Abstract
Proof of concept for MERTK gene replacement therapy has been demonstrated using different viral vectors in the Royal College of Surgeon (RCS) rat, a well characterized model of recessive retinitis pigmentosa that contains a mutation in the Mertk gene. MERTK plays a key role in renewal of photoreceptor outer segments (OS) by phagocytosis of shed OS tips. Mutations in MERTK cause impaired phagocytic activity and accumulation of OS debris in the interphotoreceptor space that ultimately leads to photoreceptor cell death. In the present study, we conducted a series of preclinical potency and GLP-compliant safety evaluations of an adeno-associated virus type 2 (AAV2) vector expressing human MERTK cDNA driven by the retinal pigment epithelium–specific, VMD2 promoter. We demonstrate the potency of the vector in RCS rats by improved electroretinogram (ERG) responses in treated eyes compared with contralateral untreated controls. Toxicology and biodistribution studies were performed in Sprague–Dawley (SD) rats injected with two different doses of AAV vectors and buffer control. Delivery of vector in SD rats did not result in a change in ERG amplitudes of rod and cone responses relative to balanced salt solution control–injected eyes, indicating that administration of AAV vector did not adversely affect normal retinal function. In vivo fundoscopic analysis and postmortem retinal morphology of the vector-injected eyes were normal compared with controls. Evaluation of blood smears showed the lack of transformed cells in the treated eyes. All injected eyes and day 1 blood samples were positive for vector genomes, and all peripheral tissues were negative. Our results demonstrate the potency and safety of the AAV2-VMD2-hMERTK vector in animal models tested. A GMP vector has been manufactured and is presently in clinical trial.
Conlon and colleagues report results from a series of preclinical potency and safety evaluations of an AAV2 vector expressing human mer proto-oncogene tyrosine kinase (MERTK) cDNA driven by a retinal pigment epithelium-specific VMD2 promoter (AAV2-VMD2-hMERTK). They demonstrate that a single injection of this vector is effective in a rat model of recessive retinitis pigmentosa, whereas biodistribution studies indicate that vector is not spread outside the eye.
Introduction
Retinitis Pigmentosa (RP) is a genetically heterogeneous disorder that causes progressive loss of rod and then cone photoreceptor cells. It affects approximately one in 3,500 people and is a major cause of inherited blindness in the Western world. It may result from mutations in any one of over 50 genes identified (http://www.sph.uth.tmc.edu/retnet/). The majority are expressed exclusively in either photoreceptor or retinal pigment epithelium (RPE) (Rivolta et al., 2002; Hartong et al., 2006).
One of the identified genes is mer proto-oncogene tyrosine kinase (MERTK) that encodes a transmembrane receptor of tyrosine kinases (Gal et al., 2000; Strick and Vollrath, 2010). Two phagocytic cell types, RPE and macrophages, are the major sites of MERTK expression (Strick and Vollrath, 2010). MERTK plays a critical role in the physiological renewal of photoreceptor outer segments (OS), in which membrane discs shed on a daily basis are phagocytized by RPE cells. MERTK triggers photoreceptor OS ingestion by the RPE. Mutations in MERTK cause impaired phagocytic activity of RPE cells and accumulation of OS debris in the interphotoreceptor space and ultimately result in retinal degeneration (Bok and Hall, 1971; Mullen and LaVail, 1976). Nearly all MERTK mutations identified in human patients are associated with early-onset retinal dystrophy. Affected individuals display dot-like autofluorescent retinal deposit early in the disease, a hallmark of the MERTK form of RP (Ksantini et al., 2012). Patients usually exhibit night vision loss in the first decade of life, followed by reduced cone vision (Gal et al., 2000; Thompson et al., 2002; Tschernutter et al., 2006; Charbel et al., 2009; Mackay et al., 2010; Shahzadi et al., 2010; Ostergaard et al., 2011). Early macular atrophy is also often reported (Tschernutter et al., 2006; Charbel et al., 2009; Mackay et al., 2010; Ostergaard et al., 2011).
