Abstract
Baculovirus-insect cell technologies are applied in the production of complex proteins, veterinary and human vaccines, gene delivery vectors‚ and biopesticides. Better understanding of how baculoviruses and insect cells interact would facilitate baculovirus-based production. While complete genomic sequences are available for over 58 baculovirus species, little insect genomic information is known. The release of the Bombyx mori and Plutella xylostella genomes, the accumulation of EST sequences for several Lepidopteran species, and especially the availability of two genome-scale analysis tools, namely oligonucleotide microarrays and next generation sequencing (NGS), have facilitated expression studies to generate a rich picture of insect gene responses to baculovirus infections. This review presents current knowledge on the interaction dynamics of the baculovirus-insect system‚ which is relatively well studied in relation to nucleocapsid transportation, apoptosis, and heat shock responses, but is still poorly understood regarding responses involved in pro-survival pathways, DNA damage pathways, protein degradation, translation, signaling pathways, RNAi pathways, and importantly metabolic pathways for energy, nucleotide and amino acid production. We discuss how the two genome-scale transcriptomic tools can be applied for studying such pathways and suggest that proteomics and metabolomics can produce complementary findings to transcriptomic studies.
Keywords: baculovirus, insect virus, virus-host interactions, microarray, RNA sequencing, next generation sequencing, genome scale, expression profile
1. Introduction
Baculoviruses are enveloped viruses that contain a circular double stranded DNA genome (80–180 kb) [1]. Baculoviruses are specifically infectious to invertebrates, most commonly insect species belonging to the Lepidoptera order, and there is no evidence of cross-order transmission [2]. Baculoviruses can also enter a range of mammalian cell types including human, primates, rodent, rabbit, porcine, bovine, and other animals such as fish and avian species, but neither replicate nor transpose baculovirus DNA into host chromosomes in these cell types [3,4]. Hence, the viruses are safe to vertebrates and have been approved by the US Food and Drug Administration and European Medicine Agency for manufacturing of veterinary and human vaccines [5].
Over 40 years of research on molecular biology of baculoviruses has produced a rich knowledge about baculovirus gene expression profiles and gene functions during infection, including: virus entry, movement into the cell nucleus, DNA unpackaging, early transcription, DNA replication, late transcription, translation, budded virus (BV) assembly, BV budding, occlusion derived virus (ODV) assembly, occlusion, and release of occlusion bodies (for an overview of baculovirus molecular biology studies, refer to Rohrmann [6]). However, little is known about host responses towards baculovirus infections.
Baculoviruses exert vigorous effects on host cells. Within a short infection time frame, the viruses rapidly propagate and change host cells by arresting host cell cycle progression, creating a “viral pseudo S phase”, remodeling the cellular cytoskeleton, forming virogenic stroma in the nucleus as a specialized compartment for replication, undertaking transcription and packaging, and eventually breaking-down the host nuclei. In the Helicoverpa armigera nucleopolyhedrovirus (HearNPV)-H. zea system, within 48 hours post infection (h.p.i), baculoviruses can produce over 250,000 genomes per cell, which is 20 times more DNA than that produced in an uninfected host cell, and the total viral mRNA from 134 virus genes is higher than the total cellular mRNA produced from over 15,000 genes during normal cell growth [7]. Similarly, in the Autographa californica multicapsid nucleopolyhedrovirus (AcMNPV) infected Spodoptera frugiperda cell line (Sf9) and AcMNPV infected Trichoplusia ni cell line (Tnms42), as high as one million viral genomes per haploid host insect genome was reported [8].
Although infected cells are controlled by baculoviruses, and a global reduction of host proteins and mRNAs occurs, host cells also respond by up-regulating or repressing certain genes and cellular pathways. A number of genes escape from the global expression shutoff and several host cellular pathways such as DNA damage responses, heat shock responses, pro-survival responses, apoptosis, energy metabolism, iron-ion transport, ubiquitin-proteasomal degradation and miRNA pathways have been shown to be up-regulated following infections by recent studies [9,10,11,12,13].
Transcriptomics is a powerful tool to study baculovirus-insect systems at the mRNA level, which is arguably the most important level of regulation during a baculovirus infection. Previous studies by Iwanaga et al. [14] using BmNPV infected cells suggested that infection events in the host cell were strongly regulated at the mRNA expression level rather than at the translational level. Gatehouse et al. [15] proposed that protein and RNA degradation are the key processes for controlling the global shutoff pattern induced by a baculovirus infection. In addition, polysome profiles, which were mRNA-ribosome complexes extracted by sucrose gradient centrifugation of cytosolic fractions of infected cells, were similar between Sf9 cells with and without an AcMNPV infection, indicating that active translation activities take place in infected cells even at late infection stages (16 h.p.i) [16]. Usually, it was found that changes in protein levels were due to prior changes in mRNA levels. Huynh et al. [17] showed that for high cell density infections in cultures, a reduction in the expression of the β-galactosidase protein in Sf9 cells infected by a recombinant AcMNPV virus was due to early infection events, most likely leading to a decrease in mRNA levels following a reduction in virion genome numbers.
To study host responses, the lack of insect genome sequences is a challenge. To overcome this challenge, rapid developments of RNA sequencing (RNA-seq) technologies for generating genome-scale transcript sequences in non-model organisms can be utilized [18,19]. This review shows that most of our understanding about insect host responses to baculovirus infections so far has been derived from microarray and RNA-seq studies in the last five years. These studies, however, investigated different host-baculovirus systems, under different infection conditions, and hence identified different sets of regulated genes. This review identifies possible conservative changes and knowledge gaps relating to host-baculovirus interactions, and proposes the further use of transcriptomics to investigate genome-scale interactions, with more focus on host responses. Finally‚ the review briefly discusses challenges and opportunities for genetic engineering of host genes, determined by transcriptomic studies, to moderate the infection process in order to increase product yields using insect cell technology, which is important for commercialization of the technology. For expression of some recombinant proteins, efficacy remains the key challenge in relation to commercial production but for many protein products and certainly for wild type baculoviruses to be used as biopesticides, yield improvements are essential for insect cell technology to be competitive in relation to alternative manufacturing systems.
2. Host Genes/Pathways that Baculoviruses Require for Successful Infection Processes in Insect Cells
2.1. Transcriptomic Studies
Transcriptomic studies of baculovirus gene expression have expanded current understanding extensively. One of the first microarray studies for baculovirus genes was by Yamagishi et al. [20], in which PCR amplified fragments of virus genes were printed on 192 spots of a microarray chip and used as probes for hybridization. The study found four virus genes, namely p10, p35, lef-3 and lef-6, were involved in differential responses of host cells to AcMNPV infection, which would lead to different productivities by two insect cell lines Sf9 and High-Five. Also, by applying DNA microarrays with cDNA probes from PCR, Jiang et al. [21] characterized sequential expression patterns of all 155 AcMNPV genes and found 12 virus genes that depended on the virus pe38 gene for expression. Recently, the use of NGS for analysis of baculovirus gene expression patterns produced unprecedented genome-scale analysis of baculovirus infection processes, including mapping of all transcription start sites and polyadenylation sites, splicing variants, and putative interactions of virus proteins expressed at certain infection phases with host proteins [8,11]. A recent study using NGS for differential gene expression analysis and bioinformatic prediction of protein-protein interaction network suggested 22 viral proteins that could interact with 2,326 host proteins, totaling almost 8,907 interactions [11]. Another RNA-seq study comprehensively characterized 218 transcription start sites and 120 polyadenylation sites of all AcMNPV virus genes [8]. The authors also detected the possible encapsidation of viral mRNAs for late genes, suggesting a novel mechanism that the virus potentially uses to establish early infection, which is currently believed to depend entirely on the host RNA polymerase. Their study also suggested that 12 virus genes carry splicing variants, rather than only one gene as previously thought. The study also found an unexpected scale of antisense transcription for 50 virus genes. Such genome-scale transcriptomic studies offer great capacity to answer many unanswered questions on baculovirus gene regulation. Table 1 outlines the current knowledge on functions of a number of baculovirus genes that interact with host genes, which have been identified, mainly by molecular experiments, to interact with insect hosts.
Table 1.
Virus genes with known interactions with host genes or known effects on host functions.
