Abstract
Summary
Our randomized controlled trial in prematurely menopausal breast cancer survivors showed that impact + resistance training prevented increases in percentage of body fat compared with controls and also improved BMD at the hip and prevented BMD loss at the spine among exercise-trained women who were menopausal for >1 year.
Introduction
Cancer treatment-related menopause worsens bone health and body composition in breast cancer survivors (BCS). We investigated whether impact + resistance training could improve bone mineral density (BMD), reduce bone turnover, build muscle, and decrease fat mass in BCS with premature menopause.
Methods
We conducted a randomized controlled trial in 71 BCS (mean age, 46.5 years) within 5 years of treatment-related menopause. Women were randomly assigned to one of two groups: (1) impact + resistance training (prevent osteoporosis with impact + resistance (POWIR)) or (2) exercise placebo (FLEX) 3×/week for 1 year. Outcomes were hip and spine BMD (in grams per square centimeter) and body composition (percent body fat (%BF) and lean and fat mass (in kilograms)) by DXA and bone turnover markers (serum osteocalcin (in nanograms per milliliter) and urinary deoxypryrodinoline (in nanomoles per milliliter).
Results
There were no significant group × time interactions for bone outcomes when using an intent-to-treat approach on the full sample. In analyses restricted to BCS who were menopausal for ≥1 year, POWIR increased BMD at the hip and slowed BMD loss at the spine compared with FLEX (femoral neck—POWIR, 0.004±0.093 g/cm2 vs. FLEX, −0.010±0.089 g/cm2; p<0.01; spine—POWIR, −0.003±0.114 g/cm2 vs. FLEX, −0.020±0.110 g/cm2; p=0.03). POWIR prevented increases in %BF (POWIR, 0.01 % vs. FLEX, 1.3 %; p<0.04). Women with attendance to POWIR at ≥64 % had better improvements in %BF than women attending less often (p<0.03).
Conclusion
Impact + resistance training may effectively combat bone loss and worsening body composition from premature menopause in BCS.
Keywords: Chemotherapy, Neoplasm, Obesity, Osteoporosis, Physical activity
Introduction
Bone loss and unfavorable shifts in body composition (e.g., decreased muscle mass and increased fat mass) are known consequences of breast cancer treatment [1–3]. Adjuvant hormone manipulation therapy and chemotherapy contribute to bone and body composition changes during treatment and these shifts can persist long term [4]. Premenopausal patients may experience chemotherapy-induced amenorrhea or undergo pharmacologic ovarian suppression treatment, subjecting them to further bone loss [3] and fat gain [5] from estrogen deprivation. Nearly 25 % of women with breast cancer are premenopausal at diagnosis and up to 70 % of those treated with chemotherapy may experience premature menopause [6, 7].
The collective impact of adjuvant treatments on body composition may be substantial. Adjuvant chemotherapy has been associated with a 1–2 % loss of bone density [8], a 1- to 2-kg loss of lean body mass and a 1–4 % increase in percent body fat [5, 9]. Chemotherapy-induced ovarian failure accelerates bone loss to 5–8 %/year [4, 10] and exacerbates fat gain [5]. Selective estrogen receptor modulators (SERM) preserve bone in post-menopausal breast cancer survivors (BCS) but cause fat gain [1, 2], while aromatase inhibitors (AI) cause bone loss of 2 to 3 %/year [2, 8] but may increase lean mass and prevent fat gain by about 1 kg [11].
Prematurely menopausal BCS face multiple treatment-related threats to healthy body composition but have seldom been the subjects of lifestyle interventions to restore healthy body composition after treatment. Resistance exercise can build muscle and reduce body fat in adult women without cancer [12] and when combined with impact loading may optimally target bone [13, 14]. The aim of this study was to determine whether our program of impact + resistance exercise, shown to stop bone loss in older BCS [15] and to build bone and decrease body fat in middle-aged women without cancer [16, 17], could also improve bone and body composition in prematurely menopausal BCS. We also evaluated intervention effects on bone turnover markers because elevated bone turnover is associated with increased fracture risk independent of bone mineral density (BMD) [18], and these markers increase with amenorrhea [19] and AI therapy [20].
Methods
Trial design
We conducted a 12-month randomized controlled trial comparing a progressive, supervised impact + resistance exercise (prevent osteoporosis with impact + resistance (POWIR)) to an exercise placebo (FLEX). All testing and exercise training took place between October 2006 and January 2009. The Oregon Health & Science University Institutional Review Board approved the study protocol and procedures.
Participants
The target sample in this study was BCS recently menopausal from cancer treatment. Eligible women met the following criteria: stages I–IIIa breast cancer, completion of chemotherapy ≥6 months and <5 years prior to study enrollment, prematurely menopausal, hip and spine T-scores>−2.5, no medication to treat bone loss, physician clearance for moderate-intensity exercise, and participation in <60 min/week of resistance training. Premature menopause was defined as being premenopausal at diagnosis and meeting one of the following: (1) amenorrheic for ≥6 consecutive months and within 1 year after starting chemotherapy, (2) confirmed menopause by blood tests within 1 year prior to enrollment, or (3) on LHRH-agonist therapy for ≥6 months before enrollment.
Women were recruited through mailings from the Oregon State Cancer Registry, clinician referral, community events, and advertisements. Sample size determination was based on a 2×3 mixed design analysis of covariance (MD-ANCOVA) using data from our previous intervention [16]. Group sizes of n=35 provided power of 0.81–0.99 to detect significant group × time interactions for bone, lean and fat mass measures at ∝=0.01 with effect sizes of 0.014 g/cm2, 0.9 kg, and 2 kg, respectively. We planned to recruit a sample at least 20 % larger (n=88) to allow for attrition.
Procedures
After providing written consent, women completed questionnaires, physical testing, and biological specimen collection. Blood and urine samples were collected in the morning after a 12-h fast and stored at −70 °C for analysis. Questionnaires, tests and specimen collection were repeated at 6 and 12 months. Test protocols were administered by trained technicians blinded to group assignment. Randomization was stratified by adjuvant hormone therapy use (AI or SERM vs. none) and current aerobic activity (≥ vs. <90 min/week). Group assignments for each combination of strata generated were placed in sealed, sequentially numbered envelopes and opened after baseline testing.
