Abstract
Some natural and synthetic protease inhibitors (PI), such as the Bowman-Birk PI from soybean, have anticancer effects. We previously purified and characterized a Bowman-Birk-type PI from Tepary bean (Phaseolus acutifolius) seeds (TBPI). A semipure protein fraction containing this inhibitor, when tested its in vitro effect on transformed cells, showed a differential cytotoxic effect, as well as an increase in cell attachment to culture dishes. In this article we report that lectins were responsible for the cytotoxic effect previously observed, exhibiting a differential, antiproliferative effect on nontransformed cells and on different lineages of cancer cells. Although the purified TBPI lacked cytotoxicity, it was found to be responsible for the increase in cell adhesion, decreasing culture dishes’ extracellular matrix degradation, leading to a decrease of the in vitro cell invasion capacity. This effect coincided with the suppression of Matrix Metalloproteinase-9 activity. These results indicate that Tepary bean seeds contain at least 2 different groups of bioactive proteins with distinct effects on cancer cells.
INTRODUCTION
Cancer is a complex process in which changes in proliferation rate, adhesion patterns, and migration play important roles in tumorogenesis and metastasis. Proteolysis of extracellular matrix (ECM) and cell surface proteins results in crucial changes in cell–cell and cell–ECM interactions. New signals generated from the cell surface can affect gene expression and ultimately influence critical cell behavior. Transformed cells lose their strong intercellular adhesive properties and new cell–ECM interactions are produced. As a consequence, several cell surface-associated adhesion molecules change their function and are the source of signals that promote growth, motility, ECM degradation, and metastasis (1).
Natural protease inhibitors (PIs) are proteins with an interesting potential against cancer development and dissemination, mainly because of their high selectivity to inhibit specific enzymes. Soybean Bowman-Birk protease inhibitor (SBBI) has been the most studied natural PI. Its evaluation at nanogram levels to cell cultures on in vitro and in vivo assays has indicated no secondary effects, showing a potential to differentially affect cancer cells (2–9). They have also been found to enhance cisplatin-induced cytotoxicity in human mesothelioma cells (10). Treatments for short periods of time were effective, showing an ability to protect cells in later inductions (11). In some cases the anticarcinogenic potential of SBBI has been specifically related to its antichymotrypsin activity (4–6,12). Presently there is at least a patent on 1 inhibitor of this type (13).
On the other hand, plant lectins have been extensively studied because of their effects on animal cells, such as induction of lymphocyte mitogenesis, immunoglobulin aggregation, induction of histamine release from basophils, and mast cells (14). Lectins can bind to the cell membrane through specific recognition to glycoconjugates, resulting in changes of cell function. Different studies have shown a strong correlation between certain lectin-binding models and their biological action in several tumors. Legume lectins inhibit cell adhesion, cell proliferation, and colony formation, causing hemagglutination and cytotoxic effects on human tumor cells. Particularly, plant lectins have anticancer properties in vitro and in vivo, by binding cancer cell membrane proteins or receptors causing cytotoxicity, apoptosis, and inhibition of tumor growth (15–17). It has been observed that lectins from different sources inhibit cancer cells growth depending on their concentration and in a differential way (18–20).
We previously characterized a 7 kDa Bowman-Birk PI from Tepary bean (Phaseolus acutifolius) seeds (TBPI) with antitrypsin and antichymotrypsin activities (21). In an earlier work, we tested the effect of a semipure sample of TBPI on cancer cells, founding an increase of cell adhesion to culture dishes, as well as a decrease in cancer cell growth (22). However, we later identified the presence of at least 1 lectin in that protein sample. In this work, we separately described the effect of a semipure lectin fraction and the effect of the pure TBPI on in vitro assays on murine transformed cells as well as in human cancer cell lines.
MATERIALS AND METHODS
Plant Material
Tepary bean seeds were obtained from a local market at Hermosillo, Sonora, Mexico and stored at −20°C until their use.
Lectin and TBPI Purification
TBPI was purified as described by Campos-Contreras et al. (21). Briefly, the protein present in the crude extract was precipitated with ammonium sulfate (40% to 65% saturation), centrifuged (39,200 g for 60 min); the pellet was dialyzed and lyophilized. The sample was chromatographed on a 167 × 2.25 cm Sephadex G-75 column that was equilibrated with 0.02 M ammonium bicarbonate. Two protein fractions were separated: a lectin-rich fraction (TLRF) and the semi-pure Tepary PI fraction (TPIF). Both fractions were tested for protein (23), agglutination activity (24), and protease inhibition (25).
The TLRF, which showed agglutination activity but lacked protease inhibition activity, was rechromatographed using an Econo-Pac® High Q cartridge column (1 × 5 cm) (Pharmacia Biotech; Uppsala, Suiza) in an Econo System BioRad collector, equilibrated with 0.01 M Tris-HCl, pH 8.0. Elution was carried out using a 0 to 0.4 M NaCl linear gradient in 0.01 M Tris-HCl, pH 8.0, with a flow rate of 1 mL/min, collecting 2 mL fractions, starting the collection at 140 min. Those fractions containing the lectin (TLF) were pooled, dialyzed, lyophilized, and stored at –20°C until use. The presence of lectin was corroborated by SDS-PAGE glycoprotein-staining (26).
Meanwhile, TPIF was rechromatographed as described by Campos-Contreras et al. (21). Briefly, DEAE-Sepharose column (1 cm × 15 cm) was used, eluting the protein in a linear gradient form 0.1 to 0.7 M NaCl in Tris 0.02 M pH 8.0 buffer solution (0.3 mL/min), collecting 3.0 mL fractions. All fractions with PI activity were dialyzed, lyophilized, and further purified through an RP-HPLC, using a preparative Vydac C-18 column. The fractions were then equilibrated in 0.1% trifluoroacetic acid and eluted with a linear gradient of acetonitrile from 0% to 60% in 0.1% trifluoroacetic acid (1 mL/min). Fractions with PI activity were collected (TBPI), lyophilized, and stored at −20 °C until use. To monitor the purity of the samples, SDS-PAGE (27) was done after each step.
