Abstract
Many of the causes of low back pain are still unknown; sufficient evidence indicates that both degenerative and mechanical change within the intervertebral disk (IVD) is a relevant factor. This article reviews intracellular signaling pathways related to pain receptors in the degenerated IVD. Several reports have demonstrated the number of nerve fibers in the IVD was increased in degenerated disks. In recent years, some groups have reported that an increase in nerve fibers is associated with the presence of inflammatory mediators and/or neurotrophins in the IVD. Cell signaling events, which are regulated by inflammatory mediators and neurotrophins, must be identified to clarify the mechanism underlying low back pain. Major intracellular signaling pathways (nuclear factor kappa β, mitogen-activated protein kinases, and Wnts) potentially play vital roles in mediating the molecular events responsible for the initiation and progression of IVD degeneration. These signaling pathways may represent therapeutic targets for the treatment of IVD degeneration and its associated back pain.
Keywords: nucleus pulposus, intervertebral disk degeneration, Wnt signal, low back pain, neurotrophic markers
Low back pain represents a significant social and economic burden, including direct medical costs, lost production, and disability benefits, and lowers the quality of life of many individuals.1,2 Low back pain is strongly associated with the intervertebral disk (IVD) degeneration, and IVD degeneration is also associated with sciatica and disk herniation or prolapse. It possibly adversely affects the behavior of other spinal structures such as muscles and ligaments. In the long term it can lead to spinal stenosis, a major cause of pain and disability in the elderly. Therefore, it follows that the suppression of IVD degeneration may limit the pain and disability associated with a range of pathologies of the back. Accordingly, clarification of the pathophysiology of IVD degeneration and its associated pain currently represents a major biomedical research priority. Much research is going on to understand IVD at a molecular level in hopes of creating clinically applicable options for treating IVD degeneration. Despite extensive study of the degenerative process in the IVD, the exact mechanism of diskogenic low back pain (IVD-related pain) has not been elucidated. In this review, we describe recent studies on diskogenic low back pain that have shed new light on the molecular mechanism and intracellular signaling pathways involved.
Disk Morphology
The IVD lies between the vertebral bodies, separated from them by the end plate and consisting of two main regions: an inner, soft and highly hydrated structure, the nucleus pulposus, and an outer, annulus fibrosus. Their major role is mechanical functions, as they constantly transmit loads arising from body weight and muscle activity through the spinal column. In addition, the IVD is the largest avascular tissue in the body, and the essential nutrients are supplied to the disk virtually entirely by diffusion.3 Some groups have reported that several intrinsic and extrinsic factors influence the cellular and molecular state of the IVD, including aging, genetics, transport of nutrients, and the mechanical environment.4,5,6,7 This makes it difficult to distinguish the vital cell type to target for therapeutic measures. Despite this complexity, recent studies have shown that there are considerable interspecies variations in the phenotypical characteristics of the cells. Although phenotypical similarities and characteristics exist between nucleus pulposus cells and articular chondrocytes, distinct differences have been identified.8,9 Nucleus pulposus cells of the IVD, which had generally been referred to as “chondrocyte-like” or “notochordal” cells, have been profiled and characterized in detail. The cells of the mature human nucleus pulposus are primarily chondrocyte-like cells, but in young individuals and in adults of some species, there is a second population of large cells with cytoplasmic inclusions. These are the “notochordal cells.” The notochordal cells express type IIA collagen, the differentially spliced form of type II collagen typical of prechondrocytes, rather than the alternate type IIB collagen, which is expressed by mature chondrocytes; they also express small proteoglycans mRNAs such as versican and decorin, more characteristic of fibroblast. These characteristics are of crucial importance for the success of regenerative and repair strategies, considering the structural and mechanical distinctions between IVD and cartilage tissues.
Effect of Degenerative Changes on Intervertebral Disk Cell Signaling
Major Intracellular Signaling Pathways for IVD Degeneration
With increasing age and degeneration, the most significant biochemical change to occur in IVD degeneration is loss of proteoglycans. These proteoglycans enable the nucleus pulposus to retain water, thereby cushioning and absorbing the considerable loads placed on the tissue. With increasing age and degeneration, the nucleus pulposus eventually becomes more fibrous tissue, and the peripheral anulus fibrosus is forced to carry larger loads, leading to tears, bulging, rupture, and herniation. In addition, loss of proteoglycans in degenerate disks influences the movement of molecules into and out of the IVD. Specifically, loss of aggrecan and type II collagen would allow increased penetration of large molecules such as growth factor complexes and cytokines into the IVD, affecting cellular behavior and possibly the progression of degeneration (Fig. 1). Therapeutic strategies for the biological treatment of IVD degeneration include the use of cellular components (mesenchymal stem cells, chondrocytes, and culture-expanded nucleus pulposus cells), matrix derivatives, molecules influencing disk-cell metabolism, and tissue-engineering strategies to restore function to the IVD degeneration.10,11,12,13,14
Fig. 1.
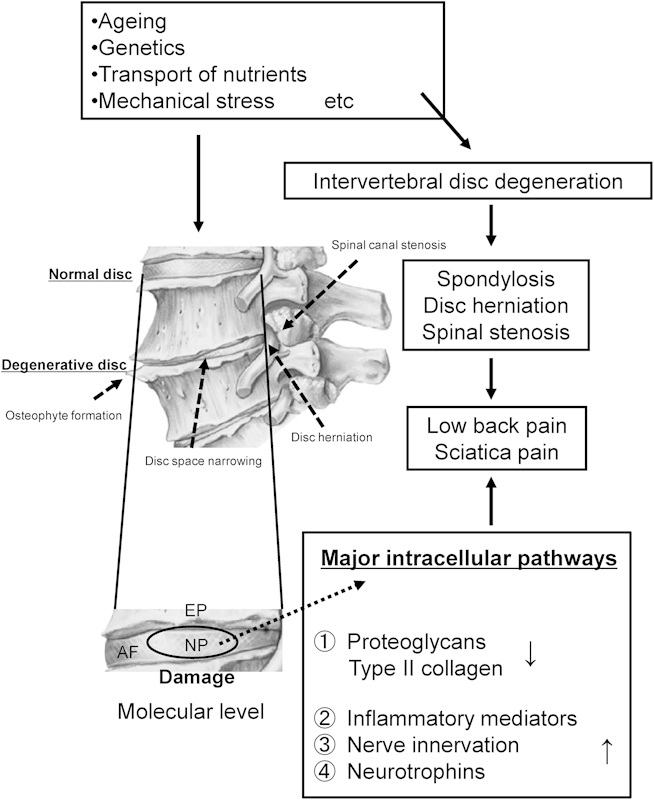
Proposed scheme of the degenerative changes on intervertebral disk cell. Abbreviations: AF, annulus fibrosus; EP, end plate; NP, nucleus pulposus.