Study programs
Participants in each group were prescribed a training program consisting of supervised classes 2 days a week plus home exercise 1 day a week for 12 months. Trained exercise instructors delivered supervised classes and participants followed a similar training program at home. Participants tracked their attendance and completion of exercises on training logs and reported any injury, side effect or symptom associated with the study program to the exercise instructor. If prescribed, women wore compression sleeves for lymphedema during training.
Impact + resistance intervention
The impact + resistance intervention (POWIR) was similar to that used in older, postmenopausal BCS [15] and was based on our prior interventions in women without cancer [17, 21]. POWIR complies with the American College of Sports Medicine guidelines for exercise in cancer survivors [22] and follows their recommendations for improving bone health in adult women [13], calling for resistance and/or impact exercise that produces varied and moderate to high bone loading forces for 30–60 min/session.
The POWIR program has been described previously [15] but important elements, including training volume and rate of training progression are summarized (Table 1). Free weights (e.g., dumbbells, barbells, resistance bands, and weighted vests) were used to apply resistance. Intensity for lower body training was prescribed as percentage of body weight loaded into a vest and for upper body training as an 8–15 repetition maximum. Adjustments in vest weight were made on a monthly basis according to the training plan and in adjustment with participant tolerance. The exercise instructor continuously monitored upper body effort and increases in weight were made when a participant could complete or exceed the highest number of specified repetitions for a given training phase. Specific resistance exercises engaged muscles attached to skeletal sites of interest (proximal femur and lumbar spine) while impact loading consisted of two-footed jumps wearing weighted vests.
Table 1.
Progression of training in POWIR over 12 months
| Month | Jump
|
Lower body
|
Upper body
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Intensity | Sets | Reps | Intensity | Sets | Reps | Intensity | Sets | Reps | |
| 1 | 0–2 % BW | 3–4 | 10 | 0–2 % BW | 2–3 | 8–12 | 12–14 RM | 2–3 | 12–14 |
| 2 | 3–5 % BW | 5–6 | 10 | 3–5 % BW | 2–3 | 8–12 | 10–12 RM | 2–3 | 10–12 |
| 3 | 6–8 % BW | 7–8 | 10 | 6–8 % BW | 2–3 | 8–12 | 8–10 RM | 2–3 | 8–10 |
| 4–6 | 9–10 % BW | 9–10 | 10 | 9–10 % BW | 2–3 | 8–12 | 6–8 RM | 2–3 | 6–8 |
| 7–12 | 9–10 % BW | 10 | 10 | 9–10 % BW | 2–3 | 8–12 | 6–8 RM | 2–3 | 6–8 |
Intensity for jumps and lower body exercises set as a percentage of body weight (BW) loaded into weighted vests. Intensity for upper body exercises set as a repetition maximum (RM) where a higher RM indicates a lower workload (participant is doing lower weight for more repetitions) and a lower RM indicates a higher workload
Exercise placebo
Participants in the placebo group performed a series of whole body stretching exercises aimed to improve flexibility and relaxation exercises, also previously described [15]. The prescription for FLEX called for one to two sets of eight to ten seated/lying stretching movements, lasting 15 to 60 s/stretch. FLEX was not expected to change bone or body composition because stretching is nonweight bearing, low-force and has little energy cost.
Study measures
Demographic and clinical characteristics were obtained by self-report. The Charlson comorbidity index was used to describe the overall health of study participants [23]. Menopausal status was clinically determined at each time point by self-report of menstrual cycles in the prior 6 months and then further evaluated by radioimmunoassay for follicle stimulating hormone (FSH) from stored serum collected at baseline. FSH levels >30 mIU/ml and estradiol <30 pg/ml are considered menopausal in women without cancer.
Body composition: BMD (in grams per square centimeter) of the proximal femur (hip) and anterior–posterior lumbar spine (L1–L4), was assessed by DXA (Hologic QDR Discovery Wi; software version 12.0). Bone-free lean and fat mass (in kilograms) of the whole body were determined from a whole body DXA scan. Licensed technicians conducted all DXA scans that were subsequently analyzed by a single technician. In-house coefficients of variation (CV) for BMD are 1.0–1.5 % and for %BF are 1.5–2.0 % [16].
Bone turnover was assessed by serum osteocalcin (in nanograms per milliliter), a byproduct of bone formation and urinary deoxypyrodinoline cross-links (in nanomoles per millliter), a byproduct of bone degradation adjusted urine volume (creatinine in millimoles milliliter). Bio-markers were analyzed in batch by enzyme-linked immunoassay using commercial kits (Diagnostic Systems Laboratory, Inc). Interassay CVs from our laboratory are 5.7 and 6.2 % and intraassay CVs are 8 and 4.8 % for deoxypyridinoline and osteocalcin, respectively.
Maximal muscle strength of the upper and lower body was included to validate the effectiveness of the prescribed resistance program and evaluated by a one-repetition maximum leg press and chest press (in kilograms), according to standard protocols. We have described our use of this test in older BCS [15]. Our in-house CVs are 6.6 and 7.5 % for leg and chest press, respectively.
Habitual physical activity and dietary intake in the past month were measured by the Community Healthy Activity Model Program for Seniors (CHAMPS) questionnaire [24] and the Block Food Frequency Questionnaire (FFQ) [25]. CHAMPS estimates total weekly energy expenditure (in kilocalories per week) from low-vigorous intensity physical activities in the past month. The block FFQ to estimates average daily energy intake (in kilocalories per day) and calcium intake (in milligrams per day) from food, beverages, and supplements over the prior 6 months.
Adherence and compliance data were abstracted from attendance records and training logs. Intervention adherence was the percentage of prescribed sessions attended by each participant while compliance was the percentage of completing exercises as prescribed.
Training effects on lymphedema was documented by comparing circumferences between arms measured at the base of the middle finger, wrist, and distal forearm at 0, 3, 6, and 12 months of training [26]. Women also reported symptoms of lymphedema to the trainer and in training logs.