Cell Culture
The antiproliferative effects of TLRF, TLF, and TBPI were tested on 3T3/v-mos transformed murine fibroblasts (kindly provided by Dr. C. Schweinfest of the Medical University of South Carolina, Charleston, SC). Cells were maintained in high glucose Dulbecco's modified Eagle's medium (DMEM; GIBCO BRL, Grand Island, NY) with 5% calf serum (CS; HyClone, Logan, UT), and incubated at 37°C under a 10% CO2/90% air humidified atmosphere. These cells presented high proliferation rates, high invasion capacity, and very poor adhesion to culture dishes. Nontransformed 3T3 fibroblasts were used as control.
In a separate experiment, human cancer cell lines (cervix: SiHa, HeLa and C33A; breast: MCF7 and ZR-75-1; and colon: CaCo2) were tested for TLF effect. Cells were maintained according to the American Type Culture Collection (ATCC) recommendations in each case.
Proliferation Assay
Bioassays were performed as described by Garcia-Gasca et al. (22). Briefly, cells were seeded (1 × 104 cells per well for 3T3 fibroblasts or 3 × 104 cells for human cancer cells) in 24-well plates. After 48 h, conditions were changed by adding 0.5 mL of different concentrations of TLRF, TLF, or TBPI in DMEM containing 0.5% bovine serum albumin (BSA; Serologicals, Clarkston, Georgia); control cells were maintained in 0.5% BSA-DMEM. After 72 h, cells were harvested with 0.15% trypsin in phosphate buffer solution and cell number was determined using a hemocytometer. All treatments were done in duplicate in at least 3 independent experiments.
Adhesion Assay
Cells were seeded (1 × 104 cells per well) in 24-well plates with 0.5 mL of 5% CS-DMEM. After 24 h, 0.5 mL of 1% BSA-1.5% CS-DMEM containing 0.027 μg protein/mL (equivalent to 250 IU/mL) of TBPI was added and a control treatment using 1% BSA–1.5% CS-DMEM was included. Cells were incubated at 37°C under a 10% CO2/90% air humidified atmosphere until confluence was reached. The number of cells in each of the treated and nontreated wells was estimated using a carmine-based colorimetric method as described by Garcia-Gasca et al. (28).
Invasion Assay
Cells were harvested with 0.2% EDTA and seeded as described in the adhesion assay in 24-well plates (2 × 104 cells/well) in a Matrigel invasion chamber (Biocoat, Becton Dickinson Labware, Franklin Lakes, NJ) using 5% fetal bovine serum as a chemoattractant. After 5 days of incubation, inserts were removed and cultures were incubated for 5 more days. Cells that were attached to the bottom of the well were harvested and counted using a hemocytometer.
ECM Degradation Assay
Cells were seeded on conventional 35 mm cell culture dishes (Corning Inc., Corning, NY) with 2 mL of 5% CS-DMEM. The conditions were changed the day after seeding, as described in the adhesion assay. When cell cultures reached confluence, ECM from culture dishes was extracted (29) and SDS-PAGE analysis was performed, applying 20 μg of protein per well. Protein band intensities were determined by densitometry measuring the peak area using an image processing software (IPLab, Scanalytics, Billerica, MA).
Matrix Metalloproteinases (MMP) Zymography Analysis
3T3/v-mos fibroblasts were cultured in 35 mm dishes to confluence; 12 h prior treatment cells were rinsed with PBS and incubated in 0.5% serum media. One hour before treatment medium was removed, cells were rinsed with PBS, and serum-free medium was added. For treated cells, 0.027 μg protein/mL (equivalent to 250 IU/mL, total volume of 2 mL) of TBPI was included and cells were incubated at 37°C for 24 h. Medium was collected, cells were separated by centrifugation at 13,000 rpm for 10 min and the conditioned media (CM) were transferred to new tubes. Samples were lyophilized and stored at –80°C until use. CM for electrophoresis were concentrated by ultrafiltration using a solvent resistant stirred cell (Cat. No. XFUF 076 01-76 mm) with a 3 kDa ultrafiltration membrane (Millipore Corporation, Billerica, MA). Gelatin zymography was carried out according Hawkes et al. (2010) (30). To determine whether TBPI directly inhibit the activity of some extracellular proteases, 10 μg of the inhibitor were additionally added to the CM and incubated at 37°C for 15 min before the electrophoresis was performed. To detect a possible proteolytic activation by Triton, after SDS-PAGE one on the gels was incubated 60 min in 0.05 M Tris-HCl, pH 8; 2.5% (v/v) Triton X-100, and the second one was incubated in the absence of Triton. A negative control treated with 5 mM EDTA was included to inhibit MMPs. Then, the gels were washed twice in 0.05 M Tris-HCl, pH 8 (10 min each) and incubated 24 h in the same Tris solution in the presence of 5 mM CaCl2, with the exception of the treatment with EDTA, which was incubated in Tris-EDTA solution. At the end, the gels were stained in 0.1% Coomassie Brilliant Blue R-250 (w/v) in methanol: acetic acid 45:10 (v/v) for 4 h and faded in the same solution without the Coomassie dye. Proteolytic activity was evident as clear bands against a background of stained gelatin.
Statistical Analysis
All experiments were performed in duplicate, in at least 2 independent times. SPSS Version 17 software was used for comparing different treatments against control cells (analysis of variance; Tukey or Dunnett P ≤ 0.05) or for comparing only 1 treatment against control cells (t- student P ≤ 0.05). To obtain the inhibitory concentration 50 (IC50), simple linear regressions of TLRF or TLF concentration logarithms versus proliferation percentage were done.
RESULTS
Protein Fractionation and TBPI Purification
Two protein fractions were obtained after the Tepary bean extract was chromatographed on Sephadex G-75: one rich in agglutinin activity (TLRF) and the other one containing the protease inhibitor activity (TPIF) (Fig. 1A). Although only TPIF presented antitrypsin inhibition activity (11,400 IU/mg protein), both fractions showed agglutination activity [represented as agglutination units (AU) per mg of protein]: 5565 and 1024 AU/mg for TLRF and TPIF, respectively. TLRF showed a main band of approximately 30 kDa after SDS-PAGE analysis, similar to the lectin previously reported (31,32), plus few more protein bands (Fig. 1B). TLRF was rechromatographed by ionic exchange chromatography (Fig. 1C), obtaining the TLF with a specific agglutination activity of 1,170 AU/mg protein. According to its electrophoretic profile, TLF showed only the band with an apparent molecular mass of 30 kDa (Fig. 1D). However, only 20% of the initial agglutination activity was recovered by this method. Finally, the TPIF fraction was rechromatographed by both ionic exchange chromatography and HPLC to finally obtain a pure PI fraction (TBPI) (Fig. 1E), whose electrophoretic profile showed only 2 protease inhibitor isoforms (P1 and P2) (Fig. 1F). This fraction showed specific PI activities of 9381 and 5352 IU/mg protein against trypsin and chymotrypsin, respectively.