Focusing on molecular therapy, some groups have reported that the biochemistry of IVD degeneration is commonly attributed to the dual effects of increased catabolic factors, such as matrix metalloproteinases (MMPs) and interleukin (IL)-1, and decreased anabolic factors such as transforming growth factors (TGFs) and bone morphogenetic proteins (BMPs). The balance between synthesis and breakdown of matrix macromolecules by the intracellular signaling pathways determines the quality and integrity of the matrix, and thus the mechanical behavior of the IVD.15,16,17,18 Under this paradigm, it follows that various cytokines and neurotrophic factors are secreted from the cells of the nucleus pulposus and annulus fibrosus, and these factors activate the nerve termini.19 Therefore, the gene expression and function of potential molecular mediators in IVD degeneration have been a major topic of research interest (Table 1).20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40 This review describes each major intracellular signaling that potentially plays vital roles in mediating the molecular events responsible for the initiation and progression of IVD degeneration. In a recent review, Wuertz et al suggested that major intracellular signaling factors, such as nuclear factor kappa β (NF-κβ) and mitogen-activated protein kinases (MAPKs) play vital roles in mediating the molecular events responsible for the initiation and progression of IVD degeneration.41 Indeed many genes, including several proinflammatory mediators, are regulated by NF-κβ in the IVD.42,43 For example, Wang et al demonstrated that the expression of ADAMTS4 (A Disintegrin And Metalloproteinase with Thrombospondin Motifs) and ADAMTS5, the two major aggrecanases, is controlled by NF-κβ-dependent mechanisms.44 Furthermore, stimulation by exogenous and endogenous proinflammatory mediators, such as IL-1 or tumor necrosis factor-α (TNF-α), can activate NF-κβ, in the cells of the IVD.45,46,47
Table 1. Reports of signaling pathways in IVD cells.
| Signaling pathway | Reference |
|---|---|
| Nuclear factor kappa β | Wang et al 201244 |
| Fujita et al 201225 | |
| Oh et al 201047 | |
| Yu et al 200946 | |
| Ohba et al 200945 | |
| Wako et al 200842 | |
| Hoylland et al 200843 | |
| Mitogen-activated protein kinase | Kim et al 201255 |
| Pratsinis et al 201231 | |
| Hiyama et al 201179 | |
| Xia et al 201156 | |
| Mavrogonatou and Kletsas 201032 | |
| Studer et al 200820 | |
| Uchiyama et al 200753 | |
| Tsai et al 200740 | |
| Pratsinis and Kletsas 200751 | |
| Séguin et al 200648 | |
| Risbud et al 2005, 200649,50 | |
| Protein kinase B | Pratsinis et al 201231 |
| Mavrogonatou and Kletsas 201032 | |
| Cheng et al 200933 | |
| Risbud et al 200549 | |
| Nerve growth factor | Gruber et al 201234 |
| Lee et al 201135 | |
| Richardson et al 200936 | |
| Yamauchi et al 200937 | |
| Abe et al 200793 | |
| Freemont et al 200292 | |
| Wnt | Smolder et al 201280 |
| Wang et al 201282 | |
| Ye et al 201129 | |
| Kondo et al 201130 | |
| Hiyama et al 2010, 201178,79 | |
| Fibroblast growth factor | Li et al 200859 |
| Tsai et al 200752 | |
| Peng et al 200660 | |
| Nagano et al 199558 | |
| Protein kinase C | Arai et al 201227 |
| Ellman et al 201228 | |
| Hypoxia-inducible factor | Gogate et al 201222 |
| Fujita et al 201223 | |
| Risbud et al 200624 | |
| Notch | Hiyama et al 201126 |
| Shh | Dahia et al 201238 |
| Choi and Harfe 201139 |
MAPK signaling research in the IVD has shown that the p38 and extracellular signal-regulated kinase (ERK) signaling pathways play a significant role in extracellular metabolism, because treatment with targeted kinase inhibitors significantly counteracted cytokine-induced changes in proteoglycan content, synthesis, and release.20,48 In addition, several signaling intermediates and factors of the MAPK pathway have been identified in IVD cells.49,50 Some groups have reported that TGF, nerve growth factor (NGF), platelet-derived growth factor, insulin-like growth factor-I, and basic fibroblast growth factor, all known to be overexpressed in degenerated disk tissue, can stimulate p38/ERK signaling in IVD of various species.51,52,53 Furthermore MMPs, inducible nitric oxide synthase, tissue inhibitor of metalloproteinases, aggrecan, type II collagen, Sox-9, acid-sensing ion channel, and tonicity-responsive enhancer-binding protein have all been identified as target genes of ERK in IVD cells.38,47,54,55,56 In addition, fibroblast growth factor-2 (FGF-2) has been identified as an anabolic mediator of disk homeostasis, an effect that may result from FGF-2-mediated stimulation of proteoglycan synthesis, such as seen in a canine IVD tissue culture system, or upregulation of cell proliferation such as seen in rat disks.57,58 In contrast, the findings of Li et al and Peng et al suggest that FGF-2 primarily has a catabolic role in disk homeostasis.59,60 Based on these apparently conflicting findings, it seems likely that FGF-2 plays multiple roles in disk homeostasis, and also that this may change depending on the stage of degeneration and the nature of the disease process.
Against the background of these intracellular signaling pathways, cell or growth factor therapies have recently been suggested to induce IVD repair in these signals and/or factors as target. However, one problem with the use of growth factors and/or cytokines in clinical trials is their high cost and safety. For these concern, Mwale et al have reported that Link N (DHLSDNYTLDHDRAIH) can stimulate the synthesis of aggrecan and collagen in the bovine IVD cells in vitro,61,62 as well as in the IVDs of rabbits in vivo.63 The changes in proteoglycan synthesis with Link N are similar to those reported previously with the same concentration of osteogenic protein-1 (OP-1). Link N agent can be treated with the low cost than OP-1 for the IVD degeneration. Link N therefore represents a potential economical therapeutic agent with beneficial effects. Their group has reported that Link N is also able to downregulate MMP expression in the degenerate IVDs, enhance the chondrogenic differentiation, and downregulate hypertrophic and osteogenic differentiation of human MSCs.64
Roll of Wnt Signaling in IVD Cells
In recent years, it has been also reported that Wnt signaling may play a crucial role of the cellular responses that lead to IVD inflammation and catabolism, much as they are involved in bone and cartilage metabolism. Wnts are secretory proteins of ∼40 kDa that control multiple aspects of development, including the proliferation, fate specification, polarity, and migration of cells.65,66 In addition, Chou et al reported that Wnt-1 and Wnt-3a both inhibit NGF-induced neurite outgrowth from PC12 cells.67 These results showed that sensory nerve receptors and neurotrophic markers cooperate with Wnt signals in regulating many biological processes, but the mechanisms of their interaction remain poorly defined in the IVD.