The Royal College of Surgeons (RCS) rat is a well studied model of recessive RP that contains a mutation in the Mertk gene resulting in a null allele (D'Cruz et al., 2000; Gal et al., 2000). The model develops a spontaneous retinal degeneration characterized by accumulation of OS debris in the subretinal space (Bok and Hall, 1971; Mullen and LaVail, 1976). Photoreceptor cell loss is rapid, beginning around postnatal day 20 (P20), with photoreceptor cells almost completely absent by P60 (Dowling and Sidman, 1962). The RCS rat has been widely used as a model for proof-of-concept studies of MERTK gene replacement therapy using different viral vectors. Improvement in photoreceptor survival, retinal electrophysiology, and RPE function has been demonstrated in response to treatment following gene delivery using adenovirus (Vollrath et al., 2001), adeno-associated virus (AAV) (Smith et al., 2003; Deng et al., 2012), and lentivirus (Tschernutter et al., 2005). These studies suggest that viral-mediated gene replacement therapy is a potentially efficacious treatment approach for patients with MERTK-associated RP.
In recent years, recombinant AAV (rAAV) in particular has gained prominence in the treatment of inherited retinal disorders (Boye et al., 2013). The results from three separate Phase I clinical trials for Leber's congenital amaurosis type 2 demonstrated the safety of AAV2 in human patients (Bainbridge et al., 2008; Cideciyan et al., 2008; Maguire et al., 2008).
In the present study, we conducted a series of preclinical potency and safety evaluations of an AAV2 vector expressing human MERTK cDNA driven by an RPE-specific VMD2 promoter. The −585/+38 bp version of the human VMD2 promoter has previously been shown to drive efficient and exclusive transgene expression in the RPE (Alexander and Hauswirth, 2008). We evaluated the effectiveness of the vector in RCS rats by electroretinogram (ERG) analysis. The potential effects of vector on eyes were investigated in Sprague–Dawley (SD) rats by electrophysiology and retinal morphology. The toxicology was assessed in SD rats by performing GLP-compliant experiments aimed at characterizing any toxicity as the result of vector administration based on clinical observations and histopathology. Concomitant with this, we also evaluated biodistribution of vector to areas proximal to the injected eye and blood.
Results and Discussion
Clinical trial
Our studies of efficacy, safety, and biodistribution presented here are intended to fill the gap between proof-of-concept and use of this material for human gene therapy by Dr. Fowzan Alkuraya and colleagues at the King Faisal Specialist Hospital & Research Centre, Saudi Arabia. The planned clinical trial would involve the same viral vector administered in the subretinal space in human patients with retinal dystrophy caused by MERTK mutations. We expect introduction of AAV-mediated expression of MERTK would preserve residual retinal function and prevent further loss of photoreceptors in treated patients. Detailed ophthalmic and systemic assessment before and at regular intervals after the injection will be performed. In addition, molecular and serological tests directed at the identification of the presence of the viral genome systemically and the development of immune response, respectively, will be carried out.
Objectives and study design
The goal of this preclinical study is to determine the efficacy of an AAV2-VMD2-hMERTK vector in RCS rats, and assess the potential toxicology and biodistribution of this vector in SD rats following subretinal injection.
Although photoreceptor cells in RCS rats degenerate at a faster rate than in human patients, the main features of the disease in RCS eyes closely mimic the ocular phenotype in human patients, including accumulation of OS debris in the interphotoreceptor space and photoreceptor cell degeneration. To evaluate the potency of the test article vector, RCS rats were injected at P9 before significant photoreceptor cell loss and electroretinographic analysis was performed 2 months post injection. SD rats were used for toxicology and biodistribution studies because of the availability of data that exist for this strain in terms of the health status of different organs and tissues. Potential toxicity associated with vector and/or vector delivery would be difficult to assess in RCS rats because of the progressive retinal dystrophy, the poorly characterized health status of their nonocular tissue, and the fact that, in general, they are poor breeders. The age range of the SD rats in the primary toxicology study (females, 150–300 g; males, 200–500 g) represented an adolescent to adult population corresponding approximately to the expected clinical application age range.
