Abstract
Objective:
National dosimetry audits are a fundamental part of quality assurance in radiotherapy, especially for new techniques. Intraoperative radiotherapy with a compact mobile kilovoltage X-ray source is a novel approach for the treatment of breast and other cancers. All seven current clinical sites in the UK were audited by a single visiting group and set of measurement equipment.
Methods:
Measurements of output, isotropy and depth doses were performed using an ion chamber in solid water, thermoluminescent dosemeters and radiochromic film, respectively.
Results:
The mean difference between measured and planned dose across all centres was −3.2±2.7%. Measured isotropy was within ±3% around the lateral plane of the X-ray source and +11±4% in the forward direction compared with the lateral plane. Measured depth doses were agreed within 5±2% of manufacturer-provided calibration values or a mean gamma index of 97% at a tolerance of 7%/0.5 mm.
Conclusion:
Agreement within measurement uncertainties was found for all three parameters except forward anisotropy, which is unlikely to be clinically significant. Steep dose gradients increase the sensitivity to small variations in positioning, but these tests are practical for use in interdepartmental audits and local baseline comparison.
Advances in knowledge:
The first UK interdepartmental audit of intraoperative radiotherapy builds confidence in the delivery of this treatment.
Dosimetry audits have been a fundamental part of quality assurance (QA) in UK radiotherapy departments for over 20 years [1–6]. More recently, ongoing verification of established systems has been delegated to regional audit groups; however, new techniques such as intensity-modulated radiotherapy [7,8] or volumetric modulated arc therapy [9] have been assessed on a national level. This gives confidence in the accurate, safe and consistent delivery of complex treatments, regardless of location or prior experience.
Intraoperative radiotherapy (IORT) has also been practised in the UK for nearly 20 years, using a compact mobile 50 kV X-ray device called PRS400 (Photoelectron Corporation, Lexington, MA) and, more recently, INTRABEAM® (PRS500; Carl Zeiss Surgical, Oberkochen, Germany). Initially, the system was used for intracranial stereotaxy [10] but found wider application in the delivery of a single fraction of radiation soon after surgical excision of breast tumours [11]. Most patients have been treated as part of the targeted intraoperative radiotherapy (TARGIT) international randomised controlled trial [12], although some other cases have also been successfully treated where external beam therapy was contraindicated [13]. Full calibration of the system is provided by the manufacturer, but recently, methods for independent dosimetry and quality assurance have been described [14,15]. The aim of this study was to audit all UK clinical centres in 2012 (Table 1) using these methods, and to verify the output, energy and isotropy of the different units. Such an inter-comparison of photon IORT has not been performed before in any country to our knowledge.
Table 1.
Participating hospitals
| Ninewells, Dundee, UK |
| Guy's and St Thomas', London, UK |
| North Middlesex, London, UK |
| Princess Grace, London, UK |
| Royal Free, London, UK (visiting centre) |
| St John and St Elizabeth, London, UK |
| Royal Hampshire County, Winchester, UK |
METHODS AND MATERIALS
A subgroup of the authors (DJE, BE or PF) visited each centre over the course of a year, taking with them the same set of measurement equipment, described below. Pre-treatment internal QA checks on the isotropy and output constancy of the host X-ray source unit (XRS) [15] were performed by the host centre and successfully verified against local tolerances. A room was provided by the host with adequate shielding, typically one used for superficial or brachytherapy treatments, which had greater availability and ease of access control than an operating theatre. Radiation protection principles were observed to minimise dose to the operators, e.g. by standing behind a mobile lead screen or retreating to an adjacent room.
