Abstract
Objective:
To test the feasibility of volumetric modulated arc therapy (VMAT) in breast cancer and to compare it with three-dimensional conformal radiotherapy (3D-CRT) as conventional tangential field radiotheraphy (conTFRT).
Methods:
12 patients (Stage I, 8: 6 left breast cancer and 2 right breast cancer; Stage II, 4: 2 on each side). Three plans were calculated for each case after breast-conserving surgery. Breast was treated with 50 Gy in four patients with supraclavicular lymph node inclusion, and in eight patients without the node inclusion. Multiple indices and dose parameters were measured.
Results:
V95% was not achieved by any modality. Heterogeneity index: 0.16 (VMAT), 0.13 [intensity-modulated radiotherapy (IMRT)] and 0.14 (conTFRT). Conformity index: 1.06 (VMAT), 1.15 (IMRT) and 1.69 (conTFRT). For both indices, IMRT was more effective than VMAT (p=0.009, p=0.002). Dmean and V20 for ipsilateral lung were lower for IMRT than VMAT (p=0.0001, p=0.003). Dmean, V2 and V5 of contralateral lung were lower for IMRT than VMAT (p>0.0001, p=0.005). Mean dose and V5 to the heart were lower for IMRT than for VMAT (p=0.015, p=0.002).
Conclusion:
The hypothesis of equivalence of VMAT to IMRT was not confirmed for planning target volume parameter or dose distribution to organs at risk. VMAT was inferior to IMRT and 3D-CRT with regard to dose distribution to organs at risk, especially at the low dose level.
Advances in knowledge:
New technology VMAT is not superior to IMRT or conventional radiotherapy in breast cancer in any aspect.
In Western countries, one in every eight females is diagnosed with breast cancer. Breast-conserving surgery with post-operative radiotherapy (RT) is the primary therapeutic strategy for Stages I and II of breast cancer. Systemic therapy is also part of the primary therapeutic strategy in most patients with Stage I and II breast cancer. RT substantially reduces the rate of local relapse and improves long-term survival [1]. However, RT is suggested to be associated with morbidity of the heart [2,3], lung [4,5], subcutaneous tissue and skin [6] and a risk of secondary malignancies [7–9].
A large body of available data regarding the potential toxicity of RT was published between 1980 and the end of 1990 [1]. Special clinical interest has been focused on acute and mostly transient lung and skin toxicity, axillary problems and late cardiac events, in addition to the risk of secondary malignancies. This period was characterised by RT delivery using a fluoroscopic technique with two-dimensional planning followed by three-dimensional (3D) conformal techniques with two conventional tangential field radiotherapy (conTFRT) fields. conTFRT encompassed the whole breast, skin, minor ipsilateral lung volume, a part of the axillary region at Level 1 and a part of the heart in the case of left-sided cancer [10–12]. These areas have been sites for local toxicity, because RT principles, and thus homogeneous photon flux across treatment fields, remained unchanged.
Intensity-modulated radiotherapy (IMRT) has been implemented in the past decade, permitting variation of fluence modulation across fields and allowing optimal dose administration according to an individual's anatomy. IMRT results in improved avoidance of critical structures such as the heart, skin, axillary region and lung, while facilitating necessary tumour volume coverage [13,14]. Clinical data on IMRT show an improvement in dose homogeneity within the irradiated breast and sparing of the heart and lung [14–17]. However, a disadvantage of IMRT over conTFRT is the long treatment duration owing to the higher number of fields and monitor units (MUs) involved. In addition, although IMRT reduces the volume of the heart and ipsilateral lung that receive high doses, it is associated with an increase in overall low-dose radiation. Despite the available clinical data, the wider use and specific indications for IMRT for breast cancer have not been established.
In volumetric modulated arc therapy (VMAT), technical extension of conventional fixed-field IMRT, an optimised dose distribution is possible with a single gantry rotation. Studies have shown that VMAT reduces the number of MUs and treatment delivery time [18–22], with similar or better planning target volume (PTV) coverage and sparing of organs at risk (OARs) than IMRT. Reports on VMAT for breast cancer are few and mainly concern planning comparisons [20,23–28] and very preliminary clinical data [29].
The RapidArc® system (Varian Medical Systems, Palo Alto, CA) has recently been introduced in our department. Accordingly, we have begun examining the potential of RapidArc VMAT for breast cancer treatment in a prospective clinical setting to adequately evaluate dosimetric parameters, treatment planning and clinical implications as well the disadvantages.
The present study aimed to compare the use of RapidArc VMAT with IMRT and conTFRT for breast cancer therapy. We hypothesised that the use of RapidArc under routine clinical circumstances would be equivalent to or better than IMRT and conTFRT in terms of PTV coverage and OAR sparing, while reducing both treatment time and MUs.
METHODS AND MATERIAL
Patient characteristics, affected breast and tumour stage
We selected 12 patients at Stage I (8 patients: 6 with left breast cancer and 2 with right breast cancer) and Stage II (4 patients: 2 each with left and right breast cancer) of the disease. All patients provided written informed consent, and the study was approved by the local review board. All patients underwent breast-conserving surgery and none received boost RT, owing to their advanced age (>60 years) or refusal. Whole-breast RT was given with (4 patients) and without (8 patients) supraclavicular lymph node inclusion (50 Gy to both target volumes) (Table 1).