FIG. 1.
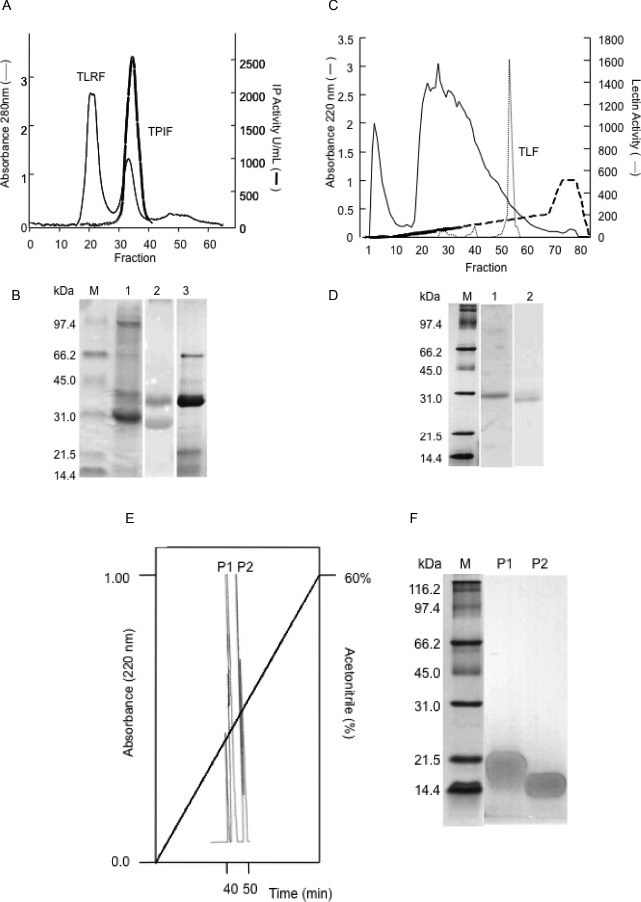
Protein fractionation. A: Tepary lectin-rich fraction (TLRF) and Tepary protease inhibitors fraction (TPIF) obtained after molecular weight exclusion chromatography. B: SDS-PAGE profile of the protein fractions after gel exclusion chromatography. Molecular markers (M), TLRF (1), TLRF stained for glycoprotein detection (2), TPIF (3). C: TLRF ionic exchange chromatography. D: SDS-PAGE profile of the protein fractions after ionic exchange chromatography. Molecular markers (M), Tepary lectin fraction (TLF) (1), and TLF stained for glycoprotein (2). E: Tepary bean protease inhibitor (TBPI) RP-HPLC chromatogram. Two peaks, with retention times of 43 min (P1) and 47.4 min (P2), showing protease inhibitor activity. F: SDS-PAGE profile of TBPI isoforms (P1 and P2) using 13.5% polyacrylamide gels loaded with 20 μg of protein per lane (Color figure available online).
Effects of TLRF, TLF, and TBPI on Cell Proliferation
The antiproliferative effect of TLRF, TLF, and TBPI was evaluated on 3T3/v-mos cell cultures. TLF cytototoxic effect was also tested on breast, cervix, and colon cancer cell lines, whereas TBPI was additionally tested for adhesion and invasion assays on 3T3/v-mos cells.
TLRF, lacking PI activity, inhibited 3T3/v-mos cell proliferation as a function of concentration, and also in a differential manner with respect to nontransformed 3T3 cells (Fig. 2A). The IC50 values were 1.21 AU/mL and 26.27 AU/mL for transformed and nontransformed cells, respectively. Although proliferation of nontransformed cells was also affected by the lectin fraction, this effect was 21 times lower than the corresponding effect on transformed cells. This cytotoxic effect was confirmed using the more purified lectin, TLF, on 3T3/v-mos transformed cells observing that cytotoxicity was effectively related to the presence of this lectin, with an IC50 of 0.27 AU/mL (Fig. 2B). In the case of TBPI, none of the different concentrations assayed presented a negative effect on 3T3/v-mos cell proliferation (Fig. 2C), on the contrary, cell proliferation increased with the highest concentration tested.
FIG. 2.
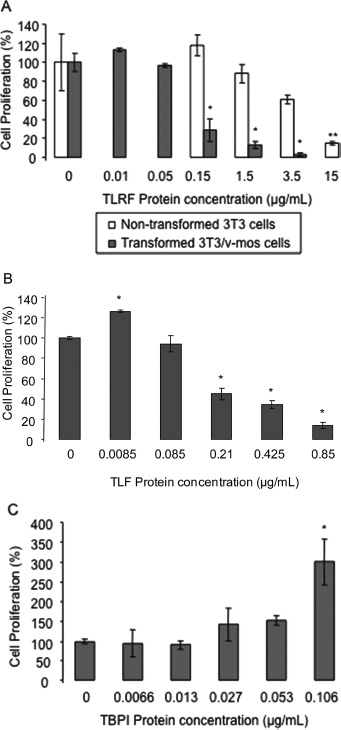
Effects of Tepary lectin-rich fraction (TLRF), Tepary lectin fraction (TLF), and Tepary bean protease inhibitor (TBPI) on cell proliferation. Non-transformed or transformed fibroblasts were seeded (1 × 104 cells/well) in 24-well plates with Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% calf serum. After 48 h, the medium was removed and different concentrations of (A) TLRF, (B) TLF, or (C) TBPI were added in 0.5% bovine serum albumin (BSA) serum-free medium. Treatment with DMEM containing 0.5% BSA was included as a control. After 72 h, cells were counted using a hemocytometer. Asterisks show statistically significant differences (Dunnett, P ≤ 0.05) with respect to controls, (∗) for transformed cells and (∗∗) for non-transformed cells.
To determine the effect of TLF on human cancer cells, dose-response curves were performed on breast cancer cells (MCF-7 and ZR-75-1), cervix cancer cells (HeLa, SiHa, and C33-A) and colon cancer cells (CaCo2). Fig. 3 shows that TLF exhibited a differential effect on cell proliferation as a function of concentration and cell type. Cervix cancer cells were the least sensible to TLF (HeLa < SiHa < C33-A), followed by breast cancer cells. Colon cancer cells were the most sensible to the antiproliferative effect of TLF with the lowest IC50.
