priatel JJ, et al. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 35.Palacios EH, Weiss A. Distinct roles for Syk and ZAP-70 during early thymocyte development. J Exp Med. 2007;204:1703–1715. doi: 10.1084/jem.20070405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negishi I, et al. Essential role for ZAP-70 in both positive and negative selection of thymocytes. Nature. 1995;376:435–438. doi: 10.1038/376435a0. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz S, et al. RasGRF2, a guanosine nucleotide exchange factor for Ras GTPases, participates in T-cell signaling responses. Mol Cell Biol. 2007;27:8127–8142. doi: 10.1128/MCB.00912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu M, et al. The role of Ras guanine nucleotide releasing protein 4 in Fc epsilonRI-mediated signaling, mast cell function, and T cell development. J Biol Chem. 2012;287:8135–8143. doi: 10.1074/jbc.M111.320580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kreslavsky T, et al. beta-Selection-induced proliferation is required for alphabeta T cell differentiation. Immunity. 2012;37:840–853. doi: 10.1016/j.immuni.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nimnual AS, et al. Coupling of Ras and Rac guanosine triphosphatases through the Ras exchanger Sos. Science. 1998;279:560–563. doi: 10.1126/science.279.5350.560. [DOI] [PubMed] [Google Scholar]
- 41.Houtman JC, et al. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 42.Gomez M, et al. The GTPase Rac-1 controls cell fate in the thymus by diverting thymocytes from positive to negative selection. Immunity. 2001;15:703–713. doi: 10.1016/s1074-7613(01)00235-7. [DOI] [PubMed] [Google Scholar]
- 43.Yuste L, et al. Overexpression of RasN17 fails to neutralize endogenous Ras in MCF7 breast cancer cells. J Biochem. 2005;137:731–739. doi: 10.1093/jb/mvi092. [DOI] [PubMed] [Google Scholar]
- 44.Marais R, et al. Requirement of Ras-GTP-Raf complexes for activation of Raf-1 by protein kinase C. Science. 1998;280:109–112. doi: 10.1126/science.280.5360.109. [DOI] [PubMed] [Google Scholar]
- 45.Marks RE, et al. Differential Ras signaling via the antigen receptor and IL-2 receptor in primary T lymphocytes. Biochem Biophys Res Commun. 2003;312:691–696. doi: 10.1016/j.bbrc.2003.10.168. [DOI] [PubMed] [Google Scholar]
- 46.Gong Q, et al. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 47.McGargill MA, et al. Cutting edge: extracellular signal-related kinase is not required for negative selection of developing T cells. J Immunol. 2009;183:4838–4842. doi: 10.4049/jimmunol.0902208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohn SJ, et al. Non-redundant function of the MEK5-ERK5 pathway in thymocyte apoptosis. EMBO J. 2008;27:1896–1906. doi: 10.1038/emboj.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rincon M, Davis RJ. Regulation of the immune response by stress-activated protein kinases. Immunol Rev. 2009;228:212–224. doi: 10.1111/j.1600-065X.2008.00744.x. [DOI] [PubMed] [Google Scholar]
- 50.Sugawara T, et al. Differential roles of ERK and p38 MAP kinase pathways in positive and negative selection of T lymphocytes. Immunity. 1998;9:565–574. doi: 10.1016/s1074-7613(00)80639-1. [DOI] [PubMed] [Google Scholar]
- 51.Jang IK, et al. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci USA. 2010;107:10620–10625. doi: 10.1073/pnas.0905039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Priatel JJ, et al. RasGRP1 regulates antigen-induced developmental programming by naive CD8 T cells. J Immunol. 2010;184:666–676. doi: 10.4049/jimmunol.0803521. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, et al. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphorylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell antigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 54.Miyaji M, et al. Genetic evidence for the role of Erk activation in a lymphoproliferative disease of mice. Proc Natl Acad Sci USA. 2009;106:14502–14507. doi: 10.1073/pnas.0903894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kortum RL, et al. A phospholipase C-gamma1-independent, RasGRP1-ERK-dependent pathway drives lymphoproliferative disease in linker for activation of T cells-Y136F mutant mice. J Immunol. 2013;190:147–158. doi: 10.4049/jimmunol.1201458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sommers CL, et al. Bam32: a novel mediator of Erk activation in T cells. Int Immunol. 2008;20:811–818. doi: 10.1093/intimm/dxn039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frost JA, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 1997;16:6426–6438. doi: 10.1093/emboj/16.21.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zang M, et al. Interaction between active Pak1 and Raf-1 is necessary for phosphorylation and activation of Raf-1. J Biol Chem. 2002;277:4395–4405. doi: 10.1074/jbc.M110000200. [DOI] [PubMed] [Google Scholar]
