Abstract
Porins are β-barrel outer membrane proteins through which small solutes and metabolites diffuse that are also exploited during cell death. We have studied how the bacteriocin colicin E9 (ColE9) assembles a cytotoxic translocon at the surface of Escherichia coli that incorporates the trimeric porin OmpF. Formation of the translocon involved ColE9’s unstructured N-terminal domain threading in opposite directions through two OmpF subunits, capturing its target TolB on the other side of the membrane in a fixed orientation that triggers colicin import. Thus an intrinsically disordered protein can tunnel through the narrow pores of an oligomeric porin to deliver an epitope signal to the cell to initiate cell death.
Porins mediate the diffusion of nutrients and ions into cells and organelles and the expulsion of xenobiotics through a central pore (1, 2). We studied the mechanism by which the endonuclease (DNase) bacteriocin ColE9 exploits the porin OmpF in the OM of Escherichia coli. OmpF is one of the most abundant proteins in the E. coli OM (>100,000 copies/cell), with related porins widespread in bacteria (3, 4). Composed of three identical 16-stranded β-barrels each with a narrow pore that traverses the OM, OmpF subunits restrict the passive diffusion of molecules to <600 Da. Nevertheless, some colicins use OmpF, or its homolog OmpC, to enter the periplasm following their initial binding to the vitamin B12 receptor BtuB (Figure 1A) (5, 6). Colicins are potent cytotoxins that are expressed and released by E. coli in response to environmental stress that target closely related organisms during interbacterial competition (7-10). ColE9 (60 kDa) is released bound to the immunity protein Im9, a high-affinity inhibitor (Kd~10−14 M), which neutralizes the DNase domain in the colicin-producing host (11). ColE9 recruits OmpF through an intrinsically unstructured translocation domain (IUTD) in order to contact TolB in the periplasm (Figure 1A), thereby forming a translocon complex that initiates cell entry (12-15). Colicin translocons are transient, unstable species involving protein-protein interactions either side of the OM (Figure S1B). We thus devised a strategy to stabilize the translocon for ColE9, enabling its purification and subsequent biophysical and structural analysis (Figure 1A).
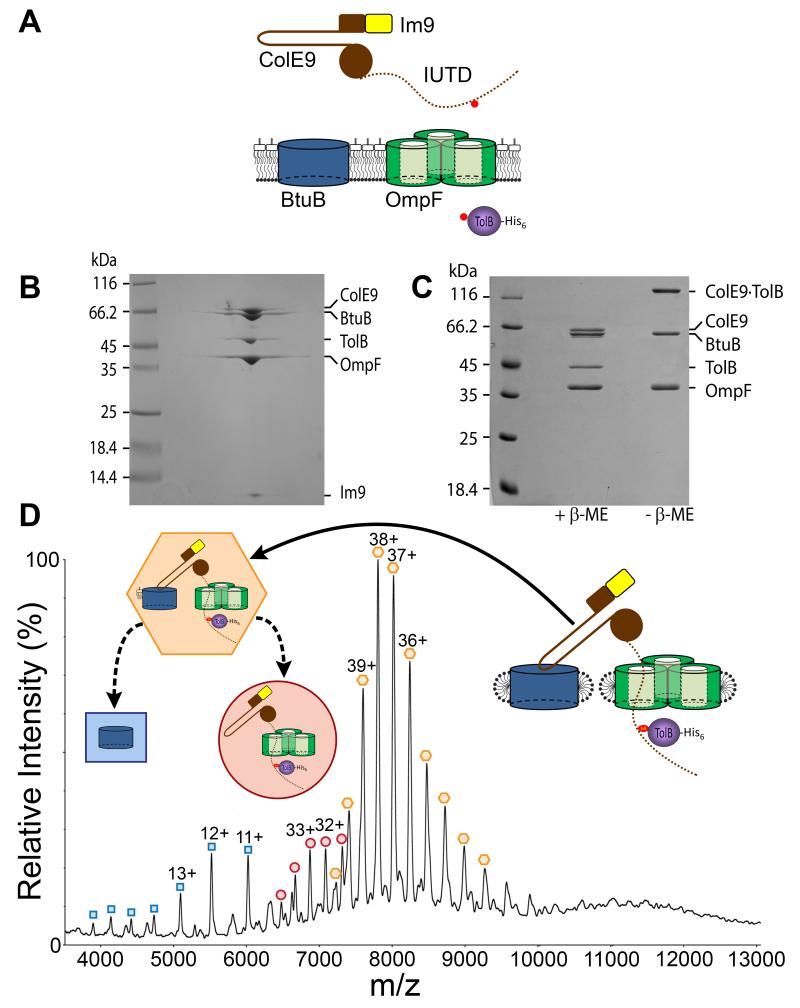
Figure 1. Isolation and characterization of the ColE9 translocon from the OM of Escherichia coli.