Table 1.
Patient characteristics
| Patient | Age (years) | Disease side | Disease stage | Tumour size (mm) |
| 1 | 66 | Left | I | 8 |
| 2 | 61 | Left | I | 12 |
| 3 | 62 | Left | I | 12 |
| 4 | 66 | Left | I | 18 |
| 5 | 59 | Left | I | 13 |
| 6 | 67 | Left | I | 23 |
| 7 | 66 | Right | I | 21 |
| 8 | 59 | Right | I | 10 |
| 9 | 60 | Right | II | 18 |
| 10 | 70 | Right | II | 20 |
| 11 | 61 | Left | II | 20 |
| 12 | 61 | Left | II | 16 |
Stages according to the Union for International Cancer Control.
Target definition
The clinical target volume (CTV), PTV and normal tissue constraints were defined. For cases at Stage I of the disease, the CTV included visible breast parenchyma, excluding the muscles (musculus pectoralis and chest wall) and ribs, retracted 5 mm from the skin into the body. The PTV comprised the CTV with a 10-mm circumferential margin to allow for daily set-up variations and potential infrafraction thoracic wall motion, also retracted by 5 mm from the skin into the body. For cases at Stage II, the CTV was extended to the supraclavicular region ipsilaterally. The OAR such as the ipsilateral and contralateral lungs, heart, oesophagus and spinal cord were contoured on CT slices. A dose–volume histogram was generated for each plan and evaluated by three senior radiation oncologists (DK, VB and HB) and an experienced medical physicist (JN) to obtain an acceptable plan.
Treatment planning software and equipment
CT was performed at 3-mm slice spacing. All treatment plans were generated using a 3D treatment planning system (RapidArc mode, Eclipse™ v. 10; Varian Medical Systems) with the dual energy linear accelerator Clinac DHX (Varian Medical Systems), using 6-MV photon energy and On-Board Imager®. Dose calculation was performed using the analytical anisotropical algorithm, a superposition–convolution algorithm implemented using the Eclipse software. The calculation of dose grid was based on a grid resolution of 2.5 mm. The accelerator was calibrated for a skin–source distance of 100 cm, 10×10 cm fields and 98 MU delivering 1 Gy.
Planning procedure
For conTFRT, the beam arrangement comprised four half-beams with two tangential beams covering the caudal part of the target volume and one anterior–posterior field (0°) and one oblique field, typically 110–115° from the anterior–posterior field, covering the cranial part. Beam angles, apertures, weights and dynamic wedges were optimised individually. Dose plans were normalised to the mean dose for the PTV. We used one isocenter for those cases receiving supraclavicular radiation. We placed it at the cranial edge of the breast fields and the caudal edge of the supraclavicular field.
For IMRT, five coplanar modulated fields were equally spaced at a 180° arc around the patient's breast and regional nodes. Dose constraints were to treat <5% of the heart with 30 Gy (V30) and <20% of the ipsilateral lung with 20 Gy (V20). Dose constraints for PTV in terms of D95 were for a minimum dose of 95% and a maximum dose of 107%. These constraints were applied to all methods, including VMAT. Inverse planning was performed. IMRT involved a sliding window multileaf collimator (MLC), and the total treatment delivery time and number of MUs were recorded. We did not account for an additional margin to the PTV outside the body.
For the RapidArc technique, arcs were configured such that the beam enters the breast before exiting through the lung, which may increase the dose volume of the lung and contralateral breast. At first, we determined the arc range according to the PTV location. Next, an area of shielding within the arc was selected with clinical consideration to avoid angles directed towards the heart, lung and contralateral breast. Then, a computerised arc optimisation algorithm was generated to determine the speed of gantry rotation, the shaping of the MLC, the MU at each gantry position and the speed of leaf motion across the arc. Optimisation specifically uses an aperture-based method, which predefines a series of beam apertures according to the geometric shapes of both the target and the OARs. MLC leaf positions and MU weights were incorporated as optimisation parameters. To assess deliverability, the cost (objective) function was based on dose–volume constraints individually specified for each target and OAR.
The VMAT optimisation technique allows a compromise between optimisation flexibility and efficiency of delivery time by varying the MLC leaf motion speed, gantry rotation speed and dose rate. The entire gantry rotation is described in the optimisation process by a sequence of 177 control points (one every 2°).
Prescribed dose
Prescription dose to the breast and the regional nodes was 50 Gy in 25 fractions (PTV50). Boost dose distribution will be analysed in a subsequent study.
Dosimetric evaluation parameters
The maximum dose (Dmax), the maximum dose to 99% of the PTV (Dmax99%) to avoid point dose influence and mean dose (Dmean) and minimum dose (Dmin) within the PTV were evaluated. D95 (the dose distributed in ≥95% of the PTV), D5 (the dose distributed in 5% of the PTV) and V95% (volume receiving 95% of the prescribed dose) were explicitly calculated. Additionally, the conformity index (CI), conformity number (CN), homogeneity index (HI) and integral dose (ID) were reconstructed.