FIG. 3.
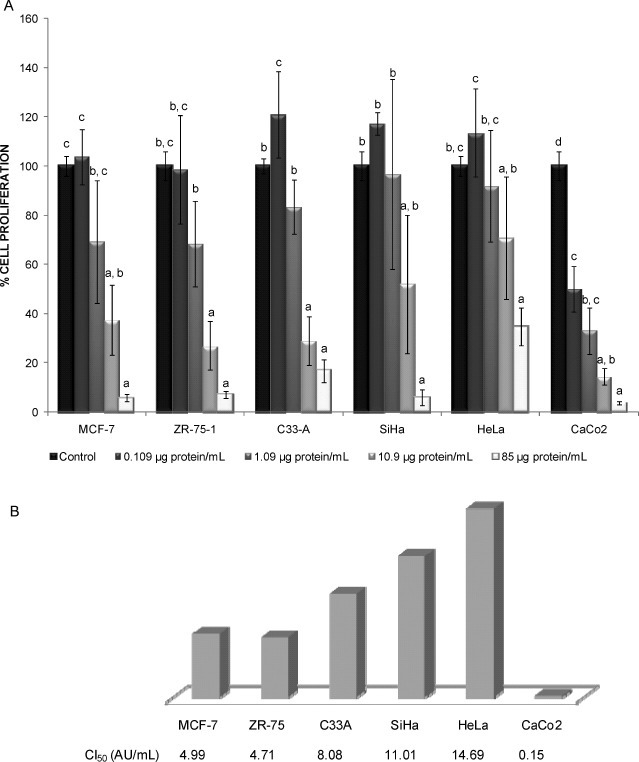
Effect of Tepary lectin fraction (TLF) on cancer cells proliferation. A: Dose-response curve for 72 h TLF treatment on human cancer cells. Cells were seeded (3 × 104 cells/well) in 24-well plates with Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% calf serum. After 48 h, the medium was removed and different concentrations of TLF were added in 0.5% bovine serum albumin (BSA) serum-free medium. Treatment with DMEM containing 0.5% BSA was included as a control. After 72 h, cells were counted using a hemocytometer. Small letters show statistically significant differences (Tukey, P × 0.05) with respect to controls in each case. B: IC50 for human cancer cells treated with TLF. IC50 was calculated by linear regression of the logarithm for TLF (AU/mL) vs proliferation percentage (Color figure available online).
Effect of TBPI on Cell Adhesion and Cell Invasion
Considering that wild-type 3T3/v-mos fibroblasts exhibit poor adhesion to cell culture dishes, they can be easily detached with a simple washing of the plate, representing a good model to study cell attachment. The number of detached cells was determined using a colorimetric carmine-staining-based assay. As we previously showed, TPIF (250 IU/mL) increased cell adhesion of 3T3/v-mos cells after 13 days in culture (22). In this experiment, treatment with 0.027 μg protein/mL (250 IU/mL) of purified TBPI during 8 days increased cell attachment (Fig. 4). This result confirms that such effect was directly related to PI activity, showing an enhanced effect of 2.5 times more cell adhesion at 8 days of treatment.
FIG. 4.
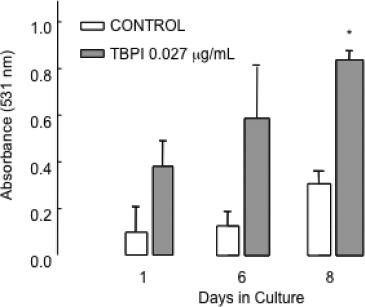
Effect of Tepary bean protease inhibitor (TBPI) on cell adhesion. NIH 3T3/v-mos fibroblasts were seeded (1 × 104 cells/well) with Dulbecco's modified Eagle's medium with 5% calf serum. After 24 h, the medium was changed and supplemented with 1% bovine serum albumin (BSA)/1.5% calf serum as a control, while adding 0.027 μg protein/mL (equivalent to 250 IU/mL) of TBPI to the experimental group. Every third day, 2 wells of each treatment were fixed with absolute ethanol until cells reached confluence. Fixed wells were stained with carmine/ethanol-HCl, and absorbance of the extracted colorant was determined at 531 nm. The asterisk shows a statistically significant difference with respect to control cells for each day (t student, P ≤ 0.05).
Cell invasion is one of the metastatic processes highly dependent on proteolysis. To determine the effect of TBPI on ECM degradation, cell invasion was measured using reconstituted basement membrane (Matrigel) coated inserts. These membranes were used to determine the ability of cells to move in response to chemotactic signals. 3T3/v-mos cells were able to pass through Matrigel, while nontransformed 3T3 fibroblasts were not. Treatment with TBPI negatively affected the cells ability to pass through Matrigel, decreasing 70% with respect to untreated cells (P ≤ 0.05) (Fig. 5).
FIG. 5.
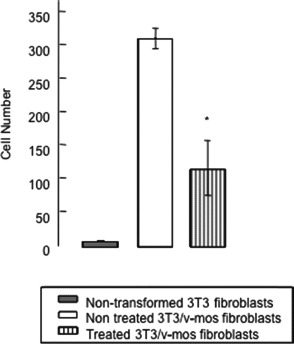
Effect of Tepary bean protease inhibitor (TBPI) on cell invasion. NIH 3T3/v-mos fibroblasts were seeded (2 × 104 cells/well) on Matrigel-coated inserts with Dulbecco's modified Eagle's medium supplemented with 1% bovine serum albumin/1.5% calf serum as a control while adding 0.027 μg protein/mL (equivalent to 250 IU/mL) of TBPI for the experimental group. After 5 days incubation, the inserts were removed and the cells were incubated 5 more days and counted with a hemocytometer. The asterisk shows a statistically significant difference with respect to wild type transformed fibroblasts (t student, P ≤ 0.05).
Effect of TBPI on ECM Degradation
Considering that TBPI is a serine PI (21) that could affect the ECM degradation process and that cultured fibroblasts can produce some ECM proteins as collagen, glycoproteins, and proteoglycans (29), we examined by SDS-PAGE the effect of treatment on ECM degradation from culture dishes. The ECM extracted from both TBPI-treated and -untreated cells showed 2 main protein bands with apparent molecular mass of 112 and 60 kDa (Fig. 6A), suggesting that they were produced by cultured fibroblasts, because no such proteins were present in noncultured dishes. The protein bands from untreated cells were 3.3 and 1.5 times lower in intensity than those observed in treated cells (Fig. 6B), indicating a partial inhibition of the ECM degradation by TBPI.