Statistical analysis
Descriptive statistics were used to characterize the sample (SPSS, v.19). Intent-to-treat (ITT) analysis was performed using Hierarchical Linear Modeling (6.08 software) analyzing each participant according to her originally assigned group and regardless of missing 6- or 12-month data. In addition to ITT analysis, data from participants with complete baseline and 12-month data (per protocol (PP)) were considered to evaluate intervention effects using separate 2 (group)×3 (time) MD-ANCOVA for each outcome. Age, time since diagnosis and adjuvant hormone therapy were included as covariates.
We also examined potential effect modification from adjuvant hormone therapy use on primary outcomes. We explored the impact of adherence on study outcomes within POWIR by comparing women whose adherence was greater than or equal to the mean value to those who were below the mean. We also performed separate sensitivity analyses to determine whether outcomes changed when excluding: (1) women with high estradiol levels (>100 pg/ml) at any time point; (2) women who switched, discontinued, or initiated adjuvant hormone therapy; or (3) women who were menopausal for <1 year based on the rationale that the osteogenic potential of exercise may be reduced during the early phase of estrogen deprivation [27, 28].
Results
Sample
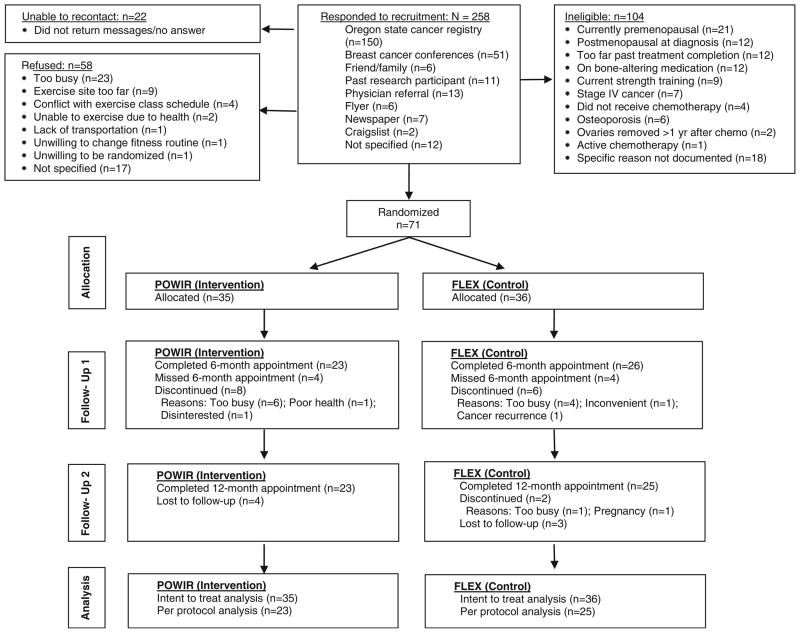
Twenty-six percent of interested women were eligible and willing to enroll in the trial (Fig. 1). After baseline testing women were randomized to POWIR (n=36) or FLEX (n= 35) (Fig. 1) and 68 % provided baseline and final data (POWIR, n=23; FLEX, n=25). Compared with women who did not withdraw, study drop outs reported higher caloric intake (completed, 1,556±506 kcal/day; dropped, 1,926±588 kcal/day; p<0.05), were more likely to be His-panic (completed, 0 %; dropped, 13 %; p<0.01), and were less likely to have received radiation therapy (completed, 69 %; dropped, 38 %; p<0.03).
Fig. 1.
Participant flow through the trial
On average, women were in their mid-40s, in good health, overweight, and menopausal by FSH levels (Table 2). Mean estradiol level in FLEX was slightly higher (35.0 pg/ml) but not significantly different from POWIR. Two women in FLEX had baseline estradiol levels more than 3 standard deviations above the mean, yet reported amenorrhea for 6 months prior to enrollment. Excluding these outliers did not change study outcomes. Groups did not significantly differ at baseline on any demographic, study outcome or other clinical characteristic. Across the study period, energy intake decreased more over time in FLEX than in POWIR (Table 3; p<0.05). Because changes in caloric intake could confound intervention effects on body composition, this variable was included as a covariate in analyses of body composition outcomes.
Table 2.
Baseline clinical characteristics of randomized participants
| Characteristic | POWIR (N=35) Mean (SD) |
FLEX (N=36) Mean (SD) |
p value* |
|---|---|---|---|
| Age (years) | 46.5 (5.0) | 46.4 (4.9) | 0.90 |
| Comorbidity index | 1.9 (1.6) | 2.1 (1.6) | 0.66 |
| Body mass index (kg/m2) | 27.0 (5.4) | 25.8 (4.6) | 0.32 |
| Spine T-score | −0.5 (1.1) | −0.4 (1.0) | 0.86 |
| Femoral neck T-score | −0.4 (0.9) | −0.5 (0.9) | 0.46 |
| FSH (IU) | 79.5 (45.0) | 62.9 (33.9) | 0.09 |
| Estradiol (pg/ml) | 15.0 (20.1) | 35.0 (79.3) | 0.16 |
| Time since diagnosis (months) | 30.1 (18.3) | 30.1 (15.5) | 0.99 |
| Time since menopause (months) | 24.1 (17.4) | 26.1 (15.4) | 0.61 |
| Stage I (%) | 22.9 % | 33.3 % | 0.41** |
| Stage II (%) | 65.7 % | 50.0 % | |
| Stage IIIa (%) | 11.4 % | 16.7 % | |
| Received radiation therapy (%) | 62.9 % | 61.1 % | 0.88 |
| Currently taking AI (%) | 40.0 % | 30.6 % | 0.70** |
| Currently taking SERM (%) | 37.1 % | 44.4 % |
Data presented as mean (SD) for continuous data or percentage of sample for categorical data
AI aromatase inhibitor; SERM selective estrogen receptor modulator
p value from unpaired t test for continuous data and from chi-squares test for categorical data;
p value for differences in the distribution of cancer stage (I, II, or IIIa) or adjuvant hormone therapy (none, AI, and SERM) between study groups
Table 3.