The HI was calculated as a quality parameter for magnitude and uniformity of dose distribution in the breast parenchyma. D2% and D98% are surrogates for dose minimum and maximum. A lower value indicates a more homogeneous dose distribution within this volume, with the ideal HI being zero:
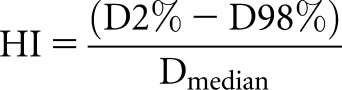 |
where D2% and D98% are the doses to 2% and 98% of the PTV.
The CI was calculated as a quality parameter. V47.5 Gy or V95% in this definition is the volume of the body receiving >47.5 Gy or 95% of the prescribed dose. The best value is 1:
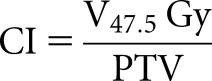 |
where V47.5 Gy represents the volume receiving 47.5 Gy or 95% of the prescribed dose.
The CN was calculated as another quality parameter for dose distribution, with a value between zero and 1:
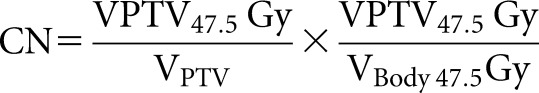 |
The ID was calculated to show how dose deposition in healthy tissue outside tumour regions might differ. To simplify the interpretation, we show results for ID in Gy×litre instead of the usually applied Gy×cm3. Higher the ID, higher the potential exposure of non-tumour healthy tissue to radiation, thus the risk for damage and secondary malignancies. The simplified formula used was
After analysing different modality plans, we looked at MUs for each modality, so as to compare the output of the machine to deliver accurate dose for each modality, and we compared the time of treatment.
Statistics
We conducted statistical analyses for all values to estimate the significance of differences.
Differences in dosimetric parameters were analysed, using the paired two-tailed Student's t-test. p-values <0.05 were considered statistically significant. The two-dimensional graphing and statistics software GraphPad Prism® v. 5.02 (GraphPad Software, Inc., La Jolla, CA) was used for statistical calculations.
RESULTS
Planning target volume
The calculated PTV ranged from 393.5 cm3 (Stage I cases) to 1500.8 cm3 (Stage II cases).
V95% could not be achieved by VMAT, IMRT or conTFRT (Table 2), although IMRT yielded the most favourable results of 93.6% in relation to VMAT (p<0.0001).
Table 2.
The PTV dose parameters of three plans (n=12, arithmetical mean)
| Parameters | 3D-CRT | IMRT | VMAT | 3D-CRT vs IMRT | IMRT vs VMAT | 3D-CRT vs VMAT |
| Total volume (cm3) | 896 | 896 | 896 | |||
| D95 (Gy) | 46.37±2.60 | 47.08±0.67 | 46.19±0.67 | 0.391 | 0.001 (**) | 0.816 |
| D5 (Gy) | 52.07±3.29 | 51.98±0.35 | 52.41±0.49 | 0.929 | 0.003 (**) | 0.721 |
| Dmin (Gy) | 38.96±5.01 | 34.82±4.05 | 37.44±1.83 | 0.024 (*) | 0.079 | 0.415 |
| Dmax (Gy) | 53.80±3.40 | 55.28±1.03 | 54.97±1.16 | 0.208 | 0.455 | 0.237 |
| Dmean (Gy) | 49.15±2.91 | 50.03±0.09 | 49.99±0.03 | 0.318 | 0.338 | 0.339 |
| V95% (%) | 92.73±2.80 | 93.60±2.28 | 88.79±3.08 | 0.462 | <0.0001 (****) | 0.015 (*) |
| HI | 0.14±0.2 | 0.13±0.03 | 0.16±0.03 | 0.170 | 0.009 (**) | 0.197 |
| CI | 1.69±0.52 | 1.15±0.29 | 1.06±0.26 | 0.0003 (***) | 0.002 (**) | <0.0001 (****) |
| CN | 0.63±0.19 | 0.94±0.23 | 0.92±0.23 | <0.0001 (****) | 0.360 | <0.0001 (****) |
CI, conformity index; CN, conformity number; CRT, conformal radiotherapy; D95, the dose distributed in ≥95% of the PTV; Dmax, maximum dose; Dmean, mean dose; Dmin, minimum dose; HI, homogeneity index; IMRT, intensity-modulated radiotherapy; PTV, planning target volume; V95%, volume receiving 95% of the prescribed dose; VMAT, volumetric modulated arc therapy.
D95 was not achieved by any modality. The HI was 0.16 for VMAT, 0.13 for IMRT and 0.14 for conTFRT. IMRT was more effective than VMAT (p=0.009). The CI was 1.06 for VMAT, 1.1.5 for IMRT and 1.69 for conTFRT. VMAT was better than IMRT (p=0.002) and conTFRT (p=0.0003). CN was 0.63 for conTFRT, 0.94 for IMRT and 0.92 for VMAT. Table 2 shows all parameters considered for each technique and the relation between them.
