Abstract
Increases in performance on tests of attention and learning are often observed shortly after a period of aerobic exercise, and evidence suggests that humans who engage in regular exercise are partially protected from age-related cognitive decline. However, the cognitive benefits of exercise are typically short-lived, limiting the practical application of these observations. We explored whether physical exercise would induce lasting changes in general cognitive ability if that exercise was combined with working memory training, which is purported to broadly impact on cognitive performance. Mice received either exercise (six weeks of voluntary running wheel access), working memory training, both treatments, or various control treatments. Near the completion of this period of exercise, working memory training (in a dual radial-arm maze) was initiated (alternating with days of exercise), and was continued for several weeks. Upon completion of these treatments, animals were assessed (2–4 weeks later) for performance on four diverse learning tasks, and the aggregate performance of individual animals across all four learning tasks was estimated. Working memory training alone promoted small increases in general cognitive performance, although any beneficial effects of exercise alone had dissipated by the time of learning assessments. However, the two treatments in combination more than doubled the improvement in general cognitive performance supported by working memory training alone. Unlike the transient effects that acute aerobic exercise can have on isolated learning tasks, these results indicate that an acute period of exercise combined with working memory training can have synergistic and lasting impact on general cognitive performance.
Keywords: Aerobic Exercise, Working Memory, Intelligence, Attention, Cognition, Learning
1. Introduction
Despite the apparent whole-body effects of exercise [1;2], it is only in the past two decades that interest has emerged in the effects of exercise on brain function. During this time, it has become clear that the central nervous system, like other systems of the body, experiences a diverse array of positive physiological effects in response to exercise. These effects are widespread, from gross structural changes to alterations of fine cellular morphology. Some of these changes include, but are not limited to, increases in cerebral blood flow (which may be the catalyst for subsequent effects), increased concentration of growth factors associated with angiogenesis [3;4], increases in proliferating endothelial cells associated with angiogenesis [5;6], changes in dendritic spine size and number [7], increases in brain volume in aged humans [8] and post-natal neurogenesis in regions such as the dentate gyrus of the hippocampus and the subventricular region of the forebrain [9;10].
Given the functional relationship between neural substrates and cognitive processes, it is not surprising that the above observations have led to a surge of interest in the cognitive effects of exercise. Indeed, the progress of research on the cognitive benefits of exercise has closely trailed the neurobiological observations, with discoveries in one field informing and encouraging growth in the other. A majority of the reported results have focused on the relationship between exercise and psychological well-being. To date, exercise has been shown to attenuate the effects of major depression [11], improve sleep quality [12], and protect humans from the cognitive declines that typically accompany aging [13;14].
Much recent interest has been directed at demonstrations of exercise’s ability to improve learning, memory, and attention. Across species and diverse tasks of learning and executive function, improvements in a variety of psychometric evaluations have been documented in response to exercise. After a 10 min bout of moderate intensity aerobic exercise (defined by 50% VO2 intake), human subjects ages 19–24 outperformed controls on the Stroop test, a known metric of attentional control [15]. In mice, voluntary running-wheel access for a period of 45 days resulted in shorter latencies and path lengths in the Morris water maze [16].
Despite the effectiveness of aerobic exercise in the promotion of cognitive performance, this intervention has serious limitations. Namely, broad conclusions have been complicated by variations in the duration of the treatment (chronic versus acute), the nature of the treatment (anaerobic versus aerobic), and in particular, the transient nature of the beneficial effects of exercise. At least when cognitive training closely follows a period of exercise, a preponderance of evidence suggests that acute aerobic treatments result in improvements in cognitive ability (see review in [13] for more details). However, given that the benefits of exercise on cognition require that the cognitive training closely follow the bout of exercise, it would be useful to engage in a cognitive training regimen that would subsequently transfer to other cognitive tasks. While many of the previously mentioned studies have demonstrated sustained single-domain improvements in learning and memory in response to exercise, few, if any, have suggested a protocol by which physical exercise could generate persistent improvements in general cognitive ability. To be successful in this regard, it would be necessary to engage subjects in a domain-independent cognitive training regimen that can broadly (and subsequently) impact more domain-specific cognitive abilities.
General cognitive ability, or g, has been a central focus of intelligence research since its initial proposal by Spearman in 1904 [17]. One hypothesis that has gained traction in recent years suggests that it is variations in the efficacy of working memory that underlie (or at least contribute to) variations in intelligence [18]. The attractiveness of this explanation lies in the structural and functional properties of the working memory system. Comprised of a short-term storage component and an executive control component, working memory maintains information for active use and serves a role in consolidating information for long-term storage [19]. Through the cooperation of these two (storage and processing) components, working memory is able to maintain, process, and integrate information in a goal-relevant fashion [18]. Importantly, working memory is widely regarded to play a critical role in the execution of most (if not all) cognitive tasks. These properties uniquely position working memory to serve as a principal regulator of general intelligence. Indeed, imaging studies have shown that IQ test performance is predicted by activity in brain areas engaged by working memory tasks, such as the dorsolateral and medial prefrontal cortex [20].
Noting the potential causal relationship between working memory and g, a number of studies using both human and animal subjects have yielded results indicative of such a relationship. For instance, Jaeggi et al. [21] reported that training on a complex working memory task yielded improvements in tests of human intelligence performance (also see [22]). In laboratory mice, Light et al. [23] demonstrated that extensive working memory practice promoted an improvement in animals’ aggregate performance across a battery of diverse learning tasks, and Matzel et al. observed that chronic (life-long) working memory practice protected animals from normal age-related cognitive declines [24]. Nevertheless, the beneficial effects of working memory training have typically been small and often do not transfer universally to all cognitive tasks (for reviews, see [25;26]), leading to the conclusion that working memory and intelligence should not be considered as synonymous constructs. Nevertheless, since working memory can broadly impact cognitive performance, any strategy that could facilitate the instantiation of working memory training might well have dramatic impact on general cognitive performance. Here, we will attempt to better instantiate working memory training by implementing it in close proximity to periods of aerobic exercise.
In the present study, a working memory training regimen that has previously been reported to promote modest improvements in general cognitive performance was be combined with a regimen of voluntary aerobic exercise. This treatment was compared to either working memory training or aerobic exercise alone, or control procedures wherein animals are simply exposed to the relevant training apparatus. In addition, the high degree of control and resolution imparted through the use of animal subjects will allow us to determine the degree to which varying amounts of aerobic exercise impact the cognitive improvements associated with that treatment. Finally, by combining both aerobic exercise with working memory training, we can examine the interaction of these two treatments, i.e., it will be possible to determine if the combined effects of these treatments are additive or synergysitc.
