Abstract
Objective:
The aim of our study was to compare volume change in grey matter (GM) and white matter (WM) in a group of subjects with anosmia and a healthy control group. We tried to find a regular pattern of atrophy within and between GM and WM and to determine whether any particular areas are more sensitive to olfactory injury.
Methods:
There were 19 anosmic patients and 20 age- and sex-matched control subjects. We acquired MR images on a 3-T scanner and performed voxel-based morphometry using the VBM8 toolbox and SPM8 in a MATLAB® (MathWorks®, Natick, MA) environment.
Results:
Patients with anosmia showed a significant decrease in GM volume, mainly in the anterior cingulate cortex, middle temporal gyrus, superior temporal gyrus, fusiform gyrus, supramarginal gyrus, superior frontal gyrus, middle frontal gyrus, middle occipital gyrus, anterior insular cortex and cerebellum. In addition, we observed volume decreases in smaller areas such as the piriform cortex, the inferior temporal gyrus, the precuneus and the subcallosal gyrus. All WM areas with atrophy were near those GM areas that experienced volume loss. There was more volume atrophy in GM areas corresponding to WM areas with more volume loss. Atrophy increased with disease duration.
Conclusion:
There is simultaneous atrophy in GM and WM, and the degree of atrophy is greater with longer disease duration. Different GM and WM areas have different sensitivities to olfactory injury.
Advances in knowledge:
This study examines the atrophy pattern in and between GM and WM—a subject that has not been widely researched previously.
Chronic olfactory disorders are common and are usually induced by olfactory infections, sinonasal diseases, head trauma, toxins and drugs or congenital olfactory loss [1]. There are relatively few published studies using functional MRI for detailed investigation of olfactory disorders compared with the number of published studies of disorders of other sensory systems. The few existing studies found that the olfactory bulb (OB) exhibits high plasticity, depending on the olfactory input [2–5]. Only a few studies have focused on cortical brain areas beyond the OB. We know little about the typical patterns and variability in anosmia. Bitter et al [6] recently reported grey matter (GM) atrophy associated with anosmia and concluded that atrophy is more extensive with longer disease duration. These authors found that some GM areas with atrophy correspond to functional olfactory areas in the brains of healthy subjects, which supports the conclusion that the structural changes lead to dysfunction in anosmics. They also found GM and white matter (WM) atrophy in hyposmics [7]. WM atrophy was spatially connected to areas of GM volume loss. However, these studies did not investigate WM change or changes in the relationship between GM and WM in anosmia.
The aim of our study was to determine whether there were structural changes in areas of human brain in anosmic patients using voxel-based morphometry (VBM). We also sought to compare our findings concerning areas of GM and WM atrophy with those reported previously, to discover regular patterns of atrophy within and between GM and WM areas and to determine whether some areas are more sensitive to olfactory injury. Our hypothesis was that we would find that structural alterations may occur in areas involved in olfactory information. We predicted a decrease of GM and WM and a relationship between GM and WM changes. We also predicted that atrophy would be greater with longer disease duration.
EXPERIMENTAL PROCEDURE
Subjects
All participants in the study gave their written consent, and the study was approved by the ethical committee of our hospital. There were 19 anosmic patients (5 males and 14 females) and 20 sex- and age-matched control subjects (6 males and 14 females). All participants were right-handed. The threshold discrimination identification score for the anosmic patients was determined by the T&T test. The quantitative analysis using a T&T olfactometer (Daiichi Sankyo Pharmaceutical Co. Ltd; Tokyo, Japan) was based on the dilution ratio of five odourants (rose, scorched, rotten, fruit and stool); each substance at eight concentrations (10−2 to 105) represented 8° (−2 to 5). For birhinal testing, different odourants were placed 1–2 cm in front of the nostrils for two or three sniffs. Averaged odour threshold, which was obtained by dividing the sum of the identification threshold for five olfactory substances by five, was used to judge the degree of olfaction. All scores for the anosmic patients were >5.5, whereas scores for the control subjects ranged from −1.0 to 1.2. The 19 anosmic subjects included 1 who was idiopathic, 13 who were post-infectious, and 4 who were post-traumatic after a minor head injury. Each participant was healthy, breathed normally through each nostril and had no subjective nasal pathology. No subjects had structural brain lesions, other neurological or psychiatric deficits or chronic rhinosinusitis. The duration of olfactory loss ranged from 2 months to 20 years with a mean of 3.12 years. The anosmic subjects were aged between 28 and 57 years with a mean of 45.3±10.2 years. The control subjects were aged from 24 to 59 years with a mean of 43.6±14.8 years.