Dose distribution in organs at risk for the whole group
Lungs
Dmean in ipsilateral lung was similar for IMRT and conTFRT (p=0.32), but worse for VMAT (p<0.0001). For V20 in ipsilateral lung, IMRT results show better numbers than VMAT (p=0.003). For low dose levels (V5, V10), conTFRT had the best of all three numbers; however, IMRT was more effective than VMAT (p=0.0002 and p<0.0001, respectively). Contralateral lung showed the lowest Dmean, V2 and V5 for conTFRT; however, IMRT was more effective than VMAT (p=0.023, p<0.0001, p=0.005). V10 was similar for all modalities (Table 3).
Table 3.
Dose comparison of the ipsilateral lung and the contralateral lung between the three plans (n=12 patients; arithmetical mean)
| Parameters | 3D-CRT | IMRT | VMAT | 3D-CRT vs IMRT | IMRT vs VMAT | 3D-CRT vs VMAT |
| Lung ipsilateral | ||||||
| Dmean (Gy) | 12.097±3.00 | 12.097±3.00 | 12.097±3.00 | 0.320 | <0.0001 (****) | <0.0001 (****) |
| V5 (%) | 40.710±11.71 | 68.317±19.56 | 94.241±5.63 | <0.0001 (****) | 0.0002 (***) | <0.0001 (****) |
| V10 (%) | 28.369±6.96 | 43.461±13.88 | 70.222±9.91 | 0.0001 (***) | <0.0001 (****) | <0.0001 (****) |
| V20 (%) | 22.486±5.61 | 19.908±3.45 | 29.181±9.00 | 0.072 | 0.003 (**) | 0.008 (**) |
| V30 (%) | 19.485±5.25 | 12.965±1.70 | 14.417±4.18 | 0.0004 (***) | 0.251 | 0.001 (**) |
| V40 (%) | 14.637±6.59 | 5.748±1.63 | 6.246±2.42 | 0.0003 (***) | 0.544 | 0.0003 (***) |
| Lung contralateral | ||||||
| Dmean (Gy) | 0.692±1.23 | 0.692±1.23 | 0.692±1.23 | 0.018 (*) | 0.023 (*) | 0.008 (**) |
| V2 (%) | 0.685±1.05 | 22.960±27.06 | 81.762±19.60 | 0.014 (*) | <0.0001 (****) | <0.0001 (****) |
| V5 (%) | 0.001±0.003 | 5.318±9.57 | 19.512±12.88 | 0.081 | 0.005 (**) | 0.0003 (***) |
| V10 (%) | 0.000±0.00 | 0.893±1.66 | 0.962±1.278 | 0.09 | 0.87 | 0.024 (*) |
3D, three dimensional; CRT, conformal radiotherapy; Dmean, mean dose; IMRT, intensity-modulated radiotherapy; V10, volume receiving 10 Gy; V2, volume receiving 2 Gy; V20, volume receiving 20 Gy; V30, volume receiving 30 Gy; V40, volume receiving 40 Gy; V5, volume receiving 5 Gy; VMAT, volumetric modulated arc therapy.
Integral dose for healthy tissue
The probability of dose deposition in healthy tissue increased from conTFRT to IMRT to VMAT (79 094 to 93 409 to 122 205, all in Gy×litre), with VMAT being associated with the highest risk (Table 4).
Table 4.
Integral dose comparison of the normal tissue between the three plans (n=12; arithmetical mean)
| Parameters | 3D-CRT | IMRT | VMAT | 3D-CRT vs IMRT | IMRT vs VMAT | 3D-CRT vs VMAT |
| Dintegral (Gy×l) | 79.094±25.26 | 93.409±29.81 | 122.205±28.29 | 0.0003 (<0.01) | <0.0001 (<0.0001) | <0.0001 (<0.0001) |
3D, three dimensional; CRT, conformal radiotherapy; Dmean, mean dose; IMRT, intensity-modulated radiotherapy; V10, volume receiving 10 Gy; V2, volume receiving 2 Gy; V20, volume receiving 20 Gy; V30, volume receiving 30 Gy; V40, volume receiving 40 Gy; V5, volume receiving 5 Gy; VMAT, volumetric modulated arc therapy.
Dose distribution in organs at risk for left-side lesions
The mean dose to the entire heart was 12.41, 8.78 and 6.55 Gy, and V5 was 85%, 46.6% and 18.5% for VMAT, IMRT and conTFRT, respectively. For both factors, VMAT was worse than IMRT (p=0.015 and p=0.002). No difference was seen for V20 between all techniques. For V40, however, conTFRT resulted in a higher dose to the heart.
The left (ipsilateral) lung received a significantly higher dose (Dmean, V5 and V20) with VMAT than with the other techniques, as was the case for the entire cohort. For the contralateral lung, VMAT was associated with the highest radiation dose (Table 5).
Table 5.