2. Materials & Methods
2.1 Animals and Housing
The use of animals in this work was approved by the Rutgers Animal Care and Facilities Committee in accordance with NIH guidelines. 66 CD-1 outbred mice, chosen for their established genetic diversity and consequent individual variability, were ordered from Harlan Laboratories at approximately 10 weeks of age. Owing to illness (intolerance of food deprivation) and apparatus failure, eight animals were lost during the course of the experiment, resulting in a final sample of 58 mice. Animals were given access to food and water ad libitum, except during food deprivation periods (see below). Animals were housed in standard shoebox style cages except during exercise treatments, in which modified wheel-mounted shoebox style cages were utilized. Through the duration of the experiment, animals were maintained on a 12-hour light/dark cycle in a temperature- and humidity-controlled colony room that was adjacent to rooms containing behavioral testing apparatus. Upon arrival in our laboratory, animals were removed from their home cages and held by an experimenter for 60 sec every day for a period of two weeks in order to acclimate to handling. Animals were then randomly divided into seven different groups (balancing for body weights), each of which received a different experimental treatment. Groups included an exercise-only (E) training group (ninitial = 10, nfinal = 7), a group that received working memory-only (WM) training (ninitial = 10, nfinal = 8), a combination (WM/E) group that received both of these treatments (ninitial = 10, nfinal = 9), sham training exposure (E-C, WM-C, WM/E-C) control groups for each of the respective treatments ([ninitial = 9, 9, 9 nfinal = 7, 9, 9, respectively], and a home cage (HC) control group (ninitial = 9, nfinal = 9).
2.2. Working Memory Training Apparatus
For this purpose, we utilized a Dual Radial Arm Maze (DRAM). Figure 1 provides a detailed description of this apparatus. Training in this task has previously been determined to improve both attentional and general learning performance [23;24]. The DRAM featured 16 arms (length: 40 cm) extending radially from a central hub (diameter: 20 cm). Arms were constructed of either black or white Plexiglas, in alternation, such that each arm was between two arms of the other color. Arms were flanked by clear Plexiglas walls on one side (length: 16 cm) that extended outward from the central hub. These walls served to prevent animals from entering adjacent arms without first returning to the central hub. The central hub consisted of two removable, cylindrical components. The first component was a clear Plexiglas cylinder with a black Plexiglas base. This component could be rotated around its center fixation point. Eight semicircular openings were located at the intersection of this cylinder’s wall and base and were spaced so as to allow entrance to every second arm. Thus, depending on the orientation of this cylinder, either the black or white maze was accessible to an animal. The second component of the central hub was a cylindrical unit without a base that was mounted directly inside the outer cylinder. This cylinder could both rotate and be raised up and down. Slightly less wide than the exterior cylinder (with the eight exit doors), this inner cylinder fit inside the outer cylinder with approximately a millimeter of space between the two. Along the bottom edge of the inner cylinder was a single semicircular opening. Thus, given the range of motion of the inner cylinder, it could be placed to provide the animal with access to only a single arm, to all arms (if raised), or no arms (if the exit opening was placed between two doors in the outer cylinder).
Figure 1.
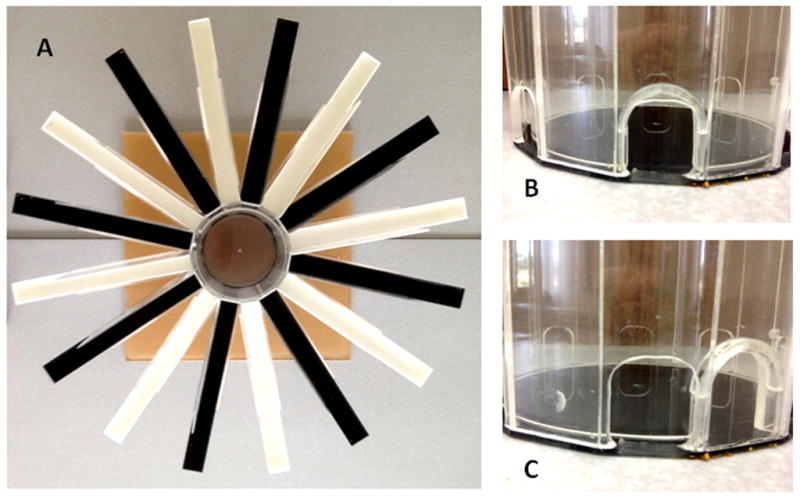
Dual Radial Arm Maze (DRAM; working memory training) apparatus. A) An aerial view of the DRAM. Alternating black and white arms radiated from a central hub. The black and white arms were segregated by a rotating inner cylinder such that animals could operate independently in either a black or white maze. B) The central hub has been removed to illustrate its design. The outer component, which contains 8 exits, is currently aligned with the inner component, which only has one. C) To completely block entry into any arm, the inner component of the central hub could be rotated by one entrance-length.
2.3. Exercise Apparatus
The exercise apparatus was a standard shoebox style cage fitted with an internally mounted freely rotating running wheel and an externally mounted electronic counter. Series 38 N10 shoebox style cages (dimensions: 7.5″ × 11.5″ × 5″) were ordered from the Ancare online catalog (product code: N10HT). Upon arrival, a single hole was drilled at approximately half of the cage’s height on one of the 7.5″ sides to allow interior mounting of the running wheel. Two holes, oriented vertically to each other, were drilled on the other side to accommodate for an externally mounted drip water bottle and a structure to hold it in place. Removable wall-hanging, open-top food containers were mounted on the interior of each cage. 38 “Super Pet Comfort Wheels” (diameter: 5 ½″) were ordered from the Petsmart online catalog (Item: 2753360) and each included a peg upon which the wheel rotated. Pegs were mounted on the interior of the cages and wheels were mounted on the pegs. All components were removable to accommodate cleaning. The exterior edge of the backside of each wheel was fitted with a magnet and counterbalanced by a weight 180 degrees away. In order for the externally mounted electronic counter to track wheel revolutions, a small circuit was wired such that the closure of a magnetically controlled switch increased the number on the counter by +1. Thus, each time the magnet on the wheel revolved past the switch, the device counted a revolution. Sham exposure apparatuses were identical in composition but featured fixed wheels (i.e., wheels that would not turn).