Wnt signaling can occur via the canonical Wnt/β-catenin-dependent pathway (hereafter called Wnt) or the noncanonical β-catenin-independent pathway, which itself can be divided into the planar cell polarity pathway and the Wnt/Ca2+ pathway.68 Among these, the canonical pathway is the most well characterized. Wnt proteins are glycoproteins that bind to the N-terminal extracellular cysteine-rich domain of the Frizzled (Fz) receptor family, of which there are 10 variants in humans. Fz is a seven-transmembrane-span protein with topological homology to G-protein-coupled receptors. In addition to the interaction between Wnt and Fz, co-receptors are also required for mediating the Wnt signaling. For example, the low-density-lipoprotein-related protein 6 is required to mediate Wnt signaling via the canonical pathway. On activation of this pathway, the cytoplasmic β-catenin is translocated to the nucleus, where it promotes binding of the transcription factor, T-cell factor/lymphocyte-enhancing factor, thereby accelerating the expression of target genes.69,70,71 It is well known that Wnt signaling plays a major role in bone metabolism. It has been shown that although the Wnt signaling suppresses differentiation of mesenchymal stem cells into chondrocytes and adipocytes, it accelerates their differentiation into osteoblasts and osteocytes. Following activation of the Wnt signaling, osteoprotegerin, which is a decoy for the receptor activator of NF-κβ ligand, is generated from osteoblasts, and ossification is accelerated as a result of inhibiting the activation of osteoclasts. Moreover, cross talk between Wnt signaling and other pathways that regulate ossification, such as the BMP pathway, is also known.72,73 In cartilage metabolism, it has been suggested that β-catenin is involved in the modulation of both anabolic and catabolic activities, suggesting that Wnt signaling also modulates inflammatory cytokine expression.74,75,76 Furthermore, it also appears that the activation of Wnt signaling may cause cellular senescence and thereby contribute to the process of IVD degeneration.77
Hiyama et al reported that rat nucleus pulposus cells undergo aging when treated with LiCl, consistent with the fact that LiCl is an activator of Wnt signaling that suppresses cell proliferation.78 Moreover, it has been reported that the activation of Wnt signaling in the IVD induces other pathways, including the TGF/BMP pathway, and that it is the cross talk between these pathways that ultimately controls the turnover of the extracellular matrix.79 Moreover, Smolders et al have reported that the activation of Wnt signaling occurs primarily in the notochordal cells during the initial stage of IVD degeneration.80 Similarly, Ukita et al have shown that Wnt signaling in the notochord progenitor cells (NPCs) is essential for posterior extension of the notochord in Not-Cre; β-cateninflox/flox embryos. In addition, they demonstrated both the expression of notochord-specific genes and the size of the notochord decline in the absence of Wnt signaling, and this is probably because Wnt signaling is essential for stabilization of the NPC phenotype.81 Wang et al also investigated the gene expression of catabolic factors with β-catenin conditional (cAct) mice and found that the expression of both MMP13 and ADAMTS-5 was increased in the IVDs of β-catenin knockouts, consistent with the observed IVD degeneration. Moreover, they found that IVD degeneration was suppressed when an inhibitor of MMP13 was administered to β-catenin cAct mice. From these results, it was concluded that β-catenin is a key factor responsible for the maintenance of the IVD tissue structure.82
To date, although there are a lot of studies of the intracellular signaling pathways in IVD, we should keep in mind that the final effect of the activation of signaling depends on their “signaling cross talk” with other activated signaling pathways. Therefore, it is necessary to investigate the importance of elucidating the cellular mechanisms of IVD degeneration and to build map of intracellular signaling pathways in IVD.
Pain and Innervations for the IVD
The normal IVD is considered to be a poorly innervated organ that is supplied only by sensory and sympathetic perivascular nerve fibers. Furthermore, there is widespread agreement that an important priority is to identify factors sensitizing the sensory nerves of the degenerated IVD. Regarding the neuropathology of IVDs studied in Japan, the analysis performed on surgical samples in 1970 by Shinohara was the first to describe a novel nerve terminal in the process of invading the annulus fibrosus.83 Subsequently, Yoshizawa et al and Bogduk et al searched for nerve cells inside degenerated IVDs and reported their presence in the outer layers of the annulus fibrosus.84,85 Moreover, Freemont et al examined nerve growth in degenerate IVDs associated with chronic low back pain.86 They collected 46 IVDs from 38 patients during spinal fusion and used standard immunohistochemical techniques to describe the abundance and location of a general nerve marker, a nociceptive neurotransmitter (substance-P), and a protein expressed during axonogenesis (growth-associated protein 43 [GAP43]). They reported that although nerve fibers were not present in the inner layer of the annulus fibrosus (or the nucleus pulposus) of normal human IVDs, they were present in the inner layer of the annulus fibrosus of degenerate IVDs.86 Subsequently, Roberts et al reported the presence of mechanoreceptors in the IVD and anterior longitudinal ligament.87 Burke et al have compared the levels of L-6, IL-8, and prostaglandin E2 in disk tissue from patients undergoing diskectomy for sciatica (n = 63) with that from patients undergoing fusion for diskogenic low back pain (n = 20). They suggested that the presence of inflammatory mediators IL-6 and IL-8 in the IVD might be involved in axonal ingrowth.88 Le Maitre et al demonstrated that herniated disks and degenerated disks from patients with chronic back pain showed a higher expression of IL-1β and TNF-α than nondegenerated disks derived from normal postmortem tissue.89 Weiler et al also demonstrated that surgical disk tissue from symptomatic back pain patients contained more TNF-α-positive cells than asymptomatic autopsy samples, with a positive correlation to the degree of IVD degeneration.90 These findings suggest that inflammation promotes the growth of afferent fibers in the disk and that this growth might be the cause of low back pain. To elucidate the cause of diskogenic low back pain, we also think that these points are very important.
Furthermore, the predominant nerve cells of the IVD have been characterized in recent years using activating transcription factor 3 (ATF3) as a marker of nerve injuries and GAP43 as a marker of axonal growth. In one study, Inoue et al characterized nerve cells in a rat model of IVD disease by creating a punch hole connecting the outside of the annulus fibrosus and the nucleus pulposus. They concluded that the procedure induced nerve injury and resulted in nerve ingrowth into the disks.91 A role for NGF in the ingrowth was indicated by subsequent studies showing enhanced levels in degenerated IVDs. Indeed, the study of neural and neurotrophic markers has been a very active area of IVD research in recent years.92,93,94,95,96 For example, neurotrophins have been shown to enhance the survival and differentiation of discrete populations of peripheral nerve neurons. The neurotrophins NGF and brain-derived neurotrophic factor (BDNF), which are associated with stimulation of axonal outgrowth and nociception by neuronal cells, are both expressed by nucleus pulposus cells, with BDNF levels increasing with disease severity.
