Abstract
Background:
Fluid resuscitation is widely practiced in intensive care units for the treatment of sepsis. A comparison of the evidence base of different fluids may inform therapeutic choice.
Methods:
The risks of mortality and morbidity (the need for renal replacement therapies (RRT)) were assessed in patients with severe sepsis. A network meta-analysis compared trials for crystalloids, albumin and hydroxyethyl starch (HES). A literature search of human randomized clinical trials was conducted in databases, the bibliographies of other recent relevant systematic reviews and data reported at recent conferences. Mortality outcomes and RRT data with the longest follow up period were compared. A Bayesian network meta-analysis assessed the risk of mortality and a pair-wise meta-analysis assessed RRT using crystalloids as the reference treatment.
Results:
13 studies were identified. A fixed-effects meta-analysis of mortality data in the trials demonstrated an odds-ratio (OR) of 0.90 between crystalloids and albumin, 1.25 between crystalloids and HES and 1.40 between albumin and HES. The probability that albumin is associated with the highest survival was 96.4% followed by crystalloid at 3.6%, with a negligible probability for HES. Sub-group analyses demonstrated the robustness of this result to variations in fluid composition, study source and origin of septic shock. A random-effects pairwise comparison for the risk of RRT provided an OR of 1.52 favoring crystalloid over HES.
Conclusion:
Fluid therapy with albumin was associated with the highest survival benefit. The higher morbidity with HES may affect mortality and requires consideration by prescribers.
Keywords: Albumin, crystalloid, hydroxyethyl starch, meta-analysis, resuscitation, sepsis, septic shock, severe sepsis.
INTRODUCTION
Sepsis is a systemic, deleterious inflammatory host response to infection. It can evolve to severe sepsis (acute organ dysfunction secondary to documented or suspected infection) and septic shock (severe sepsis plus hypotension not reversed with fluid resuscitation) [1]. Severe sepsis and septic shock are major healthcare problems increasing in incidence [2-6]. Mortality is about 33.3% in the patients with severe sepsis who are hospitalized [7] while treated with the current standard of care in the US. Hypovolemia is a feature of sepsis which is treated with fluids. Among several fluid treatments available for treating sepsis, the first line of treatment is generally crystalloids in a range of formulations [1, 8], followed by colloid if large amounts of crystalloid are administered.
A wide range of colloids are used [9]. They include human albumin solutions which have been used in acute care for decades [10]. Albumin's repertoire of molecular functions includes correction of hypoalbuminaemia [11] and antioxidant properties which are sustained in septic patients [12]. Synthetic colloids include hydroxyethyl starches (HES)which have been used in a range of clinical applications in lieu of albumin [8]. Recent large randomized clinical trials (RCTs) provide insight in determining the efficacy of the fluids and have compared albumin with crystalloid [11, 13, 14] and HES with crystalloid [15-17] but no large head to head randomized controlled trials comparing the two colloids have been reported. Estimates of relative efficacy for all treatments are required to inform clinical decisions, treatment guidelines and economic studies such as cost effectiveness analyses.
A number of meta-analyses have also indicated different mortality and morbidity outcomes with the different drugs [18-23]. None of these standard meta-analytic techniques, which evaluate the relative efficacy of one treatment compared with a single comparator, have compared different colloids or compared crystalloid with albumin. Network meta-analytic techniques have recently been developed that estimate the relative efficacy of any number of different treatments by taking account of the entire network of RCT evidence [24-26]. Given the paucity of head-to -head trials comparing HES and albumin or comparing crystalloid with both colloids [27, 28], the network method of meta-analysis may inform comparisons between fluid treatments. The present study proposes a network meta-analysis [29] utilizing direct and indirect treatment comparison of randomized trials with common comparators to assess the primary outcome of risk of mortality and a secondary outcome in the form of a need for renal replacement therapy. Using this approach allowed the inclusion of trials comparing the two colloids, albumin and HES, and concurrent comparison of each colloid with crystalloid.
METHODS
Systematic Review
A literature search of human clinical trials was conducted in PubMed, ClinicalTrials.gov and within the bibliographies of other recent relevant systematic reviews [23, 30]. In addition, data for mortality for treatment and control arms from the Early Albumin Resuscitation for Sepsis and Septic Shock (EARSS) trial [14] and the Albumin Italian Outcomes Study (ALBIOS) trial [31] for albumin in septic shock were extracted from the results reported as referenced. The search terms used were sepsis, septicemia, systemic inflammatory response syndrome, septic shock, fluid therapy, resuscitation, plasma substitute, albumin, serum albumin, starch, hydroxyethyl starch, hetastarch, pentastarch, tetrastarch and mortality. “Crystalloids” was not included in the initial search strategy as all colloid trials have included crystalloid as a control fluid; including “crystalloids” in a subsequent search did not yield any differences or additional studies.
Inclusion Criteria
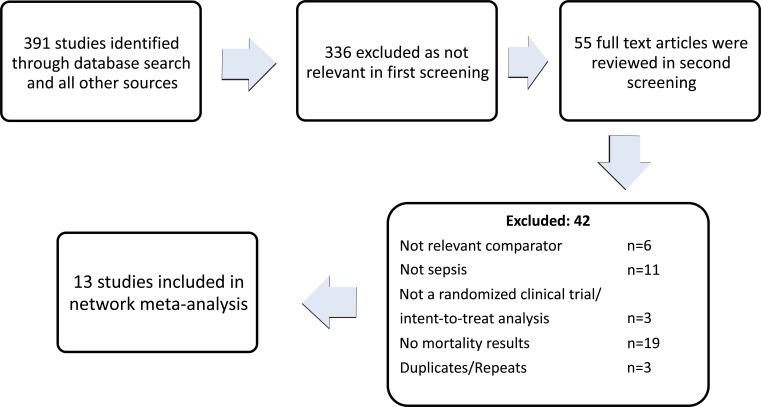
Only randomized controlled trials based on an intent-to- treat criterion and providing mortality outcomes were included. Trials with all formulations of crystalloids, HES and albumin (see Discussion) comparing two or all three treatments were included. All age groups were included. In trials with multiple endpoints, mortality outcomes after the longest follow up period were used. Only trials which reported specific data for patients with severe sepsis or septic shock were included. Trials which reported such data as a result of a pre-defined sub-group were also included. Only English language publications from 2000 to date were included. In total, 391 studies were extracted of which 13 were chosen to be included in the analysis as shown in Fig. (1). The studies used are listed in Table 1.