| Functional groups | Baculovirus genes/pathways | Baculovirus strains [6] | Functions | Ref. |
|---|---|---|---|---|
| Virus genes interacting with host cell receptors | GP-64, F protein | GP-64 only in group I alpha NPV‚ while the F protein in group II alpha-, beta- and delta- NPV | Virus-cell receptor attachment, facilitate entry by clathrin-mediated endocytosis processes | [34,35] |
| Per OS infectivity factors (Pif-1, 2, 3, p-74)‚ and possibly Pif-4 and 5 | All 5 Pifs and p-74 are core baculorius genes, and found in other invertebrate DNA viruses that replicate in the nucleus | Pif-1, 2, and 3 and p74 form a complex and facilitate ODV binding to midgut epithelial cell receptors | [36] | |
| Apoptosis | IAP-1, IAP-2, IAP-3, IAP-4, and IAP-5 | IAP1-4 in both NPVs and Granulosis viruses (GVs), while IAP-5 in GVs only. Each baculovirus strain has several/not all IAPs. IAP orthologs found in a number of hosts | Baculovirus IAPs mediate protein-protein interactions to block selected caspases. The IAP RING domain functions as an E3 ubiquitin ligase to trigger proteasome degradation of targeted caspases | [37] |
| P35 | AcMPNV, BmNPV, Culex nigripalpus NPV, Leucania separata MNPV, Maruca vitrata MNPV, T. ni MNPV and Clostera anachoreta | Binds to and inactivates host’s effector caspases | [38] | |
| p49 | SpltMNPV, LsMNPV, SlNPV, AcMNPV, and HearNPV | Inhibits host’s initiator caspases (upstream of p35 but downstream of IAPs) and several host’s effector caspases | [39] | |
| Replicative lefs (lef-1, 2, 3, and 11) p-143, DNA pol and IE1/IE0 | Lef-3 in Lepidopteran NPV and GV; Lef-1, Lef-2, Lef-11, and p-143 in all baculoviruses (except for Lef-11 not in CuniNPV), IE1/IE0 in all group I and II alpha baculoviruses | Trigger host DNA damage response and induce apoptosis | [40] | |
| Cell cycle | ODV-EC27 (A virus multifunctional Cyclin) | In all baculoviruses | Interacts with host’s cdc-2 for cell cycle arrest at G2/M phase, or with host’s cdc-6 to override host check-point to allow DNA replication | [41] |
| P33-sulfhydryloxidase (SOX) | In all baculoviruses | Forms stable complex with host’s p53 protein, preventing p53-induced apoptosis | [42] | |
| Cytoskeleton and nucleocapsid transport | Protein kinase-1, Protein kinase-2 | PK-1 found in Lepidopteran NPVs, and GVs, similar to some insect PK; PK-2 found in AcMNPV, BmNPV, PlxyNPV and RoMNPV | Actin cytoskeleton remodeling (protein-protein interaction prediction) | [11] |
| Arif-1 | All group I and most group II Alpha baculoviruses | Accumulates F-actin at the plasma membrane | [43] | |
| VP80 (a Paramyosin-like protein)/P78-83/VP39 | VP80 and p78/83 in all group I and II Lepidopteran NPVs; VP39 in all baculovirus genomes | Interact with host’s F-actin filaments to transport nucleocapsids in the cytoplasm | [44,45] | |
| VP80 | Vp80 found in all group I and II Lepidopteran NPVs | Interact with myosin motor proteins and F-actin to transport nucleocapsids to the nucleus periphery | [44] | |
| IE-1, PE38, HE65, Ac004, Ac102, Ac152 | IE1 all baculoviruses; PE38 in all Group I NPV and four GV genomes | Accumulate host’s monomeric G-Actin into nucleus | [46] | |
| P78/83 (N-WASP-homologous protein) and ODV-C42 | p78/83 all group I and II Lepidopteran NPVs | At early infection, transports nucleocapsid into nucleus by activating nuclear actin polymerization via an actin related protein (Arp2/3) complex. At late infection, facilitates actin assembly to form F-filament inside nucleus | [47,48] | |
| EXON0 | In all Lepidopteran NPVs | Interacts with β-tubulin to facilitate binding of nucleocapsids to microtubules | [49] | |
| P10 | In all group I and II NPVs and most GVs | Interacts with α-tubulin and mediates nuclear disintegration and cell lysis | [50] | |
| Nucleo-cytoplasmic transport of viral proteins | FP25K and E26 | FP25K in all Lepidopteran NPVs and GVs. E26 in group I Lepidopteran NPV | Together with host Importin-α-16, transport viral proteins into the inner nuclear membrane (INM) | [51] |
| Metabolism | ADP ribose pyrophosphatase (Ac38) | All Lepidopteran NPVs and GVs | The enzyme hydrolyzes ADP-ribose, an intermediate of metabolism of NAD+, mono- or poly-ADP-ribosylated proteins and cyclic ADP-ribose, thereby conferring detoxification effects | [52] |
| P33-sulfhydryl oxidase (SOX) | In all baculoviruses | Flavin adenine dinucleotide (FAD)-binding sulfhydryl oxidase can play roles in protein disulphide bond formation and protection from oxidative stress | [53,54] | |
| Super oxide dismutase (SOD) | In most Lepidopteran baculoviruses | Converts superoxide into Hydrogen peroxide (possibly active in BmNPV, but this activity is not confirmed in AcMNPV) | [55,56] | |
| Replication | Ribonucleotide reductase | Three GVs, 10 NPVs group II, OpMNPV and LdMNPV | Catalysis of ribonucleotides to deoxyribonucleotides for DNA synthesis | [57,58] |
| DNA polymerase complex (Dnapol, helicase, primase, SSB, and LEF-2) | All baculoviruses | May require host’s DNA topoimerases and DNA ligases | [6,59] | |
| dUTPase | In nine group II NPVs, OpMNPV, and two GV genomes | Prevents incorporation of dUTP into DNA | [58,60] | |
| Transcription | IE1/IE0, IE2, hrs, ADPRase (ADP-ribose pyrophosphatase) | IE1/IE0, hrs and ADPRase in all baculoviruses. IE2 in all Group I Lepidopteran NPVs but not others. pe38 in all Group I NPV and four GV genomes | Bind to host transcription factors | [61] |
| Lef-6 | All Lepidopteran NPVs and GVs | Lef-6 has a TAP (TIP associating domain), which can interact with nuclearporins for mRNA export to the cytoplasm | [6,62] | |
| Ac98-38 K protein | All baculoviruses | Predicted to have carboxyl terminal domain (CTD) phosphatase activities that negatively regulate RNA polymerase II by inhibiting RNA elongation | [6] | |
| Translation arrest | P35, IAPs and P49 | As mentioned before | Enhance early host translation arrest | [63] |
| Protein kinase 2 (Pk-2) | PK2 found in AcMNPV, BmNPV, PlxyNPV and RoMNPV | Represses translation arrest, which is caused by host eIF2α kinase, by blocking eIF2α access to translation initiation factors | [64,65] | |
| Host range factor 1 (Hrf-1), | Only found in viruses of Lymantria dispar host, including LdMNPV and Orgyia pseudotsugata MNPV | Inhibits translational arrest by an unknown mechanism | [66] | |
| Hycu-ep32 gene | Hyphantri acunea NPV and OpMNPV | Induces host translation arrest by an unknown mechanism | [67] | |
| IAP-1 and IAP-2 | As mentioned before | IAP1 and IAP2 possess ubiquitin ligase activities, enabling polyubiquitination of insect proteins, thus marking them for degradation | [68] | |
| Growth & development | Protein tyrosine phosphatase (PTP) | All Lepidopteran Group I NPVs, not others, orthologs found in insect host | Induces host hyperactive behaviours | [69,70] |
| Viral Fibroblast growth factor (vFGF) | All baculoviruses, orthologs found in insect hosts | Increases host larvae motility by facilitating systemic infection | [71,72] | |
| Chitinase and Cathepsin | Chitinase and Cathepsin in all Group I (except AgMNPV), all Group II (except AdhoNPV for Chitinase) and four GVs | Chitinase breaks larvae chitin layer, Cathepsin is a viral proteinase | [73] | |
| Ecdysteroid UDP glucosyl transferase (EGT) | All Lepidopteran Group I NPV, not others | Prevents moulting to extend insect life and virus propagation time (transfers glucose group to inactivate insect molting hormone ecdysteroids), induces host hyperactive behaviours | [74,75] | |
| MicroRNA | BmNPV-miR-1 | Conserved in AcMNPV, BomaNPV, PxMNPV, RoMNPV, and MaviNPV | Down-regulates the transport of host small-RNA from the nucleus to the cytoplasm, thereby reducing active population of host small RNAs | [76] |
| BmNPV-miR-2 to 4 | Conserved in AcMNPV, BomaNPV, PxMNPV, RoMNPV, and MaviNPV | Potentially targets 8 viral genes and 64 host genes | [77] |
For transcriptomic studies in an insect host, genome-scale sequences of coding genes are valuable resources for studying insect responses to baculovirus infections. With international efforts, only two Lepidopteran genomes are available to date, namely the B. mori genome [22] and the recently sequenced Diamondback moth (Plutella xylostella) genome [23]. To overcome challenges due to the lack of genomic sequences, several research groups have applied the traditional Sanger sequencing method to different collections of expressed sequence tag (EST) libraries such as those from the fall armyworm S. frugiperda [24,25], or from H. virescens [26], but these efforts could generate sequences for only a portion of the genome, usually fewer than 5,000 coding genes. Recently‚ more powerful RNA-sequencing technology has revolutionized the exome sequencing of a variety of non-model organisms, including insect species such as Manduca sexta [27], H. virescens [28], Galleria mellonella [29], L. dispar [30], and H. zea [18]. These sequence resources have been contributing to the expression analysis of baculovirus-insect interactions.
Expression analysis using microarrays have been applied to different baculovirus infection setups to compare different virus-host interactions, including: permissive vs. non-permissive hosts [31], infection of fat body vs. haemocyte [31], infection of high-yield vs. low-yield cell lines [20], uninfected vs. infected cells [15,18,32,33] or to compare infections at different time-points [7,10]. RNA-sequencing experiments are also useful for the analysis of RNA differential expression of all insect genes and of non-coding RNA [8,11,19]. Reviewing findings by these studies may show common genes and pathways being regulated by baculovirus infections (Table 2).
Table 2.
Host genes that respond to/are affected by baculovirus infections*.