Outcome and process variables across the study period
| Characteristic | POWIR (n=23)
|
FLEX (n=25)
|
p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | 12 months | % change 0–12 | Baseline | 6 months | 12 months | % change 0–12 | ||
| Outcome variables | |||||||||
| Spine BMD (g/cm2) | 0.983 (0.113) | 0.984 (0.111) | 0.972 (0.119) | −1.1 | 0.988 (.118) | 0.977 (.117) | 0.970 (0.126) | −1.8 | 0.18 |
| Total hip BMD (g/cm2) | 0.909 (0.095) | 0.907 (0.098) | 0.899 (0.096) | −1.1 | 0.892 (.119) | 0.891 (.120) | 0.887 (0.119) | −0.56 | 0.65 |
| Trochanter BMD (g/cm2) | 0.689 (0.065) | 0.688 (0.065) | 0.683 (0.066) | −.87 | 0.666 (.099) | 0.666 (0.102) | 0.662 (0.101) | −0.60 | 0.90 |
| Femoral neck BMD (g/cm2) | 0.809 (0.11) | 0.803 (0.11) | 0.804 (0.108) | −.62 | 0.781 (.093) | 0.776 (0.099) | 0.773 (0.095) | −1.0 | 0.68 |
| Osteocalcin (ng/ml) | 10.6 (4.07) | 10.1 (5.2) | 9.78 (4.5) | −7.7 | 14.0 (3.78) | 10.8 (4.0) | 11.9 (5.5) | −15.0 | 0.22 |
| Deoxypyrodinoline (nmol/mmol creatinine) | 13.0 (4.95) | 11.8 (6.8) | 15.8 (12.1) | 21.5 | 14.2 (4.57) | 15.5 (10.4) | 15.0 (8.6) | 5.6 | 0.39 |
| Body weight (kg) | 72.3 (13.4) | 73.5 (14.1) | 73.6 (14.9) | 1.8 | 70.4 (12.7) | 71.0 (12.7) | 72.3 (13.3) | 2.7 | 0.34 |
| Bone-free lean mass (kg) | 45.3 (5.0) | 46.0 (5.0) | 46.0 (5.4) | 1.5 | 44.7 (4.9) | 44.6 (4.7) | 45.1 (5.0) | 0.89 | 0.16 |
| Fat mass (kg) | 25.8 (9.6) | 26.4 (10.2) | 26.5 (10.6) | 2.7 | 24.4 (8.7) | 25.0 (9.1) | 26.0 (9.3) | 6.6 | 0.17 |
| % Body fat | 35.3 (6.8) | 35.4 (7.1) | 35.4 (7.3) | .28 | 34.3 (6.8) | 35.4 (7.1) | 35.6 (6.6) | 3.8 | 0.04c |
| Process variables/covariates | |||||||||
| Upper body strength (lbs) | 66.1 (13.8) | 73.0 (16.1) | 74.8 (15.7) | 11.6 | 67.7 (15.4) | 72.5 (13.8) | 69.4 (14.7) | 2.5 | 0.03* |
| Lower body strength (lbs) | 209.1 (66.1) | 229.8 (63.4) | 237.4 (68.0) | 13.5 | 199.2 (41.5) | 213.8 (39.9) | 214.8 (36.8) | 7.8 | 0.23 |
| Energy expenditure (kcal/day)a | 2,716 (1,332) | 3,497 (1,782) | 3,178 (1,793) | 17.0 | 2,703 (1,447) | 3,444 (2,066) | 2,812 (1,490) | 4.0 | 0.68 |
| Energy intake (kcal/d)b | 1,583 (323) | 1,589 (372) | 1,510 (417) | −4.6 | 1,608 (559) | 1,417 (486) | 1,313 (399) | −18.3 | 0.04* |
| Calcium intake (mg/day)b | 797 (237) | 720 (228) | 745 (253) | −6.6 | 802 (340) | 726 (329) | 646 (209) | −19.5 | 0.19 |
Data presented as unadjusted mean (SD) from participants with complete datasets
BMD bone mineral density
p value from RM-ANCOVA of baseline, 6- and 12-month time points, controlled for age, time since diagnosis, adjuvant hormone therapy use
Energy expenditure calculated from CHAMPS physical activity survey and includes energy expended in activities ranging from low to vigorous intensity per week
Energy intake and calcium intake were calculated from the Block Food Frequency questionnaire and includes calcium obtained both from dietary sources and from dietary supplements
Change in energy intake from 0–12 months included added as covariate
Intervention
In POWIR, adherence to supervised and home-based sessions averaged 64 and 26 %, respectively, and in FLEX averaged 72 % for supervised and 41 % for home-based sessions. Group differences for adherence approached significance for home-based (p=0.05), but not supervised sessions. Adherence to supervised sessions dropped by about 20 % in both groups from the first to second half of the intervention, while home-based adherence dropped by 10 % in POWIR and 3 % in FLEX. Three participants in POWIR stopped increasing vest weight at month 6 due to back (N= 2) or knee (N=1) pain, and one participant stopped lower body exercises at month 5 due to pain, resulting in a protocol compliance of 84 % in POWIR and 100 % in FLEX. There were no significant changes in upper-extremity circumference measures within or between groups over time (data not shown), indicating neither condition affected lymphedema. Maximal upper and lower body strength increased progressively in POWIR (Table 3) compared with nonlinear changes in FLEX (Table 3) with significant group differences for upper body strength (ITT results—coefficient on slope of time=3.12, SE=1.25, t(64)=2.49, and p<0.02).