Fig. (1).
Study Flow diagram.
Table 1.
Basic Characteristics of the Included Randomized Clinical Trials
| Study | Population | Mean Age (Years) |
Treatment Arms |
Type of Crystalloid (If Any) |
Albumin/ HES Composition |
Follow- Up Duration |
Crystalloid | Albumin | HES | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deaths/ RRT |
Total | Deaths | Total | Deaths/ RRT |
Total | |||||||
| ALBIOS 2012 [31] |
Sepsis or septic shock |
66.3- A, 66.3-S |
Crystalloid, Albumin |
crystalloids | 20% A | hospital discharge |
342 | 907 | 326 | 908 | ||
| Charpentier et al., (EARSS) 2011 [14] |
Septic shock |
66 for both |
Crystalloid, Albumin |
0.9% saline |
20% A | 28 days |
103 | 393 | 96 | 399 | ||
| Finfer S (SAFE study) 2006 [11] |
Severe sepsis |
58.6-A, 58.5-S |
Crystalloid, Albumin |
0.9% saline |
4% A | 28 days |
217 | 615 | 185 | 603 | ||
| Maitland 2005 [42] |
Children w severe malaria and sepsis as a sub-group |
1.6 (for all txs) |
Crystalloid, Albumin |
0.9% saline |
4.5% A | hospital discharge |
3 | 20 | 4 | 23 | ||
| Maitland 2005 [43] |
Children w severe malaria and moderate or severe metabolic acidosis (sepsis as a sub-group) |
2.8 (for all txs) |
Crystalloid, Albumin |
0.9% saline |
4.5% A | hospital discharge |
11 | 61 | 2 | 56 | ||
| Maitland K, (FEAST Trial Group) 2011 [13] |
Patients with severe febrile illness and impaired perfusion (sepsis as a sub-group) |
23-A, 23-S |
Crystalloid, Albumin |
0.9% saline |
5% A | 30 days |
126 | 1047 | 128 | 1050 | ||
| Myburgh (CHEST) 2012 [17] |
Adult patients with sepsis (subgroup) |
63.1-H, 62.9-S |
Crystalloid, HES |
0.9% saline |
6% H (130/0.4) |
90 days |
224/196* | 958 | 248/235* | 979 | ||
| Brunkhorst (VISEP) 2008 [45] |
Patients with severe sepsis |
64.9-S, 64.4-H |
Crystalloid, HES |
ringer's lactate |
10% H (200/0.5) |
28 days |
66 | 275 | 70 | 262 | ||
| 90 days |
93/51 | 275 | 107/81 | 262 | ||||||||
| Guidet (CRYSTMAS) 2012 [15] |
Severe sepsis | 65.8-H, 65.9-S |
Crystalloid, HES |
NaCl fluid |
6% H (130/0.4) |
28 days |
24 | 96 | 31 | 100 | ||
| 90 days |
32/19 | 96 | 40/24 | 100 | ||||||||
| Mcintyre (FINESS) 2008 [46] |
Patients with septic shock |
63.6-S, 63.1-H |
Crystalloid, HES |
normal saline |
ICU | 6 | 19 | 6 | 21 | |||
| 28 days |
6/1 | 19 | 9/3 | 21 | ||||||||
| Perner (6S) 2012 [16] |
Patients with severe sepsis |
66-H, 67-S |
Crystalloid, HES |
ringer's acetate |
6% H (130/0.4) |
28 days |
144 | 400 | 154 | 398 | ||
| 90 days |
172/65 | 400 | 201/87 | 398 | ||||||||
| Dolecek 2009 [41] |
Adult patients with severe sepsis |
47-A, 43-H |
Albumin, HES |
6% H (130/0.4), 20% A |
28 days |
4 | 30 | 6 | 26 | |||
| Veneman 2004 [27] |
Severely ill patients with sepsis and post- surgical patients with systemic inflammatory response syndrome |
67-S, 72-A, 68-H |
Crystalloid, Albumin, HES |
0.9% saline |
20% A, 10% H |
30 days |
5 | 16 | 8 | 15 | 18 | 30 |
In CHEST 2012 study, RRT is analyzed for all patients in the ICU. Sepsis is a sub-group of those patients. Total ICU patients under crystalloids: 3375, HES:3352.
S - Crystalloid, A- albumin, H - Hydroxyethyl starch (HES), RRT - Renal Replacement Therapy, ICU- Intensive care unit.
Data Extraction and Validity Assessment
The studies were first screened by their titles and abstracts by a reviewer (MB). In the second screening, two reviewers (AF, MB) were involved. In this screening, full texts were reviewed and articles were excluded on the basis of no relevant comparators, not a randomized clinical trial, not an intent-to-treat analysis, no outcomes for only sepsis patients or no mortality results (Fig. 1). The chosen studies were validated, shown in Table 2, for the method of randomization and allocation concealment, blinding, presentation of an intention to treat analysis and loss to follow-up of >5% of patients for the primary outcome [32]. Any discrepancy was resolved by consulting with a third reviewer (SB).
Table 2.