| Functional groups | Insect genes/pathways | Functions | Virus-host systems and expression time | Ref. |
|---|---|---|---|---|
| Immune genes | Gloverin | An antibacterial and antiviral protein that interacts with the lipid envelope surrounding viral nucleocapsids | BmNPV-B. mori larvae and AcMNPV-S. exigua larvae (induced from 6–12 h.p.i) | [19,31,78] |
| Cecropin | A cationic antimicrobial peptide that has positively charged regions in its α-helical peptide and interferes with the lipid membrane | H. virescens larvae-HzSNPV and HzAM1 cells-HearNPV(induced from 12 h.p.i) | [7,33,79] | |
| Apoptosis genes | Down regulation of host IAPs, and up-regulation of host apoptosis enhancers and Caspases trigger apoptosis upon virus infections | Epiphyas postvittana larvae-EppoNPV (5 d.p.i); HzAM1 cells-HearNPV (12 h.p.i); and Sf21-AcMNPV (before 24 h.p.i) | [15,18,80] | |
| Signal transduction | Phosphatidylinositol 3 kinases (Pi3K)-Akt pathway | Elevation of this pathway prevents apoptosis and creates inductive environment for virus propagation | AcMNPV-Sf9 (induced from 1–18 h.p.i) | [81,82] |
| MAPK pathways | Extracellular signal-regulated kinase (ERK) and c-Jun NH2-terminal kinase (JNK) pathways are activated at late infection and important for virus production | BmN4 cells-BmNPV (induced from 4–24 h.p.i) | [11,83] | |
| DNA damage response kinases | Triggered by virus replication‚ leading to cell death | AcMNPV-Sf21 (induced from 2–24 h.p.i) | [9] | |
| Metabolic genes | ABC transporters and sugar transporters | ABC transporters transport a broad spectrum of substrates, including degradation products from cytosol to ER | BmNPV-B. mori cell line and AcMNPV-S. exigua larvae (induced from 2–12 h.p.i); HearNPV-HzAM1 (induced from 12–18 h.p.i) | [7,19,32,84] |
| Citrate synthetase | Important for energy generation (a key enzyme in the Citric acid cycle, TCA) | AcMNPV-Sf9 and BmNPV-B. mori cell line (induced from 2 h.p.i) | [14,85] | |
| Pyruvate dehydrogenase/Aldehyde dehydrogenase | Important for energy generation and diversion of substrates to lipid biosynthesis | AcMNPV-Sf9 induced from (6 h.p.i) | [12] | |
| Lipid reductases and lipid desaturases | Fatty acid metabolism | HzNPV-H. virescens (induced from 12 h.p.i) | [33] | |
| Genes involved in cellular iron (iron ion transport, ferric iron binding, and cellular iron ion homeostasis) | Iron is important for processes such as DNA replication and ATP generation | BmNPV-B. mori cell line (induced from 3–6 h.p.i); H. virescens larvae-HzSNPV (induced from 24–72 h.p.i) | [11,86] | |
| Mitochondrial respiratory genes | Important for energy generation | BmNPV-B. mori cell line (induced from 1.5–24 h.p.i) | [7,11] | |
| Translation | Heat shock protein (HSP) 70, HSP90, and Heat shock protein cognate (HSC) 70 | Protein folding and facilitate several cellular processes conducive for virus replication such as ubiquitin-proteasome pathway | BmNPV-B. mori larvae (6–12 h.p.i); AcMNPV-Sf21 cell line (induced from 6–48 h.p.i);AcMNPV-S. exigua larvae (induced from 12 h.p.i) | [10,19,31,87] |
| ER proteins (reduced) | ER stress | AcMNPV-Sf21 and AcMNPV-Sf9 (induced from 12–48 h.p.i) | [10,12] | |
| Translation initiation factors (TIFs) | Enhance translation | BmNPV-B. mori cell line (induced from 1.5–24 h.p.i) | [11,19] | |
| eIF2α | Phosphorylation of eIF2α causes translation arrest | AcMNPV-Sf9 cell line (Before 36 h.p.i) | [64,88] | |
| Replication | Histone genes | Regulate host chromatin structure, which affects DNA replication | BmNPV-B. mori cell line (induced from 1.5–24 h.p.i) | [11] |
| Transcription | Host’s polyhedrin promoter binding protein (PPBP) | Binds to promoters of both p10 and polyhedrin genes to enhance their transcription | AcMNPV-Sf9 (induced late) | [89] |
| Transcription initiation factors | Enhance transcription | BmNPV-B. mori cell line (induced from 6–24 h.p.i); HearNPV-H.zea (induced from 18 h.p.i) | [7,11] | |
| mRNA and protein degradation | Alkaline nuclease | mRNA degradation | E. postvittana larvae-EppoNPV (induced from 5 d.p.i); BmNPV-B. mori cell line (induced from 6 h.p.i) | [15,32] |
| Ubiquitin-proteasome pathway | Protein degradation | BmNPV-B. mori cell line (induced from 2–6 h.p.i); Sf9-AcMNPV (induced from 1.5–48 h.p.i) | [ 11,14,90] | |
| Cytoskeleton | Dynein | A motor protein involved in microtubule transport | BmNPV-B. mori cell line (induced from 6–12 h.p.i) | [32] |
| Development | Juvenile hormones | Maintaining juvenile hormones at high level extends growth and inhibits moulting | H. virescens larvae-HzSNPV (induced from 12 h.p.i); E. postvittana larvae-EppoNPV (induced from 5 d.p.i) | [15,33] |
| MicroRNAs | miRNA (90 miRNAs in Sf, 114 in B.mori) | Play roles in antiviral response by degrading viral transcripts (e.g. bmo-miR-8 potentially targets IE1) | Sf9-AcMNPV (Usually induced at late infection, 24–72 h.p.i) | [13,91] |
| Dicer 2 | Produce viral short interfering siRNAs that degrade viral transcripts | H. armigera larvae—HearNPV (induced from 48 h.p.i) | [92] | |
| Transposition of host DNA | Host transposable elements (retrotransposons), reverse transcriptase, gag/pol-like proteins | DNA transposition into baculovirus genomes, contributing to virus genome instability. | Sf9 cells, T.ni cells, H. virescens larvae-HzSNPV and AcMNPV-S. exigua larvae (During in vitro serial passaging) | [19,33,93,94] |
| Detoxification | Glutathione S-transferase (GST) | Convert glutathione into water-soluble, less toxic metabolites | AcMNPV-S. exigua larvae (induced from 12 h.p.i) | [19] |
* In contrast to Table 1‚ which shows mostly findings from molecular studies based on the analysis of virus genes‚ this Table shows that a majority of current understanding on insect responses to baculovirus infections have been obtained from genome scale transcriptomic expression studies (those highlighted in bold and red text).
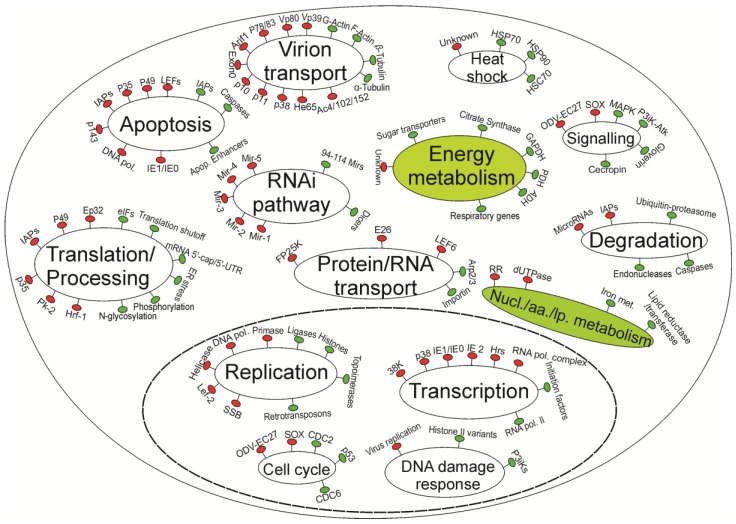
The following subsections review in more detail the virus-insect interactions presented in Table 1, Table 2, and summarized in Figure 1 according to major pathways and cellular processes.
Figure 1.
A simplified model of current understanding of host-baculovirus interactions. Virus genes/processes are in red, and insect genes/processes are in green. Major pathways are indicated by ellipses. The left half of the diagram represents pathways that are relatively well understood. The right half shows pathways that deserve more research, especially those indicated by filled ellipses. The ellipse with dashed boundary represents the nuclei. The nucl./aa./lp. abbreviation stands for nucleotide/amino acid/lipid metabolism processes.
2.2. Metabolism
In relation to energy, no gene for an enzyme involved in energy metabolism has been found in any sequenced baculoviruses. However, a number of processes during virus infection require a high energy supply. For example, DNA packaging into nucleocapsids requires Adenosine triphosphate (ATP) for motor protein activities [95], elongation during translation uses Guanosine triphosphate (GTP) energy for binding of tRNA onto ribosomes, and active substrate transport, such as that provided by ABC transporters, the uptake of amino acids into cells, the export of viral RNA and the import of viral proteins cross the nuclear membrane, plus the directional transport of nucleocapsids all consume extensive energy [44]. Therefore, baculoviruses rely on host enzymes for energy metabolism. Transcriptomic studies show that ABC transporters and Citrate synthetase increased in Sf9 cells infected by AcMNPV [32,84]. These studies also identified an increase in the level of Glyceryldehyde 3-phosphate dehydrogenase (GAPDH) in HzAM1 cells upon infection by HearNPV [7], and in other hosts infected by DNA viruses‚ including Vaccinia virus infected human monocytes [96] and Rock bream Iridovirus infected marine teleost cells [97]. Similarly, up-regulation of Aldehyde dehydrogenase was found in both S. exigua cells infected by AcMNPV [19] and AcMNPV infected Sf9 cells [12]. Importantly, how the virus manipulates host energy generation and metabolic pathways to foster virus replication is still largely unknown.
For synthesis of virus building blocks such as nucleotides and amino acids, there are known virus genes for nucleotide metabolism, encoding a Ribonucleotide reductase (RR) large subunit and a small subunit, and a dUTPase protein, which prevents incorporation of mutagenic dUTP into DNA, suggesting that nucleotide metabolism may be especially important for baculovirus infections [57,58]. Via transcriptomics, Breitenbach et al. [33] found an increase in fatty acid metabolism in infected insects, including genes encoding lipid reductases and lipid desaturases in H. virescens larvae infected by HzSNPV. However, an extensive range of responses by metabolic genes have not been identified in baculovirus infected cells, especially those related to nucleotide and amino acid metabolism, which are important for production of new viruses in infected cells.
2.3. Translation
For translation, baculoviruses are entirely dependent on the host translational machinery including ribosomes, tRNAs, amino acid metabolism and transport, chaperones for protein folding, endoplasmic reticulum (ER) for glycosylation/phosphorylation/transport, and other translation factors. Several mechanisms allowing baculovirus mRNAs to compete with insect host mRNAs for translation have been found. These include the presence of AT-rich regions and unstructured 5' untranslated (UTR) regions [8,98], which confer a higher binding affinity for host translation factors by virus mRNAs compared to that of host mRNAs; the relative independence of virus late mRNAs on the 5'-cap-binding eukaryotic initiation factor (eIF4E) for the ribosomal recruitment process, in contrast to most insect mRNAs, which are dependent on cap-binding proteins for being translated [98,99]; and the ability of several virus genes, namely protein kinase 2 (pk-2) and host range factor 1 (hrf-1), to inhibit translational shutoff [64,66]. Emerging evidence also suggests that baculoviruses up-regulate translation initiation factors to accelerate the translation process [7,11].
Up-regulation of host genes for protein folding, translation initiation, and translational arrest have been reported. Like many other viruses, baculoviruses utilize host chaperones to facilitate the rapid synthesis of a large amount of viral proteins [87]. Heat shock proteins (HSPs) and heat shock protein cognates (HSCs) were commonly up-regulated in AcMNPV-S. frugiperda, HearNPV-H. zea, HzSNPV-H. virescens, B. mori-BmNPV, and S. exigua-AcMNPV infections [10,18,19,32]. Similarly, the up-regulation of other translation factors was commonly found, including: eukaryotic initiation factors (eIF3-6, eIF1A, eIF3-2b) and an elongation factor (EF1d) in B. mori-BmNPV infections [11]; eIF in S. exigua-AcMNPV infections [19]; and IF-2 and IF-3 in H. zea-HearNPV infections [7]. In addition, baculovirus infections trigger phosphorylation of the Eukaryotic initiation factor (eIF2α), which potentially causes translation arrest, but the host protein synthesis machinery is still functional at the late infection stage, as shown by detection of polysomal translation activities [16]. Protein degradation is one of the major responses of the host cell to a baculovirus infection. Proteasome 26S subunit and the ubiquitin-proteosomal degradation pathway in general were up-regulated in silkworm cells infected by BmNPV [11,32]. There is also evidence for baculovirus infections causing Endoplasmic reticulum (ER) stress [33]. More research is needed to better understand the roles of the ER, ribosomes, and translation factors needed for facilitating viral protein production.