Meanwhile, the pain transmitters in the sensory nerves dominating the IVDs have also been characterized, and it has been shown that neuropeptides such as substance-P and calcitonin gene-related peptide are expressed in the outer layer of the annulus fibrosus.97 In addition, sensory nerve receptors such as NGF receptors, tyrosine kinase A, p75 neurotrophin receptor, and transient receptor potential V1 are present in IVDs.98 Moreover, degenerative conditions have been shown to induce the expression of receptor ligands and could modify the perception of pain.
Regarding modulation of the afferent pathway of IVD-related low back pain, it is generally accepted that nerve excitation is enhanced by inflammation of the nerves and by mechanical stress. In terms of the specific afferent pathways involved, in 1993 Takahashi et al reported that the L5-6 IVD in the rat is innervated bilaterally from the L1 and L2 dorsal root ganglia through the paravertebral sympathetic trunk.99 The authors found that injection of capsaicin, a C-fiber stimulator, into the anterior portion of a lumbar IVD of rats pretreated intravenously with Evans blue caused dye extravasation of the pigment in the groin skin. Evans Blue is an azo dye which has a very high affinity for serum albumin. Vascular permeability was assessed by measuring Evans blue dye extravasation. These results suggested the presence of dichotomizing sensory C-fibers, which innervate both the IVDs and the groin skin in the L2 spinal nerve.99 Subsequently, to elucidate the afferent pathways of diskogenic low back pain in humans, Nakamura et al hypothesized that low back pain was transmitted mainly by sympathetic afferent fibers in the L2 nerve root, and selective local anesthesia of this nerve was employed in 33 patients. Low back pain disappeared or decreased significantly in all patients after the injection.100 Furthermore, to investigate the innervation of annulus fibrosus in the posterior region of the IVD, Ohtori et al used a 21-gauge needle with the tip filled with the nerve tracer, Fluoro-Gold (FG) crystals.15,101 The tracer was injected along the axis connecting a point 4 mm from the ventral surface of the disk to the most dorsal region, and it was found that sensory fibers from the upper dorsal root ganglions innervated the dorsal portion of the disks via the paravertebral sympathetic trunks. However, fibers from the lower dorsal root ganglions were found to be innervated via the sinuvertebral nerves, showing that the innervation of the dorsal portion of the disks was under the control of both the paravertebral sympathetic trunks and the sinuvertebral nerves.15,101 Moreover, Kurokawa et al employed double fluorescent labeling using two types of neurotracers, 1,1-dioctadecyl-3,3,3,3- tetramethylindocarbocyanine perchlorate and FG, in rat lumbar disks and reported that one sensory nerve was innervating lumbar disks at multiple levels.16
From these basic research results, it is believed that when IVD degeneration occurs, such as inflammation within the annulus fibrosus and/or herniation of the nucleus pulposus, nerves with mainly C-fibers germinate from the annulus fibrosus inward, thereby causing neurogenic inflammation and leading to aggravation of pain.
Accordingly, clarifying the pathophysiologic mechanism by which IVD degeneration occurs will be required for any improvement in the current treatments for diskogenic low back pain.
Summary
Low back pain is attributed to several medical disorders including lumbar herniation, scoliosis, and muscle and ligament damage, although most importantly, IVD degeneration is associated with up to 40% of individuals under 30 years of age.102
In recent years, attention has begun to focus on the cellular and molecular mechanism and signaling of IVD in the search for an understanding of the pathophysiology of low back pain (Fig. 2). In terms of molecular alterations, the balance between matrix anabolism and catabolism that exists in the nondegenerate IVD is shifted toward catabolism in degeneration, due to the production of matrix-degrading enzymes MMPs and ADAMTSs by MAPK and/or NF-κβ pathways, among others. The most significant molecular alteration is thought to be the upregulated expression of inflammatory cytokines and/or neurotrophins. In addition, it was demonstrated recently that Wnt signaling is a key pathway to regulate and maintain the IVD homeostasis. However, degeneration of the IVD is an incredibly complex disorder; moreover, many of the results implicating molecular signaling were obtained with cells and tissues from rodent models. Therefore, much research is needed to gain a better understanding of these signals and how their “cross talk” with other pathways will be likely to influence the outcome of treatments for IVD degeneration in vivo. Recent studies have demonstrated the presence of cell niches and progenitor cells in the IVD.103,104 Kim et al have reported that end plate chondrocytes of the IVD, like articular chondrocytes, are capable of migration and that soluble factors produced by notochordal cells stimulate the migration.105 The identification of progenitor cells within IVD indicates that natural repair mechanisms exist within the IVD and may be activated for regeneration, although the signal mechanisms and function of these cells need to be elucidated. Sakai et al also showed for the first time an experimental model of nucleus pulposus differentiation induced from functional progenitor cells in vivo. They suggested that Tie2 is a sensitive marker of aging and degeneration of IVDs and will be a useful marker for the diagnosis of IVD degeneration.106 Future studies are needed to examine whether progenitor cells can be used to inhibit nerve ingrowth or nociceptor formation in degenerate IVDs. Novel biological strategies will be more successful to treat and prevent disk degeneration and finally contribute to treatment of the low back pain if the specific disk cell phenotypes, functions, and molecular signaling are taken into consideration.
Fig. 2.
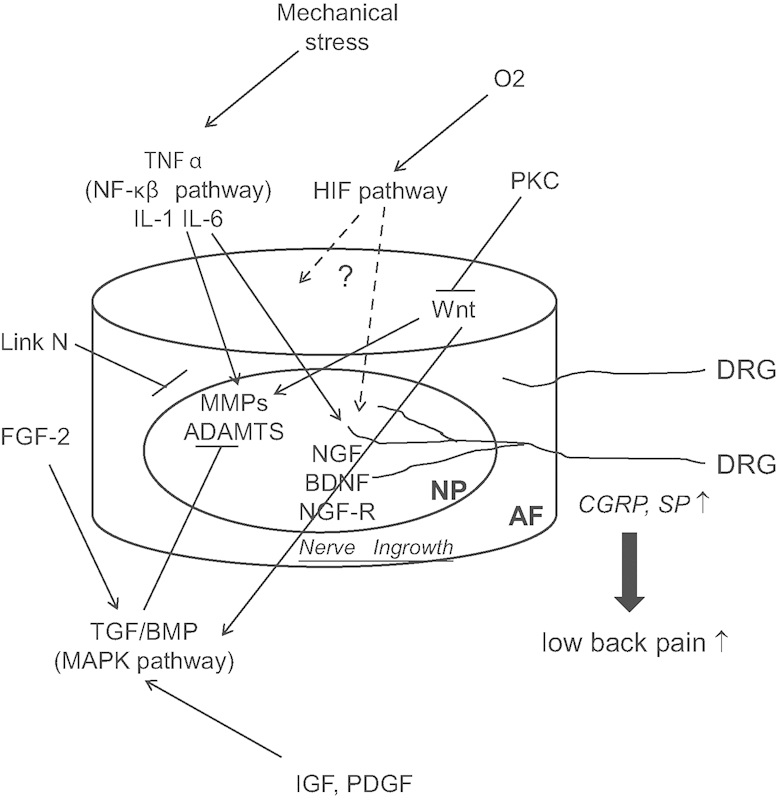
Signal pathway implicated in disk intervertebral disk degeneration. Abbreviations: ADAMTS, A Disintegrin And Metalloproteinase with Thrombospondin Motifs; AF, annulus fibrosus; BDNF, brain-derived neurotrophic factor; BMP, bone morphogenetic protein; CGRP, calcitonin gene-related peptide; DRG, dorsal root ganglion; FGF-2, fibroblast growth factor-2; HIF, hypoxia-inducible factor; IGF, MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; NF-κβ, nuclear factor kappa β; NGF, nerve growth factor; NP, nucleus pulposus; PDGF, platelet-derived growth factor; PKC, protein kinase C; SP, substance P; TGF, transforming growth factor; TNF α, tumor necrosis factor-α.