(A) Strategy for the capture and isolation of the ColE9 translocon. Nuclease colicins such as ColE9 (brown) contain a hairpin receptor-binding (R−) domain, an N-terminal translocation (T−) domain that includes the intrinsically unstructured translocation domain (IUTD), shown as a dotted line, and a C-terminal cytotoxic DNase domain to which is bound the immunity protein Im9 (yellow). The engineered cysteines for disulfide bond formation between the TolB binding epitope of ColE9 and histidine-tagged TolB (purple) are shown as red circles. (B) BN-PAGE of the isolated translocon showing the complex contains the ColE9-Im9 complex, BtuB, OmpF and TolB. (C) 12% SDS-PAGE of the translocon ± β-mercaptoethanol (β-Me) confirming the presence of the disulfide between ColE9 and TolB. (D) Native-state ESI-MS spectrum of the ColE9 translocon. Coloured inserts give assignments for species observed in the spectrum, all devoid of detergent: orange hexagon, the intact complex that includes a single molecule of lipopolysaccharide; red circle, translocon from which BtuB and lipopolysaccharide have dissociated in the gas phase; blue square, dissociated BtuB. See Table S2 for masses.
We designed a disulfide bond trap that would form between the periplasmically-located TolB-binding epitope (TBE) of ColE9 and TolB following recruitment of OmpF in the OM. The covalent bond did not induce any structural changes relative to the wild type complex (Figure S2 and Table S1) (13). The ColE9 cysteine mutant was allowed to form its translocon at the OM of E. coli cells, trapped by disulfide bond formation with the corresponding TolB cysteine mutant, and the entire complex extracted from the membrane and purified, exploiting a histidine tag on the C-terminus of TolB (Figure S1B). The ColE9 translocon was deemed homogeneous by blue native- (BN−)PAGE (Figure 1B). The individual components of the translocon were identified by matrix-assisted laser desorption/ionization mass spectrometry of protein bands excised from SDS-PAGE gels, the latter also confirmed the presence of the trapping disulfide bond (Figure 1C). The isolated translocon was a heptameric assembly composed of single copies of the ColE9-Im9 complex, BtuB, OmpF trimer and TolB as determined by size-exclusion chromatography-multiangle light scattering (SEC-MALS) (Figure S3A) and native-state electrospray ionization mass spectrometry (ESI-MS) (16, 17) (Figure 1D). The mass of the translocon obtained by ESI-MS (296,983 (± 80) Da) agreed closely with that calculated for the complex, but included additional mass of ~3.5 kDa which is likely to be a single molecule of lipopolysaccharide bound non-covalently to the BtuB receptor (Figure S4).