FIG. 6.
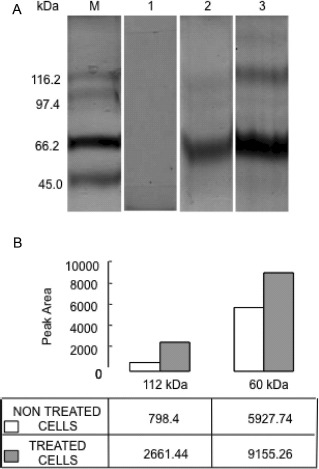
SDS-PAGE profile of extracellular matrix degradation assay. A: NIH 3T3/v-mos fibroblasts were seeded (1 × 104 cells/well) with Dulbecco's modified Eagle's medium with 5% calf serum. After 24 h, the medium was changed and supplemented with 1% bovine serum albumin/1.5% calf serum as a control while adding 0.027 μg Protein/mL (equivalent to 250 IU/mL) of TBPI to the experimental group. When confluence was reached, extracellular matrix was extracted and analyzed by SDS-PAGE at 5% polyacrylamide gel loaded with 20 μg of protein per lane, (1) non-cultured dishes, (2) untreated cells, and (3) treated cells. B: The histogram represents the band intensities evaluated in terms of relative proportion respect to pixel number by a densitometry analyzer.
Separately, CM of cells treated with TBPI clearly showed a different pattern of gelatinolytic activity compared with the control. Two specific differences were found: First, an 83 kDa MMP was inhibited and, second, 2 new proteases of 98 and 116 kDa were detected (Fig. 7). The addition of Triton X-100 to the development procedure allowed the detection of a MMP of 66 kDa apparent molecular mass. Also, when both media were incubated with TBPI before SDS-PAGE, a 104 kDa protease appeared.
FIG. 7.
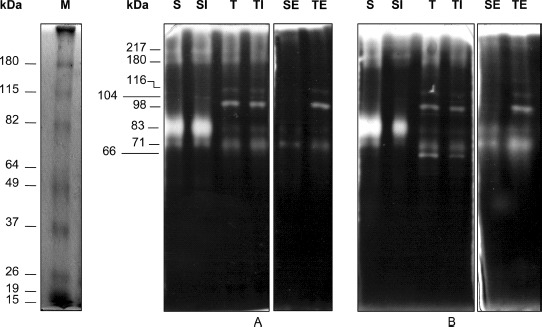
Gelatin zymography of conditioned media of 3T3/v-mos fibroblasts treated and no treated with Tepary bean protease inhibitor (TBPI). M = molecular mass markers; S = control cells conditioned media (CM); SI = control cells CM pre-incubated with 10 μg of TBPI before running the gel; T = TBPI treated cells CM; TI = TBPI treated cells CM preincubated with 10 μg of TBPI before running the gel; SE = control cells CM incubated with 5 mM EDTA before running the gel; TE = TBPI treated cells CM incubated with 5 mM EDTA before running the gel; A: Activity in the absence of Triton. B: Activity in the presence of Triton.
DISCUSSION
Different research groups have worked on legume lectins. In the case of Tepary bean lectins purification, different chromatographic methods have been used, particularly based on affinity chromatography (31,33–35), where they reported a lectin with an apparent molecular mass ranging from 21 to 40 kDa for the single subunits. Pratt et al. (32) reported 4 isolectins with apparent molecular mass of 30 kDa each. We obtained an enriched fraction of Tepary bean lectin by gel filtration chromatography followed by ion exchange chromatography. The positive glycoprotein staining suggested that this band with an apparent molecular mass of 30 kDa could correspond to the previously described Tepary bean lectin (31,36). However, despite the fact that ionic exchange chromatography allowed the separation of the semipure lectin, 80% of the original agglutination activity was lost. We first tried unsuccessfully to purify the lectin through fetuin-agarose affinity chromatography as previously described (31,34,36). Similarly, when HPLC was used, not positive results were obtained. Lectins are not single proteins, but families of homo- or hetero-oligomers with different biological activities (37,38); therefore, the characterization of the specific isolated glycoprotein becomes important when a correlation of certain structure to a specific function is to be established.
Lectins from different sources have shown antiproliferative activities, as well as apoptotic induction effects on cancer cells. There is evidence that the apoptotic effects of some lectins, such as Viscum album L. coloratum agglutinin, involve ROS production (39). Lectins exert their effects by binding the cell surface in a noncovalent way by recognizing specific glycoproteins on the cell membranes, which are frequently altered in cancer cells. This ability allows lectins to selectively interact with cancer cells, affecting their proliferation and survival (16,40). Here we report that the Tepary bean protein fractions containing lectin activity exhibit differential antiproliferative effect on nontransformed and transformed murine 3T3 fibroblasts. The observed IC50 values for TLRF (1.21 AU/mL) and TLF (0.27 AU/mL) on 3T3/v-mos transformed fibroblasts, suggests that TLF retained its biological activity.
Tepary bean lectin fractions have been tested on animal cells. Lectins obtained by affinity chromatography have showed mitogenic activity on human peripheral lymphocytes (33,35), as well as on cultured human lymphocytes and a differential cytotoxic effect was also observed on different clones of murine 3T3 fibroblasts (41). We show now that TLF presented specific cytotoxic effect on human cancer cells, and CaCo2 cells exhibited the highest sensibility as indicated by its lowest IC50 (0.15 AU/mL). Similar cytotoxic effects were observed by Valadez-Vega et al. (42) when a Tepary bean lectin obtained by affinity chromatography was tested on Sw480 (colon cancer) and C33-A (cervix cancer) cells lines, although no IC50 were calculated in that work. In this report, we found an IC50 of 8.08 AU/mL for C33-A cells.
We previously reported that a semi-pure fraction of Tepary bean PI presented cytotoxic effects on transformed cells (22); however, such effect was due to the presence of the lectin here reported, whereas the pure protease inhibitor TBPI had no effect on cell proliferation. Even more, proliferation rate increased at the highest TBPI concentration tested (0.1 μg protein/mL, equivalent to 1000 IU/mL), effect that has previously been observed for other anticarcinogenic protease inhibitors (7).