For the toxicology and biodistribution studies, a total of 46 SD rats were assigned to three injection groups: (1) vehicle control [balanced salt solution (BSS)]; (2) AAV2-VMD2-hMERTK [total 4×108 vector genomes (vg)]; and (3) AAV2-VMD2-hMERTK (total 4×109 vg). A summary of the three treatment groups is shown in Table 1, and toxicity tests performed at different time points in these three groups are summarized in Table 2.
Table 1.
Summary of Primary Toxicology and Biodistribution Dose Groups
| Group number | Number of SD rats (sex) | Substance | Total dose (vg) | Total dose volume (μL) | Dosing regimen | Route | Observation period |
|---|---|---|---|---|---|---|---|
| 1 | 8 (4F/4M) | BSS | — | 2 | 1 eye, 1 time | SR | 90 days |
| 2 | 19 (10F/9M) | AAV2-VMD2-hMERTK | 4×108 | 2 | 1 eye, 1 time | SR | 90 days |
| 3 | 19 (10F/9M) | AAV2-VMD2-hMERTK | 4×109 | 2 | 1 eye, 1 time | SR | 90 days |
BSS, balanced salt solution; SD, Sprague–Dawley; SR, subretinal; vg, vector genomes.
Table 2.
Testing Schedule of the Primary Toxicology and Biodistribution Study
| Tests performed in SD rats | BSS-treated group | AAV2-VMD2-hMERTK (4×108 vg/mL) group | AAV2-VMD2-hMERTK (4×109 vg/mL) group |
|---|---|---|---|
| Fundoscopic evaluation | Day 30 | Day 30 | Day 30 |
| Electroretinogram analysis | Day 30 | Day 30 | Day 30 |
| Retinal morphology | Day 30 | Day 30 | Day 30 |
| Organ histopathology | Day 90 | Day 90 | Day 90 |
| Clinical pathology | |||
| CBC | Day 0, 30, 90 | Day 0, 30, 90 | Day 0, 30, 90 |
| Serum chemistry | Day 0, 90 | Day 0, 90 | Day 0, 90 |
| Biodistribution | |||
| Blood | Day 0, 1, 90 | Day 0, 1, 90 | Day 0, 1, 90 |
| Tissue | Day 90 | Day 90 | Day 90 |
| Blood smear | Day 30, 90 | Day 30, 90 | Day 30, 90 |
| Body weights | Day 0, 1, weekly, 90 | Day 0, 1, weekly, 90 | Day 0, 1, weekly, 90 |
| Clinical observations | Daily | Daily | Daily |
CBC, complete blood counts; vg, vector genomes.
Summary of data
Vector potency validation in RCS rats
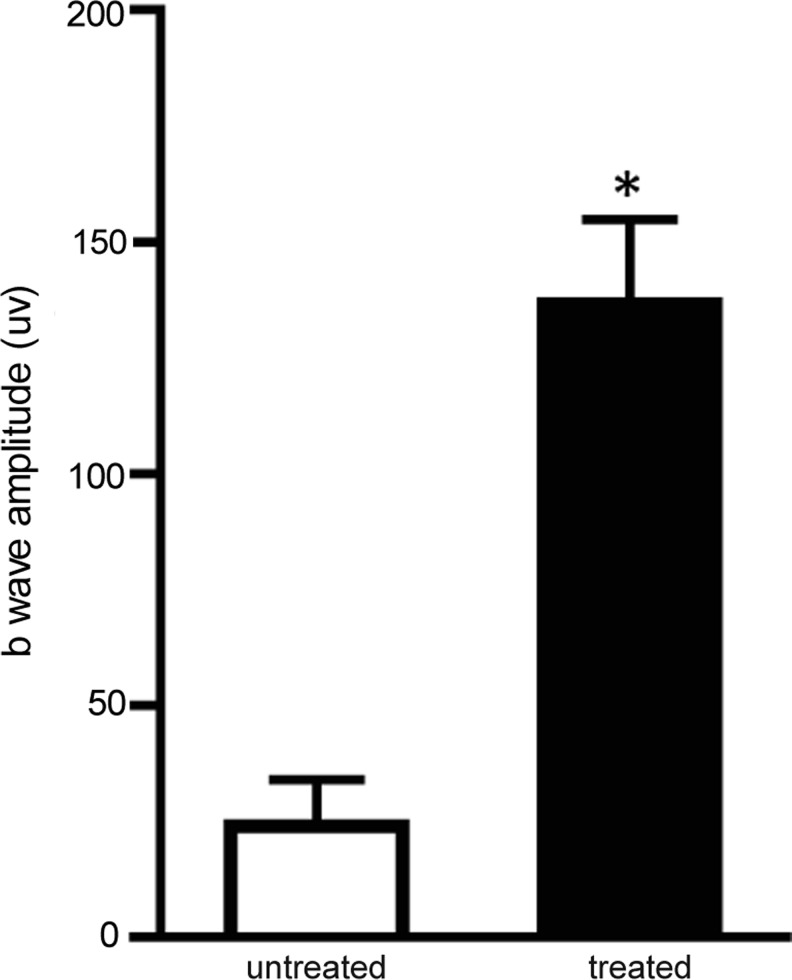