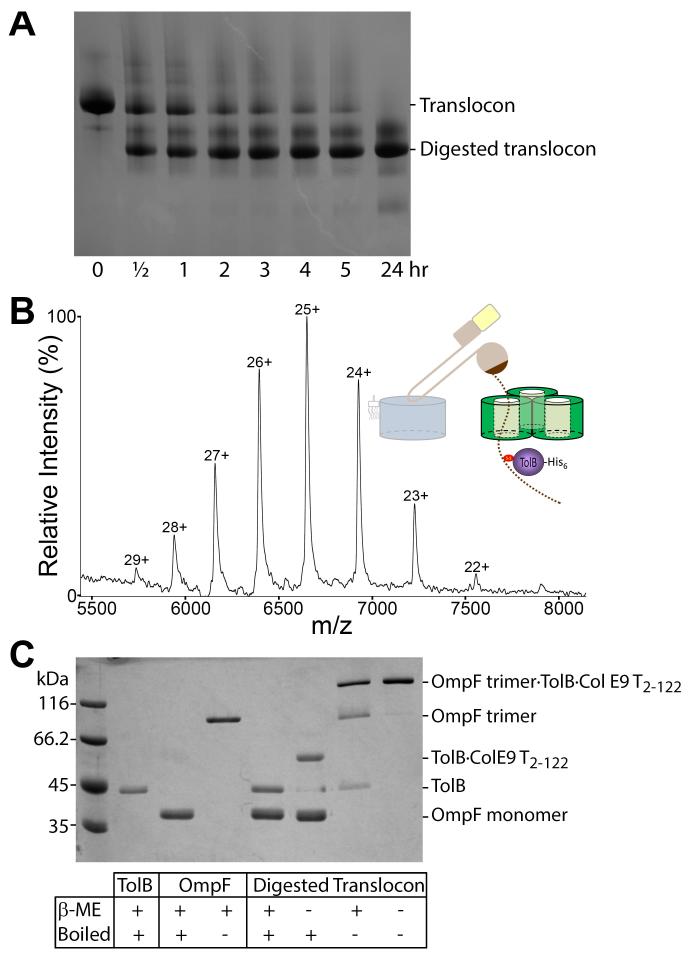
Limited proteolysis was used to probe the organization of the ColE9 translocon. Trypsin digestion yielded a stable sub-complex when analyzed by BN-PAGE (Figure 2A), which corresponded to intact TolB and OmpF and a ~11 kDa fragment of ColE9 (Figure S5A,C,E). The ColE9 IUTD is known to house two OmpF binding sites (OBS1, residues 2-18 and OBS2, residues 54-63) that flank the TolB-binding epitope (TBE; residues 32-47) (12) (Figure 3A). The ~11 kDa fragment of the digested translocon encompassed residues 2-122 from the ColE9 T-domain (ColE9 T2-122), which includes all the identified binding epitopes as well as additional sequences corresponding to extracellular regions of the colicin. ESI-MS and SEC-MALS confirmed the native mass of the digested translocon (Figure 2B; Figure S3B; Table S2). It has been proposed previously that the colicin’s IUTD passes through OmpF (Figure 1A; Figure S6A-C) (6, 12). SDS-PAGE of the digested translocon, in combination with heat treatment and reducing agent, confirmed that the colicin had indeed passed through its bound porin; TolB and OmpF only co-migrated when the disulfide between TolB and the ColE9 T2-122 fragment was intact and OmpF was a folded trimer (Figure 2C).
Figure 2. Formation of the ColE9 translocon involves passage of the colicin through OmpF.
(A) BN-PAGE showing the time course for limited proteolysis of the translocon by trypsin (100:1) at 30°C. (B) Native-state ESI-MS data for the trypsin digested translocon (containing TolB, OmpF and ColE9 T2-122) along with a graphical representation of the complex. The observed and expected masses for the complex were 166,528 (±81) Da and 166,572 Da, respectively. (C) 12% SDS-PAGE of the digested translocon and its comparison to control TolB and OmpF proteins ± reducing agent (β-Me) and/or heat denaturation. In the absence of boiling OmpF migrates as a trimer. TolB and OmpF trimer only migrate together within the proteolysed translocon when connected by ColE9 T2-122 that has passed through the trimer and formed a trapping disulfide with TolB (last lane). Reduced ColE9 T2-122 migrates off the end of this gel and so is not resolved.