Parameter for left-sided breast cancer cases, including PTV coverage, VMAT, age and dose to the lungs and heart
| Parameters | 3D-CRT | IMRT | VMAT | 3D-CRT vs IMRT | IMRT vs VMAT | 3D-CRT vs VMAT |
| PTV | ||||||
| V95% | 93.479±2.25% | 94.213±2.76% | 89.732±3.15% | 0.704 | 0.011 | 0.094 |
| D95 (Gy) | 45.689±3.68 (91.378%) | 47.197±0.88 (94.394%) | 46.289±0.71 (92.578%) | 0.382 | 0.02 (<0.05) | 0.705 |
| Lung—left | ||||||
| V40 | 13.142%±7.76 | 7.699%±2.12 | 7.126%±2025 | 0.09 | 0.661 | 0.116 |
| V20 | 21.905%±4.97 | 19.812%±2.67 | 25.06%±4.50 | 0.168 | 0.055 | 0.274 |
| V5 | 34.343%±6.34 | 60.420%±11.75 | 93.395%±4.24 | 0.002 (<0.01) | 0.0008 (<0.001) | <0.0001 (<0.0001) |
| Dmean (Gy) | 11.243±2.53 | 12.297±1.64 | 16.293±0.96 | 0.178 | 0.0096 (<0.01) | 0.009 (<0.01) |
| Lung—right | ||||||
| V5% | 0.000%±0.00 | 0.148%±0.3 | 14.796%±12.29 | 9.276 | 0.031 (<0.05) | 0.032 (<0.05) |
| Dmean (Gy) | 0.153±0.06 | 0.697±0.19 | 5.592±5.94 | 0.002 (<0.01) | 0.1 | 0.076 |
| Heart | ||||||
| V40 | 6.548%±3.85 | 1.988%±0.60 | 2.330%±1.50 | 0.02 (<0.05) | 0.0533 | 0.016 |
| V20 | 11.657%±2.50 | 12.779%±3.54 | 15.369%±6.14 | 0.232 | 0.249 | 0.126 |
| V5 | 18.568%±3.44 | 46.675%±13.01 | 85.921%±15.92 | 0.003 (<0.01) | 0.002 (<0.01) | 0.0001 (<0.001) |
| D5 (Gy) | 43.489±4.16 | 33.475±3.78 | 33.396±4.82 | 0.0004 (<0.001) | 0.970 | 0.001 (<0.01) |
| Dmean (Gy) | 6.557±1.24 | 8.782±1.49 | 12.412±2.47 | 0.004 (<0.01) | 0.015 (<0.05) | 0.001 (<0.01) |
3D, three dimensional; CRT, conformal radiotherapy; D95, the dose distributed in ≥95% of the PTV; Dmean, mean dose; IMRT, intensity-modulated radiotherapy; PTV, planning target volume; V20, volume receiving 20 Gy; V40, volume receiving 40 Gy; V5, volume receiving 5 Gy; V95%, volume receiving 95% of the prescribed dose; VMAT, volumetric modulated arc therapy.
Dose distribution in organs at risk for cases with supraclavicular region radiotherapy
Four patients with Stage II breast cancer received additional supraclavicular RT with 50 Gy. In these cases, the dose received by the spinal cord and oesophagus is of clinical importance. The spinal cord received the highest dose with conTFRT (28 Gy; IMRT, 25.3 Gy and VMAT, 26.7 Gy). The oesophageal dose was the highest with VMAT (Table 6).
Table 6.
Dose comparisons of the PRV-spinal cord and the PRV-oesophagus between the three plans and with or without irradiation of supraclavicular lymph node (arithmetical mean)
| Parameters | 3D-CRT | IMRT | VMAT | 3D-CRT vs IMRT | 3D-CRT vs VMAT | 3D-CRT vs VMAT | ||||||
| Without supraclavicular lymph node | With supraclavicular lymph node | Without supraclavicular lymph node | With supraclavicular lymph node | Without supraclavicular lymph node | With supraclavicular lymph node | Without supraclavicular lymph node | With supraclavicular lymph node | Without supraclavicular lymph node | With supraclavicular lymph node | Without supraclavicular lymph node | With supraclavicular lymph node | |
| Spinal cord | ||||||||||||
| Dmax (Gy) | 0.519±0.19 | 28.800±7.72 | 1.074±0.88 | 25.318±6.49 | 7.543±2.72 | 26.760±2.89 | 0.074 | 0.507 | 0.0001 (<0.001) | 0.639 | 0.0001 (<0.001) | 0.62 |
| Oesophagus | ||||||||||||
| Dmax (Gy) | 0.911±0.29 | 36.660±20.37 | 3.662±3.50 | 39.928±12.15 | 10.521±2.35 | 41.108±12.94 | 0.058 | 0.62 | 0.004 (<0.01) | 0.612 | <0.0001 (<0.0001) | 0.360 |
3D, three dimensional; CRT, conformal radiotherapy; D95, the dose distributed in ≥95% of the PTV; Dmean, mean dose; IMRT, intensity-modulated radiotherapy; PTV, planning target volume; VMAT, volumetric modulated arc therapy.
Monitor units and treatment time
The mean values for MUs in 8 patients with treatment for breast alone (i.e. without supraclavicular region) were 260 for conTFRT, 930 for IMRT and 407 for RapidArc. In the subgroup of 4 cases with supraclavicular region treatment, the following numbers were measured: 529 for conTFRT, 1186 for IMRT and 393 for RapidArc.