Outcomes
There were no significant group×time interactions for BMD at any skeletal site (Table 3), based on either ITT or PP analyses. There was no effect of adjuvant hormone therapy, adherence, or exclusion of women with high estradiol on outcomes. However, when women in the very early phase of menopause (<12 months postmenopausal; n=13) were removed from either ITT or PP analyses (Fig. 2), significant group × time interactions emerged for both spine (ITT results—coefficient on slope of time=0.009, SE=0.004, t (48)=2.21, and p=0.032) and femoral neck BMD (ITT results—coefficient on slope of time=0.011, SE=0.004, t (48)=3.19, and p=0.004). Among women who were 1+ years past the onset of menopause (n=49), spine BMD steadied and femoral neck BMD increased in POWIR compared with losses in FLEX (Fig. 2). Women who were 1+ years past menopause onset were significantly older and further from diagnosis than recently menopausal women (age, 47.4±4.6 vs. 43.4±4.8; p<0.01 and time since diagnosis, 35.3±15.7 vs. 13.6±6.2; p<0.01, for women 1+ vs. <1 year postmenopausal, respectively); however, these groups did not differ on any other clinical measure nor on baseline values of study outcomes, process variables, or potential covariates (data not shown). There were no significant group × time differences, effect modification, or change with sensitivity analysis for either bone turnover marker, with osteocalcin decreasing and deoxypyrodinoline cross-links increasing in both groups over time (Table 3).
Fig. 2.
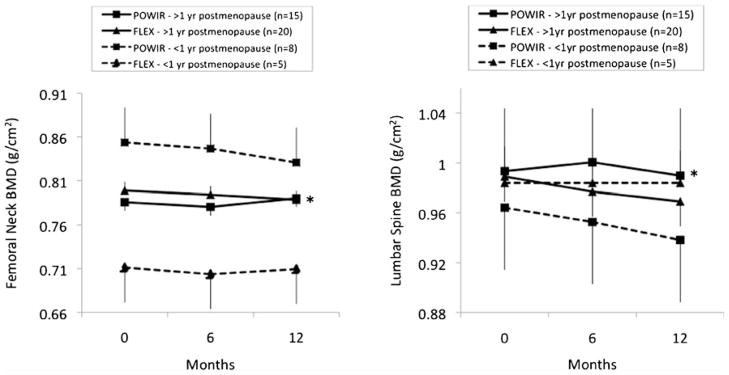
Change in femoral neck and spine bone mineral density (BMD) among participants ≥1 year postmenopausal and participants <1 year postmenopausal. Data presented as mean and standard errors are presented as positive or negative for clarity. *p<0.05, group × time interaction within each menopausal group
For body composition outcomes, there was a significant group x time interaction for %BF (ITT results—coefficient on slope of time=−0.672, SE=0.319, t(60)=−2.11, and p= 0.039) but not for absolute lean or fat mass (Table 3). Despite a slight increase in body weight, participants in POWIR maintained %BF whereas FLEX participants increased by 1.3 %. Group differences in %BF were independent of adjuvant hormone therapy use and were unaffected when excluding women who switched their hormone therapy regimens. POWIR participants whose adherence to supervised classes was at or above the mean (64 %) became leaner than women with lower adherence (p<0.05; Fig. 3).
Fig. 3.
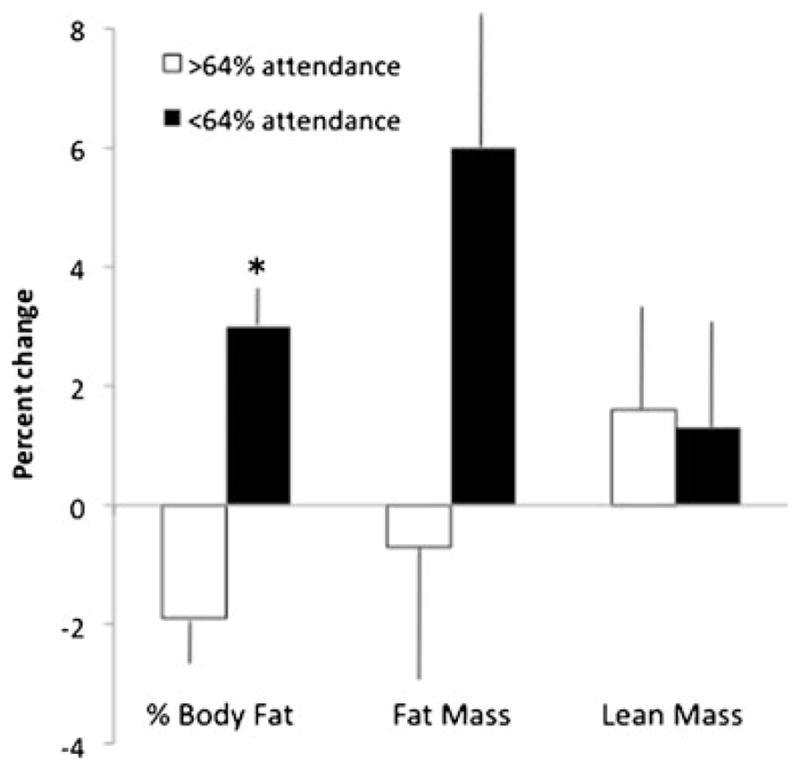
Percent change in body composition among women in POWIR who attended more than or equal to vs. lesser than the mean attendance for supervised classes. Data presented as mean ± SE. *p<0.05, group × time interaction
Discussion
Among BCS with recent treatment-related menopause, participation in a program of combined impact + resistance exercise (i.e., POWIR) prevented increases in body fat percentage and was more effective when participation was more frequent. POWIR did not appear to affect either index of bone health, e.g., BMD or bone turnover markers; however, the phase of menopause may have masked an underlying benefit of exercise on the skeleton. When considering only women who were a year or more past the onset of menopause, POWIR effectively stopped bone loss at the spine and increased BMD at the hip compared with bone loss at both sites in FLEX participants performing low-intensity stretching (Fig. 2).