Quality Assessment of the Trial
| Allocation Concealment | Intention-to-Treat | Blinding | No Loss of Follow Up* | Randomization | |
|---|---|---|---|---|---|
| ALBIOS 2012 [31] | No | Yes | No | Yes | Yes |
| Myburgh (CHEST) 2012 [17] | Yes | Yes | Yes | Yes | Yes |
| Brunkhorst (VISEP) 2008 [45] | No | Yes | No | Yes | Yes |
| Charpentier (EARSS) 2011 [14] | No | Yes | No | Yes | Yes |
| Dolecek 2009 [41] | No | Yes | No | Yes | Yes |
| Finfer (SAFE) 2010 [11] | Yes | Yes | Yes | Yes | Yes |
| Guidet (CRYSTMAS) 2012 [15] | Yes | Yes | Yes | Yes | Yes |
| Maitland 2005 [42] | Yes | Yes | No | Yes | Yes |
| Maitland 2005 [43] | Yes | Yes | No | Yes | Yes |
| Maitland (FEAST) 2011 [13] | Yes | Yes | No | Yes | Yes |
| McIntyre (FINESS) 2008 [46] | Yes | Yes | Yes | Yes | Yes |
| Perner (6S) 2012 [16] | Yes | Yes | Yes | Yes | Yes |
| Veneman 2004 [27] | Yes | Yes | No | Yes | Yes |
Loss to follow-up of >5% of patients.
Statistical Methods
The indirect comparison was conducted using the Bayesian method for a binomial likelihood, fixed effects network meta-analysis [29]. The analysis was conducted using OpenBUGS version 3.2.1. This package uses Bayesian Markov chain Monte Carlo Gibbs sampling methods [33]. The program code used is available in NHS Evidence Synthesis documents [29]. As conducted in previous research, [25, 34, 35] non-informative prior distributions were used for all the treatment effects; see program code for more details (Program 1 (d): http://www.nicedsu.org.uk/TS D2%20General%20meta%20analysis%20corrected%20Mar2 013.pdf).
Studies were included using crystalloids as a reference treatment compared to any composition of colloid fluid (albumin or HES) treatment in septic patients and head-to-head trials of colloid fluids; no trials originating from the group of Joachim boldt, which have been retracted from the peer-reviewed literature because of scientific misconduct, [36] were included.
The choice for fixed or random effect model was made by assessing model fit using Deviance Information Criteria (DIC) and heterogeneity between the trials [37]. The consistency of the model was assessed as described by National Institute for Health and Clinical Excellence (NICE) [26].
The Bayesian analysis ranked the treatments and provided probability of attaining that rank based on the proportion of Markov chain iterations in which treatment had the highest probability of lowering risk of mortality. The OR and corresponding 95% credible intervals were obtained with 50,000 iterations and convergence was seen at around 15,000 iterations. The 95% credible interval is used to assess statistical difference between comparators and can be interpreted as a 95% probability that true mean change falls within that range. In Bayesian statistical analysis, p-values are not provided [38].
Secondary Outcome
The secondary outcome was the need for renal replacement therapy for the 90-day follow up period only. The trials sourced through the search for mortality data were reviewed for data relating to RRT. In addition, a specific search was conducted for RRT within the PubMed database. The Bayesian, two treatments, pair-wise meta-analysis was used as this outcome was only reported in HES vs crystalloid RCTs. The analysis was conducted in OpenBUGS version 3.2.1; see code for details (Program 1 (a) in [37]. Other adverse events associated with colloids including hypotension, bleeding and pruritus [39] were considered for inclusion in the analysis but were not fully reported or were associated with low incidence (<1%) in the trials chosen. Hence, analysis was limited to the widely reported issue of renal dysfunction as assessed through the need for renal replacement therapy.
Sub-group Analysis
Sub-group analyses with different formulations of HES and albumin were performed, in order to assess recent conclusions that the drugs behaved as classes with no therapeutic differences between different compounds [40]. Most of the included RCTs had a population above 60 years of age. The small trial of Dolecek et al., [11, 41] had a population between 40-50 years of age. The studies of Maitland et. al. had a much younger population with infants in two trials [42, 43] and youth in the FEAST trial [13]. Further analysis was performed with patients in different age groups, as most of the included RCTs had populations of elderly (> 60 years) patients, and also without the inclusion of the malaria trials of Maitland et al., [13, 42, 43]. All the trials assessed severe sepsis or septic shock except the trials of Maitland et al., which were done on patients with malaria
and where the pathophysiology mimics that of sepsis [44]. As two of the largest trials selected - ALBIOS and EARSS - have only been reported as conference proceedings [14, 31], a subgroup analysis excluding these two studies was performed. Further analysis explored outcomes for in-hospital, 28-day vs 90-day mortality to see the effect of follow-up period on the mortality outcomes.
RESULTS
Studies Identified
In the literature search, 391 studies were identified. 336 of these were excluded in the first screening. Fig. (1) shows that 13 studies were finally included in the analysis. As shown in Fig. (2), there was only one qualifying head-to-head trial that compared albumin and HES and only one other small trial that included all three fluid treatments. Out of the remaining 11 crystalloid controlled trials, 6 were with HES and rest with albumin. The larger trials included CHEST, ALBIOS, VISEP, SAFE, EARSS, FEAST and 6S [11, 13, 14, 16, 17, 31, 45] and all were quite recent studies. The mortality data were provided for only the sepsis sub-group but RRT data was presented for all the ICU patients in the CHEST trial. The trial characteristics are summarized in Table 1.
Fig. (2).
Network diagram of type of fluid used in treatment of sepsis. Each intervention is a node in the network. The links between the nodes are trials of the study arms. The numbers represent number of studies found for each connection. The number in the center represents the number of trials studying all arms together.
Choice of Model - Random Effect vs Fixed Effect
The DIC was 182.5 for the random effects model and 180.7 for the fixed effect. The posterior mean of the residual deviance for random effects model was more than the fixed effect at 17.2 vs 15.1. The standard deviation with random effect was also quite small at 0.09 thus exhibiting very low heterogeneity between trials. These statistical outcomes indicate that the fixed effect model produced the best fit for the data [26].