2.4. Transport
For transport processes in infected cells, nucleocapsid movement is relatively well understood, while there is less knowledge on transport of viral proteins for virus DNA packaging, nucleocapsid assembly, and occlusion processes. Baculoviruses have devised effective transport mechanisms that are dependent on host Actin and cause a cytoskeleton remodeling phenomenon, in which both microfilaments and microtubules are used [44,45,46,49,100]. Nucleocapsid transport was detected within the cytoplasm as early as 5–30 min post infection, and inside the nucleus within one hour post infection [45]. A number of virus genes have been found to be involved in the nucleocapsid transport process, including: VP80, P78/83, EXONO, p10, VP39, ODV-c42, Ac66 and Arif-1 (Actin rearrangement inducing factor) [46]. For assembly, traditional protein techniques using yeast-two-hybrid screening for studying ODV protein-protein interactions have identified five triple-interactions, eight double- and nine self-interactions among virus ODV proteins [101]. Interactions of virus proteins with nuclear actin filaments and microtubule systems are essential for nucleocapsid assembly [44,45]. For transport of ODV proteins from the ER to the inner nuclear membrane, the host Importin-α-16, the viral FP25K (interacts with all known ODV proteins) and the ODV-E26 protein (interacts with some ODV proteins) play important roles [51]. The nuclear importins and exportins are also important for exporting viral mRNA to the cytoplasm, but their specific roles for transporting viral mRNA have not been investigated. BV envelope proteins are N-glycosylated and are transported from the ER to the cellular membrane, where nucleocapsids bud out. DNA packaging involves a packaging motor complex, possibly contains a virus Ac66 protein which interacts with host myosin and ATPase, and likely involves host actin and microtubules [6]. The occlusion process uses micro vesicles derived from the inner nuclear membrane [102]. Besides temporal differences in relation to BV versus ODV formation, it is unknown how virus genomes are directed to BV assembly rather than to the ODV occlusion process. The finding that a number of host proteins are incorporated into BV structures indicates that a number of unknown host proteins may be important for virus assembly, or for BV functions when entering new cells [11]. Thus, although the movement and remodeling of the host cytoskeleton system has been well studied, the roles of host genes in viral mRNA and viral protein transport and in virion assembly are still poorly understood.
2.5. Replication
For virus replication, baculoviruses have evolved a near complete replication machinery, which concentrates in a separate compartment within the nucleus, called the virogenic stroma [103]. Known replication enzymes that baculoviruses possess include: DNA polymerase (capable of both leading and lagging strand synthesis as well as proof reading by the 3'–5' exonuclease activities), helicase (p143), late expression factor LEF-1 (late expression factor, primase), LEF-2 (primase accessory factor), LEF-3 (single stranded DNA binding protein), p143 (helicase), origin binding protein IE1 (immediate early), and origin of replication with homologous regions (hrs) and non-homologous regions (non-hrs) (reviewed by Rohrmann [6] and Vanarsdall et al. [59]). Compared to virus transcription, replication may require more host factors, such as host DNA topoimerases (to untwist the double stranded DNA, thereby facilitating helicase activities) and DNA ligases (to link Okazaki fragments at the lagging strand). However, the host DNA polymerase is unable to substitute the viral DNA polymerase to complete virus DNA replication [103]. On the other hand, a baculovirus DNA polymerase can be interchanged with that from another baculovirus or even with a distantly related ascovirus DNA polymerase [104], suggesting that there is flexibility for baculovirus DNA polymerase activities, and hence it is possible that host DNA replication factors can facilitate viral replication. It remains unknown which host replication factors are used for virus replication, an efficient process that eventually leads to over 20 times more virus DNA than total host DNA in an infected cell [7].
For host DNA replication, cellular DNA replication is arrested, but viruses can utilize the host replication machinery to facilitate viral replication. Under baculovirus infection conditions, while cell cycle progression is abandoned at the G1/S or G2/M checkpoint (by 4 h.p.i, or 10 h.p.i in AcMNPV and 24 h.p.i for HearNPV systems), virus DNA replication increased rapidly [105]. Baculovirus replication, by a rolling cycle and recombination process, causes a cellular DNA damage response (DDR) following phosphorylation of several Phosphoinositide 3-kinases (Pi3K), which phosphorylate targeted substrates in the nucleus, for example the Histone 2A variant, and eventually causes cell cycle arrest or apoptosis [9]. The host DDR is a conserved response found by many host cells following infection by DNA viruses [106]. The up-regulation of Histone 2A variants was also found in B. mori cells infected by BmNPV [11]. Interestingly, even though the DDR is considered as a defence response by the cells, baculoviruses require this process for promoting virus replication and inhibition of DDR reduced virus production by 100,000 times [9]. Cellular replication factors, such as DNA polymerase subunits, could also be utilized by the virus for viral replication [7]. Knowing how the virus makes full use of the cellular replication machinery is important for maximizing virus production.
2.6. Transcription
For virus late transcription, baculoviruses possess a complete and effective transcription system to express viral genes under stringent temporal patterns. The viruses have evolved a unique RNA polymerase complex that can transcribe genes and process mRNAs at both ends by capping and polyadenylation. The complex contains 4 subunits‚ which are: LEF-4, an RNA capping enzyme; LEF-8, similar to the large subunit β in Bacteria; LEF-9, similar to the large subunit β’ in Eukaryotes; and p147 similar to the alpha subunit in Bacteria. Virally encoded transcription enhancers include: a very late factor VLF-1‚ which binds to a burst sequence to make very late promoters accessible to RNA polymerase; a LEF-5 gene as a transcription initiation factor; and different DNA sequence motives within early and late promoters. Virus genes for mRNA processing include: the LEF-4 and an RNA cap 2’O-methyltransferase that are responsible for capping by RNA triphosphatase and Guanine transferase activities; an ADP-ribose pyrophosphatase for decapping; and a gene for polyadenylation‚ which is the viral RNA polymerase II with a distinct mechanism different to the host’s polyadenylation process [107]. The viruses also encode a putative mRNA exporter (LEF-6). Nevertheless, the host factors are essential for immediate early transcription, when the virus transcription machinery has not been established. In addition, it is likely that the viruses also utilize host factors to facilitate its late transcription processes, such as a polyhedrin promoter binding protein (PPBP), which binds to promoters of both p10 and polyhedrin genes to enhance their transcription [89]. Xue et al. [11] predicted that all five virus proteins, including protein kinases (PK-1 and PK-2), CG-30, IE-2 and PE38, which display the most extensive interactions with over 1,000 insect genes, are related to transcription regulation, indicating that a high number of host proteins may contribute to virus transcription. Up-regulation of host transcription factors to facilitate the viral infection process deserves to be explored further.
2.7. Immune responses
For apoptosis and cell cycle arrest‚ it is well known that baculoviruses have evolved anti-apoptosis genes (virus inhibitor of appoptosis IAPs, p25, and p49) to inhibit cell death responses and they carry a cell cycle arrest gene, a Cyclin homolog, to block G2/M progression and create a “pseudo S” phase conducive for virus replication. On the other hand, there is little research on virus infection and host signaling pathways to identify cellular modulators and mediators involved in apoptosis and translation arrest [108]. There is evidence that the virus DNA replication process via a rolling cycle and recombination mechanism trigger a host DNA damage response, via the P3i-Akt pathway, which leads to an apoptotic response. The virus anti-apoptosis counter-defense is by direct binding of virus anti-apoptotic proteins to host caspases or by triggering proteasomal degradation of caspases. It has been found for AcMPNV and BmNPV that one of the mechanisms for the viruses to trigger cell cycle arrest at G2/M phase is by a virus Cyclin, a homolog of the host Cylin-B, which stably binds to the host cell division cycle (cdc-2) protein, leading to prolonged cdc-2 activities and preventing cell cycle progression to anaphase [41,109].
Over-expression of antimicrobial peptides and apoptosis are common host defense responses found in insect-baculovirus systems. Gloverin, encoding an antiviral peptide‚ increased in BmNPV infected B. mori and in S. exigua larvae infected by AcMPNV [19,31]. Another antiviral peptide, Cecropin, is also commonly found up-regulated, such as in H. virescens infected by Helicoverpa zea single nucleopolyhedrovirus (HzSNPV) [33] and in the H. zea-HearNPV system [7]. Similarly, induction of apoptosis by baculovirus infections has been found in many expression studies using baculovirus-insect systems [10,11,18].
Recently‚ microRNA (miRNA) and small interfering RNA (siRNA) responses have been identified as important control mechanisms for insect-virus interactions. Rapid discovery of miRNAs is aided by the development of NGS technologies and computational analysis followed by validation of selected putative miRNAs. For baculoviruses, only four viral miRNAs have been detected by using NGS to sequence small RNAs extracted from BmNPV-infected B. mori cells [77]. These four miRNAs are evolutionarily conserved among many baculoviruses. On the other hand, many insect miRNAs, from 90–62 miRNAs, have been discovered. Using Illumina NGS, over 90 host insect miRNAs were found in S. frugiperda [13]. By sequencing 6,720 clones of small RNAs in B. mori, Yu et al. [91] found 35 miRNAs, while the computational prediction identified 262 genes for miRNAs. Comparing outputs by the two approaches, NGS generated more miRNAs than traditional sequencing. For siRNA, NGS also discovered siRNA responses in the H. armigera insect [92]. The insect cells encode a Dicer 2, which cuts double stranded viral dsRNA to generate viral small interfering siRNAs that target viral mRNA products during late infection periods [92]. In addition, some virus miRNAs may regulate host miRNA populations by inhibiting nuclear export of host miRNAs [76].
In relation to signaling pathways, the BmNPV virus induced the B. mori mitogen protein kinase (MAPK) pathways, including extracellular signal-regulated kinase (ERK), and c-Jun NH2-terminal kinase activity (JNK), possibly via the virus PK-1 protein [83]. AcMNPV infection increased Akt phosphorylation from 2–6 h.p.i and activated the Pi3-Akt pathway in infected cells [81]. The MAPK and Akt pathways are pro-survival pathways, often being activated before cells commit to apoptosis. Sagisaka et al. [32] found up-regulation of a Toll protein in B. mori cells infected by BmNPV (BmToll10-3, a surface protein receptor), but a change in the Toll pathway was not identified. Similarly, there have been no findings of changes to the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) (JAK-STAT) pathway or the immune deficiency (IMD) pathway in baculovirus infected insect cells to date. Therefore how baculoviruses control, and insect cells respond, via signaling pathways‚ especially those involved in innate immune responses‚ remain to be explored.