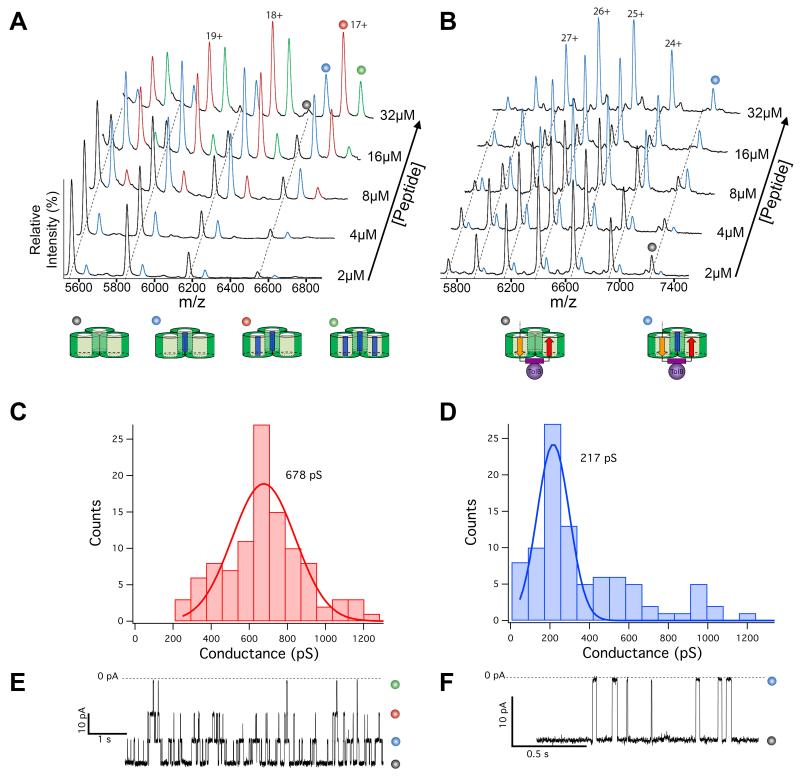
Figure 3. ColE9 occupies two subunits of an OmpF trimer.
(A) ESI-MS titration showing binding of up to three OBS1 peptides to OmpF in the gas phase (see Table S2 for masses). Peak assignments are shown below the data (OBS1 represented as a blue rectangle). (B) ESI-MS titration showing the binding of one OBS1 peptide to the proteolysed translocon. Peak assignments are shown below the data (see Fig. S6 for colour coding of ColE9 IUTD binding epitopes). (C) Histogram showing a Gaussian distribution of unitary conductance (pS) measurements for OmpF trimers in planar lipid bilayers. (D) Histogram showing the Gaussian distribution of unitary conductance (pS) measurements for the digested ColE9 translocon, where the mean value is one third the OmpF conductance observed in C. (E) Electrical recording (−50 mV) for a single OmpF trimer showing the blockade of its three channels by the OBS1 peptide, states assigned as in A. (F) Electrical recording (−50 mV) for the trypsin digested translocon showing the blockade of its single OmpF channel by the OBS1 peptide, states assigned as in B.
Two trypsin sites, one in each OBS, remained undigested when the translocon was treated with trypsin whereas the same sites were readily proteolysed in a disulfide-bonded complex containing just ColE9 and TolB (Figure S5B,D,F). These differential protease accessibility results are incompatible with a simple sequential translocation model in which OBS2 is anchored in the porin and OBS1 merely passes unhindered to the periplasm (Figure S6A-C). Instead, a single OmpF trimer may bind to both OBS sequences simultaneously (Figure S6A,B,D,E). To test this hypothesis we used ESI-MS and electrical recordings in planar lipid bilayers to compare how many pores were available in OmpF and the proteolysed translocon to bind a 17-residue ColE9 OBS1 peptide. ESI-MS data of OmpF yielded well-resolved spectra and a mass very close to that predicted for the trimer (Figure 3A and Table S2). When the OBS1 peptide was titrated into OmpF additional m/z states appeared corresponding to non-covalent binding of OBS1 in all three of its pores. By contrast, when the OBS1 peptide was added to the trypsin digested translocon a single peptide was bound indicating that only one of the OmpF pores remained accessible (Figure 3B). Furthermore, we were able to determine a Kd for the digested translocon complex with the OBS1 peptide by ESI-MS, which matched that for the OmpF-OBS1 complex obtained by ITC under the same buffer conditions (Kd~1 μM) (Figure S7). Thus, the interactions of the OBS1 sequence within the porin remain fundamentally the same in the two complexes.