Consistency of the Model
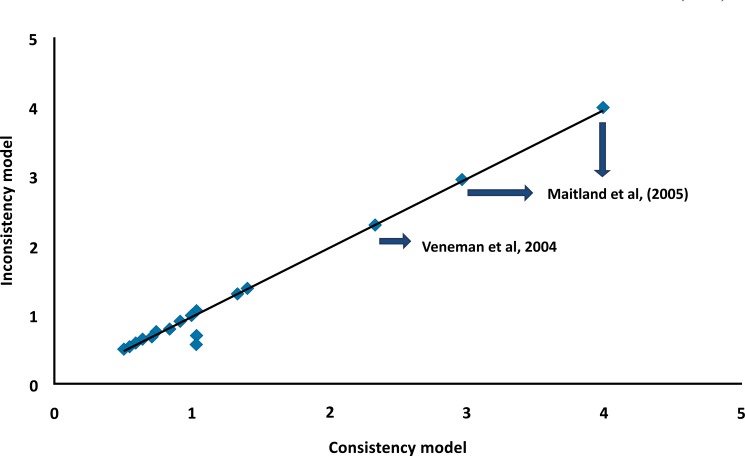
The assessment showed similar effect sizes and DICs for the consistency (DIC=180.9) and inconsistency (DIC = 182.9) models in case of the fixed effect analysis. As shown in Fig. (3), most of the posterior mean deviances of the individual data points lie around one on the line of equality suggesting consistency.
Fig. (3).
Plot of individual mortality data points’ posterior mean deviance contributions for the consistency model (horizontal axis) and the inconsistency model (vertical axis) along with the line of equality. Each data point is expected to have a posterior mean deviance contribution of about 1.0, with higher contributions suggesting a poorly fitting model [26]. Since Maitland et al., [43] and Veneman et al.,, [27] had data points much higher than 1.0, a sub-group analysis was conducted without the studies of Maitland et al.,, [13, 42, 43] and another analysis without Veneman et al., [27].
Baseline Results
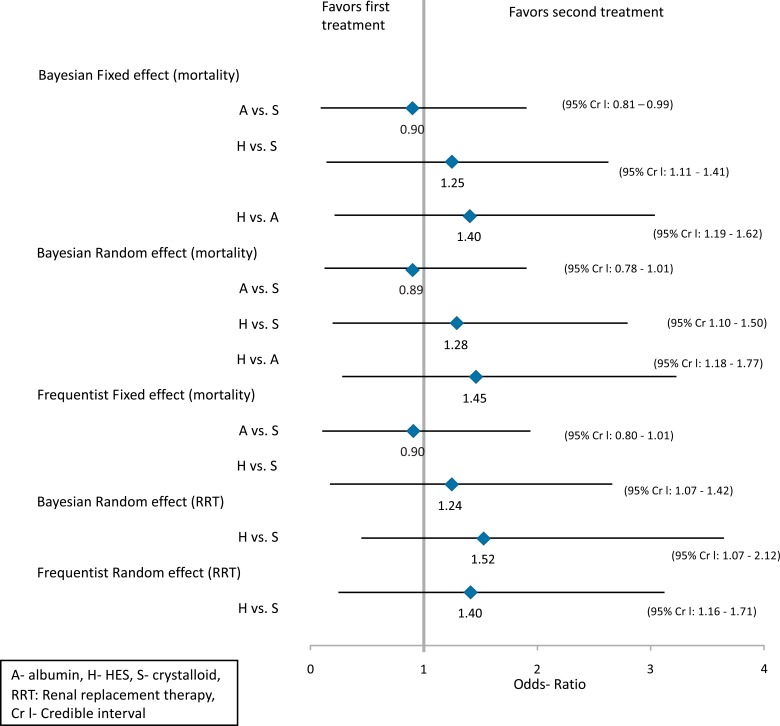
The pairwise ORs and their respective 95% credible intervals are presented in Fig. (4). The fixed effect network meta-analysis in the forest plot (Fig. 4) resulted in ORs of 0.90, 1.25 and 1.40 favoring albumin versus crystalloid, crystalloid versus HES and albumin versus HES, respectively. The random effects model showed similar results (0.89 for albumin vs crystalloid, 1.28 for HES vs crystalloid and 1.45 for HES vs albumin). The Frequentist pairwise fixed effect meta-analysis showed an OR of 0.90 favoring albumin versus crystalloid and 1.24 favoring crystalloid over HES. The baseline 95% credible intervals showed no significant statistical differences between the treatments.
Fig. (4).
Forest plot of results of Bayesian network meta-analysis of mortality and renal replacement therapy outcomes in severe sepsis and septic shock. 95% Cr I which does not include the null value, 0.00, indicates <5% probability that there is no difference between the two comparators [38].
Ranking of the Treatment
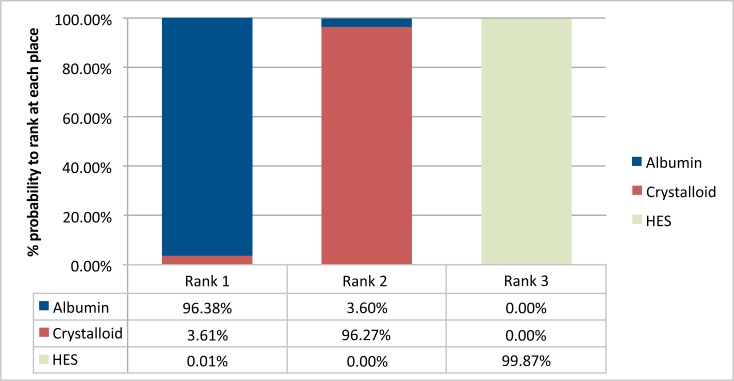
The Bayesian framework ranked the treatments and also assigned a probability to each rank that a treatment can achieve in terms of lowering the risk of mortality. Fig. (5) displays the share of these distributions under each rank. The higher the share in the distribution under a rank, the more likely the treatment will hold that rank. Albumin ranks first 96% of the times versus the other two treatments. The second place is shared in a majority by crystalloid and HES populates mostly the third place. Thus, according to the shares occupied by the treatments, albumin is the most effective treatment followed by crystalloid, and HES is the least effective. The odds-ratio (1.52) for RRT favored crystalloids in comparison to the HES in the random effects Bayesian analysis.
Fig. (5).
Barplots for the ranking probabilities of competing fluid treatments. Horizontal axis is the possible rank of each treatment (from best to worse). The size of each bar corresponds to the probability of each treatment to be at specific rank.