One of the important features of some serine PIs for their anticancer effect is their ability to inhibit chymotrypsin-like activity (6). In the case of TBPI, high inhibitory activities against both trypsin and chymotrypsin-like enzymes have been reported (21–22). Tumor development is a process heavily dependent on proteolysis that affects ECM degradation, changes in cell adhesion, migration, invasion, and chemical modification of the environment, including the growth factors production (1). Several serine proteases have been implicated in different processes in cancer, among them cathepsins, chymase, trypsin, and chymotrypsin-like enzymes, elastase, plasmin, plasmin activators, matriptase, and human tissue kallikreins (43–46).
Proteinase expression/activity and subsequent tumor cell behavior are regulated via differential engagement of cell–matrix and cell–cell adhesion receptors (47). Interference with the activities of proteases such as gelatinase A, which degrades α5β1 integrin (48), could be implicated. The effect of TBPI on cell adhesion of transformed fibroblasts could be due principally to the suppression of the activity of an apparent molecular mass of an 83 kDa protein. This activity could correspond to the MMP-9 whose molecular mass for the active enzyme is 84 kDa (49). Then, the results suggest that the presence of TBPI in the media suppressed the activity of the MMP-9 of 3T3/v-mos murine fibroblasts, perhaps by inhibiting a serine protease involved in its processing, such as plasmin or urokinase plasminogen activator (50). The inhibition of MMP-9 could promote a decrease in the degradation of adhesion proteins (51). In addition, the presence of a 66 kDa MMP found in the TBPI-treated conditioned media coincided with the molecular mass reported for the active form of MMP-2 (49) that is also involved in ECM degradation. The presence of TBPI in the culture of transformed fibroblast, also allowed the appearance of 2 new gelatinolytic bands, indicating the possibility that inhibition of certain serine proteases could prevent the degradation of these enzymes or that could also participate in the activation of other proteases (52).
Proteolytic activity changes might result in modifications of tumor cell behavior, including the loss of migration capacity. For cells to move through the ECM, they must be able to form new cell–matrix and cell–cell attachments, as well as cleaving the existing ones (53). Therefore, PIs could play a pivotal role in controlling such processes. For example, maspin, which is a cytosolic cell surface-associated and secreted protein belonging to the serine PI super family, has been associated with increased and sustained levels of mature focal adhesion contacts by quenching localized uPA/uPAR complexes before uPA activation (54).
Serine proteases are considered key enzymes for cancer development, mainly because of their role in the degradation of some components of ECM, such as laminin and its participation in the activation of several MMPs, involved in the cleavage of most ECM proteins (52,55–57). The effect of MMP activity has also been supported by in vitro studies measuring invasion through Matrigel (58). Kennedy and Wan (59) observed that SBBI decreased the ability of LNCaP cells to invade across Matrigel. Our results showed that cells treated with TBPI diminished their invasion ability most probably due to the suppression of MMP2 and MMP9.
Here, we presented evidence that 2 proteins from Tepary bean seeds have negative effects on different steps of cancer progression and promotion stages in vitro. Current studies in our group are focusing on the mechanism of action of both proteins on murine and human cancer cells as well as in vivo systems.
ACKNOWLEDGMENTS
We would like to thank CONACYT (Ciencia Básica 82349) and CONCYTEG for the fellowships and the supplementary fellowship for José L. Castro-Guillén and Rosely Marlen García-Cruz. Also to CONCYTEQ and PROMEP (103.5/04/2830) for their financial support and fellowship to Elisa Hernandez-Rivera and Francisco Josué López-Martinez, respectively. We also would like to acknowledge Rosario Botello for her technical support, Alicia Chagolla for her useful advice during the experimental work, and Yolanda Rodriguez and Cristina Elizarraráz for their technical assistance.
REFERENCES
- 1.DeClerck YA, Mercurio AM, Snack MS, Chapman HA, Zutter MM, et al. Proteases, extracellular matrix, and cancer. Am. J. Path. 2004;164:1131–1139. doi: 10.1016/S0002-9440(10)63200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yavelow J, Findlay HT, Kennedy AR, Troll W. Bowman-Birk soybean protease inhibitor as an antiarcinogen. Cancer Res. 1983;43(Suppl.):2454S–2459S. [PubMed] [Google Scholar]
- 3.Kennedy AR, Randner BS, Nagasawa H. Protease inhibitors reduce the frequency of spontaneous chromosome abnormalities in cells from patients with Bloom syndrome. Proc Natl Acad Sci (USA) 1984;81:1827–1830. doi: 10.1073/pnas.81.6.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy AR. Cancer prevention by protease inhibitors. Prev Med. 1993;22:796–811. doi: 10.1006/pmed.1993.1073. [DOI] [PubMed] [Google Scholar]
- 5.Gueven N, Dittman K, Mayer C, Rodemann HP. The radioprotective potential of the Bowman-Birk protease inhibitor is independent of its secondary structure. Cancer Lett. 1998;125:77–82. doi: 10.1016/s0304-3835(97)00481-3. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy AR. Chemopreventive agents: protease inhibitors. Pharmacol Ther. 1998;78:167–209. doi: 10.1016/s0163-7258(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy AR. The Bowman-Birk inhibitor from soybeans as an anticarcinogenic agent. Am J Clin Nutr. 1998;68(Suppl.):1406S–1412S. doi: 10.1093/ajcn/68.6.1406S. [DOI] [PubMed] [Google Scholar]
- 8.Du X, Beloussow K, Shen W. Bowman-Birk protease inhibitor and its palmitic acid conjugate prevent 7, 12 dimethylbenz(a)anthracene induced transformation in culture mouse mammary glands. Cancer Lett. 2001;164:135–141. doi: 10.1016/s0304-3835(01)00381-0. [DOI] [PubMed] [Google Scholar]
- 9.Witschi H, Espiritu I. Development of tobacco smoke-induced lung tumors in mice fed Bowman-Birk protease inhibitor concentrate (BBIC) Cancer Lett. 2002;183:141–146. doi: 10.1016/s0304-3835(02)00156-8. [DOI] [PubMed] [Google Scholar]
- 10.Kashiwagi K, Virgona N, Yamada J, Sato A, Ota M, et al. Bowman-Birk protease inhibitor from soybeans enhances cisplatin-induced cytotoxicity in human mesothelioma cells. Exp Ther Med. 2011;2:719–724. doi: 10.3892/etm.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yavelow J, Collins M, Birk Y, Troll W, Kennedy AR. Nanomolar concentration of Bowman-Birk soybean protease inhibitor suppresses x-ray-induced transformation in vitro. Proc Natl Acad Sci (USA) 1985;86:9871–9875. [Google Scholar]
- 12.Lippman SM, Matrisian LM. Protease inhibitors in oral carcinogenesis and chemoprevention. Clin Cancer Res. 2000;6:4599–4603. [PubMed] [Google Scholar]
- 13.Compositions Comprising Bowman-Birk Protease Inhibitors and Variants Thereof. USA patent number 7696173 Available online: http://www.patentgenius.com/patent/7696173.html.