The effect of AAV2-VMD2-hMERTK (4×109 vg dose only) treatment on retinal function was evaluated in RCS rats by ERG at 2 months post injection. ERGs were performed at a light intensity of 2.5 candelas (cd)/m2, which records responses from both rods and cones. The treated eyes showed significantly improved ERG responses (Fig. 1). The mixed rod-cone ERG b wave amplitude was 137±18 μV (average±SEM) in treated eyes versus 24±10 μV in untreated eyes (n=5, p<0.005). The improved ERG response in treated eyes established vector potency.
FIG. 1.
ERG analysis of RCS rats following AAV2-VMD2-hMERTK delivery. A mixed rod-cone ERG response showed significantly improved ERG amplitude in treated eyes compared with contralateral untreated controls (n=5, p<0.005).
Vector safety studies in SD rats
Relevant tests and results used to evaluate the safety of the AAV2-VMD2-hMERTK vector are presented in the following section. Expected and nondetermining results are further outlined in the Supplementary Results (Supplementary Data are available online at www.liebertonline.com/humc).
Ocular examination
The gross in vivo morphology of the retinas was evaluated by fundus photography in all three treated SD rat groups at 1 month post injection and further by ophthalmoscopy with a 28-diopter lens, if necessary, to identify any lens changes, cortical opacification, cataracts, and resolved retinal detachment. The fundoscopy examination revealed that lesions were present in 50% (2/4) of the control animals injected with BSS, 70% (7/10) of the low-dose AAV group, and 50% (5/10) of the high-dose AAV group (data not shown). Almost all lesions were characterized as procedure-associated opacities, and there was no significant difference between the low-dose and high-dose groups, suggesting that the vector was not the source of the observed lesions. Gross retinal anatomy visualizing retinal vessels, optic disc, and posterior retina was largely unremarkable between vector-treated and control eyes (Fig. 2).
FIG. 2.
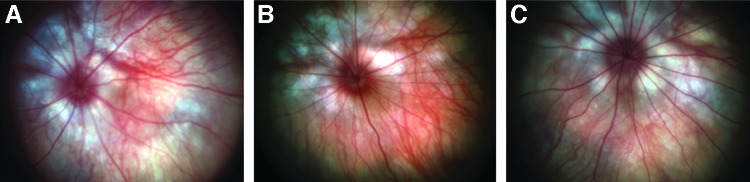
Fundoscopic images of treated eyes from the three experimental groups. (A) The treated eye of a group 1 animal (BSS-injected). (B) A treated eye from group 2 (low dose; 4×108 vg/ml). (C) A treated eye from group 3 (high dose; 4×109 vg/ml). These eyes showed no complications from receiving treatment.