MRI data acquisition
All MR data were obtained using a 3.0-T scanner (MAGNETOM® TrioTim System; Siemens Medical Solutions, Erlangen, Germany) using a standard receive 8-channel head coil. Three-dimensional whole-brain high-resolution T1 weighted (1 mm3) images were acquired. Repetition time = 1900 ms, echo time = 2.52 ms, flip angle = 9°, 176 slices, slice thickness=1 mm, matrix = 250×250; in-plane voxel size = 1×1×1 mm, total acquisition time, 5:40 min.
Voxel-based morphometry and statistical analysis
Data were processed using Statistical Parametric Mapping software v. 8 (SPM8) (Wellcome Department of Imaging Neuroscience Group, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm), where we applied VBM implemented in the DARTEL Tools (http://resting-fmri.sourceforge.net/) with default parameters. Images were preprocessed, which included bias-corrected, tissue-classified and registered data using linear (12-parameter affine) and non-linear transformations (warping), within a unified model. Analyses were subsequently performed on GM and WM segments. Finally, the modulated volumes were smoothed with a gaussian kernel of 8 mm full width at half maximum. Voxel-wise GM and WM differences between anosmic patients and controls were examined using independent sample t-tests. We used a significance level of p<0.001 for all comparisons, and only clusters exceeding a size of 100 voxels were reported. We divided the anosmic patients into two groups to investigate the effects of disease duration. One subgroup had disease duration of less than 1 year and consisted of nine patients (eight females and one male; mean age, 41.3±1.0 years; mean time since olfactory loss, 4.8±2.1 months). The other subgroup, with disease duration of more than 1 year, consisted of 10 patients (6 females and 4 males; mean age, 48.9±9.16 years; mean time since olfactory loss, 6.4±6.7 years). We used a level of significance of p<0.001 for all comparisons.
RESULTS
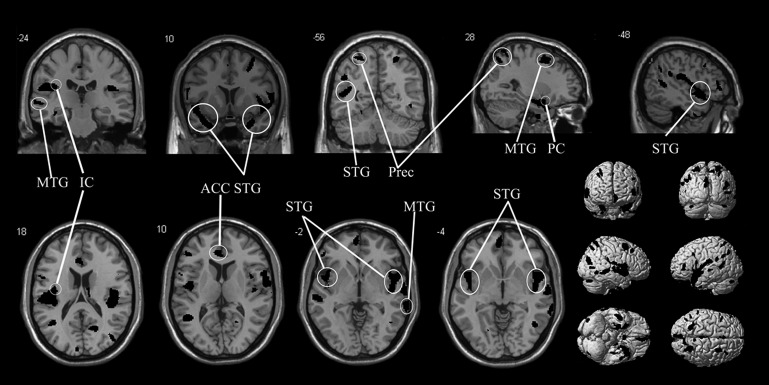
We found many areas of significant volume loss in GM in the anosmic group (Figure 1 and Table 1). The main atrophic areas were in the anterior cingulate cortex (ACC), orbitofrontal cortex (OFC), middle temporal gyrus (MTG), superior temporal gyrus, fusiform gyrus, supramarginal gyrus, superior frontal gyrus, middle frontal gyrus, middle occipital gyrus (MOG), cerebellum and the anterior insular cortex (IC). Smaller atrophic areas were seen in the piriform cortex (PC), the inferior temporal gyrus, the precuneus (Prec) and the subcallosal gyrus (SCG). IC, PC, Prec and SCG atrophy were found only on the right side (Table 1).
Figure 1.
This image shows the reductions in white matter in 19 patients with anosmia compared with 20 healthy controls. The level of significance is p<0.001 and images overlay onto the average T1 image of all subjects. The top row shows two sagittal slices and three coronal slices, whereas in the bottom row shows four axial slices and exterior views. ACC, anterior cingulate cortex; IC, anterior insular cortex; MTG, middle temporal gyrus; PC, piriform cortex; Prec, precuneus; STG, superior temporal gyrus.