In a parallel approach, the electrical activity of OmpF and the proteolysed ColE9 translocon were compared. OmpF produces voltage-gated ion channels when incorporated into planar lipid bilayers that can be blocked by a variety of molecules, including colicin IUTD sequences (18). Purified OmpF had a mean conductance of ~679 (±13) pS, representing the ion conductivity of all three pores within the trimer (Figure 3C). In contrast, the mean unitary conductance for the trypsin-digested translocon was one-third this value (217 (±7) pS), consistent with the translocon having only one of its three OmpF subunits able to conduct ions (Figure 3D). When the OBS1 peptide was added to OmpF we saw sequential blockades and re-openings of each of its three conducting subunits (Figure 3E and Figure S8). The proteolysed translocon channel was also blocked by OBS1, but its blockade was manifest as a simple two-state current trace consistent with the peptide occluding one OmpF pore (Figure 3F). Thus, the ColE9 translocon involves its porin binding sequences occupying two of the three subunits of OmpF (Figure S6E). As a consequence, the colicin’s IUTD must traverse the hydrophobic bilayer of the OM twice, its many charged and polar residues, typical of intrinsically disordered protein sequences (19), sequestered within the solvated pores of the oligomeric porin. This threading mechanism also implies that OBS1 has the ability to insert into an OmpF subunit in either orientation; N-to-C when the colicin first docks (Figure S6A), as observed in the crystal structure of the OmpF-OBS1 complex (12), and then C-to-N when it passes into a neighboring subunit from the periplasmic side of the porin (Figure S6E).
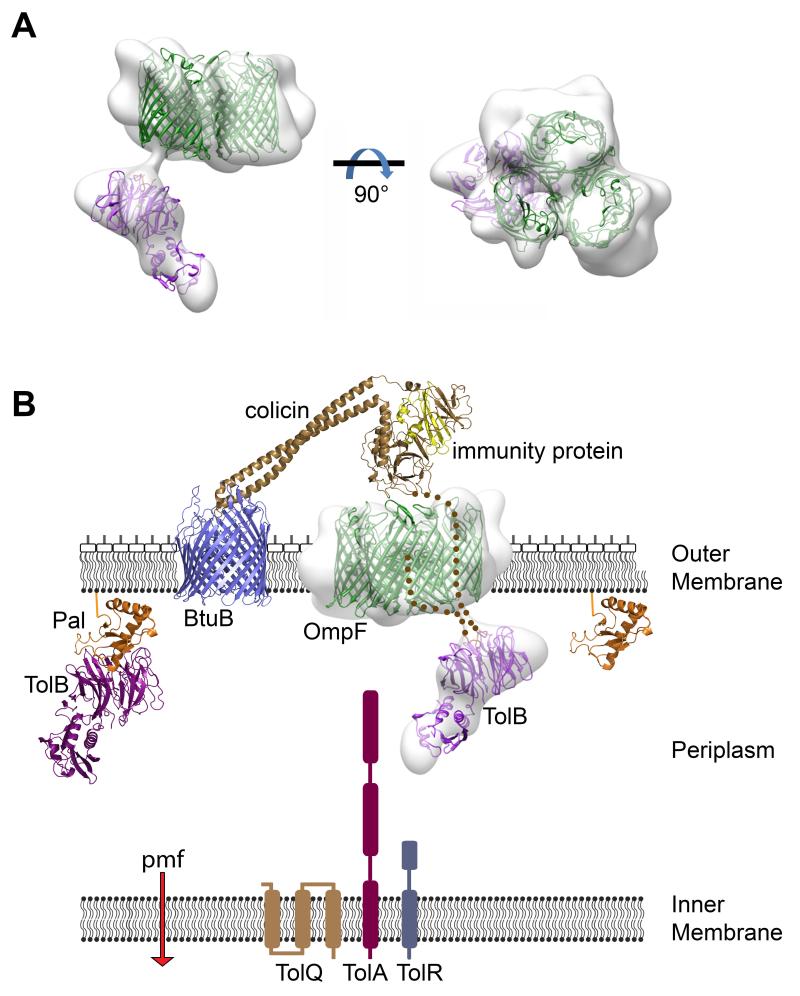
Negative stain electron microscopy (EM) structural analysis of the proteolysed translocon highlighted several important features of the assembly (Figure 4A). First, TolB was well resolved, held ~20 Å from OmpF by ColE9 for which connecting density was also observed. Second, TolB was positioned asymmetrically relative to OmpF, tilted ~45° with respect to the membrane. TolB is part of the Tol-Pal system that includes the inner membrane TolQ-TolR-TolA complex and the lipoprotein Pal, which together help stabilize the OM (7). The ColE9 TBE is an allosteric activator of TolB, inducing its association