DISCUSSION
Data from planning comparisons and dosimetric studies on VMAT in breast cancer are rather limited. Accordingly, in this study, we compared VMAT using the RapidArc system with conTFRT and IMRT. The hypothesis was that VMAT, as performed in our clinical setting, is equivalent or superior to the other techniques in terms of PTV coverage and sparing of OAR, while reducing MUs.
The merit of this study is that it analyses different real-world clinical scenarios, including cancer of the right and left sides and the treatment of breast only and breast plus supraclavicular region. In all cases, the same physicians (HB and DK) arranged target definition and plan evaluation and the same physicist (JN) calculated plans under constant and homogeneous conditions regarding hardware and software. A limitation of this retrospective study is the small number of cases and lack of clinical parameters. In this section, we discuss our main findings on using RapidArc VMAT for breast cancer.
Planning target volume coverage
Regarding PTV coverage, especially V95%, VMAT did not confer any advantage, and IMRT provided the best coverage (93.6%). The HI was better for IMRT, but CI tended to be slightly better for VMAT.
In a comparison of the three RT techniques in eight cases, Johansen et al [25] showed that RapidArc VMAT was better in terms of the PTV parameters of homogeneity and conformation. The least dose to the PTV was observed with the conventional treatment, whereas no difference was observed for the minimum significant doses D (98%) and D (99%). Nicolini et al [26] showed that the V90% was 97.8±3.4% for RapidArc VMAT and 94.0±3.5% for IMRT in breast cancer patients receiving 50 Gy. The D5–D95% value (homogeneity) was 7.3±1.4 Gy and 11.0±1.1 Gy with RapidArc VMAT and IMRT, respectively, whereas CI (V95%/VPTVII) was 1.10±0.06 and 1.14±0.09, respectively. Differences between the techniques were small; accordingly, the minor advantage with RapidArc VMAT in terms of CI and CN will not, in our judgment, influence clinical decisions.
Dose distribution in organs at risk
In our study, VMAT was associated with the most unfavourable dose deposition in the ipsilateral lung in entire cohort and in the left-sided disease subgroup, in terms of the Dmean, V5 and V10. The results were the same for the contralateral lung, in terms of mean, V2 and V5. At V20, V30 and V40, IMRT was the most favourable modality for the ipsilateral lung. V20 has been a valid clinical parameter, and IMRT was the best technique in this regard for the entire group and the left-sided disease subgroup, by contrast to the currently available information; although this is limited and heterogeneous. Qui et al [23] showed that the volumes of lung receiving >10 and 20 Gy were significantly smaller with VMAT than with conTFRT. However, no difference was found when the irradiated dose increased to 30 Gy. In a study on left-sided breast cancer, Nicolini et al [26] calculated a mean V20 of 9.7% with RapidArc VMAT and 12.8% with IMRT for the left lung, values similar to those for the right lung. In the present study, the mean V20 for the ipsilateral lung was 29.1% with RapidArc VMAT and 19.9% with IMRT for the entire group and 25.9% and 19.9% for left-sided disease subgroup.
The dose to the heart is of clinical importance for left-sided breast lesions. In the present study, the mean dose to the entire heart was 12.4, 8.7 and 6.5 Gy, for VMAT, IMRT and conTFRT, respectively, with VMAT providing the poorest outcome. This disadvantage was also seen for VMAT at the low dose level of V5. The Nicolini study reported a mean dose to the heart of 6.0 and 7.4 Gy, and Pasler et al [28] reported a mean dose to the heart of 8.8–8.9 Gy and 8.4–8.6 Gy with VMAT and IMRT, respectively. In the present study, the reasons for the higher doses to the heart with RapidArc VMAT are unclear, because we used standard definitions of the heart, and the patients did not have specific deviations in anatomy. Some specific aspects of dose constraints set prior to RT or discrepancies in weighting of the heart during planning may be associated with the higher dose received by the heart with RapidArc VMAT.
Integral dose for healthy tissue
Calculation of the integral dose outside the target volumes might be helpful to understand the potential damage caused by RT leading to morphological and functional organ changes and secondary cancers. In the present study, RapidArc VMAT showed relevant dose deposition in the body, with increasing risk of conTFRT to IMRT to VMAT. The issue of high integral dose might be of concern for young females and those patients with a low risk for systemic relapse who are likely to live for many years after breast cancer treatment [30]. Radiotherapy, especially IMRT and RapidArc, in breast may cause sarcoma and lung cancer [31]. The risk of sarcoma in the treated volume is likely to be similar with IMRT or standard techniques, but it is possible that second primary lung cancers might be increased by IMRT or RapidArc, especially in smokers [32]. Balancing the short to medium term benefits of reducing the volume of heart and left lung receiving a high dose against the risk of late malignancy requires an individual assessment of the treatment volume goals and the patient's longevity prospects with and without radiotherapy. Evaluation of 121 patients treated with IMRT compensation found a 3% rate of secondary malignancy after 7 years, which was not significantly different from the 4% rate observed by conventional radiation therapy[33]. Based of our results, one could assume that the risk situation might be worse for RapidArc than IMRT, although there is no clinical data or direct immediate scientific proof.