Footnotes
Disclosures Akihiko Hiyama, None Daisuke Sakai, None Joji Mochida, None
References
- 1.Rizzo J A, Abbott T A III, Berger M L. The labor productivity effects of chronic backache in the United States. Med Care. 1998;36:1471–1488. doi: 10.1097/00005650-199810000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Guo H R, Tanaka S, Cameron L L. et al. Back pain among workers in the United States: national estimates and workers at high risk. Am J Ind Med. 1995;28:591–602. doi: 10.1002/ajim.4700280504. [DOI] [PubMed] [Google Scholar]
- 3.Urban J P, Smith S, Fairbank J C. Nutrition of the intervertebral disc. Spine. 2004;29:2700–2709. doi: 10.1097/01.brs.0000146499.97948.52. [DOI] [PubMed] [Google Scholar]
- 4.Antoniou J, Steffen T, Nelson F. et al. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan D Song Y Sham P Cheung K M Genetics of disc degeneration Eur Spine J 20061503S317–S325., 98, 996-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grunhagen T, Wilde G, Soukane D M, Shirazi-Adl S A, Urban J P. Nutrient supply and intervertebral disc metabolism. J Bone Joint Surg Am. 2006;88 02:30–35. doi: 10.2106/JBJS.E.01290. [DOI] [PubMed] [Google Scholar]
- 7.Iatridis J C, MacLean J J, Roughley P J, Alini M. Effects of mechanical loading on intervertebral disc metabolism in vivo. J Bone Joint Surg Am. 2006;88 02:41–46. doi: 10.2106/JBJS.E.01407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai D, Nakai T, Mochida J, Alini M, Grad S. Differential phenotype of intervertebral disc cells: microarray and immunohistochemical analysis of canine nucleus pulposus and anulus fibrosus. Spine. 2009;34:1448–1456. doi: 10.1097/BRS.0b013e3181a55705. [DOI] [PubMed] [Google Scholar]
- 9.Rutges J, Creemers L B, Dhert W. et al. Variations in gene and protein expression in human nucleus pulposus in comparison with annulus fibrosus and cartilage cells: potential associations with aging and degeneration. Osteoarthritis Cartilage. 2010;18:416–423. doi: 10.1016/j.joca.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Richardson S M, Walker R V, Parker S. et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 11.Sakai D, Mochida J, Iwashina T. et al. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 12.Yoon S T, Patel N M. Molecular therapy of the intervertebral disc. Eur Spine J. 2006;15 03:S379–S388. doi: 10.1007/s00586-006-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson S M, Curran J M, Chen R. et al. The differentiation of bone marrow mesenchymal stem cells into chondrocyte-like cells on poly-L-lactic acid (PLLA) scaffolds. Biomaterials. 2006;27:4069–4078. doi: 10.1016/j.biomaterials.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Roughley P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, Alini M. The potential of chitosan-based gels containing intervertebral disc cells for nucleus pulposus supplementation. Biomaterials. 2006;27:388–396. doi: 10.1016/j.biomaterials.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 15.Ohtori S, Takahashi K, Chiba T, Yamagata M, Sameda H, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral discs in rats. Spine. 2001;26:946–950. doi: 10.1097/00007632-200104150-00020. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa M DRG neurons innervating three levels of lumbar intervertebral discs in rats Abstracts of 30th Annual Meeting of International Society for the Study of the Lumbar Spine; Vancouver, Canada; May 13-17, 2003
- 17.Cui Y, Yu J, Urban J P, Young D A. Differential gene expression profiling of metalloproteinases and their inhibitors: a comparison between bovine intervertebral disc nucleus pulposus cells and articular chondrocytes. Spine. 2010;35:1101–1108. doi: 10.1097/BRS.0b013e3181c0c727. [DOI] [PubMed] [Google Scholar]
- 18.Roberts S, Caterson B, Menage J, Evans E H, Jaffray D C, Eisenstein S M. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Weiler C, Nerlich A G, Zipperer J, Bachmeier B E, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Studer R K, Gilbertson L G, Georgescu H, Sowa G, Vo N, Kang J D. p38 MAPK inhibition modulates rabbit nucleus pulposus cell response to IL-1. J Orthop Res. 2008;26:991–998. doi: 10.1002/jor.20604. [DOI] [PubMed] [Google Scholar]
- 21.Koshi T, Ohtori S, Inoue G. et al. Lumbar posterolateral fusion inhibits sensory nerve ingrowth into punctured lumbar intervertebral discs and upregulation of CGRP immunoreactive DRG neuron innervating punctured discs in rats. Eur Spine J. 2010;19:593–600. doi: 10.1007/s00586-009-1237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogate S S, Fujita N, Skubutyte R, Shapiro I M, Risbud M V. Tonicity enhancer binding protein (TonEBP) and hypoxia-inducible factor (HIF) coordinate heat shock protein 70 (Hsp70) expression in hypoxic nucleus pulposus cells: role of Hsp70 in HIF-1α degradation. J Bone Miner Res. 2012;27:1106–1117. doi: 10.1002/jbmr.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita N, Chiba K, Shapiro I M, Risbud M V. HIF-1α and HIF-2α degradation is differentially regulated in nucleus pulposus cells of the intervertebral disc. J Bone Miner Res. 2012;27:401–412. doi: 10.1002/jbmr.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Risbud M V, Guttapalli A, Stokes D G. et al. Nucleus pulposus cells express HIF-1 alpha under normoxic culture conditions: a metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 25.Fujita N, Gogate S S, Chiba K, Toyama Y, Shapiro I M, Risbud M V. Prolyl hydroxylase 3 (PHD3) modulates catabolic effects of tumor necrosis factor-α (TNF-α) on cells of the nucleus pulposus through co-activation of nuclear factor κB (NF-κB)/p65 signaling. J Biol Chem. 2012;287:39942–39953. doi: 10.1074/jbc.M112.375964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hiyama A, Skubutyte R, Markova D. et al. Hypoxia activates the notch signaling pathway in cells of the intervertebral disc: implications in degenerative disc disease. Arthritis Rheum. 2011;63:1355–1364. doi: 10.1002/art.30246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arai F, Hiyama A, Sakai D, Yokoyama K, Mochida J. The expression and role of non-canonical (PKC) signaling in nucleus pulposus cell metabolism. J Orthop Res. 2012;30:1478–1485. doi: 10.1002/jor.22095. [DOI] [PubMed] [Google Scholar]