The beneficial effect of our combined impact + resistance exercise program on hip and spine BMD, albeit specific to women outside of the acute phase of menopause, is congruent with our original trial of this program in premenopausal women without cancer [17, 29]. POWIR also prevented a similar degree of bone loss at the spine in older, postmenopausal BCS but failed to affect hip BMD. Others have reported that age may alter the effectiveness of exercise at the hip [30, 31]. We have previously shown that the osteogenic effects of POWIR at the spine are attributed to the resistance component and the effects at the hip are attributed to the combination of resistance + impact (jumping) [17]. Meta-analyses of exercise trials in women without cancer also support our observations that the site-specific effects of exercise on bone depend upon loading type [14, 32, 33]. In this study, the positive effect of POWIR on both upper body strength and spine BMD suggests that the resistance component of POWIR provided the vertebral load, whereas the discordant effects of POWIR on lower body strength (e.g., no significant effect) and hip BMD suggests that the impact component loaded the hip. Saarto et al. studied impact loading, via vigorous aerobic exercise, in posttreatment BCS and reported maintenance of femoral neck BMD in premenopausal patients but no effect at the spine [34]. The jumping program common to both our study and Saarto’s confirms that impact loading can be osteogenic at the hip in women with breast cancer; however, it appears that more direct loading of the spine, i.e., via upper body resistance training, is necessary to prevent bone loss at this site. The additional load incurred from jumping with weighted vests on probably explains why hip BMD increased from POWIR compared with a maintenance effect at this site from jumping without added loads [34].
An important caveat to our BMD outcomes is that POWIR was only effective in BCS past the acute phase of menopause. The positive influence of exercise on bone mass may be influenced by the estrogen environment. The net impact of hormonal, environmental (i.e., mechanical loading) and dietary influences on BMD result from the summation of bone resorption and bone formation processes over time. Both Lanyon and Dalsky argue that during estrogen deficiency, the osteogenic influence of exercise must compete with resorptive processes resulting from low estrogen and that exercise may be unable to influence bone during periods of high turnover [26, 35]. Increases in bone resorption are highest in the first year of menopause but slow thereafter, which may then permit exercise to exert its osteogenic effect [26]. Bone loss and resorption are particularly high in the first year of treatment-related menopause among BCS [4, 36] but then slow and may explain the differential effectiveness of POWIR on BMD in our sample. However, because the number of women in our sample who were recently menopausal was small (POWIR, N=8; FLEX, N=5), we could not explore a moderating effect of time since menopause on the bone response to exercise. The small sample likely contributed to the greater variance among recently menopausal women compared with women 1+years past menopause (Fig. 2), suggesting that future studies that include bone as an outcome need to carefully consider time since menopause in the research design and sample determination.
The POWIR intervention maintained body fat percentage over time compared with an increase of just over 1 % body fat among controls, a similar magnitude of difference reported in our trial in premenopausal women without cancer [16]. Schwartz reported a similar maintenance effect from resistance training during adjuvant treatment in female cancer patients [37], whereas Schmitz reported decreases of about 1 % body fat from resistance training in posttreatment BCS [38, 39]. While studies differ in the timing of the intervention relative to treatment, types of treatment, menopausal status, and specific training protocols, a consistent mean difference between groups of 1–2 % was found [16, 38, 39]. Though our study was not specifically designed to test for a dose–response, we found that participants who attended about two thirds of prescribed classes had significantly better improvements in body fat percentage than those attending less often. Increasing exercise duration at a given intensity is more effective for fat loss than increasing intensity and maintaining total volume (i.e., by decreasing duration) [40]. The ideal exercise prescription for optimizing body composition in BCS must be determined by appropriately designed trials.
A strength of this study is that it was the first to target an exercise program to prematurely menopausal BCS—a group at risk for bone loss and poor body composition [4, 41, 42]. Our exercise program was specifically designed to target clinically relevant skeletal sites based on the theoretical foundations for designing osteogenic loading regimens [13, 14] and results of our prior empirical work [17]. We also used an exercise placebo group rather than usual care to protect against unequal attrition, contamination by increased physical activity in controls, and risks associated with inactivity. Limitations of our study were the modest sample size and poor compliance to home-based training. Though our sample size was lower than other exercise studies in BCS with a bone outcome [34, 43], our sample had sufficient power to detect differences in BMD, even using an ITT analysis. Adherence to our study classes was comparable to [39, 44] or greater than other center-based trials [34]; however, adherence to the home program was low. We thought home-based exercise would be a reasonable strategy to deliver a thrice-weekly dose of exercise by allowing women to exercise one time a week at home, but supervised classes were better attended. Possibly the women gained added value from exercising with a group, a hypothesis that can be explored in a future trial.
The combined effect of treatments that alter body composition could predispose younger BCS to early onset health problems such as osteoporosis, fractures, sarcopenia, functional decline, falls, obesity-related diseases, and increased risk of breast cancer recurrence. Increased fat and reduced muscle mass could partly explain reports from younger BCS that their body image, physical function, and quality of life worsen after treatment [45]. Impact + resistance training may provide an optimal loading pattern to protect bone health at both clinically relevant skeletal sites and a metabolic stimulus to stop fat gain. Notably, we have shown that an exercise training program that improves bone and body composition in women without cancer can be safely performed and similarly beneficial in prematurely menopausal BCS, even during adjuvant hormone therapy.
Acknowledgments
Funding was provided by an American Cancer Society Research Scholar Grant (RSGPB-06-092-01-CPPB). We thank the Oregon State Cancer Registry for their assistance with recruitment; Thera-band, Inc. for providing elastic exercise bands; and our research assistants, exercise trainers, and participants.