In conclusion, it has been shown that our hypothesis of equivalence of VMAT to IMRT was not confirmed; neither with respect to PTV coverage nor for dose distribution in organs at risk. On the contrary, VMAT was inferior to IMRT and conTFRT with regard to the dose distribution in organs at risk, especially for low dose levels (V2, V5 und V10) and mean dose. This was true also for healthy tissue integral dose. Our study shows that IMRT may be in this setting and, for these patients, a more sophisticated modality. More prospective studies with clinical data acquisition are indeed necessary.
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011;378:1707–16 10.1016/S0140-6736(11)61629-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hooning MJ, Botma A, Aleman BM, Baaijens MH, Bartelink H, Klijn JG, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. J Natl Cancer Inst 2007;99:365–75 10.1093/jnci/djk064 [DOI] [PubMed] [Google Scholar]
- 3.Harris EE, Correa C, Hwang WT, Liao J, Litt HI, Ferrari VA, et al. Late cardiac mortality and morbidity in early-stage breast cancer patients after breast-conservation treatment. J Clin Oncol 2006;24:4100–6 [DOI] [PubMed] [Google Scholar]
- 4.Rancati T, Wennberg B, Lind P, Svane G, Gagliardi G. Early clinical and radiological pulmonary complications following breast cancer radiation therapy: NTCP fit with four different models. Radiother Oncol 2007;82:308–16 [DOI] [PubMed] [Google Scholar]
- 5.Henson KE, McGale P, Taylor C, Darby SC. Radiation-related mortality from heart disease and lung cancer more than 20 years after radiotherapy for breast cancer. Br J Cancer 2013;108:179–82 10.1038/bjc.2012.575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller SR, Mondry T, Reed JS, Findley A, Johnstone PA. Delayed cellulitis associated with conservative therapy for breast cancer. J Surg Oncol 1998;67:242–5 [DOI] [PubMed] [Google Scholar]
- 7.Morgan EA, Kozono DE, Wang Q, Mery CM, Butrynski JE, Baldini EH, et al. Cutaneous radiation-associated angiosarcoma of the breast: poor prognosis in a rare secondary malignancy. Ann Surg Oncol 2012;19:3801–8 10.1245/s10434-012-2563-4 [DOI] [PubMed] [Google Scholar]
- 8.Rubino C, Shamsaldin A, Le MG, Labbe M, Guinebretiere JM, Chavaudra J, et al. Radiation dose and risk of soft tissue and bone sarcoma after breast cancer treatment. Breast Cancer Res Treat 2005;89:277–88 10.1007/s10549-004-2472-8 [DOI] [PubMed] [Google Scholar]
- 9.Sachs RK, Brenner DJ. Solid tumor risks after high doses of ionizing radiation. Proc Natl Acad Sci U S A 2005;102:13040–5 10.1073/pnas.0506648102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paszat LF, Mackillop WJ, Groome PA, Boyd C, Schulze K, Holowaty E. Mortality from myocardial infarction after adjuvant radiotherapy for breast cancer in the surveillance, epidemiology, and end-results cancer registries. J Clin Oncol 1998;16:2625–31 [DOI] [PubMed] [Google Scholar]
- 11.Gyenes G, Rutqvist LE, Liedberg A, Fornander T. Long-term cardiac morbidity and mortality in a randomized trial of pre- and postoperative radiation therapy versus surgery alone in primary breast cancer. Radiother Oncol 1998;48:185–90 [DOI] [PubMed] [Google Scholar]
- 12.Cuzick J, Stewart H, Rutqvist L, Houghton J, Edwards R, Redmond C, et al. Cause-specific mortality in long-term survivors of breast cancer who participated in trials of radiotherapy. J Clin Oncol 1994;12:447–53 [DOI] [PubMed] [Google Scholar]
- 13.Dayes I, Rumble RB, Bowen J, Dixon P, Warde P; Members of the IMRT Indications Expert Panel Intensity-modulated radiotherapy in the treatment of breast cancer. Clin Oncol 2012;24:488–98 10.1016/j.clon.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 14.Pignol JP, Olivotto I, Rakovitch E, Gardner S, Sixel K, Beckham W, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008;26:2085–92 [DOI] [PubMed] [Google Scholar]
- 15.Donovan E, Bleakley N, Denholm E, Evans P, Gothard L, Hanson J, et al. Randomised trial of standard 2D radiotherapy (RT) versus intensity modulated radiotherapy (IMRT) in patients prescribed breast radiotherapy. Radiother Oncol 2007;82:254–64 [DOI] [PubMed] [Google Scholar]
- 16.Vicini FA, Sharpe M, Kestin L, Martinez A, Mitchell CK, Wallace MF, et al. Optimizing breast cancer treatment efficacy with intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:1336–44 [DOI] [PubMed] [Google Scholar]