- 28.Ellman M B, Kim J S, An H S. et al. The pathophysiologic role of the protein kinase Cδ pathway in the intervertebral discs of rabbits and mice: in vitro, ex vivo, and in vivo studies. Arthritis Rheum. 2012;64:1950–1959. doi: 10.1002/art.34337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ye S, Wang J, Yang S. et al. Specific inhibitory protein Dkk-1 blocking Wnt/β-catenin signaling pathway improve protectives effect on the extracellular matrix. J Huazhong Univ Sci Technolog Med Sci. 2011;31:657–662. doi: 10.1007/s11596-011-0577-y. [DOI] [PubMed] [Google Scholar]
- 30.Kondo N, Yuasa T, Shimono K. et al. Intervertebral disc development is regulated by Wnt/β-catenin signaling. Spine. 2011;36:E513–E518. doi: 10.1097/BRS.0b013e3181f52cb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratsinis H, Constantinou V, Pavlakis K, Sapkas G, Kletsas D. Exogenous and autocrine growth factors stimulate human intervertebral disc cell proliferation via the ERK and Akt pathways. J Orthop Res. 2012;30:958–964. doi: 10.1002/jor.22017. [DOI] [PubMed] [Google Scholar]
- 32.Mavrogonatou E, Kletsas D. Effect of varying osmotic conditions on the response of bovine nucleus pulposus cells to growth factors and the activation of the ERK and Akt pathways. J Orthop Res. 2010;28:1276–1282. doi: 10.1002/jor.21140. [DOI] [PubMed] [Google Scholar]
- 33.Cheng C C, Uchiyama Y, Hiyama A, Gajghate S, Shapiro I M, Risbud M V. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc. J Cell Physiol. 2009;221:668–676. doi: 10.1002/jcp.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gruber H E, Hoelscher G L, Bethea S, Hanley E N Jr. Interleukin 1-beta upregulates brain-derived neurotrophic factor, neurotrophin 3 and neuropilin 2 gene expression and NGF production in annulus cells. Biotech Histochem. 2012;87:506–511. doi: 10.3109/10520295.2012.703692. [DOI] [PubMed] [Google Scholar]
- 35.Lee J M, Song J Y, Baek M. et al. Interleukin-1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29:265–269. doi: 10.1002/jor.21210. [DOI] [PubMed] [Google Scholar]
- 36.Richardson S M, Doyle P, Minogue B M, Gnanalingham K, Hoyland J A. Increased expression of matrix metalloproteinase-10, nerve growth factor and substance P in the painful degenerate intervertebral disc. Arthritis Res Ther. 2009;11:R126. doi: 10.1186/ar2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamauchi K, Inoue G, Koshi T. et al. Nerve growth factor of cultured medium extracted from human degenerative nucleus pulposus promotes sensory nerve growth and induces substance p in vitro. Spine. 2009;34:2263–2269. doi: 10.1097/BRS.0b013e3181a5521d. [DOI] [PubMed] [Google Scholar]
- 38.Dahia C L, Mahoney E, Wylie C. Shh signaling from the nucleus pulposus is required for the postnatal growth and differentiation of the mouse intervertebral disc. PLoS ONE. 2012;7:e35944. doi: 10.1371/journal.pone.0035944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi K S, Harfe B D. Hedgehog signaling is required for formation of the notochord sheath and patterning of nuclei pulposi within the intervertebral discs. Proc Natl Acad Sci U S A. 2011;108:9484–9489. doi: 10.1073/pnas.1007566108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai T T, Guttapalli A, Agrawal A, Albert T J, Shapiro I M, Risbud M V. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22:965–974. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- 41.Wuertz K Vo N Kletsas D Boos N Inflammatory and catabolic signalling in intervertebral discs: the roles of NF-κB and MAP kinases Eur Cell Mater 201223103–119., discussion 119-120 [DOI] [PubMed] [Google Scholar]
- 42.Wako M, Ohba T, Ando T. et al. Mechanism of signal transduction in tumor necrosis factor-like weak inducer of apoptosis-induced matrix degradation by MMP-3 upregulation in disc tissues. Spine. 2008;33:2489–2494. doi: 10.1097/BRS.0b013e318186b343. [DOI] [PubMed] [Google Scholar]
- 43.Hoyland J A, Le Maitre C, Freemont A J. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008;47:809–814. doi: 10.1093/rheumatology/ken056. [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Markova D, Anderson D G. et al. TNF-α and IL-1β promote a disintegrin-like and metalloprotease with thrombospondin type I motif-5-mediated aggrecan degradation through syndecan-4 in intervertebral disc. J Biol Chem. 2011;286:39738–39749. doi: 10.1074/jbc.M111.264549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohba T, Haro H, Ando T. et al. TNF-alpha-induced NF-kappaB signaling reverses age-related declines in VEGF induction and angiogenic activity in intervertebral disc tissues. J Orthop Res. 2009;27:229–235. doi: 10.1002/jor.20727. [DOI] [PubMed] [Google Scholar]
- 46.Yu Z G, Xu N, Wang W B, Pan S H, Li K S, Liu J K. Interleukin-1 inhibits Sox9 and collagen type II expression via nuclear factor-kappaB in the cultured human intervertebral disc cells. Chin Med J (Engl) 2009;122:2483–2488. [PubMed] [Google Scholar]
- 47.Oh I S, Park S E, Son J M. et al. Glucocorticoid mechanism of inhibition of the inflammatory cells in lumbar intervertebral disc cells stimulated by TNF-alpha production of nuclear factor-kappaB. J Korean Orthop Res Soc. 2010;13:80–87. [Google Scholar]
- 48.Séguin C A, Bojarski M, Pilliar R M, Roughley P J, Kandel R A. Differential regulation of matrix degrading enzymes in a TNFalpha-induced model of nucleus pulposus tissue degeneration. Matrix Biol. 2006;25:409–418. doi: 10.1016/j.matbio.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 49.Risbud M V, Fertala J, Vresilovic E J, Albert T J, Shapiro I M. Nucleus pulposus cells upregulate PI3K/Akt and MEK/ERK signaling pathways under hypoxic conditions and resist apoptosis induced by serum withdrawal. Spine. 2005;30:882–889. doi: 10.1097/01.brs.0000159096.11248.6d. [DOI] [PubMed] [Google Scholar]
- 50.Risbud M V, Guttapalli A, Albert T J, Shapiro I M. Hypoxia activates MAPK activity in rat nucleus pulposus cells: regulation of integrin expression and cell survival. Spine. 2005;30:2503–2509. doi: 10.1097/01.brs.0000186326.82747.13. [DOI] [PubMed] [Google Scholar]