Table 1.
Reduction in grey matter in 19 patients with anosmia compared with 20 healthy controls
| Region | Side | MNI coordinates (mm) | Z-score | Cluster size (voxels) | ||
| x | y | z | ||||
| Anterior cingulate cortex | R | 14 | −12 | 36 | 6.23 | 48 |
| L | −3 | 40.5 | 9 | 4.98 | 519 | |
| Orbitofrontal cortex | R | 20 | 45 | −9 | 2.40 | 11 |
| L | −3 | 51 | −6 | 5.39 | 463 | |
| Middle temporal gyrus | R | 72 | −36 | −3 | 6.05 | 679 |
| L | −57 | −36 | 4 | 3.78 | 279 | |
| Inferior temporal gyrus | R | 72 | −40.5 | −9 | 5.51 | 60 |
| L | −63 | −21 | −21 | 3.82 | 160 | |
| Superior temporal gyrus | R | 27 | 11 | −27 | 6.25 | 1987 |
| L | −36 | 12 | −23 | 7.27 | 2287 | |
| Middle frontal gyrus | R | 59 | 21 | 27 | 4.41 | 731 |
| L | −39 | 7.5 | 57 | 4.53 | 477 | |
| Superior frontal gyrus | R | 12 | 39 | 55.5 | 5.53 | 158 |
| L | −14 | 24 | 49 | 4.42 | 34 | |
| Middle occipital gyrus | R | 57 | −73 | 6 | 4.21 | 164 |
| L | −19.5 | −66 | −9 | 4.20 | 78 | |
| Parahippocampal gyrus/fusiform gyrus | R | 25.5 | 1.5 | −19.5 | 7.25 | 398 |
| L | −21 | 5 | −32 | 5.64 | 345 | |
| Supramarginal gyrus | R | 50 | −33 | 25 | 5.39 | 249 |
| L | −51 | −22.5 | 21 | 7.32 | 862 | |
| Cerebellum | R | 39 | −73.5 | −25.5 | 4.23 | 262 |
| L | −42 | −75 | −28.5 | 4.56 | 151 | |
| Anterior insular cortex | R | 46.5 | 9 | −4.5 | 3.01 | 1023 |
| Piriform cortex | R | 24 | 10 | −10 | 2.81 | 25 |
| Subcallosal gyrus | R | 7 | 6 | −15 | 2.76 | 28 |
| Precuneus | R | 20 | −52 | 15 | 2.93 | 133 |
L, left; MNI, Montreal Neurological Institute; R, right.
Level of significance is p<0.001 and only clusters exceeding a size of 100 voxels are reported. All coordinates are given in MNI space.
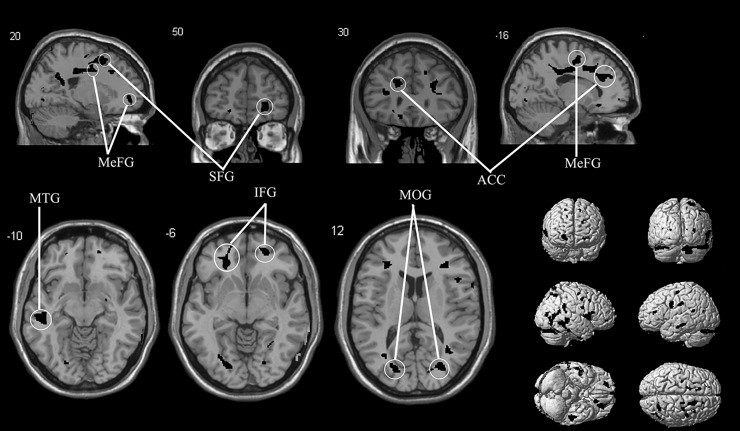
In the VBM WM analysis, various atrophic areas were found in areas corresponding to GM areas and additionally in the inferior frontal gyrus and medial frontal gyrus (Figure 2 and Table 2). WM atrophic areas occurred mainly near or around the corresponding GM atrophy (Table 2). No significant GM or WM volume increases were observed. Patients with a disease duration of more than 1 year showed more extensive atrophy in these areas than did patients with disease duration of less than 1 year (Figure 3 and Figure 4).