Retinal function
SD rats were further examined by electroretinography at 1 month post injection to determine if vector treatment resulted in any functional deficits. Rats were dark-adapted overnight. ERGs were first performed under scoptopic conditions to target rod-mediated responses, followed by photobleaching and ERG performed under photopic conditions targeted to cone responses (Table 3). The scotopic response (average a-wave amplitude to b-wave amplitude) and photopic ERG response (b-wave amplitude only) analysis revealed an approximately 20% reduction in all three treatment groups that closely matched our previously observed injection-related damage (Pang et al., 2012). The only exception was an approximately 30% reduction of photopic b wave in the BSS-treated group, which is most likely due to the small animal numbers tested.
Table 3.
A Comparison of Scotopic and Photopic ERG Amplitudes Between Treated and Untreated Eyes Across Treatment Groups
| Scotopic b wave | Scotopic a wave | Photopic b wave | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Group | Untreated eye | Treated eye | Treated/untreated | Untreated eye | Treated eye | Treated/untreated | Untreated eye | Treated eye | Treated/untreated |
| BSS (n=4) | 456 | 338 | 0.74 | 202 | 142 | 0.7 | 90 | 51 | 0.57 |
| Low dose (n=9) | 488 | 387 | 0.79 | 195 | 154 | 0.79 | 75 | 59 | 0.79 |
| High dose (n=9) | 491 | 397 | 0.81 | 203 | 173 | 0.85 | 70 | 57 | 0.81 |
The amplitude ratio of treated/untreated is unchanged by increasing dosage.
Retinal morphology
One control-injected SD rat and two SD rats from each vector treatment group were sacrificed, and retinal morphology was analyzed by hematoxylin and eosin staining (Fig. 3). Retinal lamination was consistent across samples with 11–12 rows of outer nuclei present and normal OS length observed in all samples. There was no apparent difference between control, low-dose, and high-dose samples.
FIG. 3.
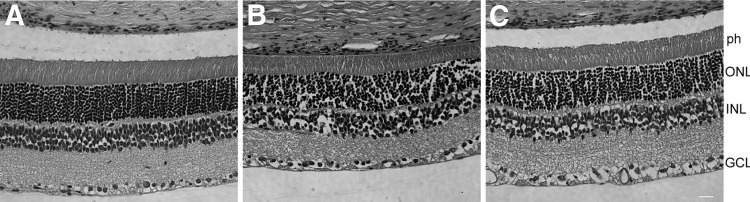
Retinal morphology of treated eyes from the three experimental groups. (A) BSS-injected eye. (B) A low-dose (4×108 vg/ml) treated eye. (C) A high-dose (4×109 vg/ml) treated eye. All eyes showed normal morphology. ph, photoreceptor cells; ONL, outer nuclei layer; INL, inner nuclei layer; GCL, ganglion cell layer.
Histopathology findings
All 24 animals survived to the scheduled necropsy time point. Upon histopathology exam, neither the vector-injected eyes nor optic nerves of both low- and high-dosed males, nor the uninjected eye and optic nerves from all other contralateral uninjected subjects, were found to have any findings by the pathologist. Additional findings, minimal to mild, in the peripheral organs were considered background for the strain, sex, age, and status of the animals in a laboratory setting and are summarized in Table 4.
Table 4.
Summary of Histopathology Findings
| Group 1, BSS control | |
| Pancreas | |
| Granuloma, mild | ++ (1) |
| Group 2, 4×108 vg | |
| Kidney | |
| Ectasia, tubular | + (1), ++ (3) |
| Skeletal muscle, diaphragm | |
| Infiltration, mononuclear cell | + (2) |
| Skeletal muscle, quadriceps | |
| Infiltration, mononuclear cell | + (2) |
| Pancreas | |
| Infiltration, mononuclear cell | + (1) |
| Group 3, 4×109 vg | |
| Kidney | |
| Ectasia, tubular | + (2), ++ (1) |
| Skeletal muscle, quadricep | |
| Infiltration, mononuclear cell | + (2) |
| Lung | |
| Accumulation, macrophage | + (1) |
| Pancreas | |
| Granuloma | + (1) |
+, minimal; ++, mild; +++, moderate; ++++, marked. The number of subjects is shown in parentheses.