3. Manipulation of Host Cells or Larvae to Study Baculovirus-Insect Interactions
Common methods applied for studying pathways in insects use chemical inhibitors to moderate a pathway, for example: using 5-fluoro-29deoxyuridine (FdUrd) for cell cycle arrest at G1/S [105], using U0126 and PD98059 for moderation of Extracellular signal regulated kinases (ERK) [69], using SP600125 for moderation of the JNK pathway [69], and using PS-341 for proteasome modifications [11]. Similarly; to trigger a pathway, Actinocyn D is used for inducing apoptosis [110]. Molecular biology methods for testing roles of a pathway or a gene to baculovirus infection in insects most commonly use gene knockdown by RNA-interfering (RNAi) technology, for example using RNAi to repress the MAPK pathway (JNK and ERK) [69] or apoptotic caspases [111]. Until recently, RNAi silencing technology has been used successfully for studies involving 15 Lepidopteran species [112]. A technique for targeted gene knockout, using Zinc Finger Nuclease technology, was also successfully applied for studies with B. mori [113]. For over-expressing a gene or introducing a heterologous gene into insect cells, transient transfections have been widely applied with a range of transfection reagents, including inexpensive and effective reagents such as polyethylenimine (PEI) [114]. Tools are also available for stable transfection, most commonly using antibiotics for selecting cells containing heterologous DNA randomly inserted into insect cell chromosomes [115]. More details about methods for genetic engineering of the baculovirus-insect system are reviewed elsewhere [116,117].
4. Conclusions and Future Perspectives
Overall, while baculoviruses have evolved to control insect hosts‚ there is increasing evidence of host cell responses against baculovirus infections. Figure 1 summarizes many of the bidirectional interactions between cell and baculovirus genes/proteins that have been discussed in this review. However, host responses are relatively poorly understood compared to current knowledge on virus genes. Metabolic pathways including energy and nucleotide/amino acid/lipid metabolism are expected to be up-regulated‚ but little is known about which genes, if any, are affected. Likewise‚ how the viruses control various pathways such as signaling pathways are still largely unanswered questions. Similarly‚ although heat shock responses are now known to be a conserved mechanism beneficial to baculoviruses and have been found in all studied baculovirus-insect cell systems, the trigger of the response by these host genes to baculovirus infections remains unknown.
“Omics” tools can greatly improve the knowledge landscape of baculovirus-insect interactions by investigating cellular responses at a genome scale. It can be seen from Figure 1 that the right half of the diagram represents cellular pathways rather than virus-related processes, indicating a lack of research on cellular processes affected by virus infections. This lack of understanding of cellular responses is mainly due to the unavailability of genomic sequences as well as the longer time required to engineer an insect genome than the time required to edit a virus genome. To date, RNA-seq technology is revolutionizing research in organisms without genomic sequences by generating transcript sequences and allowing the analysis of expression levels of these transcripts [118,119]. Transcriptomic approaches enable more comprehensive studies, which are at a whole genome scale (involving over 15,000 genes), generally far more genes compared to the number of proteins or metabolites being measured by proteomic or metabolomic approaches.
A number of studies comparing microarray and RNA-sequencing (RNA-seq) have shown that the two tools produce highly correlated results (r > 0.91) and they complement each other [119,120]. RNA-seq has advantages over microarrays in the discovery of novel transcripts, analysis of isoforms, and detection of antisense, splice junctions, siRNA, and miRNA. However, for non-model organisms that do not have reference genomes, accurately assembling transcripts and mapping of reads onto transcripts are fundamentally important for determining differential expression results using RNA-seq [119]. In addition, it is speculated that RNA-seq might produce bias for highly expressed virus genes at late infection stages when the polyhedrin and p10 genes are hyper-expressed, and detection of low abundant genes could be compromised [11]. Combining results from microarray and RNA-seq analysis can increase detection power and accuracy [120].
Compared to transcriptomics, proteomics and metabolomics are in an early stage of development, but rapid progress is being made with these tools. Proteomics with mass spectroscopy based approaches and activity-based functional validation procedures can provide insights into host-virus interactions [121]. A proteomic study of infections in permissive, semi-permissive and non-permissive cell lines, H. virescens and H. zea, by AcMNPV and HzSNPV identified 21 proteins, among which the up-regulation of two Calreticulin proteins, two DNA supercoiling factors, and two heat shock proteins by 5-fold were found [122]. Courtiade et al. [123] carried out a proteomic study using H. armigera cells following induction of apoptosis and identified changes in 13 proteins compared to control cells. Chen et al. [124] found nine proteins being changed in susceptible and resistant B. mori midgut tissues following virus infections. The current metabolomics technology only allows measurements of a part of the metabolome [125]. Among a few metabolic studies in insects so far, measurements of amino acids, sugars and several redox agents have been undertaken. Tran et al. [126] measured amino acids and adenosine phosphates Bernal et al. [127] measured glucose, glutamine, glutamate, lactate, ammonia, maltose, sucrose, glucose, NAD+/NADH and performed assays for 10 enzyme activities. At this stage, the rapid development of quantitative proteomics allows identification and measurements of thousands of proteins [128]. Targeted metabolomics is also powerful in unraveling changes in host metabolism [129], an area that remains largely unexplored for baculovirus-insect systems.
Engineering insect cells for functional analysis of target genes in insect-virus interactions can use high throughput screening technologies, involving the use of RNAi systems [130]. Although no high throughput gene knocking technologies have been established to date in insect-baculovirus systems, temporary expression by transient transfection of expression vectors or creating stably transfected polyclonal cells can be good options to validate genes that require up-regulation or over-expression to improve production outcomes using insect cell technology. These technologies together with the power of “omics” tools, especially the two well established transcriptomic tools, can unravel many host pathways that are yet to be fully explored.
Acknowledgments
The authors wish to acknowledge the financial support from the Australian Research Council (Linkage grant LP0989824), Agrichem Pty Ltd, and The University of Queensland.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Van Oers M.M., Vlak J.M. Baculovirus genomics. Curr. Drug Targets. 2007;8:1051–1068. doi: 10.2174/138945007782151333. [DOI] [PubMed] [Google Scholar]
- 2.Thiem S.M., Cheng X.-W. Baculovirus Host-Range. Virol. Sin. 2009;24:436–457. doi: 10.1007/s12250-009-3058-8. [DOI] [Google Scholar]
- 3.Chen C.Y., Wu H.H., Chen C.P., Chern S.R., Hwang S.M., Huang S.F., Lo W.H., Chen G.Y., Hu Y.C. Biosafety assessment of human mesenchymal stem cells engineered by hybrid baculovirus vectors. Mol. Pharm. 2011;8:1505–1514. doi: 10.1021/mp100368d. [DOI] [PubMed] [Google Scholar]
- 4.Chen C.Y., Lin C.Y., Chen G.Y., Hu Y.C. Baculovirus as a gene delivery vector: Recent understandings of molecular alterations in transduced cells and latest applications. Biotechnol. Adv. 2011;29:618–631. doi: 10.1016/j.biotechadv.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mena J.A., Kamen A.A. Insect cell technology is a versatile and robust vaccine manufacturing platform. Expert Rev. Vaccines. 2011;10:1063–1081. doi: 10.1586/erv.11.24. [DOI] [PubMed] [Google Scholar]
- 6.Rohrmann G.F. Baculovirus Molecular Biology. 2nd. National Library of Medicine, National Center for Biotechnology Information; Bethesda, MD, USA: 2011. [(accessed 2 June 2013)]. [Online] Available online: http://www.ncbi.nlm.nih.gov/books/NBK49500/ [Google Scholar]
- 7.Nguyen Q., Chan L.C., Nielsen L.K., Reid S. Genome scale analysis of differential mRNA expression of Helicoverpa zea insect cells infected with a H. armigera baculovirus. Virology. 2013;444:158–170. doi: 10.1016/j.virol.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y.-R., Zhong S., Fei Z., Hashimoto Y., Xiang J.Z., Zhang S., Blissard G.W. The transcriptome of the baculovirus Autographa californica Multiple Nucleopolyhedrovirus (AcMNPV) in Trichoplusia ni cells. J. Virol. 2013;87:6391–6405. doi: 10.1128/JVI.00194-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell J.K., Friesen P.D. Baculoviruses modulate a proapoptotic DNA damage response to promote virus multiplication. J. Virol. 2012;86:13542–13553. doi: 10.1128/JVI.02246-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salem T.Z., Zhang F., Xie Y., Thiem S.M. Comprehensive analysis of host gene expression in Autographa californica nucleopolyhedrovirus-infected Spodoptera frugiperda cells. Virology. 2011;412:167–178. doi: 10.1016/j.virol.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xue J., Qiao N., Zhang W., Cheng R.-L., Zhang X.-Q., Bao Y.-Y., Xu Y.-P., Gu L.-Z., Han J.-D.J., Zhang C.-X. Dynamic interactions between Bombyx mori nucleopolyhedrovirus and its host cells revealed by transcriptome analysis. J. Virol. 2012;86:7345–7359. doi: 10.1128/JVI.07217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carinhas N., Robitaille A.M., Moes S., Carrondo M.J.T., Jenoe P., Oliveira R., Alves P.M. Quantitative proteomics of Spodoptera frugiperda cells during growth and baculovirus infection. PLoS One. 2011;6:e26444. doi: 10.1371/journal.pone.0026444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehrabadi M., Hussain M., Asgari S. MicroRNAome of Spodoptera frugiperda cells (Sf9) and its alteration following baculovirus infection. J. Gen. Virol. 2013;94:1385–1397. doi: 10.1099/vir.0.051060-0. [DOI] [PubMed] [Google Scholar]