Figure 2.
This image shows the reductions in grey matter in 19 patients with anosmia compared with 20 healthy controls. The level of significance is p<0.001 and images overlay onto the average T1 image of all subjects. The top row shows two sagittal slices and two coronal slices, whereas the bottom row shows three axial slices and exterior views. ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; MeFG, medial frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; SFG, superior frontal gyrus.
Table 2.
Reduction in white matter in 19 patients with anosmia compared with 20 healthy controls
| Region | Side | MNI coordinates (mm) | Z-score | Cluster size (voxels) | ||
| x | y | z | ||||
| Anterior cingulate cortex | R | 14 | 14 | 28 | 4.60 | 44 |
| L | −17 | 32 | 25 | 5.67 | 123 | |
| Orbitofrontal cortex | R | 40 | 26 | −15 | 4.78 | 109 |
| L | −15 | 27 | −14 | 6.09 | 295 | |
| Middle temporal gyrus | R | 71 | −33 | −2 | 4.89 | 79 |
| L | −48 | −28 | −12 | 6.44 | 403 | |
| Inferior temporal gyrus | R | 62 | −44 | −9 | 4.23 | 108 |
| L | −47 | −43 | −12 | 3.21 | 25 | |
| Superior temporal gyrus | R | 27 | 11 | −29 | 5.92 | 162 |
| L | −49.5 | −46.5 | 18 | 4.99 | 152 | |
| Middle frontal gyrus | R | 23 | 43.5 | −0 | 7.23 | 362 |
| L | −21 | 47 | −8 | 4.91 | 416 | |
| Superior frontal gyrus | R | 13 | 22 | 45 | 5.21 | 324 |
| L | −12 | 18 | 54 | 4.16 | 150 | |
| Middle occipital gyrus | R | 30 | −76.5 | 10.5 | 5.77 | 327 |
| L | −32 | −78 | 3 | 3.55 | 39 | |
| Cerebellum | R | 41 | −76 | −26 | 2.54 | 4 |
| L | −51 | −76 | −32 | 3.19 | 43 | |
| Insular cortex-thalamus | R | 47 | 11 | −6 | 3.90 | 105 |
| L | −45 | 10 | −8 | 3.81 | 28 | |
| Inferior frontal gyrus | R | 45 | −1 | 25 | 5.63 | 399 |
| L | −54 | 40.5 | 3 | 4.71 | 625 | |
| Medial frontal gyrus | R | 17 | 30 | 39 | 5.04 | 354 |
| L | −16.5 | −3 | 52.5 | 6.55 | 303 | |
L, left; MNI, Montreal Neurological Institute; R, right.
Level of significance is p<0.001 and only clusters exceeding a size of 100 voxels are reported. All coordinates are given in MNI space.
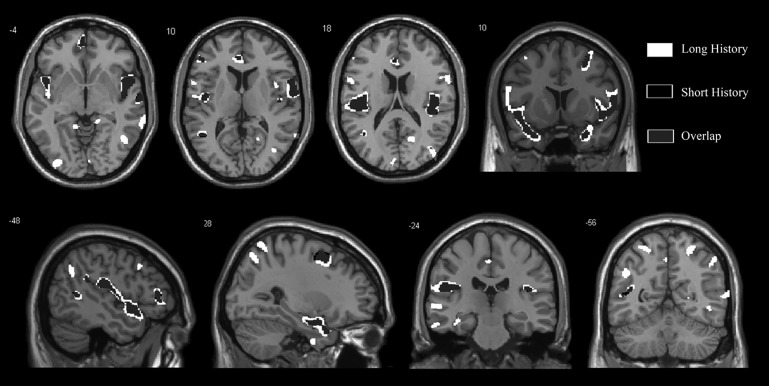
Figure 3.