Sub-Group Results
Table 3 summarizes the results of sub-group analyses when excluding the trials of Maitland et al., [13, 42, 43], when follow up was for 28days compared to the base case (longest follow-up period), when elderly patients were studied and when different formulations of the colloid solutions were studied. The relative rankings in the survival benefit were not affected in any of the sub-groups (Table 3). In addition, the exclusion of the hitherto not fully published EARSS and ALBIOS trials also did not affect the key outcome.
Table 3.
Sub-Group Analyses
| Sub-Group | Odds- Ratio (95%Cr I) | ||
|---|---|---|---|
| A vs S | H vs S | H vs A | |
| Excluding trials (mortality) | |||
| All trials except Maitland et al. trials | 0.88 (0.77-1.01) | 1.25 (1.08-1.44) | 1.42 (1.16-1.72) |
| All trials except Veneman et al., trial | 0.88 (0.66-1.05) | 1.28 (1.03-1.60) | |
| All trials except ALBIOS and EARSS trials | 0.88 (0.76-1.01) | 1.25 (1.11-1.41) | 1.44 (1.19-1.71) |
| Follow-up time | |||
| Trials with 28-day* or in-hospital mortality | 0.90 (0.80-1.01) | 1.21(0.98-1.49) | 1.36 (1.06-1.71) |
| Trials with 28-day* or in-hospital mortality except Maitland et al., | 0.89 (0.77-1.01) | 1.21(0.98-1.48) | 1.38 (1.07-1.74) |
| Trials with 28-day* mortality | 0.85 (0.72-0.99) | 1.21 (1.01-1.43) | 1.44 (1.13-1.80) |
| Trials with in-hospital mortality | 1.12 (0.23-1.94) | ||
| Trials with 90 days of mortality | 1.29 (0.90-1.81) | ||
| Formulation | |||
| Trials with 130/0.4 HES on mortality | 1.37 (0.90-1.93) | ||
| Trials with 130/0.4 HES on RRT | 1.80 (0.73-2.35) | ||
| Trials with 20% albumin on mortality | 0.93 (0.81-1.06) | 2.17 (0.97-4.00) | 2.33 (1.05-4.30) |
| Trials with 4-6% albumin on mortality | 1.05 (0.21-1.99) | ||
| Population (mortality) | |||
| Trials with 60+ age population | 0.93 (0.79-1.09) | 1.25 (1.08-1.44) | 1.36 (1.08-1.68) |
A-albumin, S-crystalloid, H-hydroxyethyl starch, Cr I- Credible interval, RRT- Renal replacement therapy.
Includes both 28-day and 30-day mortality data.
DISCUSSION
The present study found a 96.4% probability that, of the fluid treatments assessed, albumin provides the highest survival benefit in patients with sepsis through lowering the risk of mortality. In this analysis, the longest follow-up mortality data in trials were included. The trials with longer observation periods found more cases of adverse effects such as renal toxicity in patients treated with HES [16, 17, 45]. This finding is augmented by the sub-group analysis in this study, which showed that there is higher risk of mortality in the long term for patients treated with HES.
The recent meta-analyses compared HES either with other colloids or crystalloids in critically ill patients. Gattas et al., [18] concluded that with HES there is a 6% increase in relative risk of death and a significant 25% increase in relative risk of being treated with RRT. Zarychanski et al., [20] also showed in their analysis that removing the studies by boldt, there was a clear survival benefit with other control fluids in critically ill patients. Patel et al., [47] conducted a meta-analysis on trials with severe septic patients treated with 6% HES (130/0.4 or 130/0.42). The control fluid showed a higher survival benefit in the 90-day follow up period. Haase et al., [19] found higher rate of RRT and blood transfusion in patients treated with HES 130/0.38-0.45 but no significant difference in risk of death. Delaney et al., [23] compared albumin with any control fluid and found albumin to be superior in reducing the risk of mortality.
The methodology of these routine meta-analyses does not allow the assessment of comparative effectiveness and safety of specific treatments in the absence of direct head to head trials. The present study applied network meta-analysis using a Bayesian approach to compare and rank the different fluid therapies available for severe sepsis so as to inform clinical decision making. This approach has been used to compare therapies in similar circumstances, in areas including pain management, diabetic neuropathy [48] and antifibrinolytic therapy in cardiac surgery [49], and has been shown to be not increase the bias relative to routine meta-analysis [50].
The inclusion of data from two recently reported large trials - ALBIOS and EARSS - which assessed the effect of albumin versus crystalloid in severe sepsis from the reported conference proceedings required caution as such data can lead to inconsistencies [51], and sensitivity analysis without this data was performed and confirmed the robustness of the analysis. Similarly, further sub-group analysis was performed in order to assess the effect of including the trials of Maitland et al., on children with malaria [13, 42, 43] which has a pathophysiology with many features in common with sepsis [44]. The sub-group analysis confirmed that the outcomes of the base case were not affected by the incision of these trials. Maitland et al., also demonstrated a survival benefit in children receiving albumin compared to gelofusine [52] but the present study was restricted to HES as this is the predominant synthetic colloid globally [9].
An underlying presumption in comparing outcomes in different clinical trials through techniques like meta-analysis is that different therapeutic preparations within each arm are biopharmaceutically equivalent. Hence, the different compositions of HES and albumin are assumed to have the same therapeutic effect when compared in meta-analyses such as that in the present study. Crystalloids also form a very broad category with differently formulated solutions. Any differences in formulation, manufacturing method etcetera between different fluids within the same broad class (crystalloids, albumin and HES) are assumed to not affect their therapeutic properties. Equivalent efficacy and safety profiles have been shown for albumin produced by different methods [53]. The most recent HES products have been claimed to be associated with fewer adverse events than earlier products [54] but the outcomes of meta-analysis [30, 55] and recent trials [16,17] have disputed this, and have led the US Food and Drug Administration (FDA) to conclude that in relation to these effects, all HES products behave as a class [40]. This has led the European Medicines Agency to conclude that the negative risk-benefit balance associated with these products justifies the removal of their marketing authorization [56]. Similarly, crystalloid solutions formulated to approximate physiological conditions have been claimed to be therapeutically superior to normal saline [57]. This has not been supported in a systematic review of mortality and morbidity in patients [58]. To test this assumption further, we performed the analysis with different formulations of albumin and HES and obtained similar results. Hence, we propose that the results of previous analyses, [30, 55] and the current study are not affected by any differences in the preparations within each treatment arm.