2.4. Overview of Procedure
Figure 2 graphically details the progression of the experiment. Animals arrived and were housed in standard shoebox cages for a period of two weeks, during which they were handled for 60 seconds every day for two weeks. After two weeks of handling, animals that had been assigned to exercise-relevant groups (E, E-C, WM/E, WM/E-C) began their initial exercise treatment. These animals were housed in individual running-wheel equipped shoebox style cages. Concurrently, all remaining groups were housed in standard shoebox style cages. Exercise-relevant groups were given 24 hour/day, 6 day/week access to these wheels for a period of 6 weeks, during which time running wheel revolution counts (for a 24 hr period) were taken every day between 3:00 and 4:00 PM. Animals were placed in standard shoebox style cages on the 7th day of each week to allow cleaning of each cage/exercise apparatus. In order to prevent loose food or other detritus from blocking the rotation of the wheels, bedding and food were changed every day (for all animals) after counts had been taken. Animals’ body weights were taken once weekly in order to see if there were any differential effects on body weight related to wheel counts and other measures of cognitive performance. Throughout this period, any time at which exercise animals were handled (e.g., for cage cleaning), all non-exercise animals were handled for a comparable amount of time. After completion of the 6-week exercise treatment, E, E-C, WM/E, and WM/E-C animals were placed back into standard shoebox style cages and were allowed to rest for 24 hours.
Figure 2.
Graphic illustration of the experimental design. Animals were received and handled for a period of two weeks, after which they were randomly assigned to the groups detailed in the methods section. Treatments that are depicted in vertical parallel occurred concurrently.
Upon completion of the initial exercise treatment, we began the working memory training treatment stage. In this task, animals operated simultaneously (making alternating choices) in two radial arm mazes that share an overlapping set of extramaze visual cues. Since the animals must maintain a memory of choices in the presence of strong task-relevant interference, this task is believed to tax processing aspects of working memory, i.e., capacity and selective attention [27] (Kolata et al., 2005, 2007). Unlike performance in a single radial arm maze (which primarily taxes short-term memory), extensive experience in the DRAM promotes improvements in both selective attention and learning performance across diverse tasks [23;24]. Sham exposure controls were exposed to the maze and rewarded in a manner equivalent to that of the training groups, but without the relevant task demands (see below).
Animals in working memory training-relevant groups (W, W-C, WM/E, WM/E-C) received training in the DRAM or relevant sham exposure. While the DRAM is structurally a radial 16-arm maze, training proceeded in such a manner as to selectively isolate animals in one set of arms (i.e., black or white) or the other, depending on the demands of that particular day. Given the large number of animals to be trained on the DRAM, animals were divided into two cohorts (n = 33). All working memory relevant groups (WM, WM-C, WM/E, WM/E-C) were trained in or received sham exposure to the DRAM. One day prior to the start of training, animals were food deprived. Throughout the remainder of the experiment, animals were given a 2 hr free feeding period at the end of every day’s light cycle. Body weights were recorded each day to assess each animal’s percent of their free-feeding body weight, and if percentages dropped below 90%, animals were given an additional 2-gram pellet of food at the start of the dark cycle.
Prior to actual DRAM training, several weeks of preliminary training were necessary in order to ensure that the animals had achieved proficiency in the individual mazes. Initially, animals were given two days of acclimation exposure to the mazes. During the first day, animals were confined for 180 sec to one black arm of the 16-arm maze, with food reward at its end. During the second day, animals were confined to one arm of the maze (with a food reward at the end) for a period of 90 sec, after which they were moved to the next arm for another 90 seconds. This was repeated for all 16 arms.
On the day following acclimation exposure, animals began training in the black maze. During training, all eight arms of the maze were baited, and the animal was placed in an enclosed cylinder in the center of the maze. Once opened, a timer was started and the animal was allowed to make a free choice. Once the choice had been made, the animal was enclosed in the arm until the food reward had been consumed, after which it was allowed back into the center, enclosed for two seconds, and then allowed to make another decision. This procedure was followed until the animal had successfully entered all 8 arms and had consumed the eight reinforcers. An error was recorded each time that an animal entered an arm in which the food reward had already been retrieved. This first stage of training continued for four days, followed by four days of rest during which time the remaining animals received the same training. During the first cohort’s off days, exercise-relevant groups were placed back into exercise apparatuses and were allowed continuous running wheel access in addition to ad libitum food access. This pattern was continued throughout the remaining training and testing, i.e., four days of training alternated with four days of running wheel access.
After initial training in the black maze, animals began training on the white maze, the second of two radial 8-arm mazes built into the structure of the DRAM. All procedures here were identical to those used for the black maze. Once completed (and animals had received four days of training in both the black and white mazes), the third training stage began, during which animals received additional training in both mazes during each day, i.e., training in the black maze occurred each morning, followed 4 hr later by training in the white maze. All animals received a total of eight days of training in this manner (i.e., training in both the black and white mazes in the course of one day) over a total of 16 days (including two cycles of four consecutive rest days).
Upon completion of the above stages of training, every animal had received 12 days of experience in both the black and white mazes, the last eight days of which included performance in both mazes each day. Upon completion of these preliminary treatments, the critical working memory training began in the DRAM. During this stage, animals were placed in the center unit of the DRAM, with the exterior unit of the central hub set so that only the black maze was accessible to animals. The unit was opened and the animals were allowed to choose an arm. After three correct choices (and, consequently, consumption of three food rewards), doors to the black maze were closed, and doors to the white maze opened. Each animal was then allowed to make three correct choices and consume the respective food rewards in the white maze, after which, the doors to the white maze were closed and doors to the black maze opened. Animals continued in this pattern until all eight food rewards were collected in each maze. Through each trial, latency to finish, number of errors (returns to an arm where the food reward had been collected), and streak of correct arm choices were recorded. This phase of the experiment also lasted for 16 total days. At the completion of working memory training, animals were returned to their standard home cages and had no further access to the running wheels.
2.5. Learning Tasks
Two weeks after completion of the working memory training (and the termination of animals’ access to the running wheels), the performance of all groups was assessed on a five-task learning battery in order to assess their aggregate (general) learning performance. The learning battery consisted of the Morris Water Maze, the Lashley III Maze, an Odor Discrimination Task, and a passive avoidance task. Each of these tasks was chosen due to the different cognitive abilities they are believed to tax as well as their distinct sensory and motor requirements. Task-specific details were as follows, and are described in more detail in [28].