with TolA (20) thereby connecting the colicin to the proton motive force across the inner membrane (Figure 4B) (21). Formation of this network initiates colicin entry by triggering release of the tightly bound Im9 in a step that likely involves remodeling of the nuclease (22, 23). Tethering TolB to OmpF via the colicin’s two porin binding sites ensures TolB does not rotate but rather is presented to TolA in a fixed orientation. Although neither OBS sequence is essential for ColE9 activity (at least one must be present) cell killing is significantly enhanced by having both present (12), suggesting that restricting TolB rotation increases the efficiency of translocation. Third, the intrinsically disordered ColE9 TBE has a kinetic advantage in binding TolB relative to Pal, the endogenous binding partner of TolB with which it competes (24, 25). Constraining the ColE9 TBE through two OmpF attachment points might further enhance such competition by lowering the entropic penalties associated with binding TolB.
Figure 4. ColE9 uses OmpF to capture TolB on the other side of the membrane in a defined orientation.
(A) Negative stain EM structure at ~20 Å resolution of the trypsin digested ColE9 translocon comprising OmpF trimer and TolB connected by ColE9 T2-122, seen in side (left) and face views (right) of the membrane plane. OmpF and TolB crystal structures have been manually docked into the density as rigid bodies. (B) Structural representation of the ColE9 translocon – the structure for the related ColE3-Im3 bound to BtuB is shown (pdb accession code 1UJW) – and its protein-protein interaction network across the Gram-negative cell envelope (see text for details) (14, 21)
Supplementary Material
Acknowledgements
We thank S. Johnson (Pathology, Oxford) for help with SEC-MALS experiments and D. Ashford (Biology, York) for LC-MS data. NGH acknowledges the Department of Biochemistry (Oxford) for financial support. DRL was supported by the NIH (ROI HG003709). CVR acknowledges the MRC, ERC Impress and the Royal Society for financial support. HRS acknowledges support from BBSRC, ERC and The Wellcome Trust. This work was supported by grants to CK from The Wellcome Trust (WT082045) and the BBSRC (BB/G020671/1). Atomic coordinates and structural amplitudes have been deposited in the Protein Data Bank under accession number 4JML.