- 17.Keller LM, Sopka DM, Li T, Klayton T, Li J, Anderson PR, et al. Five-year results of whole breast intensity modulated radiation therapy for the treatment of early stage breast cancer: the Fox Chase Cancer Center experience. Int J Radiat Oncol Biol Phys 2012;84:881–7 [DOI] [PubMed] [Google Scholar]
- 18.Palma D, Vollans E, James K, Nakano S, Moiseenko V, Shaffer R, et al. Volumetric modulated arc therapy for delivery of prostate radiotherapy: comparison with intensity-modulated radiotherapy and three-dimensional conformal radiotherapy. Int J Radiat Oncol Biol Phys 2008;72:996–1001 [DOI] [PubMed] [Google Scholar]
- 19.Scorsetti M, Fogliata A, Castiglioni S, Bressi C, Bignardi M, Navarria P, et al. Early clinical experience with volumetric modulated arc therapy in head and neck cancer patients. Radiat Oncol 2010;5:93 10.1186/1748-717X-5-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clivio A, Fogliata A, Franzetti-Pellanda A, Nicolini G, Vanetti E, Wyttenbach R, et al. Volumetric-modulated arc radiotherapy for carcinomas of the anal canal: a treatment planning comparison with fixed field IMRT. Radiother Oncol 2009;92:118–24 [DOI] [PubMed] [Google Scholar]
- 21.Stieler F, Wolff D, Lohr F, Steil V, Abo-Madyan Y, Lorenz F, et al. A fast radiotherapy paradigm for anal cancer with volumetric modulated arc therapy (VMAT). Radiat Oncol 2009;4:48 10.1186/1748-717X-4-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagerwaard FJ, Meijer OW, van der Hoorn EA, Verbakel WF, Slotman BJ, Senan S. Volumetric modulated arc radiotherapy for vestibular schwannomas. Int J Radiat Oncol Biol Phys 2009;74:610–5 10.1016/j.ijrobp.2008.12.076 [DOI] [PubMed] [Google Scholar]
- 23.Qiu JJ, Chang Z, Wu QJ, Yoo S, Horton J, Yin FF. Impact of volumetric modulated arc therapy technique on treatment with partial breast irradiation. Int J Radiat Oncol Biol Phys 2010;78:288–96 10.1016/j.ijrobp.2009.10.036 [DOI] [PubMed] [Google Scholar]
- 24.Popescu CC, Olivotto IA, Beckham WA, Ansbacher W, Zavgorodni S, Shaffer R, et al. Volumetric modulated arc therapy improves dosimetry and reduces treatment time compared to conventional intensity-modulated radiotherapy for locoregional radiotherapy of left-sided breast cancer and internal mammary nodes. Int J Radiat Oncol Biol Phys 2010;76:287–95 [DOI] [PubMed] [Google Scholar]
- 25.Johansen S, Cozzi L, Olsen DR. A planning comparison of dose patterns in organs at risk and predicted risk for radiation induced malignancy in the contralateral breast following radiation therapy of primary breast using conventional, IMRT and volumetric modulated arc treatment techniques. Acta Oncol 2009;48:495–503 [DOI] [PubMed] [Google Scholar]
- 26.Nicolini G, Clivio A, Fogliata A, Vanetti E, Cozzi L. Simultaneous integrated boost radiotherapy for bilateral breast: a treatment planning and dosimetric comparison for volumetric modulated arc and fixed field intensity modulated therapy. Radiat Oncol 2009;4:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakumi A, Shiraishi K, Onoe T, Yamamoto K, Haga A, Yoda K, et al. Single-arc volumetric modulated arc therapy planning for left breast cancer and regional nodes. J Radiat Res 2012;53:151–3 [DOI] [PubMed] [Google Scholar]
- 28.Pasler M, Georg D, Bartelt S, Lutterbach J. Node-positive left-sided breast cancer: does VMAT improve treatment plan quality with respect to IMRT? Strahlenther Onkol 2013;189:380–6 10.1007/s00066-012-0281-2 [DOI] [PubMed] [Google Scholar]
- 29.Jin GH, Chen LX, Deng XW, Liu XW, Huang Y, Huang XB. A comparative dosimetric study for treating left-sided breast cancer for small breast size using five different radiotherapy techniques: conventional tangential field, filed-in-filed, tangential-IMRT, multi-beam IMRT and VMAT. Radiat Oncol 2013;8:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Followill D, Geis P, Boyer A. Estimates of whole-body dose equivalent produced by beam intensity modulated conformal therapy. Int J Radiat Oncol Biol Phys 1997;38:667–72 [DOI] [PubMed] [Google Scholar]
- 31.Hall EJ, Wuu CS. Radiation-induced second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol Biol Phys 2003;56:83–8 [DOI] [PubMed] [Google Scholar]
- 32.McDonald MW, Godette KD, Whitaker DJ, Davis LW, Johnstone PA. Three-year outcomes of breast intensity-modulated radiation therapy with simultaneous integrated boost. Int J Radiat Oncol Biol Phys 2010;77:523–30 [DOI] [PubMed] [Google Scholar]
- 33.McDonald MW, Godette KD, Butker EK, Davis LW, Johnstone PA. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int J Radiat Oncol Biol Phys 2008;72:1031–40 10.1016/j.ijrobp.2008.02.053 [DOI] [PubMed] [Google Scholar]