Output
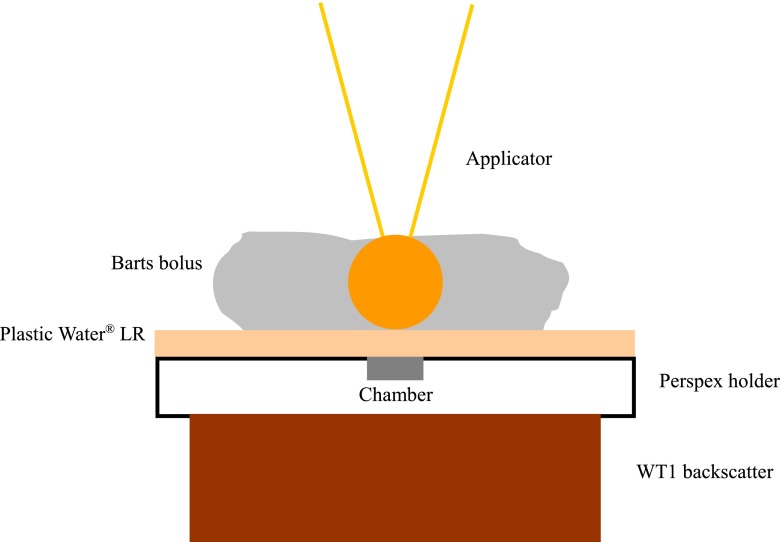
Output was measured in solid water-equivalent materials, at the common prescription depth of 1 cm from the surface of the spherical applicators used to deliver the treatment, using the set-up shown in Figure 1. A soft X-ray parallel plate ionisation chamber (type 23342-1739; PTW, Freiburg, Germany) was positioned in a poly(methyl methacrylate) (PMMA) slab holder, with a few sheets of acetate behind to align the effective measurement point (at the chamber surface) as close as possible to the surface of the slab. A block of WT1 solid water-equivalent material (St Bartholomew's Hospital, London, UK) was used for backscatter, and a 1-cm-thick sheet of Plastic Water® LR (CIRS, Norfolk, VA) placed on top. The INTRABEAM XRS with a 4.5-cm or 5.0-cm large diameter applicator attached was held in the robotic gantry arm and positioned over the centre of the chamber, using marks drawn on the surface of the blocks. A large applicator was used to minimise positional uncertainties. Specific pieces of malleable Barts bolus (St Bartholomew's Hospital) were carefully shaped around the applicator sphere to provide sufficient side scatter, but care was taken to ensure that the applicator remained in contact with the Plastic Water. In some cases, a small weight was placed on the XRS to counteract any flexion in the arm. Temperature and pressure were measured using UKAS-traceable calibrated meters (Checktemp®1; Hanna Instruments, Leighton Buzzard, UK, and DPI-700/705; Druck Ltd, Leicester, UK). Readings were acquired for a set dose of 2 Gy at 1 cm from the applicator surface (giving an approximate treatment time as calculated by the system of 10 min) using a UNIDOS® E electrometer (PTW). At least two readings were acquired, with care taken to ensure that leakage over the measurement time was minimal. Calibration of the chamber and electrometer was traceable to the National Physics Laboratory primary standard for air kerma by intercomparison with a secondary standard chamber in our centre using a superficial kilovoltage unit with similar beam quality [15].
Figure 1.
Diagram of set-up for measurement of output at depth in solid water-equivalent material. Plastic Water LR is manufactured by CIRS, Norfolk, VA.
The beam quality of this unit (0.85–1.30 mm Al [15]) lies on the boundary between two regimes of the UK kilovoltage code of practice: very low (0.04–1.0 mm Al) and low (1.0–8 mm) [16]. However, the low-energy regime uses a field-size-dependent backscatter factor, which is not readily applicable to an isotropic effective point source of radiation with no collimation to a specific field size. Therefore, the very-low-energy regime, using measurement of absorbed dose to water at the surface of a water-equivalent phantom, was chosen for this audit. Since the first clinical use of this device in the UK, an addendum to the code of practice has been published with different chamber correction factors, kch [17]. Early users did not adopt these new factors, to maintain consistency within the TARGIT trial, and subsequent centres to acquire the device have followed a similar approach to ensure consistency across the country. Therefore, calculation of dose in this audit also followed the original 1996 code, with a factor of unity for kch.