Similarly, we acknowledge that variability in the patient populations recruited in the individual trials may influence the results of this analysis, although the populations studied, consisting of severe sepsis and septic shock patients, were very similar in terms of age (all populations in the different fluid arms were in the age group 61 to 68) with the exception of the pediatric populations studied in the malaria trials of Maitland et al., We have assessed the effect of variability resulting from patient population, follow up time, product composition and patient age through sub-group analyses which demonstrated no effect on the final result of this study (Table 3), but it is possible that variability in these and other factors may influence the results, as with all meta-analyses.
Our sub-group analysis of trials with 28-day mortality showed a lower risk with HES than our analysis of trials with 90-day mortality outcomes. This suggests that more adverse outcomes from HES ensue after a prolonged period after use, as was shown in both the VISEP [45] and 6S [16] large clinical trials of different generation HES products. A high fraction of HES is taken up and deposited in tissues [59], where its long-term toxic effects on the kidney, liver, and bone marrow [60, 61] may explain the relative time frame of the mortality outcomes. There is a lower risk of mortality with HES and crystalloid in older patients in as assessed through the sub-group analysis in the present study but the relative ranking in survival benefit is not affected. This could be speculatively attributed to a higher incidence of other comorbidities in elderly patients, thus diminishing the difference between the efficacies of the fluid treatments.
All the trials with RRT outcomes had a 90-day follow up period. The indication was severe sepsis or septic shock for all included trials except the CHEST study [17] which assessed all the patients in the ICU setting. Sepsis was a sub-group in the CHEST trial, and the findings of the sub-group have not been published but have been reported (Myburgh J, Presentation at the International Symposium on Intensive Care and Emergency Medicine, Brussels March 2013). The need for RRT in all trials comparing albumin with other fluids did not show any increased risk with albumin, possibly due to albumin’s lack of renal toxicity [62, 63] compared to HES [64].
Direct data on mortality in fluid resuscitation with sepsis, comparing albumin, HES and crystalloids, are minimal and thus the present study may inform therapeutic choice. Moreover, network meta-analysis and indirect comparison is receiving increased acceptance in health care decision making (Pharmaceutical Benefits Advisory Committee in Australia, Canadian Agency for Drugs and Technologies in Health [CADTH], National Institute for Health and Clinical Excellence [NICE] in the UK) [29, 65, 66]. Even when direct evidence is available and conclusive, combining direct and indirect results may yield more refined and precise estimates of the interventions and broaden inference to the population sampled. Network meta-analysis links and maximizes existing information within the network of treatment comparisons [24, 25, 67]. We propose that such an approach should assist policy makers, manufacturers, physicians and patients, when making a choice between fluid-resuscitation treatments.
ACKNOWLEDGEMENT:
All the authors were involved in study design. Bansal M contributed to literature review, meta-analysis and manuscript preparation. Farrugia A contributed to literature review and manuscript preparation, Balboni S was involved in literature and manuscript review and Martin G reviewed the manuscript.
CONFLICT OF INTEREST
Bansal M, Farrugia A and Balboni S provide services to the plasma protein therapeutics industry, which includes the manufacturers of therapies described in this work.
PATIENT CONSENT
Declared none.
REFERENCES
- 1.Dellinger RP, Levy M, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(1):580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence. outome.and associated costs of care. Crit Care Med . 2001; 29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Dellinger RP. Cardiovascular management of septic shock. Crit Care Med. 2003;31(3):946–55. doi: 10.1097/01.CCM.0000057403.73299.A6. [DOI] [PubMed] [Google Scholar]
- 4.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 5.Linde-Zwirble WT, Angus DC. Severe sepsis epidemiology: sampling. selecion.and society. Crit Care. 2004; 8(4):222–6. doi: 10.1186/cc2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244–50. doi: 10.1097/01.CCM.0000261890.41311.E9. [DOI] [PubMed] [Google Scholar]
- 7.Healthcare Cost and Utilization Project (HCUP).HCUP Nationwide Inpatient Sample (NIS) [Internet]. Rockille MD.; Agency for Healthcare Research and Quality 2010. . Available from http://hcupnet.ahrq.gov. 2010 [Google Scholar]
- 8.Keith B. Albumin 2015 A Market Profile and Forecast of Albumin in the United States through the year 2015.USA. . The Marketing Research Bureau Inc Jun. 2011 [Google Scholar]
- 9.Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farrugia A. Albumin usage in clinical medicine: tradition or therapeutic?. Transf Med Rev. 2010;24(1):53–63. doi: 10.1016/j.tmrv.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Finfer S, Bellomo R, McEvoy S, et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006;18 333(7577):1044. doi: 10.1136/bmj.38985.398704.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quinlan GJ, Margarson MP, Mumby S, et al. Administration of albumin to patients with sepsis syndrome: A possible beneficial role in plasma thiol repletion. Clin Sci. 1998;95:459–65. [PubMed] [Google Scholar]
- 13.Maitland K, Kiguli S, Opoka RO, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364(26):2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 14.Mira JP. Facts or myths: Early albumin resuscitation during septic shock (the EARSS trial) [Internet]. Berlin [cited 2013 Jun 17]. Available from: http://www.esicm.org/flashConference/ 2011/Berlin/10438/swf/player.swf . 2011 [Google Scholar]