- 59.Hundt M, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–522. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 60.Abdoel N, et al. Linker for activation of T cells is displaced from lipid rafts and decreases in lupus T cells after activation via the TCR/ CD3 pathway. Clin Immunol. 2012;142:243–251. doi: 10.1016/j.clim.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 61.Fukumoto R, et al. Inhibition of T-cell receptor signal transduction and viral expression by the linker for activation of T cells-interacting p12(I) protein of human T-cell leukemia/lymphoma virus type 1. J Virol. 2007;81:9088–9099. doi: 10.1128/JVI.02703-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeyda M, et al. LAT displacement from lipid rafts as a molecular mechanism for the inhibition of T cell signaling by polyunsaturated fatty acids. J Biol Chem. 2002;277:28418–28423. doi: 10.1074/jbc.M203343200. [DOI] [PubMed] [Google Scholar]
- 63.Marshall AJ, et al. A novel B lymphocyte-associated adaptor protein, Bam32, regulates antigen receptor signaling downstream of phosphatidylinositol 3-kinase. J Exp Med. 2000;191:1319–1332. doi: 10.1084/jem.191.8.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chau LA, Madrenas J. Phospho-LAT-independent activation of the ras-mitogen-activated protein kinase pathway: a differential recruitment model of TCR partial agonist signaling. J Immunol. 1999;163:1853–1858. [PubMed] [Google Scholar]
- 65.Kong KF, et al. A motif in the V3 domain of the kinase PKC-theta determines its localization in the immunological synapse and functions in T cells via association with CD28. Nat Immunol. 2011;12:1105–1112. doi: 10.1038/ni.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fuller DM, et al. The importance of the erk pathway in the development of linker for activation of T cells-mediated autoimmunity. J Immunol. 2012;189:4005–4013. doi: 10.4049/jimmunol.1201380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Markegard E, et al. Basal LAT-diacylglycerol-RasGRP1 signals in T cells maintain TCRalpha gene expression. PLoS ONE. 2011;6:e25540. doi: 10.1371/journal.pone.0025540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liston A, et al. Unravelling the association of partial T-cell immunodeficiency and immune dysregulation. Nat Rev Immunol. 2008;8:545–558. doi: 10.1038/nri2336. [DOI] [PubMed] [Google Scholar]
- 69.Matsuda S, et al. Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT. EMBO J. 2004;23:2577–2585. doi: 10.1038/sj.emboj.7600268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kamioka Y, et al. Multiple decisive phosphorylation sites for the negative feedback regulation of SOS1 via ERK. J Biol Chem. 2010;285:33540–33548. doi: 10.1074/jbc.M110.135517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lapinski PE, et al. A role for p120 RasGAP in thymocyte positive selection and survival of naive T cells. J Immunol. 2011;187:151–163. doi: 10.4049/jimmunol.1100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Iborra S, et al. H-ras and N-ras are dispensable for T-cell development and activation but critical for protective Th1 immunity. Blood. 2011;117:5102–5111. doi: 10.1182/blood-2010-10-315770. [DOI] [PubMed] [Google Scholar]
- 73.Perez de Castro I, et al. Mice deficient for N-ras: impaired antiviral immune response and T-cell function. Cancer Res. 2003;63:1615–1622. [PubMed] [Google Scholar]
- 74.Chang CF, et al. Polar opposites: Erk direction of CD4 T cell subsets. J Immunol. 2012;189:721–731. doi: 10.4049/jimmunol.1103015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.D’Souza WN, et al. The Erk2 MAPK regulates CD8 T cell proliferation and survival. J Immunol. 2008;181:7617–7629. doi: 10.4049/jimmunol.181.11.7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dummler B, et al. Pak protein kinases and their role in cancer. Cancer Metastasis Rev. 2009;28:51–63. doi: 10.1007/s10555-008-9168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Parrini MC, et al. Pak1 kinase homodimers are autoinhibited in trans and dissociated upon activation by Cdc42 and Rac1. Mol Cell. 2002;9:73–83. doi: 10.1016/s1097-2765(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 78.Yablonski D, et al. A Nck-Pak1 signaling module is required for T-cell receptor-mediated activation of NFAT, but not of JNK. EMBO J. 1998;17:5647–5657. doi: 10.1093/emboj/17.19.5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ku GM, et al. A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J. 2001;20:457–465. doi: 10.1093/emboj/20.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bubeck Wardenburg J, et al. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 1998;9:607–616. doi: 10.1016/s1074-7613(00)80658-5. [DOI] [PubMed] [Google Scholar]