This image demonstrates the overlap (grey) between the atrophy in white matter in 9 patients with anosmia for less than 1 year (black) compared with 10 patients with anosmia for more than 1 year (white). The level of significance of the voxel-based morphometry results is p<0.001 and images overlay onto the average T1 image of all subjects. The top row shows one coronal slice and three axial slices, whereas the bottom row shows two coronal slices and two sagittal slices.
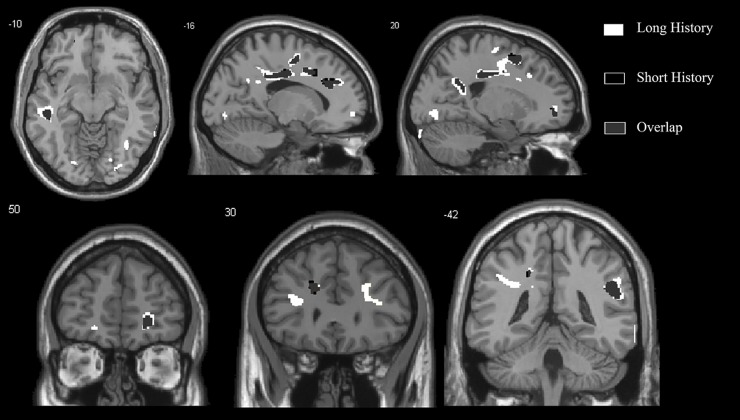
Figure 4.
This image demonstrates the overlap (grey) between the atrophy in grey mater in 9 patients with anosmia for less than 1 year (black) compared with 10 patients with anosmia for more than 1 year (white). The level of significance of the voxel-based morphometry results is p<0.001 and images overlay onto the average T1 image of all subjects. The top row shows two sagittal slices and one axial slice, whereas the bottom row shows three coronal slices.
DISCUSSION
Olfaction is traditionally thought to involve primary and secondary olfactory areas. The primary cortices consist of OB, PC, entorhinal cortex, amygdala, anterior olfactory nucleus and olfactory tubercle [8,9]. The secondary olfactory areas include IC, OFC, ACC, hippocampus and thalamus. Involvement of these structures has been confirmed by functional brain research.
For the primary olfactory cortex, we could see PC atrophy in the right GM, but we found no WM atrophy. We found no volume loss in the left PC. The reason for this unilateral volume loss remains unclear, but some reports suggest it is because of right hemispheric dominance. Using VBM, we found no other volumetric changes in other primary olfactory cortical areas. VBM may not have provided enough resolution to find changes in these small structures.
Atrophy was found in secondary olfactory areas, specifically IC, OFC and ACC. GM atrophy was found only in the right IC, and there was more left than right GM atrophy in ACC and OFC. We found more WM atrophy in the left ACC and OFC than in the right ACC and OFC, and no WM atrophy was found in the IC. Several functional imaging studies have shown stronger GM activity in the right IC than in the left IC during olfactory tasks, which might be explained by laterality in olfactory processing at the IC level [10–12]. Gottfried [8] and Shepherd [9] found that the medial prefrontal cortex (MPC), which includes the ACC, is closely related to olfactory sensation, and the ACC has been called a key node in the “flavor network” [13]. Furthermore, Varney et al [14] showed hypometabolism in the MPC as well as in the OFC in a positron emission tomography study on patients with post-traumatic anosmia. This hypometabolism could be explained by the GM and WM decreases in the MPC and OFC as measured by VBM in our study.
In addition to these well-known functional olfactory areas, we found atrophy in other areas, such as MOG and Prec, which are considered to play an important role in the recall of episodic memories during olfactory matching [15]. We also found both GM and WM atrophy in the cerebellum, with more right than left GM atrophy in the cerebellum. Sobel et al [16] suggested that the cerebellum receives olfactory information for modulating sniffing, which, in turn, modulates olfactory input. The study by Savic et al [17,18] found that the right cerebellum is activated during discrimination of odour intensity, odour quality and odour recognition memory, whereas the left cerebellum is activated by episodic odour recognition memory retrieval. Cerf-Ducastel and Murphy [19] found that a cross-modal olfactory recognition memory aroused cerebellar activation. Their data demonstrate that the cerebellum not only modulates sniffing but is also involved in olfactory cognitive processing. In addition, the fusiform gyrus showed bilateral atrophy. This function of the area in olfaction is still not clear, but its role in chemosensory processing has previously been demonstrated [20].