2.5.1. Morris Water Maze
As established by Morris [29], this task involves the use of extra-maze cues, and not fixed motor patterns, to navigate through an environment. Utilizing a round, black pool (diameter: 140 cm, depth: 56 cm), its area was divided conceptually into four quadrants. In the center of one of these quadrants was a slightly submerged platform (1.5 cm below water’s surface). The pool was filled with opaque black water. This color was achieved by blending in non-toxic black finger paint, so as to obscure the position of the platform in the water. In our protocol, three days of training (one of acclimation, one of critical training, and one of probe trials) were utilized. During the acclimation day, animals were enclosed (in a clear Plexiglas cylinder) on the escape platform for 300 sec. On the subsequent day, animals were started from one of two unique locations in each quadrant for a total of 6 trials, with an ITI of 10 min. Animals were timed for their latency to find the platform and were also video recorded in order to quantify the length of the pathways they took to find the platform. Animals were considered to have found the platform when all four paws were placed on it for duration of 10 seconds. On the final day, the submerged platform was removed. Animals were placed in the pool for a total of 90 seconds. Perseverative behavior in the quadrant in which the platform was usually located was taken to be a measure of retention (i.e., more time spent in the quadrant indicated an animal’s expectation that the platform should be there). Ratio of time spent in this quadrant to total time was taken as a measure of retention (and was an indicator of the degree to which the animals had adopted a spatial strategy to locate the platform).
2.5.2. Lashley III Maze
The Lashley III maze is designed in such a way as to promote the use of egocentric cues, as opposed to extramaze cues, for navigation around a maze. Comprised of 4 interconnected alleys (each 58 cm long and 6 cm wide) made of black Plexiglas, animals are placed in the maze in a dark room lit by a single-bulb light source and, in order to navigate its alleys must locate themselves in the maze based only on the motor patterns they have used. Animals received two days of training (acclimation and critical training), prior to which they were food deprived for one day. During acclimation, animals were placed in each of the first three alleys for a period of 4 minutes and in the last for a period of 6 minutes, wherein 3 food rewards were available. During confinement in an alley, access to adjacent alleys was blocked. On the critical training day, animals were placed in the starting alley and allowed to navigate to the maze’s end, where a food reward was located. Animals were placed back into their home cages for an ITI of 20 minutes, during which time the maze was cleaned. This sequence was repeated a total of 5 times. Latency and errors, here counted as turns in the incorrect direction, were recorded.
2.5.3. Odor Discrimination
With respect to appetitive, goal-oriented behavior, mice utilize odors to reinforce these patterns. To take advantage of this fact, we used a black, Plexiglas square (60 square cm, 30 cm high) in which 3 corners featured odors (mint, lemon, or almond). In each of these corners, a small cup, loaded with 3 pieces of food bait (30 mg of chocolate puffed rice) and cotton soaked with 25 μliters of odor under mesh wiring, was located. However, it was only in one cup that these food rewards was accessible. This food reward was reliably paired with mint, despite the location of the cups varying from trial to trial. Animals received two days of training (acclimation and critical training), and were food deprived on the day prior to first training. On the acclimation day, animals were placed in the open field for a period of 20 minutes with no cups present. At the end of their acclimation, three pieces of puffed rice food reward were placed in the animal’s home cage to acclimate them to this novel food item. On the critical training day, the three cups were loaded with food and odor and placed in three corners of the open field. The animal was started in the open corner and allowed to navigate the box until it found the food reward. On only the first trial, an additional food reward was placed on the edge of the cup to encourage the animal to explore the interior of the cups for food. During each of four trials, the location of the correct food odor pairing varied, but the odor was mint throughout all trials. After each trial, the animal was given an additional twenty seconds in the field before being moved back to its home cage for an ITI of 6 minutes. Throughout the task, latency to find food reward and errors were recorded. In this instance, errors included nose pokes into cups with incorrect odors or nose pokes into the correct cup without eating the food reward present.
2.5.4. Passive Avoidance
Utilizing an aversive sound and light stimulus, this task was a one-trial assay in which animals learned to suppress behavior in order to avoid the stimulus. The stimulus was composed of a bright (550 Lux) white light, noise, and vibration. Noise and vibration were produced by a flexible nylon rod attached to a motor outside of an exterior wall of the chamber such that the rod struck the wall of the chamber twice during each revolution (1400 rpm) of the motor, producing a noise 65 dBa above a 45 dBa background and a 46 Hz vibration of the chamber surfaces. In this assay, animals reliably have increased latencies to their second step-down off of a platform in the test chamber relative to their first, demonstrating a one-trial learned suppression of behavior. Animals were placed in a 75 × 45 × 45 cubic centimeter black Plexiglas enclosed platform raised 5 cm above a 16 × 12 cm white grid platform, which, when stepped on, initiated both of the aversive stimuli. Both of these structures were enclosed within a larger box, illuminated by red light. Animals were enclosed in the platform by a remotely operated clear plastic door for a period of five minutes. The latency to leave the platform was recorded, upon which animals left and activated the aversive stimuli. After stepping down, animals were once again placed in the platform for a period of five minutes and latency to leave the platform was taken. The ratio of post- to pre-training latencies was taken as a measure of learning on this task.
2.6. Data analysis
Statistical analyses were performed within the SPSS Statistics package. Once all the animals had been tested in all tasks, several statistical analyses were performed. First, an exploratory factor analysis was performed, both to assess any influence on general (across-task) performance, and to provide a means with which to characterize the general learning performance of individual animals. The factor analysis requires that a single summary score be provided by each animal for each learning task. For this purpose, each animal was assigned a score for each task based on the animal’s performance during the middle of the acquisition phase of learning (i.e., from the middle of the group learning curve), as it is during these trials that the greatest variability between animals is likely to be apparent, and thus individual differences play the strongest role in variations in learning. For example, if during Trials 2 and 3 (of four) of odor discrimination training we observed an interim level of learning (intermediate between first trial and asymptotic performance), an average of these two trials was calculated for each animal, and this average value was entered into the factor analysis to represent that animal’s performance on that task. In one learning task (passive avoidance), animals received only a single training trial. For this task, training conditions were used with which we have previously observed sub-asymptotic (on average) levels of learning.