Isotropy and depth dose
Isotropy was measured by taping sealed packets of 3–4 thermoluminescent dosemeters (TLD-100 rods, LiF:Mg,Ti) to the orthogonal positions around the surface of a large diameter applicator [15]. TLDs were selected at random from a small batch with sensitivity variation within ±5%. The packets were marked with their location [forward/distal (+Z), and lateral (±X, ±Y) with respect to the probe tip], submerged in water and irradiated for approximately 10 min. TLDs were read out according to standard procedures in our centre [18] and the average reading from each packet compared with the mean lateral TLD reading to determine the isotropy.
Depth doses were measured as a surrogate for beam energy using GAFCHROMIC® EBT film (International Specialty Products, Wayne, NJ), as described previously [14]. Film pieces were cut to fit around the applicator sphere, taped to hang down vertically, submerged in water and irradiated for approximately 5 min. Care was taken to ensure that the film closely conformed to the applicator surface with marks on the film to align with the centre of the effective source position. Films were read out according to standard procedures in our centre [14] and a profile in the forward direction compared with manufacturer-provided depth dose tables, normalised to 10 mm from the surface. It is common in dosimetry comparisons of two-dimensional data to calculate the gamma index (γ), a composite threshold of dose and distance to agreement [19]. Therefore, one-dimensional γ agreement levels were also calculated for each centre.
RESULTS
Measurements were performed by the visiting group at all seven clinical centres in the UK, and for an additional loaned XRS unit at our own centre, which was used during return of the original unit for calibration by the manufacturer. Each centre utilised the annual calibration service offered by the manufacturer. This involves remeasuring the forward depth dose curve and output constancy test for the XRS, and updating the internal calibration files, to take account of any changes over the previous year. All readings were taken using a 4.5-cm-diameter applicator, except in one centre where this size was not present, so the 5.0-cm-diameter applicator was used instead. No systematic differences were seen between the different applicator sizes. One centre had two clinical XRS units and applicator sets, but only one was measured during the audit. A small residual gap of 0.3 mm was present between the chamber surface and the surface of the PMMA holder, so the expected (planned) doses were adjusted based on an inverse square dependence on distance from the effective source position (approximately 2%).
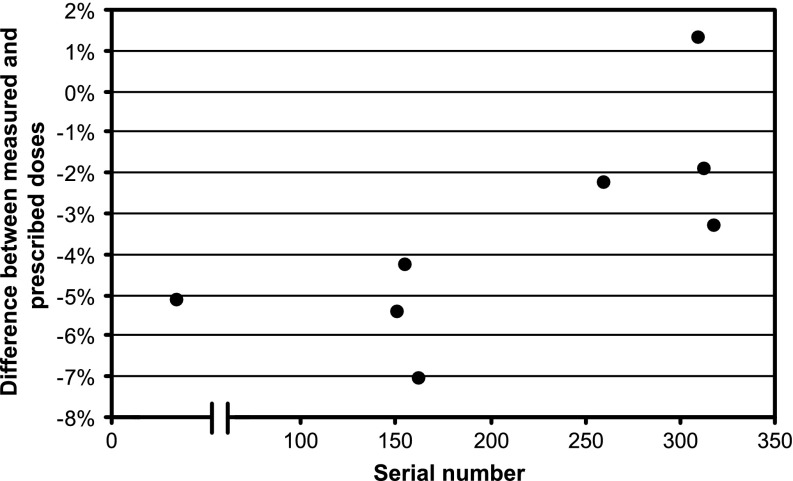
The mean difference between measured and planned dose across all centres was −3.2±2.7% (range, −7.1% to +1.3%). The difference between the original and loaned XRS units in our centre, measured 2 weeks apart, was 1.1%. No trends were seen with respect to time of audit visit or applicator size, although there was some variation with the age of the device, using the serial number as a surrogate (Figure 2).
Figure 2.