- 14.Tareq A-A. Plant Lectins. Poisonous Plants Homepage: Animal Science at Cornell University. Available online: http://www.ansci.cornell.edu/plants/toxicagents/lectins.html.
- 15.Kiss R, Camby I, Duckworth D, De-Decker R, Salmon I. In vitro influence of Phaseolus vulgaris Griffonia simplicifolia Concavalin A, wheat germ and peanut agglutinins on HCT-15, LoVo and SW 837 human colorrectal cancer cell growth. Gut. 1997;40:253–261. doi: 10.1136/gut.40.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González de Mejía E, Prisecaru VI. Lectins as Bioactive Plant Proteins: A Potential in Cancer Treatment. Crit Rev Food Sci Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 17.Ferriz-Martinez RA, Torres-Arteaga IC, Blanco-Labra A, Garcia-Gasca T. The role of plant lectins in cancer treatment. In: Mejia C, Navarro S, editors. The New Approaches in the Treatment of Cancer. NY, USA: Nova Science Publishers; 2010. pp. 71–89. [Google Scholar]
- 18.Lyu S, Choi SH, Park WB, Kim W. Involvement of caspase-3 in apoptosis induced by Viscum album var. coloratum agglutinin in HL-60 cells. Biosci Biothecnol. Biochem. 2001;65:534–541. doi: 10.1271/bbb.65.534. [DOI] [PubMed] [Google Scholar]
- 19.Pryme IF, Bardocz S. Anti-cancer therapy: Diversion of polyamines in the gut. Eur J Gastroenterol Hepatol. 2001;13:1041–1046. doi: 10.1097/00042737-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Lyu S, Choi SH, Park WB. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway independent of p-53. Arch Pharm Res. 2002;25:93–101. doi: 10.1007/BF02975269. [DOI] [PubMed] [Google Scholar]
- 21.Campos J, Martínez-Gallardo N, Mendiola-Olaya E, Blanco-Labra A. Purification and partial characterization of a proteinase inhibitor from Tepary bean (Phaseolus acutifolius) seeds. J Food Biochem. 1997;21:203–218. [Google Scholar]
- 22.García-Gasca T, Salazar-Olivo LA, Mendiola-Olaya E, Blanco-Labra A. The effects of a protease inhibitor fraction from Tepary bean (Phaseolus acutifolius) on in vitro cell proliferation and cell adhesion of transformed cells. Toxicol In Vitro. 2002;16:229–233. doi: 10.1016/s0887-2333(02)00006-1. [DOI] [PubMed] [Google Scholar]
- 23.Bradford M. Arapid and sensitive method for the quantitationof microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 24.Jaffé W. Toxic Constituents of Plant Foodstuffs. New York: Academic Press; 1980. Hemagglutinins (lectins) pp. 73–102. [Google Scholar]
- 25.Schwertz G, Takenaka Y. Spectrophotometric determination of trypsin and chymotrypsins activity. Biochim Biophys Acta. 1955;16:571–575. doi: 10.1016/0006-3002(55)90280-8. [DOI] [PubMed] [Google Scholar]
- 26.Gerard C. Purification of glycoproteins. In: Deutscher MP, editor. Guide to Protein Purification. San Diego, CA: Academic Press; 1990. pp. 535–536. [Google Scholar]
- 27.Laemmli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.García-Gasca T, Paz-González V, Moncada-Álvarez M, Blanco-Labra A, Salazar-Olivo L. Colorimetric quantitation of in vitro cell density using carmine a chromosome-specific stain. Toxicol In Vitro. 2002;16:573–579. doi: 10.1016/s0887-2333(02)00044-9. [DOI] [PubMed] [Google Scholar]
- 29.Ignotz RA, Messague J. Transforming growth factor? stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem. 1986;261:4337–4345. [PubMed] [Google Scholar]
- 30.Hawkes SP, Li H, Taniguchi GT. Zymography and reverse zymography for detecting MMPs and TIMPs. In: Clark IM, Young DA, Rowan AD, editors. Matrix Metalloproteinase Protocols. Methods in Molecular Biology. 2nd ed. New York: Humana Press; 2010. pp. 257–269. [DOI] [PubMed] [Google Scholar]
- 31.Pusztai A, Watt WB, Stewart JC. Erithro- and lymphoaglglutinins of Phaseolus acutifolius. Phytochem. 1987;26:1009–1013. [Google Scholar]
- 32.Pratt RC, Singh NK, Shade RE, Murdock LL, Bressan RA. Isolation and partial characterization of a seed lectin from tepary bean that delays bruchid beetle development. Plant Physiol. 1990;93:1453–1459. doi: 10.1104/pp.93.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vargas-Albores F, de la Fuente G, Agundis C, Córdoba F. Purification and characterization of a lectin from Phaseolus acutifolius var. latifolius. Prep Biochem. 1987;17:379–396. doi: 10.1080/00327488708062503. [DOI] [PubMed] [Google Scholar]
- 34.Reynoso-Camacho R, Gonzalez de Mejia E, Loarca-Piña G. Purification and acute toxicity of a lectin extracted from Tepary bean (Phaseolus acutifolius) Food Chem Toxicol. 2003;41:21–27. doi: 10.1016/s0278-6915(02)00215-6. [DOI] [PubMed] [Google Scholar]
- 35.Castillo-Villanueva A, Caballero-Ortega H, Abdullaev-Jafarova F, Ganfias Y, Jiménez-Martínez M, et al. Lectin from Phaseolus acutifolius var. escumite: chemical characterization, sugar specificity, and effect on human T-lymphocytes. J Agric Food Chem. 2007;55:5781–5787. doi: 10.1021/jf063644k. [DOI] [PubMed] [Google Scholar]
- 36.de Mejía EG, Hankin C, Paredes-López O, Shannon L. The lectins and lectin-like proteins of Tepary beans (Phaseolus acutifolius) and Tepary-common bean (Phaseolus vulgaris) hybrids. J Food Biochem. 1990;14:117–126. [Google Scholar]
- 37.Sharon N. Lectin-carbohidrate complexes of plants and animals: an atomic view. TIBS. 1996;18:221–226. doi: 10.1016/0968-0004(93)90193-q. [DOI] [PubMed] [Google Scholar]