- 15.Guidet B, Martinet O, Boulain T, et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0. versus 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit Care. 2012;16 (R94):1–33. doi: 10.1186/cc11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perner A. 6S Trial Group and the Scandinavian Critical Care Trials Group.Hydroxyethyl Starch 130/0.4 versus Ringer's Acetate in Severe Sepsis. N Engl J Med. 2012;367(5):481. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 17.Myburgh JA, Finfer S, Bellomo R, et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N Engl J Med. 2012;367(20):1901–11. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 18.Gattas DJ, Dan A, Myburgh J, Billot L, Lo S, Finfer S. Fluid resuscitation with 6 % hydroxyethyl starch 130/0. and 130/0.42) in acutely ill patients: systematic review of effects on mortality and treatment with renal replacement therapy. Intensive Care Med. 2013;9(4):782–3. doi: 10.1007/s00134-013-2840-0. [DOI] [PubMed] [Google Scholar]
- 19.Haase N, Perner A, Hennings LI, et al. Hydroxyethyl starch 130/0.8-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. BMJ. 2013;346:f839. doi: 10.1136/bmj.f839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309(7):678–88. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 21.Perel P, Roberts I. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2012;6:CD000567. doi: 10.1002/14651858.CD000567.pub5. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Waheed U, Brett SJ. Randomised trials of 6 % tetrastarch (hydroxyethyl starch 130/0. or 0.42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Med. 2013;39(5):811–22. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 23.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: A systematic review and meta-analysis. Crit Care Med. 2011;39(2):386–91. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 24.Jansen JP, Fleurence R, Devine B, et al. Interpreting Indirect Treatment Comparisons and Network Meta-Analysis for Health-Care Decision Making: Report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: Part 1. Val in Health. 2011;14(4):417–28. doi: 10.1016/j.jval.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331(7521):897–900. doi: 10.1136/bmj.331.7521.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials. Tech Supp Doc Ser. 2011;(4):1–98. [PubMed] [Google Scholar]
- 27.Veneman TF, Nijhuis JO, Woittiez AJJ. Human albumin and starch administration in critically ill patients: a prospective randomized clinical trial. Wiener klinische Wochenschrift. 2004;116(9):305–9. doi: 10.1007/BF03040900. [DOI] [PubMed] [Google Scholar]
- 28.Rackow E, Falk J, Fein I. Fluid resuscitation in circulatory shock: A comparison of the cardiorespiratory effects of albumin hetastarch and saline solutions in patients with hypovolemic and septic shock. Crit Care Med. 1983;11:839–50. [PubMed] [Google Scholar]
- 29.Dias S, Welton N, Sutton A, Ades A. NICE DSU Technical Support Document 2: A Generalized Linear Modelling Framework for Pairwise and Network Meta-analysis of Randomised Controlled Trials [Internet]. National Institute for Health and Clinical Excellence (NICE) 2012 Apr. Available from: http://www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%20Mar2013.pdf . [PubMed] [Google Scholar]
- 30.Zarychanski R, Turgeon AF, Fergusson DA, et al. Renal outcomes and mortality following hydroxyethyl starch resuscitation of critically ill patients: systematic review and meta-analysis of randomized trials. Open Med. 2009;3(4):e196. [PMC free article] [PubMed] [Google Scholar]
- 31.Gattinoni L. Albumin in septic shock: The ALBIOS study Lisbon [cited 2013 Jun 19]. Available from: http://www.albumin-ppta.com/-sepsis-literature-database/304-albumin-administration-improves-survival-in-severe-sepsis-and-septic-shock-the-albios-study. 2012 [Google Scholar]
- 32.Juni P, Altman D, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ. 2001;(323):42–6. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andrew T. OpenBUGS 3 2 1 (Bayesian inference Using Gibbs Sampling). http://www.openbugs.info/w/ [Google Scholar]
- 34.Lu G, Ades AE. Assessing Evidence Inconsistency in Mixed Treatment Comparisons. J Am Stat Assoc. 2006;101(474):447–59. [Google Scholar]
- 35.Warn DE, Thompson SG, Spiegelhalter DJ. Bayesian random effects meta-analysis of trials with binary outcomes: methods for the absolute risk difference and relative risk scales. Stat Med. 2002;21(11):1601–23. doi: 10.1002/sim.1189. [DOI] [PubMed] [Google Scholar]
- 36.Rasmussen LS, Yentis SM, Van Aken H, et al. Editors-in-Chief statement regarding published clinical trials conducted without IRB approval by Joachim Boldt. Minerva Anestesiol. 2011;77(5):562–3. [PubMed] [Google Scholar]
- 37.Dias S, Sutton A, Welton N, Ades A. NICE DSU Technical Support Document 3: Heterogeneity: Subgroups Meta-Regression Bias and Bias-Adjustment. National Institute for Health and Clinical Excellence (NICE) Apr. http://www.nicedsu.org.uk. 2012 [PubMed] [Google Scholar]
- 38.Naci H, Brugts JJ, Fleurence R, Ades A. Dose-comparative effects of different statins on serum lipid levels: a network meta-analysis of 256 827 individuals in 181 randomized controlled trials. Eur J Prev Cardiol. 2013;20(4):658–70. doi: 10.1177/2047487313483600. [DOI] [PubMed] [Google Scholar]
- 39.Farrugia A. Safety of plasma volume expanders. J Clin Pharmacol. 2011;51(3):292–300. doi: 10.1177/0091270010372107. [DOI] [PubMed] [Google Scholar]
- 40.Food and Drug Administartion. Risks and Benefits of Hydroxyethyl Starch Solutions. Bethesda Maryland [cited 2013 Jan 29]. http://www.fda.gov/downloads/BiologicsBloodVacc ines/NewsEvents/WorkshopsMeetingsConferences/UCM321588.pdf . 2012 [Google Scholar]
- 41.Dolecek M, Svoboda P, Kantorova I. Therapeutic influence of 20% albumin versus 6% hydroxyethylstarch on extravascular lung water in septic patients: A randomized controlled trial. Hepato-gastroenterology. 2009;56:1622–8. [PubMed] [Google Scholar]