Graph of output agreement with respect to serial number (approximate age) of device, in arbitrary units. The break in the abscissa relates to devices produced by the previous and current manufacturer, although the basic technology was unchanged.
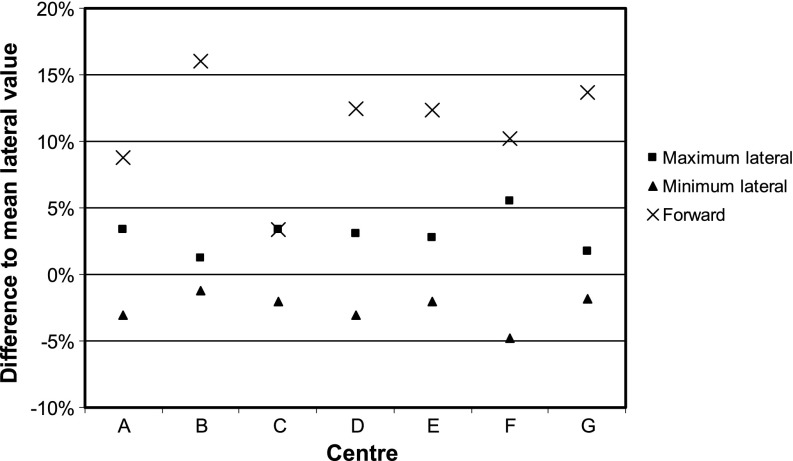
Isotropy variation was quantified relative to the mean lateral value. In the lateral plane (±X or ±Y directions), the mean variation across all centres was −2.6% to +3.0% (range, −4.8% to +5.5%). In the forward direction (+Z), the mean difference was+11.0±4.1% (range, +3.4% to +16.0%) (Figure 3).
Figure 3.
Graph of isotropy variation compared with mean lateral value for each centre.
Percentage depth dose differences were quantified relative to the local value for distances <10 mm from the applicator surface, and relative to the absolute value at 10 mm for distances >10 mm, to avoid large apparent differences between small numbers far from the effective source position. Overall, the difference between measured and calibration depth dose data was 4.9±1.9% (range, 2.6–8.1%). Mean γ agreement was 97% of points on the depth dose curve (range, 88–100%) at a tolerance level of 7%/0.5 mm and 100% for all centres at a level of 10%/1 mm.
DISCUSSION
Output
Kilovoltage audits typically aim to demonstrate an accuracy within 5% in dose delivery [5]. However, the fall off of dose with depth for this unit is very rapid, approximately 10%/mm around the prescription point at 1 cm depth in tissue, so a tolerance of 5% with an action level of 10% has been proposed for independent checks [15]. The majority of centre results fall within this tolerance and all within the action level. The uncertainties in measurement depend primarily on the positional accuracy, with a value of 6% estimated for measuring output using an ion chamber in solid water-equivalent material [15]. Therefore, the results found in this audit show good agreement between different centres (all within 4.5% of the overall mean) with a small systematic reduction in measured dose compared with the planned system value (−3.2%). There is currently no direct link to a primary standard for “electronic brachytherapy” units such as these, so this systematic difference is not definitive. Some of the difference can probably be attributed to the intercomparison-based chamber calibration factor compared with the PTW certificate value (−1.1%), the use of solid water-equivalent material (about 2% for this combination of materials [20]) and the one-sided nature of positional errors (i.e. it is not physically possible for the effective source position to be too close).
An intercomparison phantom using GAFCHROMIC XR film (International Specialty Products) in a fixed geometry PMMA holder was previously designed and tested on four XRS units in one centre [21]. Differences of ±3.9% were found between the four units with an estimated uncertainty of 4.7%. It is encouraging that the variation found in this audit is similar, so is not greatly increased by transporting and setting up the equipment in multiple locations. Intercentre variation would have been higher with a “peer-to-peer” (round robin) approach or a composite chamber factor, although the single visiting group approach requires more resources from that centre [5].