Footnotes
References and Notes
- 1.Schulz GE. Bacterial porins: structure and function. Curr Opin Cell Biol. 1993;5:701. doi: 10.1016/0955-0674(93)90143-e. [DOI] [PubMed] [Google Scholar]
- 2.Zeth K, Thein M. Porins in prokaryotes and eukaryotes: common themes and variations. Biochem J. 2010;431:13. doi: 10.1042/BJ20100371. [DOI] [PubMed] [Google Scholar]
- 3.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schabert FA, Henn C, Engel A. Native Escherichia coli OmpF porin surfaces probed by atomic force microscopy. Science. 1995;268:92. doi: 10.1126/science.7701347. [DOI] [PubMed] [Google Scholar]
- 5.Housden NG, Loftus SR, Moore GR, James R, Kleanthous C. Cell entry mechanism of enzymatic bacterial colicins: porin recruitment and the thermodynamics of receptor binding. Proc Natl Acad Sci U S A. 2005;102:13849. doi: 10.1073/pnas.0503567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kurisu G, et al. The structure of BtuB with bound colicin E3 R-domain implies a translocon. Nat. Struct. Biol. 2003;10:948. doi: 10.1038/nsb997. [DOI] [PubMed] [Google Scholar]
- 7.Cascales E, et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158. doi: 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirkup BC, Riley MA. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature. 2004;428:412. doi: 10.1038/nature02429. [DOI] [PubMed] [Google Scholar]
- 9.Stecher B, et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl. Acad. Sci. USA. 2012;109:1269. doi: 10.1073/pnas.1113246109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cornforth DM, Foster KR. Competition sensing: the social side of bacterial stress responses. Nat Rev Microbiol. 2013;11:285. doi: 10.1038/nrmicro2977. [DOI] [PubMed] [Google Scholar]
- 11.Kleanthous C, et al. Structural and mechanistic basis of immunity toward endonuclease colicins. Nat Struct Biol. 1999;6:243. doi: 10.1038/6683. [DOI] [PubMed] [Google Scholar]
- 12.Housden NG, et al. Directed epitope delivery across the Escherichia coli outer membrane through the porin OmpF. Proc Natl Acad Sci U S A. 2010;107:21412. doi: 10.1073/pnas.1010780107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loftus SR, et al. Competitive recruitment of the periplasmic translocation portal TolB by a natively disordered domain of colicin E9. Proc. Natl. Acad. Sci. USA. 2006;103:12353. doi: 10.1073/pnas.0603433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleanthous C. Swimming against the tide: progress and challenges in our understanding of colicin translocation. Nat Rev Microbiol. 2010;8:843. doi: 10.1038/nrmicro2454. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita E, Zhalnina MV, Zakharov SD, Sharma O, Cramer WA. Crystal structures of the OmpF porin: function in a colicin translocon. EMBO J. 2008;27:2171. doi: 10.1038/emboj.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrera NP, Di Bartolo N, Booth PJ, Robinson CV. Micelles protect membrane complexes from solution to vacuum. Science. 2008;321:243. doi: 10.1126/science.1159292. [DOI] [PubMed] [Google Scholar]
- 17.Laganowsky A, Reading E, Hopper JTS, Robinson CV. Mass spectrometry of intact membrane protein complexes. Nat Protocols. 2013 doi: 10.1038/nprot.2013.024. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zakharov SD, et al. Colicin occlusion of OmpF and TolC channels: outer membrane translocons for colicin import. Biophys.J. 2004;87:3901. doi: 10.1529/biophysj.104.046151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat.Rev.Mol.Cell Biol. 2005;6:197. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 20.Bonsor DA, et al. Allosteric beta-propeller signalling in TolB and its manipulation by translocating colicins. EMBO J. 2009;28:2846. doi: 10.1038/emboj.2009.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jakes KS, Cramer WA. Border crossings: colicins and transporters. Annu Rev Genet. 2012;46:209. doi: 10.1146/annurev-genet-110711-155427. [DOI] [PubMed] [Google Scholar]
- 22.Vankemmelbeke M, et al. Energy-dependent immunity protein release during tol-dependent nuclease colicin translocation. J Biol Chem. 2009;284:18932. doi: 10.1074/jbc.M806149200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrance OE, et al. A force-activated trip switch triggers rapid dissociation of a colicin from its immunity protein. PLoS Biol. 2013;11:e1001489. doi: 10.1371/journal.pbio.1001489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadakos G, Housden NG, Lilly KJ, Kaminska R, Kleanthous C. Kinetic basis for the competitive recruitment of TolB by the intrinsically disordered translocation domain of colicin E9. J Mol Biol. 2012;418:269. doi: 10.1016/j.jmb.2012.01.039. [DOI] [PubMed] [Google Scholar]