BSS, balanced salt solution; vg, vector genomes.
Clinical pathology
Complete blood count assessments were performed on samples collected at baseline, on day 30, and at sacrifice. All samples were analyzed on the day of collection. All parameters measured in the three treatment groups at the specified time points were within normal range and showed no statistical significance except for two parameters. The platelet counts in the high-dose group and the control group at sacrifice were both below the normal range (685–1,436 K/μL established by the manufacturer), and a statistically higher level (p<0.05) than that observed in the high-dose group (575±374 K/μL), compared with the control group (328±162 K/μL). Increased platelet counts (thrombocytosis) may be indicative of myeloproliferative disorder. A careful review of the blood smear slides showed no evidence of such proliferation, or any hematological abnormalities (see the following section). The other statistically significant (p<0.05) decrease was observed for mean corpuscular volume in the high-dose group compared with the control group at sacrifice. However, although statistically different, measurements for both groups were within normal range, which suggested the difference was not clinically significant.
Whole blood smears
Because up-regulation or ectopic MERTK expression is associated with a number of human cancers, including those of the blood (Linger et al., 2008), blood smears were prepared on days 30 and 90 post injection and reviewed by an independent clinical veterinary pathologist for the presence of morphological abnormalities and transformed cells with complete blood count parameters available. Blood cell morphology was within normal variation on all blood samples collected, and there was no evidence of transformed cells.
Vector dissemination in the eye, blood, and peripheral organs
Quantitative real-time PCR was performed to assess the biodistribution of AAV2-VMD2-hMERTK vector DNA in peripheral blood at baseline, at 24 hr, and at sacrifice (day 90±3 days) after administration, as well as in the tissues stated in the Materials and Methods (see Supplementary Data). Vector distribution in the eye is summarized in Table 5. The presence of vector was detected in both low-dose and high-dose groups and showed a dose-response trend. Optic nerves of injected and contralateral uninjected eyes were all negative.
Table 5.
Summary of Vector Biodistribution in the Eye
| Injected eye (vg/μg gDNA) | Uninjected eye (vg/μg gDNA) | |||
|---|---|---|---|---|
| Dose (vg) | Eye | Optic nerve | Eye | Optic nerve |
| 4×108 | 27,520±17,036 | neg | neg | neg |
| 4×109 | 624,338±625,103 | nega | neg | neg |
gDNA, genomic DNA; neg, group mean below limit of detection (<100 copies/μg gDNA); vg, vector genomes.
Conclusions
Our results clearly demonstrated that AAV2-VMD2-hMERTK vector was effective and safe in the animal models tested. We validated vector potency in RCS rats and performed a series of safety studies in normal SD rats that included retinal function, eye anatomy, retinal morphology, tissue histopathology in various organs, clinical pathology by serial blood tests, and biodistribution in different organs at various time points. No local or systemic toxicity was detected after either dose of AAV2-VMD2-hMERTK delivery. There is no indication of vector spread outside the treated eye. The dosage used in these studies would give us a foundation for an initial trial in human patients. We have shown previously that an AAV8 vector containing a tyrosine-to-phenylalanine mutation at position 733 (AAV8 Y733F) conferred a more profound and longer-term rescue in RCS rats (Deng et al., 2012); however, unlike AAV2, safety of the AAV8 Y733F capsid has not been established as of yet in toxicology models or human eyes. The VMD2 promoter used in this vector restricts the MERTK expression exclusively in the RPE cell layer (Alexander and Hauswirth, 2008; Deng et al., 2012) and should prevent any potential adverse effect from off-target MERTK expression. In this case, higher titer vector could be considered in human trials if initial doses prove to be safe.
Supplementary Material
Acknowledgments
The authors would like to thank Andy Neeley for his technical assistance with ERGs and Vince Chiodo for discussion on AAV vector purification. We would also like to thank Douglas Vollrath and Mathew LaVail for providing RCS rat breeders and Kang Zhang for providing MERTK cDNA. This work was supported in part by NIH EY021721, Foundation Fighting Blindness, Macula Vision Research Foundation, and NIH/NCATS Clinical and Translational Science Award to the University of Florida UL1 TR000064.