Our primary interest is in relationships between GM and WM atrophy. We found that greater volume atrophy in some GM areas was accompanied by greater volume atrophy in corresponding WM areas. Using SPSS® v. 16.0 (SPSS Inc., Chicago, IL) to analyse the relationship between GM and WM, we did indeed find positive correlations between atrophy in corresponding GM and WM areas. We can thus assume that GM and WM alteration is interrelated, not independent. GM changes are proportional to WM changes. We speculate that GM is injured more seriously and earlier than WM in anosmia, resulting in larger GM atrophies.
As predicted, we did find a significant positive correlation between disease duration and the degree of the GM and WM atrophy. We separated the anosmic patients into two subgroups using disease duration of less or more than 1 year and found that those with duration of more than 1 year had significantly more atrophy than those with duration of less than 1 year. We conclude that GM and WM atrophy becomes more serious as anosmia progresses.
There was no compensatory increase of GM or WM in the anosmic group. By contrast, auditory or visual dysfunction always leads to a compensatory volume increase in other sensory areas [21,22]. That is why a blind man will have more sensitive tactile sensation and hearing. However, there is no compensation for an olfactory disorder by the other senses. Conversely, patients with hyposmia show a decrease in gustatory function [23].
Our research confirmed Bitter's results that anosmia can lead to some primary and secondary olfactory cortex atrophy, that the degree of atrophy is positively related to the duration of anosmia and that there is no compensation in other sensory areas. However, we found atrophy in different areas. For instance, Bitter found more right GM atrophy in the ACC and MTG and left cerebellum atrophy in hyposmics, whereas there was more right cerebellum atrophy and left ACC and MTG atrophy in anosmic patients [6,7]. We found more right MTG atrophy, more right cerebellar atrophy and more left ACC atrophy in anosmics. One possible reason for these differences is that a relatively small number of subjects was used in both studies. Use of more subjects might yield comparable results. The studies may also differ in disease duration. There was a mean disease duration of 3.12 years in our research compared with Bitter's 4.15 years. It may be that most of the anosmic patients in our study had a shorter history of anosmia. Our patients were intermediate in the degree of atrophy with regard to those of Bitter's two studies on anosmic patients and hyposmic patients [6,7]. We found that the volume loss in the ACC in our study was smaller than that in Bitter's anosmic study and larger than that in Bitter's hyposmic study. This is additional evidence that MPC volume is strongly correlated with olfactory performance. We thus presume that some brain areas are differentially sensitive to olfaction injury at different stages of the disease process. This hypothesis needs to be confirmed by further research using more patients at different stages of the disease process.
In brief, our study demonstrates that anosmia does induce brain structure changes similar to those caused by other sensory defects, but without compensatory mechanisms. GM and WM atrophy simultaneously and the degree of atrophy are related to disease duration. Our future research will explore whether different GM and WM areas have different sensitivity to olfactory injury.