The variation with serial number (age) of the devices is intriguing, but there is no known reason why there should be a positive trend or two clusters of values. To our knowledge, the design of the XRS has not changed over the time span of the audited units. The annual manufacturer calibration replaces the internal dose rate files, and the internal QA checks include an output constancy measurement, so any expected reduction in output with age will be accounted for during treatment delivery [15]. The differences are comparable to the uncertainties of the method, and the agreement of our own XRS (serial number 155) in 2011 was −1.2%, which does not fit the pattern in Figure 2. Therefore, perceived trends are not likely to be significant.
It is also tempting to suggest that use of an updated chamber correction factor would improve the overall agreement (a value for kch of 1.07 was recommended in the addendum to the kV code of practice [16] and verified for this unit [14]). However, the modified mean output difference of +3.8% (−0.1% to+8.3%) is a similar magnitude to the original comparison, and therefore offers neither improvement nor any detriment.
Isotropy and depth dose
The majority of results for isotropy around the lateral plane were within a suggested tolerance of 5% [15], and all lay within an action level of 10%. There was a systematic difference, however, between the forward and lateral directions of +11% (±4%) across all centres in this audit. Data from the past 5 years in our centre showed lateral variations of ±2–4% and forward “enhancement” of +5–8% [15], which is consistent with the audit findings. Most of this anisotropy is from the solid applicator, which has a manufacturer specification of −8.3% to +0.4% (relative to the forward direction) for the 4.5-cm-diameter applicator, and a maximum range of −8% to +6% across all the applicator sizes in our centre. If this minimum is taken as the baseline, then all results for forward anisotropy across the audit are within the action level of 10%. Likewise, in each individual centre, the maximum variation between any two points ranged from ±2.7% to ±8.6%, also within the action level. It should be noted, however, that the anisotropy results in a lower dose to the sides of the applicator than in front, but the output is measured in front. Therefore, even if the output measurement is very close to expected, there will be an underdose of around 10% to other areas of the treatment volume. This is consistent with recent in vivo measurements using GAFCHROMIC film around the inside of the tumour cavity, which found mean differences compared with planned values of −12% [22] and −17% [23] for the 4.5-cm-diameter applicator.
However, the clinical effect of these variations is unlikely to be significant, since it can be assumed that all units in the TARGIT trial (which included five of the seven in this audit [12]) had this characteristic anisotropy. In addition, prescription at two alternative points was permissible within the trial protocol (either at the applicator surface or at 1-cm depth in tissue), which leads to potential dose variations of approximately 15%. Therefore, reported clinical outcomes already include these physical dose variations. It is likely that differences in geographical positioning, such as when IORT is delivered as a second surgical procedure after excision, will have a greater impact on clinical outcomes than variations in prescription or anisotropy. This should be borne in mind when considering the acceptable accuracy of this system in comparison to other kilovoltage or brachytherapy treatments.
Depth dose agreement using film was in good agreement with manufacturer-provided internal system values, given an uncertainty in this method of 7%, and suggested action level of 10% [15]. Gamma analysis has been used previously to compare a virtual source model of this unit to EBT2 film readings, with 98% of points passing a tolerance of 2%/1 mm (relative to the global maximum) [24]. However, although this tolerance would be very stringent for an external beam plan containing regions of high- or low-dose gradient, the very-high-dose gradient of this IORT unit means that the majority of points will pass on distance-to-agreement rather than dose. If the gradient is approximately 10%/mm, then a tolerance of 2%/1 mm will give similar results to 10%/1 mm, which was passed by a similarly high proportion of points in this study. A more appropriate and sensitive tolerance would be 7%/0.5 mm, which is of the same order as the measurement uncertainty.
CONCLUSIONS
External audits demonstrate the quality of radiotherapy dosimetry and add value to the patient pathway by building confidence in consistency between institutions and minimising the likelihood of errors. Novel technologies that are inherently portable may be used in remote clinics with little or no past experience of radiation. For example, an alternative miniature 50 kV X-ray device is currently being marketed in the UK for use in a mobile vehicle (www.advancedoncotherapy.com). It is imperative that such devices are included in national audits and their dosimetry is validated by independent medical physics experts.