Upon completion of the factor analysis, a factor score was then computed for each animal that represented that animal’s aggregate learning performance. A factor score is analogous to an average Z-score for each animal computed from the Z-scores obtained for that animal on each task, with the Z-score for each task weighted for the degree to which that task contributed to the principal factor. The factor scores of exercise-only, working memory training-only, and mixed group animals will be compared to assess differences in general learning abilities across the different groups. Subsequently, the groups can be compared by ANOVA followed by planned comparisons of the individual groups.
3. Results
3.1. Exercise
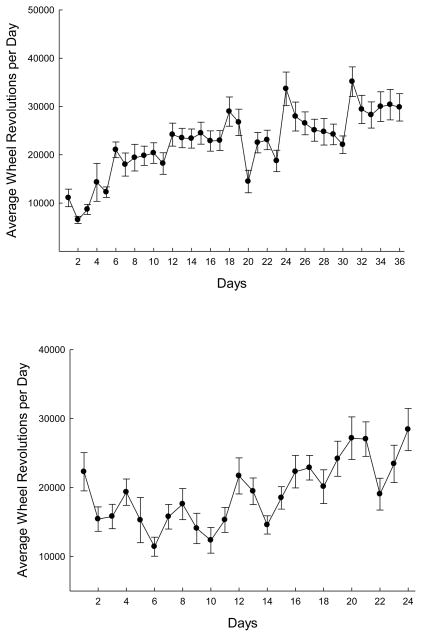
Revolutions of the running wheel were counted for all E and WM/E subjects every day for a total of 36 days at the outset of the experiment. These measurements were taken at roughly the same time every day in order to give a measure of the number of revolutions per 24-hour period. Animals’ (n = 16) daily scores were averaged and plotted against days of treatment. Throughout this period, animals exhibited a steady increase in the amount that they ran each day (See Figure 3A). These scores showed a 209% increase in wheel revolutions from Day 1 (M = 9601, SE = 1826) to Day 36 (M = 29,685 revolutions / day, SE = 2833), F(1, 18) = 41.38, p <. 001. Additionally, a strong effect of exercise over all days was observed, F (35, 630), p <. 001. Mean revolutions per day for the sample over the duration of the treatment was M = 22,254.85 (SE = 1699.16). In addition to the measurements taken at the outset of the experiment, revolutions were tracked during exercise maintenance over the course of training on the DRAM (See Figure 3B). During this phase of the experiment, a generally positive upward increase was once again observed over the duration of the treatment. While the slope of the increase was not as steep as that seen during the initial exercise treatment, there were still significant increases in number of revolutions per day across this period, F (23, 276) = 7.74, p < .001.
Figure 3.
(A) E and WM/E animals received 6 weeks of free access to running wheels, and wheel revolutions were counted for each animal each day. Animals’ scores were averaged and plotted as a function of days (brackets = standard error of the mean). (B) During DRAM training, E and WM/E animals had access to running wheels for four days beginning every fourth day. Again, scores were averaged and plotted as a function of days (brackets = standard error of the mean).
3.2. DRAM Training
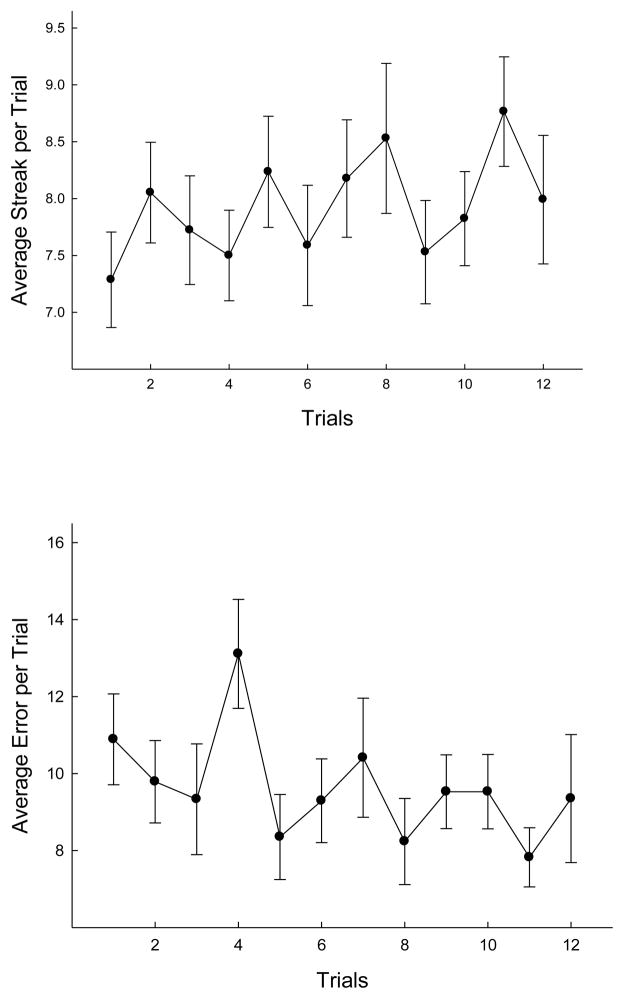
WM and WM/E animals received training on the DRAM for a total of 28 days. The DRAM was intended to tax an animal’s selective attention. However, because WM and WM/E animals were naïve to the task demands of the DRAM, the first 16 of the 28 days of training were dedicated to ensuring that each animal had learned the relevant task demands of each individual maze within the DRAM. During this time, streak length (choices prior to an error) in the black maze improved over the course of days, F (11, 176) = 2.095, P = .023, while the number of errors committed decreased during this same period of training, F (11, 176) = 3.98, p < .001. A similar pattern was observed in the white maze during this initial training period. After the initial training in the black and white mazes, the critical phase of working memory training began. Unlike the initial training period, streak length did not significantly improve over days, although a significant decrease in errors made over days was observed, F (11, 176) = 1.86, p <. 05 (see Figure 4). These results suggest an improvement in working memory/selective attention performance across the course of training.
Figure 4.
Errors and length of streaks of correct choices prior to an error were recorded during DRAM (working memory) training. (A) Average streak length over the 12 days during which the critical training trials occurred, i.e., those trial in which animals alternated choices between the black and white mazes. (B) Average number of errors over the 12 days during which the critical training trials occurred. Brackets indicate standard error of the mean.