- 14.Iwanaga M., Shimada T., Kobayashi M., Kang W. Identification of differentially expressed host genes in Bombyx mori nucleopolyhedrovirus infected cells by using subtractive hybridization. Appl. Entomol. Zool. 2007;42:151–159. doi: 10.1303/aez.2007.151. [DOI] [Google Scholar]
- 15.Gatehouse H.S., Poulton J., Markwick N.P., Gatehouse L.N., Ward V.K., Young V.L., Luo Z., Schaffer R., Christeller J.T. Changes in gene expression in the permissive larval host lightbrownapple moth (Epiphyas postvittana, Tortricidae) in response to EppoNPV (Baculoviridae) infection. Insect Mol. Biol. 2009;18:635–648. doi: 10.1111/j.1365-2583.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Oers M.M., Doitsidou M., Thomas A.A.M., de Maagd R.A., Vlak J.M. Translation of both 5'TOP and non-TOP host mRNAs continues into the late phase of baculovirus infection. Insect Mol. Biol. 2003;12:75–84. doi: 10.1046/j.1365-2583.2003.00389.x. [DOI] [PubMed] [Google Scholar]
- 17.Huynh H., Tran T.B., Chan L.L., Nielsen L., Reid S. Decline in baculovirus-expressed recombinant protein production with increasing cell density is strongly correlated to impairment of virus replication and mRNA expression. Appl. Microbiol. Biotechnol. 2013;97:5245–5257. doi: 10.1007/s00253-013-4835-8. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen Q., Palfreyman R.W., Chan L.C.L., Reid S., Nielsen L.K. Transcriptome sequencing of and microarray development for a Helicoverpa zea cell line to investigate in vitro insect cell-baculovirus interactions. PLoS One. 2012;7:e36324. doi: 10.1371/journal.pone.0036324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi J.Y., Roh J.Y., Wang Y., Zhen Z., Tao X.Y., Lee J.H., Liu Q., Kim J.S., Shin S.W., Je Y.H. Analysis of genes expression of Spodoptera exigua Larvae upon AcMNPV infection. PLoS One. 2012;7:e42462. doi: 10.1371/journal.pone.0042462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagishi J., Isobe R., Takebuchi T., Bando H. DNA microarrays of baculovirus genomes: Differential expression of viral genes in two susceptible insect cell lines. Arch. Virol. 2003;148:587–597. doi: 10.1007/s00705-002-0922-3. [DOI] [PubMed] [Google Scholar]
- 21.Jiang S.S., Chang I.S., Huang L.W., Chen P.C., Wen C.C., Liu S.C., Chien L.C., Lin C.Y., Hsiung C.A., Juang J.L. Temporal transcription program of recombinant autographa californica multiple nucleopolyhedrosis Virus. J. Virol. 2006;80:8989–8999. doi: 10.1128/JVI.01158-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The International Silkworm Genome Consortium The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008;38:1036–1045. doi: 10.1016/j.ibmb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 23.You M., Yue Z., He W., Yang X., Yang G., Xie M., Zhan D., Baxter S.W., Vasseur L., Gurr G.M., et al. A heterozygous moth genome provides insights into herbivory and detoxification. Nat. Genet. 2013;45:220–225. doi: 10.1038/ng.2524. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y., Dong Y., Thodima V., Clem R.J., Passarelli A.L. Analysis and functional annotation of expressed sequence tags from the fall armyworm Spodoptera frugiperda. BMC Genomics. 2006;7 doi: 10.1186/1471-2164-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landais I., Ogliastro M., Mita K., Nohata J., Ferber L.M., Cerutti M.D., Shimada T., Fournier P., Devauchelle G. Annotation pattern of ESTs from Spodoptera frugiperda Sf9 cells and analysis of the ribosomal protein genes reveal insect-specific features and unexpectedly low codon usage bias. Bioinformatics. 2003;19:2343–2350. doi: 10.1093/bioinformatics/btg324. [DOI] [PubMed] [Google Scholar]
- 26.Shelby K.S., Popham H.J.R. Analysis of ESTs generated from immune-stimulated hemocytes of larval Heliothis virescens. J. Invertebr. Pathol. 2009;101:86–95. doi: 10.1016/j.jip.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Pauchet Y., Wilkinson P., Vogel H., Nelson D.R., Reynolds S.E., Heckel D.G., Ffrench-Constant R.H. Pyrosequencing the Manduca sexta larval midgut transcriptome: Messages for digestion, detoxification and defence. Insect Mol. Biol. 2009;19:61–75. doi: 10.1111/j.1365-2583.2009.00936.x. [DOI] [PubMed] [Google Scholar]
- 28.Vogel H., Heidel A.J., Heckel D.G., Groot A.T. Transcriptome analysis of the sex pheromone gland of the noctuid moth Heliothis virescens. BMC Genomics. 2010;11 doi: 10.1186/1471-2164-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel H., Altincicek B., Glockner G., Vilcinskas A. A comprehensive transcriptome and immune-gene repertoire of the lepidopteran model host Galleria mellonella. BMC Genomics. 2011;12 doi: 10.1186/1471-2164-12-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparks M.E., Gundersen-Rindal D.E. The Lymantria dispar IPLB-Ld652Y cell line transcriptome comprises diverse virus-associated transcripts. Viruses. 2011;3:2339–2350. doi: 10.3390/v3112339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bao Y.Y., Lu Z.Y., Liu Z.B., Xue J., Xu Y.P., Zhang C.X. Comparative analysis of Bombyx mori nucleopolyhedrovirus responsive genes in fat body and haemocyte of B. mori resistant and susceptible strains. Insect Mol. Biol. 2010;19:347–358. doi: 10.1111/j.1365-2583.2010.00993.x. [DOI] [PubMed] [Google Scholar]
- 32.Sagisaka A., Fujita K., Nakamura Y., Ishibashi J., Noda H., Imanishi S., Mita K., Yamakawa M., Tanaka H. Genome-wide analysis of host gene expression in the silkworm cells infected with Bombyx mori nucleopolyhedrovirus. Virus Res. 2010;147:166–175. doi: 10.1016/j.virusres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Breitenbach J.E., Shelby K.S., Popham H.J.R. Baculovirus induced transcripts in hemocytes from the larvae of Heliothis virescens. Viruses. 2011;3:2047–2064. doi: 10.3390/v3112047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang M.L., Yin F.F., Shen S., Tan Y., Deng F., Vlak J.M., Hu Z.H., Wang H.L. Partial functional rescue of Helicoverpa armigera single nucleocapsid nucleopolyhedrovirus infectivity by replacement of F protein with GP64 from Autographa californica multicapsid nucleopolyhedrovirus. J. Virol. 2010;84:11505–11514. doi: 10.1128/JVI.00862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long G., Pan X., Kormelink R., Vlak J.M. Functional entry of baculovirus into insect and mammalian cells is dependent on clathrin-mediated endocytosis. J. Virol. 2006;80:8830–8833. doi: 10.1128/JVI.00880-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peng K., van Oers M.M., Hu Z., van Lent J.W., Vlak J.M. Baculovirus per os infectivity factors form a complex on the surface of occlusion-derived virus. J. Virol. 2010;84:9497–9504. doi: 10.1128/JVI.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clem R.J. Baculoviruses and apoptosis: A diversity of genes and responses. Curr. Drug Targets. 2007;8:1069–1074. doi: 10.2174/138945007782151405. [DOI] [PubMed] [Google Scholar]
- 38.Bump N.J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P., et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995;269:1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- 39.Guy M.P., Friesen P.D. Reactive-site cleavage residues confer target specificity to baculovirus P49, a dimeric member of the P35 family of caspase inhibitors. J. Virol. 2008;82:7504–7514. doi: 10.1128/JVI.00231-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schultz K.L.W., Friesen P.D. Baculovirus DNA replication-specific expression factors trigger apoptosis and shutoff of host protein synthesis during infection. J. Virol. 2009;83:11123–11132. doi: 10.1128/JVI.01199-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Belyavskyi M., Braunagel S.C., Summers M.D. The structural protein ODV-EC27 of Autographa californica nucleopolyhedrovirus is a multifunctional viral cyclin. Proc. Natl. Acad. Sci. USA. 1998;95:11205–11210. doi: 10.1073/pnas.95.19.11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu W., Passarelli A.L. Autographa californica multiple nucleopolyhedrovirus Ac92 (ORF92, P33) is required for budded virus production and multiply enveloped occlusion-derived virus formation. J. Virol. 2010;84:12351–12361. doi: 10.1128/JVI.01598-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dreschers S., Roncarati R., Knebel-Morsdorf D. Actin rearrangement-inducing factor of baculoviruses is tyrosine phosphorylated and colocalizes to F-actin at the plasma membrane. J. Virol. 2001;75:3771–3778. doi: 10.1128/JVI.75.8.3771-3778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marek M., Merten O.-W., Galibert L., Vlak J.M., van Oers M.M. Baculovirus VP80 protein and the F-Actin cytoskeleton interact and connect the viral replication factory with the nuclear periphery. J. Virol. 2011;85:5350–5362. doi: 10.1128/JVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohkawa T., Volkman L.E., Welch M.D. Actin-based motility drives baculovirus transit to the nucleus and cell surface. J. Cell Biol. 2010;190:187–195. doi: 10.1083/jcb.201001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkawa T., Rowe A.R., Volkman L.E. Identification of six Autographa californica multicapsid nucleopolyhedrovirus early genes that mediate nuclear localization of G-actin. J. Virol. 2002;76:12281–12289. doi: 10.1128/JVI.76.23.12281-12289.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li K., Wang Y., Bai H., Wang Q., Song J., Zhou Y., Wu C., Chen X. The putative pocket protein binding site of Autographa californica nucleopolyhedrovirus BV/ODV-C42 is required for virus-induced nuclear actin polymerization. J. Virol. 2010;84:7857–7868. doi: 10.1128/JVI.00174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goley E.D., Ohkawa T., Mancuso J., Woodruff J.B., D’Alessio J.A., Cande W.Z., Volkman L.E., Welch M.D. Dynamic nuclear actin assembly by Arp2/3 complex and a baculovirus WASP-Like protein. Science. 2006;314:464–467. doi: 10.1126/science.1133348. [DOI] [PubMed] [Google Scholar]