All centres using IORT clinically in the UK in 2012 were successfully audited, and differences were within acceptable limits. Tolerances are wider for this equipment owing to the steep dose gradients involved and impact of small changes in position. Inherent anisotropy of the unit leads to lower doses around the sides of the applicator than at the distal end, where the output calibration is performed, but this is unlikely to be clinically significant.
ACKNOWLEDGMENTS
The authors would like to thank all the other centres who participated in the audit: Dundee, UK (Colin Mackay and John Parry); Guy's and St Thomas', London, UK (Regina Gonzalez and Ian Honey); North Middlesex, London, UK (Richard Knott and Yanni Papastavrou); Princess Grace, London, UK (Andrew Robinson); St John and St Elizabeth, London, UK (Simon Stevens); and Royal Hampshire County, Winchester, UK (Claire Birch and Sophie Stickells).
REFERENCES
- 1.Thwaites DI, Williams JR, Aird EG, Klevenhagen SC, Williams PC. A dosimetric intercomparison of megavoltage photon beams in UK radiotherapy centres. Phys Med Biol 1992;37:445–61 [DOI] [PubMed] [Google Scholar]
- 2.Bonnett DE, Mills JA, Aukett RJ, Martin-Smith P. The development of an interdepartmental audit as part of a physics quality assurance programme for external beam therapy. Br J Radiol 1994;67:275–82 [DOI] [PubMed] [Google Scholar]
- 3.Nisbet A, Thwaites DI. A dosimetric intercomparison of electron beams in UK radiotherapy centres. Phys Med Biol 1997;42:2393–409 [DOI] [PubMed] [Google Scholar]
- 4.Blake SW, Casebow MP. A pragmatic approach to dosimetric audit in radiotherapy. Br J Radiol 2002;75:754–62 [DOI] [PubMed] [Google Scholar]
- 5.Burton NL, Brimelow J, Welsh AD. A regional audit of kilovoltage X-rays—a single centre approach. Br J Radiol 2008;81:422–6 10.1259/bjr/85252741 [DOI] [PubMed] [Google Scholar]
- 6.Palmer A, Mzenda B, Kearton J, Wills R. Analysis of regional radiotherapy dosimetry audit data and recommendations for future audits. Br J Radiol 2011;84:733–42 10.1259/bjr/18691638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark CH, Hansen VN, Chantler H, Edwards C, James HV, Webster G, et al. Dosimetry audit for a multi-centre IMRT head and neck trial. Radiother Oncol 2009;93:102–8 10.1016/j.radonc.2009.04.025 [DOI] [PubMed] [Google Scholar]
- 8.Budgell G, Berresford J, Trainer M, Bradshaw E, Sharpe P, Williams P. A national dosimetric audit of IMRT. Radiother Oncol 2011;99:246–52 10.1016/j.radonc.2011.03.016 [DOI] [PubMed] [Google Scholar]
- 9.Hussein M, Tsang Y, Thomas RA, Gouldstone C, Maughan D, Snaith JA, et al. A methodology for dosimetry audit of rotational radiotherapy using a commercial detector array. Radiother Oncol 2013;108:78–85 10.1016/j.radonc.2013.05.027 [DOI] [PubMed] [Google Scholar]
- 10.Biggs DS, Thomson ES. Radiation properties of a miniature x-ray device for radiosurgery. Br J Radiol 1996;69:544–7 [DOI] [PubMed] [Google Scholar]
- 11.Vaidya JS, Baum M, Tobias JS, D'Souza DP, Naidu SV, Morgan S, et al. Targeted intra-operative radiotherapy (Targit): an innovative method of treatment for early breast cancer. Ann Oncol 2001;12:1075–80 [DOI] [PubMed] [Google Scholar]