3.3. Learning Battery
Each task from the learning battery was scored as previously described (see Methods). In all multi-trial tasks, animals’ scores improved across trials, exhibiting learning curves similar to those previously documented [28]. As learning curves for each individual task is not relevant to the present analysis, graphs of performance on individual tasks from the battery are not illustrated here.
In order to conduct a factor analysis, a single measure representing individual animal’s rate of learning on each task was generated. Trials in the “middle” of the learning curve (i.e., exclusive of the first trial and prior to group asymptote) on each task were selected and averaged together to provide a single score for each animal to enter in the factor analysis. As the direction of change indicative of learning differed for some of the learning tasks (e.g., a more positive value [longer step-down latencies] indicated better learning in Passive Avoidance, but a negative value [reduced path length] indicated improvement in the Morris Water Maze), all values that were entered into the factor analysis were converted such that negative numbers indicated improved performance. By doing so, correlated performance measures would load in a consistent direction in our factor analysis. (This conversion is done merely to simplify the visual interpretation of factor loadings; it has no impact on either eigenvalues or the percentage of variance explained by any factor.)
The performance of animals across the individual learning tasks were positively correlated (see Table 1), suggesting that the ability to learn these diverse tasks was regulated by a single underlying source of variance (i.e., “general learning ability). This conclusion was supported by the results of a principal component analysis of the learning data from all animals across all learning tasks (Table 2). Significantly, a primary factor (eiganvalue 1.41) accounted for 35% of the variance in performance across all learning tasks, and performance on all tasks loaded in a consistent direction on this factor. Thus this factor describes “general learning ability”. A second factor accounted for 32% of the variance across tasks (eiganvalue 1.28). However, performance on the individual tasks loaded with similar strength in opposite directions on this factor, suggesting that it accounted for some aspect of task-specific performance. The interpretation of this second factor will not be considered further.
Table 1.
Pearson correlations (r) of the performance of all animals among learning tasks.
| Learning task | Passive Avoidance | Lashley Maze | Odor Discrimination | Water Maze |
|---|---|---|---|---|
| Passive Avoidance | 1 | - | - | - |
| Lashley Maze | .220 | 1 | - | - |
| Odor Discrimination | .135 | .287* | 1 | - |
| Water Maze | .235 | .045 | .212 | 1 |
Correlation is significant at the 0.05 level (2-tailed).
Table 2.
Results of a principal component analysis of animals’ performance across four learning tasks. On the principal factor, all learning tasks loaded from moderately to highly in a single direction, indicative of a general influence on diverse learning abilities. The irregular loading on Factor 2 suggests that this factor describes some aspect of task-specific performance.
| Factor 1 (General Learning) | Factor 2 | |
|---|---|---|
|
| ||
| Lashley Maze | .38 | −.71 |
| Water Maze | .51 | −.26 |
| Passive Avoid. | .68 | .27 |
| Odor Discrim | .74 | .79 |
|
| ||
| eignavalue | 1.41 | 1.28 |
|
| ||
| % Variance | 35.26 | 32.01 |
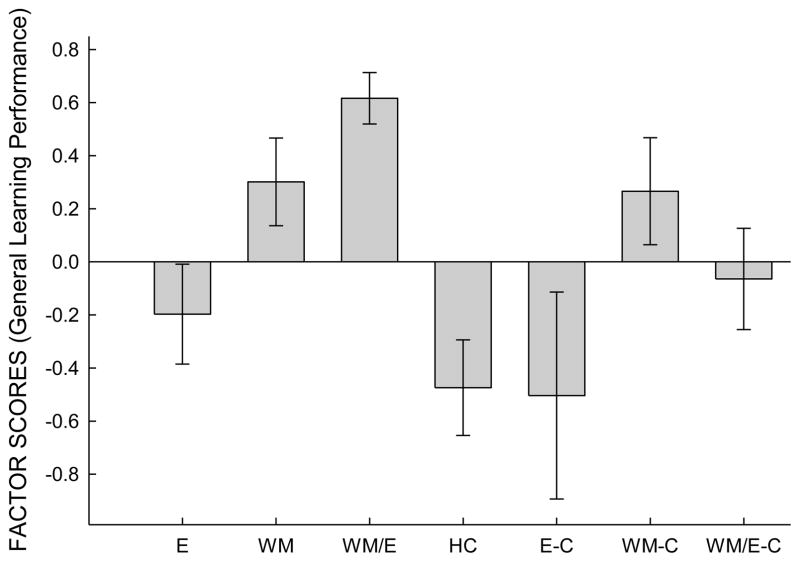
Factor scores (indicative of aggregate learning performance across all tasks) were computed for each animal and were segregated into their respective groups (E, E-C, W, W-C, WM/E, WM/E-C, HC), and a mean value was computed for each group (see Figure 5). To assess differences between all groups, a simple ANOVA with contrasts was performed. In this analysis, the only significant differences observed between groups that received either of the critical treatments was between the WM/E and E groups, t(50) = 2.850, p = .006 (Cohen’s d effect size = 0.81). A near-significant difference between WM and WM/E groups was observed, t(50) = 1.740, p = .088 (Cohen’s d effect size = 0.49). No significant difference was observed between the E and WM group. Comparisons between experimental and control groups were then conducted. A significant difference between WM/E and WM/E-C was observed, t(50) = 2.32, p = .024 (Cohen’s d effect size = 0.66), while no significant differences between E and E-C or WM and WM-C were found. Given that there was no significant difference between E and E-C, a comparison between these groups and the HC groups was conducted. Again, no significant differences were observed.
Figure 5.
Factor scores (representing aggregate performance across four learning tasks) were segregated into the seven groups. Average factors are plotted for each group (± standard error of the mean). Animals that received working memory training combined with exercise exhibited higher aggregate learning performance than all other groups.
In order to determine if a relationship existed between an animals’ total amount of exercise (mean number of revolutions during exercise treatments) and its factor scores (indicative of general learning performance), as well as between an animals’ performance on the DRAM and its factor scores, regressions for each of these comparisons were made. There was a strong correlation between the amount of aerobic exercise engaged in during the initial 6 weeks of access to the running wheels and during subsequent access interspersed with working memory training, r(16) = 0.76, p = .001, indicating a consistency of individual animal’s amount of exercise over time. Across all animals that had access to the running wheels, there was no significant correlation between running-wheel revolutions and factor scores indicative of general learning performance, r(16) = −0.11, p = .68. Additionally (for Group WM/E), the amount of exercise engaged in was not predictive of streak length during working memory training, r(16)= −0.02, p = .95, or error total during working memory training, r(16)= 0.33, p = .392. These results suggest that beyond some optimal level of running, additional running had no beneficial impact on either the instantiation of working memory training or general learning performance.