REFERENCES
- 1.Nordin S, Brämerson A. Complaints of olfactory disorders: epidemiology, assessment and clinical implications. Curr Opin Allergy Clin Immunol 2008;8:10–15 10.1097/ACI.0b013e3282f3f473 [DOI] [PubMed] [Google Scholar]
- 2.Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Olfactory function and olfactory bulb volume in patients with postinfectious olfactory loss. Laryngoscope 2006;116:436–9 [DOI] [PubMed] [Google Scholar]
- 3.Rombaux P, Mouraux A, Bertrand B, Nicolas G, Duprez T, Hummel T. Retronasal and orthonasal olfactory function in relation to olfactory bulb volume in patients with posttraumatic loss of smell. Laryngoscope 2006;116:901–5 10.1097/01.mlg.0000217533.60311.e7 [DOI] [PubMed] [Google Scholar]
- 4.Mueller A, Rodewald A, Reden J, Gerber J, von Kummer R, Hummel T. Reduced olfactory bulb volume in post-traumatic and post-infectious olfactory dysfunction. Neuroreport 2005;16:475–8 [DOI] [PubMed] [Google Scholar]
- 5.Abolmaali N, Gudziol V, Hummel T. Pathology of the olfactory nerve. Neuroimaging Clin N Am 2008;18:233–42 10.1016/j.nic.2007.10.002 [DOI] [PubMed] [Google Scholar]
- 6.Bitter T, Gudziol H, Burmeister HP, Mentzel HJ, Guntinas-Lichius O, Gaser C. Anosmia leads to a loss of gray matter in cortical brain areas. Chem Senses 2010;35:407–15 10.1093/chemse/bjq028 [DOI] [PubMed] [Google Scholar]
- 7.Bitter T, Brüderle J, Gudziol H, Burmeister H.P, Gaser C, Guntinas-Lichius O. Gray and white matter reduction in hyposmic subjects—A voxel-based morphometry study. Brain Res 2010;1347:42–7 10.1016/j.brainres.2010.06.003 [DOI] [PubMed] [Google Scholar]
- 8.Gottfried JA. Smell: central nervous processing. Adv Otorhinolaryngol 2006;63:44–69 10.1159/000093750 [DOI] [PubMed] [Google Scholar]
- 9.Shepherd GM. Smell images and the flavour system in the human brain. Nature 2006;444:316–21 10.1038/nature05405 [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson S, Berglund H, Gulyas B, Cohen E, Savic I. Brain activation during odor perception in males and females. Neuroreport 2001;12:2027–33 [DOI] [PubMed] [Google Scholar]
- 11.Djordjevic J, Zatorre RJ, Petrides M, Boyle JA, Jones-Gotman M. Functional neuroimaging of odor imagery. Neuroimage 2005;24:791–801 10.1016/j.neuroimage.2004.09.035 [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci 2005;60:510–514 [DOI] [PubMed] [Google Scholar]
- 13.Small DM, Prescott J. Odor/taste integration and the perception of flavor. Exp Brain Res 2005;166:345–57 10.1007/s00221-005-2376-9 [DOI] [PubMed] [Google Scholar]
- 14.Varney NR, Pinkston JB, Wu JC. Quantitative PET findings in patients with posttraumatic anosmia. J Head Trauma Rehabil 2001;16:253–9 [DOI] [PubMed] [Google Scholar]
- 15.Qureshy A, Kawashima R, Imran MB, Sugiura M, Goto R, Okada K, et al. Functional mapping of human brain in olfactory processing: a pet study. J. Neurophysiol 2000;84:1656–66 [DOI] [PubMed] [Google Scholar]
- 16.Sobel N, Prabhakaran V, Hartley CA, Desmond JE, Zhao Z, Glover GH, et al. Odorant-induced and sniff-induced activation in the cerebellum of the human. J Neurosci 1998;18:8990–9001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron 2000;26:735–45 [DOI] [PubMed] [Google Scholar]
- 18.Savic I, Gulyas B, Berglund H. Odorous stimuli are processed differently depending on the cranial nerves involved. Neuroimage 2000;11:S692 [Google Scholar]
- 19.Cerf-Ducastel B, Murphy C. fMRI activation in response to odorants orally delivered in aqueous solutions. Chem Senses 2001;26:625–37 [DOI] [PubMed] [Google Scholar]
- 20.Zhou W, Chen D. Encoding human sexual chemosensory cues in the orbitofrontal and fusiform cortices. J Neurosci 2008;28:14416–21 10.1523/JNEUROSCI.3148-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penhune VB, Cismaru R, Dorsaint-Pierre R, Petitto LA, Zatorre RJ. The morphometry of auditory cortex in the congenitally deaf measured using MRI. Neuroimage 2003;20:1215–25 10.1016/S1053-8119(03)00373-2 [DOI] [PubMed] [Google Scholar]
- 22.Noppeney U, Friston KJ, Ashburner J, Frackowiak R, Price CJ. Early visual deprivation induces structural plasticity in gray and white matter. Curr Biol 2005;15:R488–90 10.1016/j.cub.2005.06.053 [DOI] [PubMed] [Google Scholar]
- 23.Gudziol H, Rahneberg K, Burkert S. Anosmics are more poorly able to taste than normal persons [in German]. Laryngorhinootologie 2007;86:640–3 10.1055/s-2007-966228 [DOI] [PubMed] [Google Scholar]