- 25.Bonsor DA, Grishkovskaya I, Dodson EJ, Kleanthous C. Molecular Mimicry Enables Competitive Recruitment by a Natively Disordered Protein. J. Am. Chem. Soc. 2007;15:4800. doi: 10.1021/ja070153n. [DOI] [PubMed] [Google Scholar]
- 26.Garinot-Schneider C, Penfold CN, Moore GR, Kleanthous C, James R. Identification of residues in the putative TolA box which are essential for the toxicity of the endonuclease toxin colicin E9. Microbiology. 1997;143(Pt 9):2931. doi: 10.1099/00221287-143-9-2931. [DOI] [PubMed] [Google Scholar]
- 27.Carr S, Penfold CN, Bamford V, James R, Hemmings AM. The structure of TolB, an essential component of the tol-dependent translocation system, and its protein-protein interaction with the translocation domain of colicin E9. Structure. 2000;8:57. doi: 10.1016/s0969-2126(00)00079-4. [DOI] [PubMed] [Google Scholar]
- 28.Law CJ, et al. OmpF enhances the ability of BtuB to protect susceptible Escherichia coli cells from colicin E9 cytotoxicity. FEBS letters. 2003;545:127. doi: 10.1016/s0014-5793(03)00511-8. [DOI] [PubMed] [Google Scholar]
- 29.Dombkowski AA. Disulfide by Design: a computational method for the rational design of disulfide bonds in proteins. Bioinformatics. 2003;19:1852. doi: 10.1093/bioinformatics/btg231. [DOI] [PubMed] [Google Scholar]
- 30.Kabsch W. Xds. Acta Crystallogr D Biol Crystallogr. 2010;66:125. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evans PR. Data collection and Processing. Proceedings of the CCP4 Study Weekend. 1993:114. [Google Scholar]
- 32.Winn MD, et al. Overview of the CCP4 suite and current developments. Acta Crystallogr D Biol Crystallogr. 2011;67:235. doi: 10.1107/S0907444910045749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kantardjieff KA, Rupp B. Matthews coefficient probabilities: Improved estimates for unit cell contents of proteins, DNA, and protein-nucleic acid complex crystals. Protein Sci. 2003;12:1865. doi: 10.1110/ps.0350503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matthews BW. Solvent content of protein crystals. J Mol Biol. 1968;33:491. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- 35.Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. Journal of Applied Crystallography. 1997;30:1022. [Google Scholar]
- 36.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr. 1997;53:240. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 37.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 38.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr. 2010;66:12. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slotboom DJ, Duurkens RH, Olieman K, Erkens GB. Static light scattering to characterize membrane proteins in detergent solution. Methods. 2008;46:73. doi: 10.1016/j.ymeth.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Hernandez H, Robinson CV. Determining the stoichiometry and interactions of macromolecular assemblies from mass spectrometry. Nat Protoc. 2007;2:715. doi: 10.1038/nprot.2007.73. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 42.Maglia G, Heron AJ, Stoddart D, Japrung D, Bayley H. Analysis of single nucleic acid molecules with protein nanopores. Methods Enzymol. 2010;475:591. doi: 10.1016/S0076-6879(10)75022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mastronarde DN. Automated electron microscope tomography using robust prediction of specimen movements. J Struct Biol. 2005;152:36. doi: 10.1016/j.jsb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J Struct Biol. 1996;116:71. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 45.Nicastro D, et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- 46.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 47.Frank J, et al. SPIDER and WEB: processing and visualization of images in 3D electron microscopy and related fields. J Struct Biol. 1996;116:190. doi: 10.1006/jsbi.1996.0030. [DOI] [PubMed] [Google Scholar]
- 48.Crowther RA, Henderson R, Smith JM. MRC image processing programs. J Struct Biol. 1996;116:9. doi: 10.1006/jsbi.1996.0003. [DOI] [PubMed] [Google Scholar]
- 49.Ludtke SJ, Baldwin PR, Chiu W. EMAN: semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 50.Pettersen EF, et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 51.Potterton L, et al. Developments in the CCP4 molecular-graphics project. Acta Crystallogr D Biol Crystallogr. 2004;60:2288. doi: 10.1107/S0907444904023716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.