- 42.Maitland K, Pamba A, English M, et al. Pre-transfusion management of children with severe malarial anaemia: a random-ised controlled trial of intravascular volume expansion. Brit J Haematol. 2005;128(3):393–400. doi: 10.1111/j.1365-2141.2004.05312.x. [DOI] [PubMed] [Google Scholar]
- 43.Maitland K, Pamba A, English M, et al. Randomized trial of volume expansion with albumin or saline in children with severe malaria: preliminary evidence of albumin benefit. Clin Infect Dis. 2005;40(4):538–45. doi: 10.1086/427505. [DOI] [PubMed] [Google Scholar]
- 44.Maitland K, Marsh K. Pathophysiology of severe malaria in children. Acta Trop. 2004;90(2):131–40. doi: 10.1016/j.actatropica.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Brunkhorst FM, Engel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358(2):125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 46.McIntyre LA, Fergusson D, Cook D, Rankin N, Dhingra V, Granton J. Fluid resuscitation in the management of early septic shock (FINESS): a randomized controlled feasibility trial. Can J Anaesth. 2008;55(12):819–26. doi: 10.1007/BF03034053. [DOI] [PubMed] [Google Scholar]
- 47.Patel A, Waheed U, Brett SJ. Randomised trials of 6 % tetrastarch (hydroxyethyl starch 130/0. or 0.42) for severe sepsis reporting mortality: systematic review and meta-analysis. Intensive Care Medicine. 2013;39(5):811–22. doi: 10.1007/s00134-013-2863-6. [DOI] [PubMed] [Google Scholar]
- 48.Griebeler ML, Tsapas A, Brito JP, et al. Pharmacologic interventions for painful diabetic neuropathy: an umbrella systematic review and comparative effectiveness network meta-analysis (Protocol). Syst Rev. 2012;1:61. doi: 10.1186/2046-4053-1-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ. 2012;345:e5798. doi: 10.1136/bmj.e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salanti G. The use of network meta-analysis in Cochrane reviews: concerns and future directions [Internet] Auckland New Zealand. [cited 2013 Feb 28]. http://www.mtm.uoi.gr/PLE NARY.pdf. 2012 [Google Scholar]
- 51.Dundar Y, Dodd S, Williamson P, Dickson R, Walley T. Case study of the comparison of data from conference abstracts and full-text articles in health technology assessment of rapidly evolving technologies: does it make a difference?. Int J Technol Assess Health Care. 2006;22(3):288–94. doi: 10.1017/s0266462306051166. [DOI] [PubMed] [Google Scholar]
- 52.Akech S, Gwer S, Idro R, et al. Volume expansion with albumin compared to gelofusine in children with severe malaria: results of a controlled trial. PLoS Clin Trials. 2006;1(5):e21. doi: 10.1371/journal.pctr.0010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf M, Kronenberg H, Dodds A, et al. A safety study of Albumex 5 a human albumin solution produced by ion exchange chromatography. Vox Sang. 1996;70(4):198–202. doi: 10.1111/j.1423-0410.1996.tb01326.x. [DOI] [PubMed] [Google Scholar]
- 54.Westphal M, James M, Kozek-Langenecker S, Stocker R, Giuidet B, Aken H. Hydroxyethyl Starches. Different Products - Different Effects. Anesthesiology. 2009;111:187–202. doi: 10.1097/ALN.0b013e3181a7ec82. [DOI] [PubMed] [Google Scholar]
- 55.Dart AB, Mutter TC, Ruth CA, Taback SP. Hydroxyethyl starch (HES) versus other fluid therapies: effects on kidney function. The Cochrane Collaboration Dart AB editors Cochrane Database of Syst Rev Jan 20. [cited 2012 Jun 27] http://doi.wiley.com/10.1002/14651858.CD007594.pub2 . 2010 doi: 10.1002/14651858.CD007594.pub2. [DOI] [PubMed] [Google Scholar]
- 56.European Medicines Agency. PRAC recommends suspending marketing authorisations for infusion solutions containing hydroxy-ethyl-starch. [cited 2013 Jun 18]. http://www.ema.europa.eu/ ema/index.jsp?. curl=pages/medicines/human/referrals/Hydroxyethy l_starch-containing_solutions/human_referral_prac_000012. . 2013 [Google Scholar]
- 57.Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann's solution: a randomized double-blind crossover study. Clin Sci. 2003;104(1):17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 58.Burdett E, Dushianthan A, Bennett-Guerrero E, et al. Perioperative buffered versus non-buffered fluid administration for surgery in adults. Cochrane Database Syst Rev. 2012;12:CD004089. doi: 10.1002/14651858.CD004089.pub2. [DOI] [PubMed] [Google Scholar]
- 59.Bellmann R, Feistritzer C, Wiedermann CJ. Effect of molecular weight and substitution on tissue uptake of hydroxyethyl starch: a meta-analysis of clinical studies. Clin Pharmacokinet. 2012;51(4):225–36. doi: 10.2165/11594700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 60.Christidis C, Mal F, Ramos J, et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol. 2001;35(6):726–32. doi: 10.1016/s0168-8278(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 61.Schmidt-Hieber M, Loddenkemper C, Schwartz S, Arntz G, Thiel E, Notter M. Hydrops lysosomalis generalisatus--an underestimated side effect of hydroxyethyl starch therapy?. Eur J Haematol. 2006;77(1):83–5. doi: 10.1111/j.1600-0609.2006.00657.x. [DOI] [PubMed] [Google Scholar]
- 62.Baxter Healthcare Corporation A BUMINATE 5%. Albumin (Human) USP 5% Solution - Prescribing Information. Sep [cited 2012 De http /www.4legpharma.com/Product%20Inse> rt s/Baxter/Buminate5_9. 2009 [Google Scholar]
- 63.Baxter Healthcare Corporation BUMINATE 25% Albumin (Hu-man). USP.25% Solution - Prescribing Information. 2009 Sep [cited Dec 13]. http/www.homebybaxter.us/downloads/healt hcare_professionals/products/Buminate_25_PI.pdf . 2012 [Google Scholar]
- 64. US Food and Drug Administration. Approved Product Information for Voluven. [cited 2012 Aug 31]. http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/NewDrugApplicationsNDAs/UCM083138.pdf. 2012 [Google Scholar]
- 65. Pharmaceutical Benefits Advisory Committee.Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee Australian Government. Department of Health and Ageing Dec. 2008 [Google Scholar]
- 66.Wells G, Sultan S, Chen L, Khan M, Coyle D. Indirect Evidence: Indirect Treatment Comparisons in Meta-Analysis. Canadian Agency for Drugs and Technologies in Health . 2009 [Google Scholar]
- 67.Lu G, Ades A. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]