- 49.Fang M., Nie Y., Theilmann D.A. AcMNPV EXON0 (AC141) which is required for the efficient egress of budded virus nucleocapsids interacts with beta-tubulin. Virology. 2009;385:496–504. doi: 10.1016/j.virol.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 50.Carpentier D.C., Griffiths C.M., King L.A. The baculovirus P10 protein of Autographa californica nucleopolyhedrovirus forms two distinct cytoskeletal-like structures and associates with polyhedral occlusion bodies during infection. Virology. 2008;371:278–291. doi: 10.1016/j.virol.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 51.Braunagel S.C., Cox V., Summers M.D. Baculovirus data suggest a common but multifaceted pathway for sorting proteins to the inner nuclear membrane. J. Virol. 2009;83:1280–1288. doi: 10.1128/JVI.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ge J., Wei Z., Huang Y., Yin J., Zhou Z., Zhong J. AcMNPV ORF38 protein has the activity of ADP-ribose pyrophosphatase and is important for virus replication. Virology. 2007;361:204–211. doi: 10.1016/j.virol.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Long C.M., Rohrmann G.F., Merrill G.F. The conserved baculovirus protein p33 (Ac92) is a flavin adenine dinucleotide-linked sulfhydryl oxidase. Virology. 2009;388:231–235. doi: 10.1016/j.virol.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 54.Hakim M., Mandelbaum A., Fass D. Structure of a baculovirus sulfhydryl oxidase, a highly divergent member of the erv flavoenzyme family. J. Virol. 2011;85:9406–9413. doi: 10.1128/JVI.05149-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomalski M.D., Eldridge R., Miller L.K. A baculovirus homolog of a Cu/Zn superoxide dismutase gene. Virology. 1991;184:149–161. doi: 10.1016/0042-6822(91)90831-U. [DOI] [PubMed] [Google Scholar]
- 56.Wang W., Wu J., Wu X. Deletion of superoxide dismutase gene of Bombyx mori nuclear polyhedrosis virus affects viral DNA replication. Int. J. Ind. Entomol. 2004;9:225–228. [Google Scholar]
- 57.Kuzio J., Pearson M.N., Harwood S.H., Funk C.J., Evans J.T., Slavicek J.M., Rohrmann G.F. Sequence and analysis of the genome of a baculovirus pathogenic for Lymantria dispar. Virology. 1999;253:17–34. doi: 10.1006/viro.1998.9469. [DOI] [PubMed] [Google Scholar]
- 58.Hughes A.L., Friedman R. Genome-wide survey for genes horizontally transferred from cellular organisms to baculoviruses. Mol. Biol. Evol. 2003;20:979–987. doi: 10.1093/molbev/msg107. [DOI] [PubMed] [Google Scholar]
- 59.Vanarsdall A.L., Mikhailov V.S., Rohrmann G.F. Baculovirus DNA replication and processing. Curr. Drug Targets. 2007;8:1096–1102. doi: 10.2174/138945007782151397. [DOI] [PubMed] [Google Scholar]
- 60.Baldo A.M., McClure M.A. Evolution and horizontal transfer of dUTPase-encoding genes in viruses and their hosts. J. Virol. 1999;73:7710–7721. doi: 10.1128/jvi.73.9.7710-7721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Landais I., Vincent R., Bouton M., Devauchelle G., Duonor-Cerutti M., Ogliastro M. Functional analysis of evolutionary conserved clustering of bZIP binding sites in the baculovirus homologous regions (hrs) suggests a cooperativity between host and viral transcription factors. Virology. 2006;344:421–431. doi: 10.1016/j.virol.2005.08.036. [DOI] [PubMed] [Google Scholar]
- 62.Matzat L.H., Berberoglu S., Levesque L. Formation of a Tap/NXF1 homotypic complex is mediated through the amino-terminal domain of Tap and enhances interaction with nucleoporins. Mol. Biol. Cell. 2008;19:327–338. doi: 10.1091/mbc.E07-03-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thiem S.M., Chejanovsky N. The role of baculovirus apoptotic suppressors in AcMNPV-mediated translation arrest in Ld652Y cells. Virology. 2004;319:292–305. doi: 10.1016/j.virol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Dever T.E., Sripriya R., McLachlin J.R., Lu J., Fabian J.R., Kimball S.R., Miller L.K. Disruption of cellular translational control by a viral truncated eukaryotic translation initiation factor 2alpha kinase homolog. Proc. Natl. Acad. Sci. USA. 1998;95:4164–4169. doi: 10.1073/pnas.95.8.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y., Miller L.K. Expression and functional analysis of a baculovirus gene encoding a truncated protein kinase homolog. Virology. 1995;206:314–323. doi: 10.1016/S0042-6822(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 66.Thiem S.M., Du X., Quentin M.E., Berner M.M. Identification of baculovirus gene that promotes Autographa californica nuclear polyhedrosis virus replication in a nonpermissive insect cell line. J. Virol. 1996;70:2221–2229. doi: 10.1128/jvi.70.4.2221-2229.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shirata N., Ikeda M., Kobayashi M. Identification of a Hyphantria cunea nucleopolyhedrovirus (NPV) gene that is involved in global protein synthesis shutdown and restricted Bombyx mori NPV multiplication in a B. mori cell line. Virology. 2010;398:149–157. doi: 10.1016/j.virol.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 68.Imai N., Matsuda N., Tanaka K., Nakano A., Matsumoto S., Kang W. Ubiquitin ligase activities of Bombyx mori nucleopolyhedrovirus RING finger proteins. J. Virol. 2003;77:923–930. doi: 10.1128/JVI.77.2.923-930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsuma S., Koyano Y., Kang W., Kokusho R., Kamita S.G., Shimada T. The Baculovirus uses a captured host phosphatase to induce enhanced locomotory activity in host caterpillars. PLoS Pathog. 2012;8:e1002644. doi: 10.1371/journal.ppat.1002644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van Houte S., Ros V.I.D., Mastenbroek T.G., Vendrig N.J., Hoover K., Spitzen J., van Oers M.M. Protein Tyrosine phosphatase-induced hyperactivity is a conserved strategy of a subset of baculoVIruses to manipulate lepidopteran host behavior. PLoS One. 2012;7:e46933. doi: 10.1371/journal.pone.0046933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Means J.C., Passarelli A.L. Viral fibroblast growth factor, matrix metalloproteases, and caspases are associated with enhancing systemic infection by baculoviruses. Proc. Natl. Acad. Sci. USA. 2010;107:9825–9830. doi: 10.1073/pnas.0913582107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Detvisitsakun C., Berretta M.F., Lehiy C., Passarelli A.L. Stimulation of cell motility by a viral fibroblast growth factor homolog: Proposal for a role in viral pathogenesis. Virology. 2005;336:308–317. doi: 10.1016/j.virol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Hawtin R.E., Zarkowska T., Arnold K., Thomas C.J., Gooday G.W., King L.A., Kuzio J.A., Possee R.D. Liquefaction of Autographa californica nucleopolyhedrovirus-infected insects is dependent on the integrity of virus-encoded chitinase and cathepsin genes. Virology. 1997;238:243–253. doi: 10.1006/viro.1997.8816. [DOI] [PubMed] [Google Scholar]
- 74.Hoover K., Grove M., Gardner M., Hughes D.P., McNeil J., Slavicek J. A gene for an extended phenotype. Science. 2011;333 doi: 10.1126/science.1209199. [DOI] [PubMed] [Google Scholar]
- 75.O’Reilly D.R., Miller L.K. A baculovirus blocks insect molting by producing ecdysteroid UDP-glucosyl transferase. Science. 1989;245:1110–1112. doi: 10.1126/science.2505387. [DOI] [PubMed] [Google Scholar]
- 76.Singh C.P., Singh J., Nagaraju J. A baculovirus-encoded MicroRNA (miRNA) suppresses its host miRNA biogenesis by regulating the exportin-5 cofactor Ran. J. Virol. 2012;86:7867–7879. doi: 10.1128/JVI.00064-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singha J., Singha C.P., Bhavania A., Nagaraju J. Discovering microRNAs from Bombyx mori nucleopolyhedrosis virus. Virology. 2010;407:120–128. doi: 10.1016/j.virol.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 78.Moreno-Habel D.A., Biglang-awa I.M., Dulce A., Luu D.D., Garcia P., Weers P.M.M., Haas-Stapleton E.J. Inactivation of the budded virus of Autographa californica M nucleopolyhedrovirus by gloverin. J. Invertebr. Pathol. 2012;110:92–101. doi: 10.1016/j.jip.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Q., Liu Y., He H.-J., Zhao X.-F., Wang J.-X. Immune responses of Helicoverpa armigera to different kinds of pathogens. BMC Immunol. 2010;11 doi: 10.1186/1471-2172-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vandergaast R., Schultz K.L.W., Cerio R.J., Friesen P.D. Active depletion of host cell inhibitor-of-apoptosis IAP triggers apoptosis upon Baculovirus DNA replication. J. Virol. 2011;85:8348–8358. doi: 10.1128/JVI.00667-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xiao W., Yang Y., Weng Q., Lin T., Yuan M., Yang K., Pang Y. The role of the PI3K-Akt signal transduction pathway in Autographa californica multiple nucleopolyhedrovirus infection of Spodoptera frugiperda cells. Virology. 2009;391:83–89. doi: 10.1016/j.virol.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- 83.Katsuma S., Mita K., Shimada T. ERK- and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J. Virol. 2007;81:13700–13709. doi: 10.1128/JVI.01683-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Broehan G., Kroeger T., Lorenzen M., Merzendorfer H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genomics. 2013;14:1471–2164. doi: 10.1186/1471-2164-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bernal V., Carinhas N., Yokomizo A.Y., Carrondo M.J.T., Alves P.M. Cell density effect in the baculovirus-insect cells system: A quantitative analysis of energetic metabolism. Biotechnol. Bioeng. 2009;104:162–180. doi: 10.1002/bit.22364. [DOI] [PubMed] [Google Scholar]
- 86.Popham H., Sun R., Shelby K., Robertson J. Iron levels change in larval Heliothis virescens tissues following baculovirus infection. Biol. Trace Elem. Res. 2012;148:356–362. doi: 10.1007/s12011-012-9373-1. [DOI] [PubMed] [Google Scholar]
- 87.Mayer M.P. Recruitment of Hsp70 chaperones: A crucial part of viral survival strategies. Rev. Physiol. Biochem. Pharmacol. 2005;153:1–46. doi: 10.1007/s10254-004-0025-5. [DOI] [PubMed] [Google Scholar]
- 88.Aarti I., Rajesh K., Ramaiah K.A. Phosphorylation of eIF2 alpha in Sf9 cells: A stress, survival and suicidal signal. Apoptosis. 2010;15:679–692. doi: 10.1007/s10495-010-0474-z. [DOI] [PubMed] [Google Scholar]