Author Disclosure Statement
W.W.H. and the University of Florida have a financial interest in the use of AAV therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work. The other authors declare no conflicts of interest.
References
- Alexander J.J. Hauswirth W.W. Adeno-associated viral vectors and the retina. Adv. Exp. Med. Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- Bainbridge J.W. Smith A.J. Barker S.S., et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bok D. Hall M.O. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J. Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye S.E. Boye S.L. Lewin A.S. Hauswirth W.W. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:503–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbel I.P. Bolz H.J. Ebermann I., et al. Characterisation of severe rod-cone dystrophy in a consanguineous family with a splice site mutation in the MERTK gene. Br. J. Ophthalmol. 2009;93:920–925. doi: 10.1136/bjo.2008.147397. [DOI] [PubMed] [Google Scholar]
- Cideciyan A.V. Aleman T.S. Boye S.L., et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Cruz P.M. Yasumura D. Weir J., et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Dowling J.E. Sidman R.L. Inherited retinal dystrophy in the rat. J. Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotte T.R. Conlon T.J. Mueller C. Preclinical study design for rAAV. Methods Mol. Biol. 2011;807:317–337. doi: 10.1007/978-1-61779-370-7_14. [DOI] [PubMed] [Google Scholar]
- Gal A. Li Y. Thompson D.A., et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Hartong D.T. Berson E.L. Dryja T.P. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- Ksantini M. Lafont E. Bocquet B., et al. Homozygous mutation in MERTK causes severe autosomal recessive retinitis pigmentosa. Eur. J. Ophthalmol. 2012;22:647–653. doi: 10.5301/ejo.5000096. [DOI] [PubMed] [Google Scholar]
- Linger R.M. Keating A.K. Earp H.S. Graham D.K. TAM receptor tyrosine kinases: biologic functions, signaling, and potential therapeutic targeting in human cancer. Adv. Cancer Res. 2008;100:35–83. doi: 10.1016/S0065-230X(08)00002-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay D.S. Henderson R.H. Sergouniotis P.I., et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol. Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- Maguire A.M. Simonelli F. Pierce E.A., et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen R.J. LaVail M.M. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Ostergaard E. Duno M. Batbayli M., et al. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol. Vis. 2011;17:1485–1492. [PMC free article] [PubMed] [Google Scholar]
- Pang J.J. Deng W.T. Dai X., et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS One. 2012;7:e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivolta C. Sharon D. DeAngelis M.M., et al. Retinitis pigmentosa and allied diseases: numerous diseases, genes, and inheritance patterns. Hum. Mol. Genet. 2002;11:1219–1227. doi: 10.1093/hmg/11.10.1219. [DOI] [PubMed] [Google Scholar]
- Shahzadi A. Riazuddin S.A. Ali S., et al. Nonsense mutation in MERTK causes autosomal recessive retinitis pigmentosa in a consanguineous Pakistani family. Br. J. Ophthalmol. 2010;94:1094–1099. doi: 10.1136/bjo.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A.J. Schlichtenbrede F.C. Tschernutter M., et al. AAV-mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol. Ther. 2003;8:188–195. doi: 10.1016/s1525-0016(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Strick D.J. Vollrath D. Focus on molecules: MERTK. Exp. Eye Res. 2010;91:786–787. doi: 10.1016/j.exer.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D.A. McHenry C.L. Li Y., et al. Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. Am. J. Hum. Genet. 2002;70:224–229. doi: 10.1086/338455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernutter M. Schlichtenbrede F.C. Howe S., et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- Tschernutter M. Jenkins S.A. Waseem N.H., et al. Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br. J. Ophthalmol. 2006;90:718–723. doi: 10.1136/bjo.2005.084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D. Feng W. Duncan J.L., et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc. Natl. Acad. Sci. U.S.A. 2001;98:12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.