- 51.Pratsinis H, Kletsas D. PDGF, bFGF and IGF-I stimulate the proliferation of intervertebral disc cells in vitro via the activation of the ERK and Akt signaling pathways. Eur Spine J. 2007;16:1858–1866. doi: 10.1007/s00586-007-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai T T, Guttapalli A, Oguz E. et al. Fibroblast growth factor-2 maintains the differentiation potential of nucleus pulposus cells in vitro: implications for cell-based transplantation therapy. Spine. 2007;32:495–502. doi: 10.1097/01.brs.0000257341.88880.f1. [DOI] [PubMed] [Google Scholar]
- 53.Uchiyama Y, Cheng C C, Danielson K G. et al. Expression of acid-sensing ion channel 3 (ASIC3) in nucleus pulposus cells of the intervertebral disc is regulated by p75NTR and ERK signaling. J Bone Miner Res. 2007;22:1996–2006. doi: 10.1359/jbmr.070805. [DOI] [PubMed] [Google Scholar]
- 54.Risbud M V, Di Martino A, Guttapalli A. et al. Toward an optimum system for intervertebral disc organ culture: TGF-beta 3 enhances nucleus pulposus and anulus fibrosus survival and function through modulation of TGF-beta-R expression and ERK signaling. Spine. 2006;31:884–890. doi: 10.1097/01.brs.0000209335.57767.b5. [DOI] [PubMed] [Google Scholar]
- 55.Kim J S, Ellman M B, An H S. et al. Lactoferricin mediates anabolic and anti-catabolic effects in the intervertebral disc. J Cell Physiol. 2012;227:1512–1520. doi: 10.1002/jcp.22867. [DOI] [PubMed] [Google Scholar]
- 56.Xia M, Zhu Y. Fibronectin fragment activation of ERK increasing integrin α5 and β1 subunit expression to degenerate nucleus pulposus cells. J Orthop Res. 2011;29:556–561. doi: 10.1002/jor.21273. [DOI] [PubMed] [Google Scholar]
- 57.Thompson J P, Oegema T R Jr, Bradford D S. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 58.Nagano T, Yonenobu K, Miyamoto S, Tohyama M, Ono K. Distribution of the basic fibroblast growth factor and its receptor gene expression in normal and degenerated rat intervertebral discs. Spine. 1995;20:1972–1978. doi: 10.1097/00007632-199509150-00002. [DOI] [PubMed] [Google Scholar]
- 59.Li X, An H S, Ellman M. et al. Action of fibroblast growth factor-2 on the intervertebral disc. Arthritis Res Ther. 2008;10:R48. doi: 10.1186/ar2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peng B, Hao J, Hou S. et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine. 2006;31:560–566. doi: 10.1097/01.brs.0000201324.45537.46. [DOI] [PubMed] [Google Scholar]
- 61.Mwale F, Demers C N, Petit A. et al. A synthetic peptide of link protein stimulates the biosynthesis of collagens II, IX and proteoglycan by cells of the intervertebral disc. J Cell Biochem. 2003;88:1202–1213. doi: 10.1002/jcb.10479. [DOI] [PubMed] [Google Scholar]
- 62.Petit A, Yao G, Rowas S A. et al. Effect of synthetic link N peptide on the expression of type I and type II collagens in human intervertebral disc cells. Tissue Eng Part A. 2011;17:899–904. doi: 10.1089/ten.TEA.2010.0494. [DOI] [PubMed] [Google Scholar]
- 63.Mwale F, Masuda K, Pichika R. et al. The efficacy of Link N as a mediator of repair in a rabbit model of intervertebral disc degeneration. Arthritis Res Ther. 2011;13:R120. doi: 10.1186/ar3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Antoniou J, Wang H T, Alaseem A M, Haglund L, Roughley P J, Mwale F. The effect of Link N on differentiation of human bone marrow-derived mesenchymal stem cells. Arthritis Res Ther. 2012;14:R267. doi: 10.1186/ar4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Logan C Y, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 66.Cadigan K M, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 67.Chou A H, Howard B D. Inhibition by Wnt-1 or Wnt-3a of nerve growth factor-induced differentiation of PC12 cells is reversed by bisindolylmaleimide-I but not by several other PKC inhibitors. Oncogene. 2002;21:6348–6355. doi: 10.1038/sj.onc.1205791. [DOI] [PubMed] [Google Scholar]
- 68.Kikuchi A, Kishida S, Yamamoto H. Regulation of Wnt signaling by protein-protein interaction and post-translational modifications. Exp Mol Med. 2006;38:1–10. doi: 10.1038/emm.2006.1. [DOI] [PubMed] [Google Scholar]
- 69.Behrens J, von Kries J P, Kühl M. et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- 70.Behrens J, Jerchow B A, Würtele M. et al. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science. 1998;280:596–599. doi: 10.1126/science.280.5363.596. [DOI] [PubMed] [Google Scholar]
- 71.Korinek V, Barker N, Morin P J. et al. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC-/- colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- 72.Harada S, Rodan G A. Control of osteoblast function and regulation of bone mass. Nature. 2003;423:349–355. doi: 10.1038/nature01660. [DOI] [PubMed] [Google Scholar]
- 73.Bodine P V, Zhao W, Kharode Y P. et al. The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Mol Endocrinol. 2004;18:1222–1237. doi: 10.1210/me.2003-0498. [DOI] [PubMed] [Google Scholar]
- 74.Loughlin J, Dowling B, Chapman K. et al. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc Natl Acad Sci U S A. 2004;101:9757–9762. doi: 10.1073/pnas.0403456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu Q, Zhu M, Rosier R N, Zuscik M J, O'Keefe R J, Chen D. Beta-catenin, cartilage, and osteoarthritis. Ann N Y Acad Sci. 2010;1192:344–350. doi: 10.1111/j.1749-6632.2009.05212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu M, Tang D, Wu Q. et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu H, Fergusson M M, Castilho R M. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 78.Hiyama A, Sakai D, Risbud M V. et al. Enhancement of intervertebral disc cell senescence by WNT/β-catenin signaling-induced matrix metalloproteinase expression. Arthritis Rheum. 2010;62:3036–3047. doi: 10.1002/art.27599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hiyama A, Sakai D, Tanaka M. et al. The relationship between the Wnt/β-catenin and TGF-β/BMP signals in the intervertebral disc cell. J Cell Physiol. 2011;226:1139–1148. doi: 10.1002/jcp.22438. [DOI] [PubMed] [Google Scholar]
- 80.Smolders L A, Meij B P, Riemers F M. et al. Canonical Wnt signaling in the notochordal cell is upregulated in early intervertebral disk degeneration. J Orthop Res. 2012;30:950–957. doi: 10.1002/jor.22000. [DOI] [PubMed] [Google Scholar]