- 89.Ghosh S., Jain A., Mukherjee B., Habib S., Hasnain S.E. The host factor polyhedrin promoter binding protein (PPBP) is involved in transcription from the baculovirus polyhedrin gene promoter. J. Virol. 1998;72:7484–7493. doi: 10.1128/jvi.72.9.7484-7493.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lyupina Y.V., Abaturova S.B., Erokhov P.A., Orlova O.V., Beljelarskaya S.N., Mikhailov V.S. Proteotoxic stress induced by Autographa californica nucleopolyhedrovirus infection of Spodoptera frugiperda Sf9 cells. Virology. 2013;436:49–58. doi: 10.1016/j.virol.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 91.Yu X., Zhou Q., Li S.C., Luo Q., Cai Y., Lin W.C., Chen H., Yang Y., Hu S., Yu J. The silkworm (Bombyx mori) microRNAs and their expressions in multiple developmental stages. PLoS One. 2008;3:0002997. doi: 10.1371/journal.pone.0002997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jayachandran B., Hussain M., Asgari S. RNA Interference as a cellular defense mechanism against the DNA virus baculovirus. J. Virol. 2012;86:13729–13734. doi: 10.1128/JVI.02041-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Menzel T., Rohrmann G. Diversity of errantivirus (retrovirus) sequences in two cell lines used for baculovirus expression, Spodoptera frugiperda and Trichoplusia ni. Virus Genes. 2008;36:583–586. doi: 10.1007/s11262-008-0221-5. [DOI] [PubMed] [Google Scholar]
- 94.Giri L., Feiss M.G., Bonning B.C., Murhammer D.W. Production of baculovirus defective interfering particles during serial passage is delayed by removing transposon target sites in fp25k. J. Gen. Virol. 2012;93:389–399. doi: 10.1099/vir.0.036566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fuller D.N., Raymer D.M., Kottadiel V.I., Rao V.B., Smith D.E. Single phage T4 DNA packaging motors exhibit large force generation, high velocity, and dynamic variability. Proc. Natl. Acad. Sci. USA. 2007;104:16868–16873. doi: 10.1073/pnas.0704008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nahlik K.W., Mleczko A.K., Gawlik M.K., Rokita H.B. Modulation of GAPDH expression and cellular localization after vaccinia virus infection of human adherent monocytes. Acta Biochim. Pol. 2003;50:667–676. [PubMed] [Google Scholar]
- 97.Cho Y.S., Lee S.Y., Kim K.H., Nam Y.K. Differential modulations of two glyceraldehyde 3-phosphate dehydrogenase mRNAs in response to bacterial and viral challenges in a marine teleost Oplegnathus fasciatus (Perciformes) Fish Shellfish Immunol. 2008;25:472–476. doi: 10.1016/j.fsi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Scheper G.C., Vries R.G., Broere M., Usmany M., Voorma H.O., Vlak J.M., Thomas A.A. Translational properties of the untranslated regions of the p10 messenger RNA of Autographa californica multicapsid nucleopolyhedrovirus. J. Gen. Virol. 1997;78:687–696. doi: 10.1099/0022-1317-78-3-687. [DOI] [PubMed] [Google Scholar]
- 99.Van Oers M.M., van der Veken L.T.J.N., Vlak J.M., Thomas A.A.M. Effect of baculovirus infection on the mRNA and protein levels of the Spodoptera frugiperda eukaryotic initiation factor 4E. Insect Mol. Biol. 2001;10:255–264. doi: 10.1046/j.1365-2583.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 100.Volkman L.E. Baculovirus infectivity and the actin cytoskeleton. Curr. Drug Targets. 2007;8:1075–1083. doi: 10.2174/138945007782151379. [DOI] [PubMed] [Google Scholar]
- 101.Peng K., Wu M.Z., Deng F., Song J.J., Dong C.S., Wang H.L., Hu Z.H. Identification of protein-protein interactions of the occlusion-derived virus-associated proteins of Helicoverpa armigera nucleopolyhedrovirus. J. Gen. Virol. 2010;91:659–670. doi: 10.1099/vir.0.017103-0. [DOI] [PubMed] [Google Scholar]
- 102.Braunagel S.C., Summers M.D. Molecular biology of the baculovirus occlusion-derived virus envelope. Curr. Drug Targets. 2007;8:1084–1095. doi: 10.2174/138945007782151315. [DOI] [PubMed] [Google Scholar]
- 103.Vanarsdall A.L., Okano K., Rohrmann G.F. Characterization of the replication of a baculovirus mutant lacking the DNA polymerase gene. Virology. 2005;331:175–180. doi: 10.1016/j.virol.2004.10.024. [DOI] [PubMed] [Google Scholar]
- 104.Ahrens C.H., Rohrmann G.F. The DNA polymerase and helicase genes of a baculovirus of Orgyia pseudosugata. J. Gen. Virol. 1996;77:825–837. doi: 10.1099/0022-1317-77-5-825. [DOI] [PubMed] [Google Scholar]
- 105.Braunagel S.C., Parr R., Belyavskyi M., Summers M.D. Autographa californica nucleopolyhedrovirus infection results in Sf9 cell cycle arrest at G2/M phase. Virology. 1998;244:195–211. doi: 10.1006/viro.1998.9097. [DOI] [PubMed] [Google Scholar]
- 106.Weitzman M.D., Lilley C.E., Chaurushiya M.S. Genomes in conflict: Maintaining genome integrity during virus infection. Annu. Rev. Microbiol. 2010;64:61–81. doi: 10.1146/annurev.micro.112408.134016. [DOI] [PubMed] [Google Scholar]
- 107.Jin J., Guarino L.A. 3'-End Formation of Baculovirus Late RNAs. J. Virol. 2000;74:8930–8937. doi: 10.1128/JVI.74.19.8930-8937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ikeda M., Yamada H., Hamajima R., Kobayashi M. Baculovirus genes modulating intracellular innate antiviral immunity of lepidopteran insect cells. Virology. 2013;435:1–13. doi: 10.1016/j.virol.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 109.Baluchamy S., Gopinathan K.P. Characterization of a cyclin homolog from Bombyx mori nucleopolyhedrovirus. Virus Res. 2005;108:69–81. doi: 10.1016/j.virusres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 110.Cartier J.L., Hershberger P.A., Friesen P.D. Suppression of apoptosis in insect cells stably transfected with baculovirus p35: Dominant interference by N-terminal sequences p35(1–76) J. Virol. 1994;68:7728–7737. doi: 10.1128/jvi.68.12.7728-7737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kim E.J., Kramer S.F., Hebert C.G.V., James J., Bentley W.E. Metabolic engineering of the baculovirusexpression system via inverse “Shotgun” genomic analysis and RNA interference (dsRNA) increases product yield and cell longevity. Biotechnol. Bioeng. 2007;98:645–654. doi: 10.1002/bit.21353. [DOI] [PubMed] [Google Scholar]
- 112.Terenius O., Papanicolaou A., Garbutt J.S., Eleftherianos I., Huvenne H., Kanginakudru S., Albrechtsen M., An C., Aymeric J.-L., Barthel A., et al. RNA interference in Lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011;57:231–245. doi: 10.1016/j.jinsphys.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 113.Takasu Y., Kobayashi I., Beumer K., Uchino K., Sezutsu H., Sajwan S., Carroll D., Tamura T., Zurovec M. Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem. Mol. Biol. 2010;40:759–765. doi: 10.1016/j.ibmb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Ogay I.D., Lihoradova O.A., Azimova Sh S., Abdukarimov A.A., Slack J.M., Lynn D.E. Transfection of insect cell lines using polyethylenimine. Cytotechnology. 2006;51:89–98. doi: 10.1007/s10616-006-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee J.M., Hull J.J., Kawai T., Tsuneizumi K., Kuriharaa M., Tanokura M., Nagata K., Nagasawa H., Matsumoto S. Establishment of Sf9 transformants constitutively expressing PBAN receptor (PBANR) variants: Application to functional evaluation. Front. Endocrinol. 2012;3 doi: 10.3389/fendo.2012.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kost T.A., Condreay J.P., Jarvis D.L. Baculovirus as versatile vectors for protein expression in insect and mammalian cells. Nat. Biotechnol. 2005;23:567–575. doi: 10.1038/nbt1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Van Oers M.M. Opportunities and challenges for the baculovirus expression system. J. Invertebr. Pathol. 2011;107:S3–S15. doi: 10.1016/j.jip.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 118.Grabherr M.G., Haas B.J., Yassour M., Levin J.Z., Thompson D.A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nookaew I., Papini M., Pornputtpong N., Scalcinati G., Fagerberg L., Uhlén M., Nielsen J. A comprehensive comparison of RNA-Seq-based transcriptome analysis from reads to differential gene expression and cross-comparison with microarrays: A case study in Saccharomyces cerevisiae. Nucleic Acids Res. 2012;40:10084–10097. doi: 10.1093/nar/gks804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kogenaru S., Yan Q., Guo Y., Wang N. RNA-seq and microarray complement each other in transcriptome profiling. BMC Genomics. 2012;13 doi: 10.1186/1471-2164-13-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Berkhout B., Coombs K.M. Quantitative omics and its application to study virus-host interactions-a new frontier. Front. Microbiol. 2013;4 doi: 10.3389/fmicb.2013.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Popham H.J., Grasela J.J., Goodman C.L., McIntosh A.H. Baculovirus infection influences host protein expression in two established insect cell lines. J. Insect Physiol. 2010;56:1237–1245. doi: 10.1016/j.jinsphys.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 123.Courtiade J., Muck A., Svatos A., Heckel D.G., Pauchet Y. Comparative proteomic analysis of Helicoverpa armigera cells undergoing apoptosis. J. Proteome Res. 2011;10:2633–2642. doi: 10.1021/pr2001868. [DOI] [PubMed] [Google Scholar]
- 124.Chen H.Q., Yao Q., Bao F., Chen K.P., Liu X.Y., Li J., Wang L. Comparative proteome analysis of silkworm in its susceptibility and resistance responses to Bombyx mori densonucleosis virus. Intervirology. 2012;55:21–28. doi: 10.1159/000322381. [DOI] [PubMed] [Google Scholar]
- 125.Christians U., Klawitter J., Hornberger A., Klawitter J. How unbiased is non-targeted metabolomics and is targeted pathway screening the solution? Curr. Pharm. Biotechnol. 2011;12:1053–1066. doi: 10.2174/138920111795909078. [DOI] [PubMed] [Google Scholar]
- 126.Tran T.T.B., Dietmair S., Chan L.C.L., Huynh H.T., Nielsen L.K., Reid S. Development of quenching and washing protocols for quantitative intracellular metabolite analysis of uninfected and baculovirus-infected insect cells. Methods. 2012;56:396–407. doi: 10.1016/j.ymeth.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 127.Bernal V., Monteiro F., Carinhas N., Ambrósio R., Alves P.M. An integrated analysis of enzyme activities, cofactor pools and metabolic fluxes in baculovirus-infected Spodoptera frugiperda Sf9 cells. J. Biotechnol. 2010;150:332–342. doi: 10.1016/j.jbiotec.2010.09.958. [DOI] [PubMed] [Google Scholar]
- 128.Bantscheff M., Lemeer S., Savitski M., Kuster B. Quantitative mass spectrometry in proteomics: Critical review update from 2007 to the present. Anal. Bioanal. Chem. 2012;404:939–965. doi: 10.1007/s00216-012-6203-4. [DOI] [PubMed] [Google Scholar]
- 129.Kafsack B.F.C., Llinás M. Eating at the table of another: Metabolomics of host-parasite interactions. Cell Host Microbe. 2010;7:90–99. doi: 10.1016/j.chom.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ou L., Duan D., Wu J., Nice E., Huang C. The application of high throughput siRNA screening technology to study host-pathogen interactions. Comb. Chem. High Throughput Screen. 2012;15:299–305. doi: 10.2174/138620712799361834. [DOI] [PubMed] [Google Scholar]