- 12.Vaidya JS, Joseph DJ, Tobias JS, Bulsara M, Wenz F, Saunders C, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomised, non-inferiority phase 3 trial. Lancet 2010;376:91–102 10.1016/S0140-6736(10)60837-9 [DOI] [PubMed] [Google Scholar]
- 13.Keshtgar MR, Vaidya JS, Tobias JS, Wenz F, Joseph D, Stacey C, et al. Targeted intraoperative radiotherapy for breast cancer in patients in whom external beam radiation is not possible. Int J Radiat Oncol Biol Phys 2011;80:31–8 10.1016/j.ijrobp.2010.01.045 [DOI] [PubMed] [Google Scholar]
- 14.Eaton DJ, Duck S. Dosimetry measurements with an intraoperative x-ray device. Phys Med Biol 2010;55:N359–69 10.1088/0031-9155/55/12/N02 [DOI] [PubMed] [Google Scholar]
- 15.Eaton DJ. Quality assurance and independent dosimetry for an intraoperative x-ray device. Med Phys 2012;39:6908–20 10.1118/1.4761865 [DOI] [PubMed] [Google Scholar]
- 16.Klevenhagen SC, Auckett RJ, Harrison RM, Moretti C, Nahum AE, Rosser KE. The IPEMB code for practice for the determination of absorbed dose for x-rays below 300 kV generating potential (0.035 mm Al–4 mm Cu HVL; 10–300 kV generating potential). Phys Med Biol 1996;41:2605–258971972 [Google Scholar]
- 17.Aukett RJ, Burns JE, Greener AG, Harrison RM, Moretti C, Nahum AE, et al. Addendum to the IPEMB code for practice for the determination of absorbed dose for x-rays below 300 kV generating potential (0.035 mm Al–4 mm Cu HVL). Phys Med Biol 2005;50:2739–48 10.1088/0031-9155/50/12/001 [DOI] [PubMed] [Google Scholar]
- 18.Eaton DJ, Best B, Brew-Graves C, Duck S, Ghaus T, Gonzalez R, et al. In vivo dosimetry for single-fraction targeted intraoperative radiotherapy (TARGIT) for breast cancer. Int J Radiat Oncol Biol Phys 2012;82:e819–24 10.1016/j.ijrobp.2011.11.012 [DOI] [PubMed] [Google Scholar]
- 19.Low DA, Harms WB, Mutic S, Purdy JA. A technique for the quantitative evaluation of dose distributions. Med Phys 1998;25:656–61 [DOI] [PubMed] [Google Scholar]
- 20.Eaton DJ. Water-equivalence of solid phantom materials at low to very low kilovoltage energies. Proceedings of the Medical Physics and Engineering Conference; 10–12 September 2012; Oxford, UK. York, UK: IPEM, 2012. p. 142
- 21.Armoogum K, Watson C. A dosimetry intercomparison phantom for intraoperative radiotherapy. Z Med Phys 2008;18:120–7 [DOI] [PubMed] [Google Scholar]
- 22.Avanzo M, Rink A, Dassie A, Massarut S, Roncardin M, Borsatti E, et al. In vivo dosimetry with radiochromic films in low-voltage intraoperative radiotherapy of the breast. Med Phys 2012;39:2359–68 10.1118/1.3700175 [DOI] [PubMed] [Google Scholar]
- 23.Price C, Pederson A, Frazier C, Duttenhaver J. In vivo dosimetry with optically stimulated dosimeters and RTQA2 radiochromic film for intraoperative radiotherapy of the breast. Med Phys 2013;40:091716 10.1118/1.4819825 [DOI] [PubMed] [Google Scholar]
- 24.Nwankwo O, Clausen S, Schneider F, Wenz F. A virtual source model of a kilo-voltage radiotherapy device. Phys Med Biol 2013;58:2363–75 10.1088/0031-9155/58/7/2363 [DOI] [PubMed] [Google Scholar]