4. Discussion
With the physiological and neurobiological benefits of exercise well-documented, the present study aimed to determine whether chronic physical exercise could facilitate the instantiation of working memory training, and if so, if that better instantiation of working memory would promote improvements in general cognitive ability. This possibility contrasts with more established procedures wherein exercise has been associated with time-limited improvements in isolated learning tasks, for which there would be no expectation of transfer to other learning domains. Nine weeks of aerobic exercise, the last three of which were interspersed with working memory practice, had a positive impact on animals’ aggregate performance when they were later tested on four tests of learning. Significant differences in average group factor scores (indicative of general cognitive performance) were observed between the animals that received both aerobic exercise and DRAM (working memory) training relative to animals that received exercise or DRAM training in isolation. Not surprisingly, since a long delay (>two weeks) interceded between aerobic exercise and testing in the learning battery, exercise alone promoted no improvements in general learning ability relative to sham exercise or untreated controls. In contrast, DRAM training alone promoted improvements in general learning performance (relative to untreated controls). However, this latter conclusion should be qualified, since actual DRAM treatment and sham exposure to the DRAM apparatus produced similar levels of improvement in aggregate learning performance. This lack of difference between DRAM training and its exposure control group is somewhat paradoxical, as in previous studies, similar control groups exhibited inferior general learning ability relative to animals that received actual working memory training [23;24]. Nevertheless, a general pattern emerged that seemed to corroborate the hypothesis that working memory practice, when coupled with exercise, promoted increases in performance beyond what could be contributed additively by either treatment individually. In total, these results indicate the general and lasting impact of working memory training is better instantiated when instituted in conjunction with aerobic activity.
It is established that exercise induces a variety of neurobiological changes, including changes in synaptic plasticity, dendritic spine density, and vasculature [30]. While some doubt has been cast as to the functional significance of these changes, research has indicated that structural changes to the brain in periods after adolescence do indeed serve functional purposes, not the least of which include the formation of new, functional synapses associated with spatial learning [31], and fully integrated newly-born neurons in the hippocampus [16]. Noting that these structural changes do indeed prove fruitful for the enhancement of specific learning domains, we hoped to develop a strategy with which it would be possible to promote more general improvements in cognitive function. Having previously demonstrated that a working memory training regimen induces improvement in general cognitive performance, including on various tests of learning and selective attention [23;24], it was our expectation that coupling aerobic exercise with working memory training might enhance general cognitive ability beyond that seen in response to either treatment alone. This might occur if a modified (through aerobic exercise) neurobiological substrate would provide a better environment for the instantiation of working memory training. Thus, if the well-established neurobiological changes that follow from exercise create a substrate through which more efficient learning can occur, a combined treatment of exercise and working memory training would lead to increases in general cognitive ability, not simply the better instantiation of a single learned response (as has been previously demonstrated). The present behavioral data lends support to this general hypothesis, which is corroborated by a recent report by Langdon and Corbett [32], wherein it was determined that the combination of voluntary running and cognitive therapy was more effective than either treatment in isolation.
It is notable that the effects of exercise on both cognition and brain substrates has been found to be dose-dependent. It has been reported that the effects of exercise are graded in its ability to induce neurogenesis [33], its ability to attenuate depression [34], and with respect to decreasing rates of heart disease and improving quality of life measures [35]. Thus we anticipated that the more an animal engaged in exercise (wheel running), the greater the cognitive benefits it might receive from this experience. However, no relationship was observed between the degree to which an animal engaged in exercise and the degree of improvement in its general learning performance relative to untreated controls. This data contrasted with what were clear differences between groups that were mediated by exercise. In this regard, it is notable that although a high degree of variability in the amount of running was observed across animals, all animals made large numbers of wheel revolutions each day. Thus it is possible that all animals crossed a critical level of exercise beyond which no additional benefits were possible (also see [32]). Again though, since exercise combined with working memory training promoted improvements in general cognitive performance relative to animals that had no access to the running wheels, it follows that there is also a lower limit of exercise necessary for animals to experience these cognitive benefits. As the present study did not manipulate dosage (allowing only free access to running), future studies will be necessary to determine the dose-response effects of exercise on working memory training.
5. Conclusions
The present data indicate several conclusions about exercise and its relationship to cognitive ability. Specifically, it is clear that exercise imparts a general cognitive benefit when coupled with a working memory-training regimen (which itself, broadly impacts cognitive performance), but does not (in all circumstances) in itself provide lasting cognitive benefits. Alone, working memory training had more modest impact on general cognitive performance. The present data lend themselves to a body of literature that increasingly emphasizes the holistic importance of exercise. With these data, future research venues include further exploration into changes in neurobiological structure in regions of the brain important for executive function. Utilizing the behavioral paradigm established in this study, future research can more accurately determine to what degree recent versus chronic exercise impacts the instantiation of working memory training.
Research Highlights.
Mice received either or both working memory training and aerobic exercise.
Improvements were observed in both aerobic activity and working memory performance.
Effects of treatments were assessed across four tests of learning.
Aerobic exercise alone had no sustained effect on cognitive performing.
Aerobic exercise with working memory training promoted lasting cognitive improvements.