- 38.Sharon N, Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiology. 2004;14:53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- 39.Won-Ho K, Won BP, Bin G, Myeong HJ. Critical role of reactive oxygen species and mitochondrial membrane potential in Korean mistletoe lectin-induced apoptosis in human hepatocarcinoma cells. Mol Pharmacol. 2004;66:1383–1396. doi: 10.1124/mol.104.001347. [DOI] [PubMed] [Google Scholar]
- 40.Petrossian K, Banner LR, Oppenheimer SB. Lectin binding and effects in culture on human cancer and non-cancer cell lines: Examination of issues of interest in drug design strategies. Acta Histochem. 2007;109:491–500. doi: 10.1016/j.acthis.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valadez-Vega C, Guzmán-Partida AM, Soto-Cordova FJ, Álvarez-Manilla G, Morales-González JA, et al. Purification, biochemical characterization, and bioactive properties of a lectin purified from the seeds of white tepary bean (Phaseolus acutifolius variety Latifolius) Molecules. 2011;16:2561–2582. doi: 10.3390/molecules16032561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valadez-Vega C, Alvarez-Manilla G, Riverón-Negrete L, García-Carrancá A, Morales-González JA, et al. Detection of cytotoxic activity of lectin on human colon adenocarcinoma (Sw480) and epithelial cervical carcinoma (C33-A) Molecules. 2011;16:2107–2118. doi: 10.3390/molecules16032107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Benaud CM, Oberst M, Dickson RB, Lin CY. Deregulated activation of matriptase in breast cancer cells. Clin Exp Metastasis. 2002;19:639–649. doi: 10.1023/a:1020985632550. [DOI] [PubMed] [Google Scholar]
- 44.Uhland K. Matriptase and its putative role in cancer. Cell Mol Life Sci. 2006;63:2968–2978. doi: 10.1007/s00018-006-6298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandey R, Patil N, Rao M. Proteases and protease inhibitors: implications in antitumorogenesis and drug development. Int J Hum Genet. 2007;7:67–82. [Google Scholar]
- 46.Castro-Guillén JL, Garcia-Gasca T, Blanco-Labra A. Protease inhibitors as anticancer agents. In: Mejia C, Navarro S, editors. The New Approaches in the Treatment of Cancer. NY, USA: Nova Science Publishers, Inc.; 2010. pp. 91–124. [Google Scholar]
- 47.Ghosh S, Brown R, Jones JC, Ellerbroek SM, Stack MS. Urinary-type plasminogen activator (uPA) expression and uPA receptor localization are regulated by α3 β1 integrin in oral keratinocytes. J Biol Chem. 2000;275:23869–23876. doi: 10.1074/jbc.M000935200. [DOI] [PubMed] [Google Scholar]
- 48.Ray J, Stetler-Stevenson W. Gelatinase A activity directly modulates melanoma cell adhesion and spreading. EMBO J. 1995;14:908–917. doi: 10.1002/j.1460-2075.1995.tb07072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woessner JF., Jr . The matrix metalloproteinase family. In: Parks WC, Mecham RP, editors. Matrix Metalloproteinases. San Diego, CA: Academic Press; 1998. pp. 1–13. [Google Scholar]
- 50.Bodey B, Bodey JB, Siegel SE, Kaiser HF. Matrix metalloproteinase expression in malignant melanomas: tumor-extracellular matrix interactions in invasion and metastasis. In Vivo. 2001;15:57–64. [PubMed] [Google Scholar]
- 51.Riikonen T, Westermarck J, Koivisto L, Broberg A, Kahari VM, et al. Integrinα2β1 is a positive regulator of collagenase (MMP-1) and collagen α1 (I) gene expression. J Biol Chem. 1995;270:13548–13552. doi: 10.1074/jbc.270.22.13548. [DOI] [PubMed] [Google Scholar]
- 52.Koblinski JE, Ahram M, Sloana BF. Unraveling the role of proteases in cancer. Clin Chim Acta. 2000;291:113–135. doi: 10.1016/s0009-8981(99)00224-7. [DOI] [PubMed] [Google Scholar]
- 53.Kleiner DE, Stetler-Stevenson WG. Matrix metalloproteinases and metastasis. Cancer Chemother. Pharmacol. 1999;43(Suppl.):S42–S51. doi: 10.1007/s002800051097. [DOI] [PubMed] [Google Scholar]
- 54.Yin S, Lockett J, Meng Y, Biliran H, Jr, Blouse GE, et al. Maspin retards cell detachment via a novel interaction with the urokinase-type plasminogen activator/urokinase-type plasminogen activator receptor system. Cancer Res. 2006;66:4173–4181. doi: 10.1158/0008-5472.CAN-05-3514. [DOI] [PubMed] [Google Scholar]
- 55.Moy LY, Billings PC. A proteolytic activity in a human breast cancer cell line which is inhibited by the anticarcinogenic Bowman-Birk protease inhibitor. Cancer Lett. 1994;85:205–210. doi: 10.1016/0304-3835(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 56.Mazzieri R, Masiero L, Zanetta L, Monea S, Onisto M, et al. Control of type IV collagenase activity by components of the urokinase-plasmin system: a regulatory mechanism with cell-bound reactants. EMBO J. 1997;16:2319–2332. doi: 10.1093/emboj/16.9.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ware JH, Wan XS, Rubin H, Schechter NM, Kennedy AR. Soybean Bowman-Birk protease inhibitor is a highly effective inhibitor of human mast cell chymase. Arch Biochem Biophys. 1997;344:133–138. doi: 10.1006/abbi.1997.0182. [DOI] [PubMed] [Google Scholar]
- 58.Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- 59.Kennedy AR, Wan XS. Effects ofthe Bowman-Birk inhibitor on growth, invasion, and clonogenic survival of human prostate epithelial cells and prostate cancer cells. Prostate. 2002;50:125–133. doi: 10.1002/pros.10041. [DOI] [PubMed] [Google Scholar]