Footnotes
Conflicts of interest None
Contributor Information
K. M. Winters-Stone, Email: wintersk@ohsu.edu, School of Nursing, Oregon Health & Science University, Portland, OR, USA. Knight Cancer Center, Oregon Health & Science University, Portland, OR, USA. Oregon Health & Science University, 3455 SW US Veteran’s Hospital Rd, Mailcode: SN-ORD, Portland, OR 97239, USA
J. Dobek, School of Nursing, Oregon Health & Science University, Portland, OR, USA. School of Medicine, Oregon Health & Science University, Portland, OR, USA
L. M. Nail, School of Nursing, Oregon Health & Science University, Portland, OR, USA. Knight Cancer Center, Oregon Health & Science University, Portland, OR, USA
J. A. Bennett, School of Nursing, Oregon Health & Science University, Portland, OR, USA. Knight Cancer Center, Oregon Health & Science University, Portland, OR, USA
M. C. Leo, Kaiser Permanente Center for Health Research, Portland, OR, USA
B. Torgrimson-Ojerio, School of Nursing, Oregon Health & Science University, Portland, OR, USA
S.-W. Luoh, Portland VA Medical Center, Portland, OR, USA
A. Schwartz, Idaho State University, Pocatello, ID, USA
References
- 1.Vance V, Mourtzakis M, McCargar L, Hanning R. Weight gain in breast cancer survivors: prevalence, pattern and health consequences. Obesity Rev. 2011;12(4):282–294. doi: 10.1111/j.1467-789X.2010.00805.x. [DOI] [PubMed] [Google Scholar]
- 2.Santen RJ. Effect of endocrine therapies on bone in breast cancer patients. J Clin Endocrinol Metab. 2011;96(2):308–319. doi: 10.1210/jc.2010-1679. [DOI] [PubMed] [Google Scholar]
- 3.Cameron D, Douglas S, Brown J, Anderson R. Bone mineral density loss during adjuvant chemotherapy in pre-menopausal women with early breast cancer: is it dependent on oestrogen deficiency? Breast Cancer Res Treat. 2010;23(3):805–814. doi: 10.1007/s10549-010-0899-7. [DOI] [PubMed] [Google Scholar]
- 4.Vehmanen L, Saarto T, Elomaa I, Makela P, Valimaki M, Blomqvist C. Long-term impact of chemotherapy-induced ovarian failure on bone mineral density (BMD) in premenopausal breast cancer patients. The effect of adjuvant clodronate treatment. Eur J Cancer. 2001;37(18):2373–2378. doi: 10.1016/s0959-8049(01)00317-3. [DOI] [PubMed] [Google Scholar]
- 5.Demark-Wahnefried W, Peterson BL, Winer EP, Marks L, Aziz N, Marcom PK, Blackwell K, Rimer BK. Changes in weight, body composition, and factors influencing energy balance among premenopausal breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2001;19(9):2381–2389. doi: 10.1200/JCO.2001.19.9.2381. [DOI] [PubMed] [Google Scholar]
- 6.Bruning P, Pit M, Md J-B, Avd E, Hart A, Av E. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br J Cancer. 1990;61(2):308–310. doi: 10.1038/bjc.1990.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin PJ, Ennis M, Pritchard KI, McCready D, Koo J, Sidlofsky S, Trudeau M, Hood N, Redwood S. Adjuvant treatment and onset of menopause predict weight gain after breast cancer diagnosis. J Clin Oncol. 1999;17(1):120–129. doi: 10.1200/JCO.1999.17.1.120. [DOI] [PubMed] [Google Scholar]
- 8.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, Pritchard KI. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26(33):5465–5476. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RJ, Aziz N, Albanes D, Hartman T, Danforth D, Hill S, Sebring N, Reynolds JC, Yanovski JA. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J Clin Endocrinol Metab. 2004;89(5):2248–2253. doi: 10.1210/jc.2003-031874. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro CL, Manola J, Leboff M. Ovarian failure after adjuvant chemotherapy is associated with rapid bone loss in women with early-stage breast cancer. J Clin Oncol. 2001;19(14):3306–3311. doi: 10.1200/JCO.2001.19.14.3306. [DOI] [PubMed] [Google Scholar]
- 11.van Londen G, Perera S, Vujevich K, Rastogi P, Lembersky B, Brufsky A, Vogel V, Greenspan S. The impact of an aromatase inhibitor on body composition and gonadal hormone levels in women with breast cancer. Breast Cancer Res Treat. 2011;125(2):441–446. doi: 10.1007/s10549-010-1223-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurley BF, Hanson ED, Sheaff AK. Strength training as a countermeasure to aging muscle and chronic disease. Sports Med. 2011;41(4):289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Kohrt WM, Bloomfield SA, Little KD, Nelson ME, Yingling VR. American college of sports medicine position stand: physical activity and bone health. Med Sci Sports Exerc. 2004;36(11):1985–1996. doi: 10.1249/01.mss.0000142662.21767.58. [DOI] [PubMed] [Google Scholar]
- 14.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43(12):898–908. doi: 10.1136/bjsm.2008.052704. [DOI] [PubMed] [Google Scholar]
- 15.Winters-Stone K, Dobek J, Nail L, Bennett JA, Naik A, Schwartz A. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2011;27(2):447–456. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winters KM, Snow CM. Detraining reverses positive effects of exercise on the musculoskeletal system in premenopausal women. J Bone Miner Res. 2000;15:2495–2503. doi: 10.1359/jbmr.2000.15.12.2495. [DOI] [PubMed] [Google Scholar]
- 17.Winters-Stone K, Snow C. Site-specific response of bone to exercise in premenopausal women. Bone. 2006;39(6):1203–1209. doi: 10.1016/j.bone.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Garnero P. Biomarkers for osteoporosis management: utility in diagnosis, fracture risk prediction and therapy monitoring. Molec Diagnos Ther. 2008;12(3):157–170. doi: 10.1007/BF03256280. [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Melton LJ, 3rd, Atkinson EJ, O’Fallon WM, Klee GG, Riggs BL. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab. 1998;83 (7):2266–2274. doi: 10.1210/jcem.83.7.4924. [DOI] [PubMed] [Google Scholar]
- 20.Hadji P, Asmar L, van Nes J, Menschik T, Hasenburg A, Kuck J, Nortier J, van de Velde C, Jones S, Ziller M. The effect of exemestane and tamoxifen on bone health within the Tamoxifen Exemestane Adjuvant Multinational (TEAM) trial: a meta-analysis of the US, German, Netherlands, and Belgium sub-studies. J Cancer Res Clin Oncol. 2011;137(6):1015–1025. doi: 10.1007/s00432-010-0964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snow CM, Shaw JM, Winters KM, Witzke KA. Long-term exercise using weighted vests prevents hip bone loss in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2000;55(9):M489–M491. doi: 10.1093/gerona/55.9.m489. [DOI] [PubMed] [Google Scholar]