Acknowledgments
This work was supported by the National Institute of Aging (AG022698), the Office of Naval Research (N000141210873) and the Busch Foundation (to LDM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fletcher GF, Balady G, Blair SN, Blumenthal J, Caspersen C, Chaitman B, Epstein S, Froelicher ESS, Froelicher VF, Pina IL, Pollock ML. Statement on Exercise: Benefits and Recommendations for Physical Activity Programs for All Americans: A Statement for Health Professionals by the Committee on Exercise and Cardiac Rehabilitation of the Council on Clinical Cardiology, American Heart Associ. Circulation. 1996;94:857–862. doi: 10.1161/01.cir.94.4.857. [DOI] [PubMed] [Google Scholar]
- 2.Warburton DER, Nicol CW, Bredin SSD. Health benefits of physical activity: the evidence. Canadian medical association journal. 2006;174:801–809. doi: 10.1503/cmaj.051351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Gustafsson T, Puntschart A, Kaijser L, Jansson E, Johan C, Jansson EVA, Sundberg CJ, Carl JS. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. American Journal of Physiology - Heart and Circulatory Physiology. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- 5.Ekstrand J, Hellsten J, Tingström A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neuroscience Letters. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- 6.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jürgens K, Miche E, Böhm M, Nickenig G. Physical training increases endothelial progenitor cells, inhibits neointima formation, and enhances angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 7.Stranahan AM, Khalil D, Gould E. Running Induces Widespread Structural Alterations in the Hippocampus and Entorhinal Cortex. Hippocampus. 2007;17:1017–1022. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colcombe SJ, Erickson KI, Scalf PE, Kim JS, Prakash R, Mcauley E, Elavsky S, Marquez DX, Hu L, Kramer AF. Aerobic exercise training increases brain volume in aging humans. Journal of Gerontology: MEDICAL SCIENCES. 2006;61:1166–1170. doi: 10.1093/gerona/61.11.1166. [DOI] [PubMed] [Google Scholar]
- 9.Bednarczyk MR, Aumont A, Décary S, Bergeron R, Fernandes KJL. Prolonged voluntary wheel-running stimulates neural precursors in the hippocampus and forebrain of adult CD1 mice. Hippocampus. 2009;19:913–927. doi: 10.1002/hipo.20621. [DOI] [PubMed] [Google Scholar]
- 10.Gould E, Beylin AV, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the adult hippocampal formation. Nature neuroscience. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- 11.Martinsen EW, Medhus A, Sandvik L. Effects of aerobic exercise on depression: a controlled study. British medical journal (Clinical research ed) 1985;291:109. doi: 10.1136/bmj.291.6488.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gambelunghe C, Rossi R, MariucciI G, Tantuci M, Ambrosini MV. Effects of light physical exercise on sleep regulation in rats. Medicine and science in sports and exercise. 2001;33:57–60. doi: 10.1097/00005768-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Colcombe S, Kramer AF. Fitness Effects on the Cognitive Function of Older Adults A Meta-Analytic Study. Psychological science. 2003:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 14.Dustman RE, Ruhling RO, Russell EM, Shearer DE, Bonekat HW, Shigeoka JW, Wood JS, Bradford DC. Aerobic exercise training and improved neuropsychological function of older individuals. Neurobiology of Aging. 1984;5:35–42. doi: 10.1016/0197-4580(84)90083-6. [DOI] [PubMed] [Google Scholar]
- 15.Yanagisawa H, Dan I, Tsuzuki D, Kato M, Okamoto M, Kyutoku Y, Soya H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage. 2010;50:1702–1710. doi: 10.1016/j.neuroimage.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spearman C. General intelligence, objectively determined and measured. American Journal of Psychology. 1904;15:201–293. [Google Scholar]
- 18.Kane MJ, Hambrick DZ, Conway AR. Working memory capacity and fluid intelligence are strongly related constructs: comment on Ackerman, Beier, and Boyle (2005) Psychological Bulletin. 2005;131:66–71. doi: 10.1037/0033-2909.131.1.66. [DOI] [PubMed] [Google Scholar]
- 19.Baddeley AD. Working memory: Looking back and looking forward. Nature Reviews Neuroscience. 2003;4:829–839. doi: 10.1038/nrn1201. [DOI] [PubMed] [Google Scholar]
- 20.Conway ARA, Kane MJ, Al CET. Working memory span tasks : A methodological review and user’s guide. Psychonomic bulletin & review. 2005;12:769–786. doi: 10.3758/bf03196772. [DOI] [PubMed] [Google Scholar]
- 21.Jaeggi SM, Buschkuehl M, Jonides J, Perrig WJ. Improving fluid intelligence with training on working memory. Proc Natl Acad Sci USA. 2008;105:6829–6833. doi: 10.1073/pnas.0801268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kray J, Kipp KH, Karbach J. The development of selective inhibitory control: the influence of verbal labeling. Acta Psychol(Amst) 2009;130:48–57. doi: 10.1016/j.actpsy.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Light K, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. Working memory training promotes general cogntive abilities in genetically heterogeneous mice. Current Biology. 2010 doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzel LD, Light K, Wass C, Colas-Zelin DL, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learn Mem. 2011 doi: 10.1101/lm.2034711. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shipstead Z, Redick TS, Engle RW. Is working memory training effective? Psychol Bull. 2012;138:628–654. doi: 10.1037/a0027473. [DOI] [PubMed] [Google Scholar]
- 26.Redick TS, Shipstead Z, Harrison TL, Hicks KL, Fried DE, Hambrick DZ, Kane MJ, Engle RW. No Evidence of Intelligence Improvement After Working Memory Training: A Randomized, Placebo-Controlled Study. J Exp Psychol Gen. 2012 doi: 10.1037/a0029082. [DOI] [PubMed] [Google Scholar]
- 27.Kolata S, Light K, Townsend DA, Hale G, Grossman H, Matzel LD. Variations in working memory capacity predict individual differences in general learning abilities among genetically diverse mice. Neurobio Learn Mem. 2005;84:242–246. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Matzel LD, Han YR, Grossman H, Karnik MS, Patel D, Scott N, Specht SM, Gandhi CC. Individual differences in the expression of a “general” learning ability in mice. J Neurosci. 2003;23:6423–6433. doi: 10.1523/JNEUROSCI.23-16-06423.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris RGM. Spatial localization does not require the presence of local cues. Learn Mot. 1981;12:239–260. [Google Scholar]
- 30.Van Praag H. Exercise and the brain: something to chew on. Trends in neurosciences. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moser MB, Trommald M, Andersen P. An increase in dendritic spine density on hippocampal CA1 pyramidal cells following spatial learning in adult rats suggests the formation of new synapses. Proc Natl Acad Sci USA. 1994;91:12673–12675. doi: 10.1073/pnas.91.26.12673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langdon KD, Corbett D. Improved working memory following novel combinations of physical and cognitive activity. Neurorehabil Neural Repair. 2012;26:523–532. doi: 10.1177/1545968311425919. [DOI] [PubMed] [Google Scholar]
- 33.Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- 34.Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- 35.Martin CK, Church TS, Thompson AM, Earnest CP, Blair SN. Exercise dose and quality of life: a randomized controlled trial. Arch Intern Med. 2009;169:269–278. doi: 10.1001/archinternmed.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]