- 81.Ukita K, Hirahara S, Oshima N. et al. Wnt signaling maintains the notochord fate for progenitor cells and supports the posterior extension of the notochord. Mech Dev. 2009;126:791–803. doi: 10.1016/j.mod.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang M, Tang D, Shu B. et al. Conditional activation of β-catenin signaling in mice leads to severe defects in intervertebral disc tissue. Arthritis Rheum. 2012;64:2611–2623. doi: 10.1002/art.34469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shinohara H. A study on lumbar disc lesion. Significance of histology of free nerve endings in lumbar discs. J Jpn Orthop Assoc. 1970;44:553–570. [PubMed] [Google Scholar]
- 84.Yoshizawa H, O'Brien J P, Smith W T, Trumper M. The neuropathology of intervertebral discs removed for low-back pain. J Pathol. 1980;132:95–104. doi: 10.1002/path.1711320202. [DOI] [PubMed] [Google Scholar]
- 85.Bogduk N, Tynan W, Wilson A S. The nerve supply to the human lumbar intervertebral discs. J Anat. 1981;132(Pt 1):39–56. [PMC free article] [PubMed] [Google Scholar]
- 86.Freemont A J, Peacock T E, Goupille P, Hoyland J A, O'Brien J, Jayson M I. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350:178–181. doi: 10.1016/s0140-6736(97)02135-1. [DOI] [PubMed] [Google Scholar]
- 87.Roberts S, Eisenstein S M, Menage J, Evans E H, Ashton I K. Mechanoreceptors in intervertebral discs. Morphology, distribution, and neuropeptides. Spine. 1995;20:2645–2651. doi: 10.1097/00007632-199512150-00005. [DOI] [PubMed] [Google Scholar]
- 88.Burke J G, Watson R W, McCormack D, Dowling F E, Walsh M G, Fitzpatrick J M. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 89.Le Maitre C L, Hoyland J A, Freemont A J. Catabolic cytokine expression in degenerate and herniated human intervertebral discs: IL-1beta and TNFalpha expression profile. Arthritis Res Ther. 2007;9:R77. doi: 10.1186/ar2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiler C Nerlich A G Bachmeier B E Boos N Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls Spine 20053044–53., discussion 54 [DOI] [PubMed] [Google Scholar]
- 91.Inoue G, Ohtori S, Aoki Y. et al. Exposure of the nucleus pulposus to the outside of the anulus fibrosus induces nerve injury and regeneration of the afferent fibers innervating the lumbar intervertebral discs in rats. Spine. 2006;31:1433–1438. doi: 10.1097/01.brs.0000219946.25103.db. [DOI] [PubMed] [Google Scholar]
- 92.Freemont A J, Watkins A, Le Maitre C. et al. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 93.Abe Y, Akeda K, An H S. et al. Proinflammatory cytokines stimulate the expression of nerve growth factor by human intervertebral disc cells. Spine. 2007;32:635–642. doi: 10.1097/01.brs.0000257556.90850.53. [DOI] [PubMed] [Google Scholar]
- 94.Navone S E, Marfia G, Canzi L. et al. Expression of neural and neurotrophic markers in nucleus pulposus cells isolated from degenerated intervertebral disc. J Orthop Res. 2012;30:1470–1477. doi: 10.1002/jor.22098. [DOI] [PubMed] [Google Scholar]
- 95.Gruber H E, Ingram J A, Hoelscher G, Zinchenko N, Norton H J, Hanley E N Jr. Brain-derived neurotrophic factor and its receptor in the human and the sand rat intervertebral disc. Arthritis Res Ther. 2008;10:R82. doi: 10.1186/ar2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Orita S, Ohtori S, Nagata M. et al. Inhibiting nerve growth factor or its receptors downregulates calcitonin gene-related peptide expression in rat lumbar dorsal root ganglia innervating injured intervertebral discs. J Orthop Res. 2010;28:1614–1620. doi: 10.1002/jor.21170. [DOI] [PubMed] [Google Scholar]
- 97.Suseki K, Takahashi Y, Takahashi K, Chiba T, Yamagata M, Moriya H. Sensory nerve fibres from lumbar intervertebral discs pass through rami communicantes. A possible pathway for discogenic low back pain. J Bone Joint Surg Br. 1998;80:737–742. doi: 10.1302/0301-620x.80b4.8239. [DOI] [PubMed] [Google Scholar]
- 98.Sugiura A, Ohtori S, Yamashita M. et al. Existence of nerve growth factor receptors, tyrosine kinase a and p75 neurotrophin receptors in intervertebral discs and on dorsal root ganglion neurons innervating intervertebral discs in rats. Spine. 2008;33:2047–2051. doi: 10.1097/BRS.0b013e31817f8d58. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi Y, Nakajima Y, Sakamoto T, Moriya H, Takahashi K. Capsaicin applied to rat lumbar intervertebral disc causes extravasation in the groin skin: a possible mechanism of referred pain of the intervertebral disc. Neurosci Lett. 1993;161:1–3. doi: 10.1016/0304-3940(93)90125-5. [DOI] [PubMed] [Google Scholar]
- 100.Nakamura S I, Takahashi K, Takahashi Y, Yamagata M, Moriya H. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606–612. [PubMed] [Google Scholar]
- 101.Ohtori S, Takahashi Y, Takahashi K. et al. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295–2299. doi: 10.1097/00007632-199911150-00002. [DOI] [PubMed] [Google Scholar]
- 102.Cheung K M, Karppinen J, Chan D. et al. Prevalence and pattern of lumbar magnetic resonance imaging changes in a population study of one thousand 7forty-three individuals. Spine. 2009;34:934–940. doi: 10.1097/BRS.0b013e3181a01b3f. [DOI] [PubMed] [Google Scholar]
- 103.Risbud M V, Guttapalli A, Tsai T T. et al. Evidence for skeletal progenitor cells in the degenerate human intervertebral disc. Spine. 2007;32:2537–2544. doi: 10.1097/BRS.0b013e318158dea6. [DOI] [PubMed] [Google Scholar]
- 104.Henriksson H, Thornemo M, Karlsson C. et al. Identification of cell proliferation zones, progenitor cells and a potential stem cell niche in the intervertebral disc region: a study in four species. Spine. 2009;34:2278–2287. doi: 10.1097/BRS.0b013e3181a95ad2. [DOI] [PubMed] [Google Scholar]
- 105.Kim K W, Ha K Y, Lee J S. et al. Notochordal cells stimulate migration of cartilage end plate chondrocytes of the intervertebral disc in in vitro cell migration assays. Spine J. 2009;9:323–329. doi: 10.1016/j.spinee.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Sakai D, Nakamura Y, Nakai T. et al. Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 2012;3:1264. doi: 10.1038/ncomms2226. [DOI] [PMC free article] [PubMed] [Google Scholar]