- 22.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvao DA, Pinto BM, Irwin ML, Wolin KY, Segal RJ, Lucia A, Schneider CM, Vong VE, Schwartz AL. American college of sports medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42(7):1409–1426. doi: 10.1249/MSS.0b013e3181e0c112. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 24.Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Binkley N, Bilezikian JP, Kendler DL, Leib ES, Lewiecki EM, Petak SM. Summary of the International Society for Clinical Densitometry 2005 Position Development Conference. J Bone Miner Res. 2007;22(5):643–645. doi: 10.1359/jbmr.070204. [DOI] [PubMed] [Google Scholar]
- 26.Bicego D, Brown K, Ruddick M, Storey D, Wong C, Harris SR. Exercise for women with or at risk for breast cancer–related lymphedema. Phys Ther. 2006;86(10):1398–1405. doi: 10.2522/ptj.20050328. [DOI] [PubMed] [Google Scholar]
- 27.Dalsky GP. Effect of exercise on bone: permissive influence of estrogen and calcium. Med Sci Sports Exerc. 1990;22(3):281–285. [PubMed] [Google Scholar]
- 28.Frost HM. The role of changes in mechanical usage set points in the pathogenesis of osteoporosis. J Bone Miner Res. 1992;7 (3):253–261. doi: 10.1002/jbmr.5650070303. [DOI] [PubMed] [Google Scholar]
- 29.Winters KM, Snow CM. Body composition predicts bone mineral density and balance in premenopausal women. J Womens Health Gend Based Med. 2000;9(8):865–872. doi: 10.1089/152460900750020892. [DOI] [PubMed] [Google Scholar]
- 30.Bassey E, Rothwell M, Littlewood J, Pye D. Pre- and postmenopausal women have different bone mineral density responses to the same high-impact exercise. J Bone Miner Res. 1998;13(12):1805–1813. doi: 10.1359/jbmr.1998.13.12.1805. [DOI] [PubMed] [Google Scholar]
- 31.Kohrt WM. Aging and the osteogenic response to mechanical loading. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S137–S142. doi: 10.1123/ijsnem.11.s1.s137. [DOI] [PubMed] [Google Scholar]
- 32.Martyn-St James M, Carroll S. Progressive high-intensity resistance training and bone mineral density changes among pre-menopausal women: evidence of discordant site-specific skeletal effects. Sports Med. 2006;36(8):683–704. doi: 10.2165/00007256-200636080-00005. [DOI] [PubMed] [Google Scholar]
- 33.Martyn-St James M, Carroll S. High-intensity resistance training and postmenopausal bone loss: a meta-analysis. Osteoporos Int. 2006;17(8):1225–1240. doi: 10.1007/s00198-006-0083-4. [DOI] [PubMed] [Google Scholar]
- 34.Saarto T, Sievänen H, Kellokumpu-Lehtinen P, Nikander R, Vehmanen L, Huovinen R, Kautiainen H, Järvenpää S, Penttinen H, Utriainen M, Jääskeläinen A, Elme A, Ruohola J, Palva T, Vertio H, Rautalahti M, Fogelholm M, Luoto R, Blomqvist C. Effect of supervised and home exercise training on bone mineral density among breast cancer patients. A 12-month randomised controlled trial. Osteoporos Int. 2012;23:1601–1612. doi: 10.1007/s00198-011-1761-4. [DOI] [PubMed] [Google Scholar]
- 35.Lanyon LE. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18(1 Suppl):37S–43S. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 36.Hadji P, Ziller M, Maskow C, Albert U, Kalder M. The influence of chemotherapy on bone mineral density, quantitative ultrasonometry and bone turnover in pre-menopausal women with breast cancer. Eur J Cancer. 2009;45(18):3205–3212. doi: 10.1016/j.ejca.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz AL, Winters-Stone K. Effects of a 12-month randomized controlled trial of aerobic or resistance exercise during and following cancer treatment in women. Phys Sportsmed. 2009;37 (3):62–67. doi: 10.3810/psm.2009.10.1730. [DOI] [PubMed] [Google Scholar]
- 38.Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin-like growth factor axis proteins. Cancer Epidemiol Biomarkers Prev. 2005;14(7):1672–1680. doi: 10.1158/1055-9965.EPI-04-0736. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz KH, Ahmed RL, Troxel AB, Cheville A, Lewis-Grant L, Smith R, Bryan CJ, Williams-Smith CT, Chittams J. Weight lifting for women at risk for breast cancer-related lymphedema. JAMA. 2010;304(24):2699–2705. doi: 10.1001/jama.2010.1837. [DOI] [PubMed] [Google Scholar]
- 40.Slentz CA, Houmard JA, Kraus WE. Exercise, abdominal obesity, skeletal muscle, and metabolic risk: evidence for a dose response. Obesity. 2009;17(3):S27–S33. doi: 10.1038/oby.2009.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Winters-Stone KM, Nail L, Bennett JA, Schwartz A. Bone health and falls: fracture risk in breast cancer survivors with chemotherapy-induced amenorrhea. Oncol Nurs Forum. 2009;36 (3):315–325. doi: 10.1188/09.ONF.315-325. [DOI] [PubMed] [Google Scholar]
- 42.Gordon AM, Hurwitz S, Shapiro CL, Leboff MS. Premature ovarian failure and body composition changes with adjuvant chemotherapy for breast cancer. Menopause. 2011;18(11):1244–1248. doi: 10.1097/gme.0b013e31821b849b. [DOI] [PubMed] [Google Scholar]
- 43.Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, Berg K, Kupzyk K. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: a 24-month randomized controlled trial. Osteoporos Int. 2010;21(8):1361–1369. doi: 10.1007/s00198-009-1083-y. [DOI] [PubMed] [Google Scholar]
- 44.Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, Bryan CJ, Williams-Smith CT, Greene QP. Weight lifting in women with breast-cancer-related lymphedema. New Engl J Med. 2009;361(7):664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 45.Ganz PA, Land SR, Geyer CE, Cecchini RS, Costantino JP, Pajon ER, Fehrenbacher L, Atkins JN, Polikoff JA, Vogel VG, Erban JK, Livingston RB, Perez EA, Mamounas EP, Wolmark N, Swain SM. Menstrual history and quality-of-life outcomes in women with node-positive breast cancer treated with adjuvant therapy on the NSABP B-30 trial. J Clin Oncol. 2011;29(9):1110–1116. doi: 10.1200/JCO.2010.29.7689. [DOI] [PMC free article] [PubMed] [Google Scholar]