Abstract
Aims
The Clinical Outcomes Research Initiative database was used to evaluate ethnic trends in complicated reflux disease and suspected Barrett’s esophagus among various racial groups.
Methods
Endoscopic findings for procedures performed January 2000–December 2005 for any indication and for reflux-related indications were reviewed by racial group.
Results
Of 280,075 procedures examined, Hispanics were the most likely to have esophagitis (Hispanic 19.6%, white 17.3%, black 15.8%, Asian/Pacific Islander 9.5%, P-value < 0.0001), and white subjects were most likely to have suspected BE (white 5.0%, Hispanic 2.9%, Asian/Pacific Islander 1.8%, black 1.5%, P-value < 0.0001). Endoscopies performed for reflux-related indications had similar trends for esophagitis and esophageal stricture. Among reflux/Barrett’s screening procedures adjusted for age and gender, Hispanics were most likely to have esophagitis (OR = 1.28, P-value < 0.0001) compared to Caucasians.
Conclusion
Our results demonstrate an association of suspected Barrett’s esophagus and stricture with white patients and esophagitis with Hispanic patients. These findings need to be followed-up with further study.
Keywords: Barrett’s esophagus, Esophagitis, Stricture, Minority groups, Race
Introduction
Gastroesophageal reflux disease (GERD) is a condition characterized by abnormal reflux of gastric contents into the distal esophagus, resulting in symptoms of heartburn, regurgitation, and epigastric pain. It represents the third most common GI disorder in the U.S., with epidemiologic studies indicating a prevalence of weekly heartburn or regurgitation of approximately 20% [1–3]. According to the guidelines provided by the American College of Gastroenterology (ACG), patients with typical symptoms can be diagnosed with uncomplicated GERD and can be managed initially by antisecretory therapy [4]. However, a percentage of cases will evolve into complicated GERD, and upper endoscopy may be warranted [5]. Visualization of the distal esophagus endoscopically allows one to detectacid-related mucosal changes such as erosive esophagitis, esophageal stricture, or the development of BE, all of which have implications for further management.
Identifying those at risk for complications of GERD is imperative for proper allocation of our limited endoscopic resources. Traditionally, diagnostic endoscopy has been recommended for those with persistent symptoms despite medical treatment and those with alarm symptoms. Additionally, the updated guidelines for BE diagnosis and management have identified older Caucasian males patients with long-standing reflux symptoms as a population who may benefit from screening endoscopy [4, 6, 7]. Despite these recommendations, there are still many patient variables whose roles in predicting complicated GERD remain to be defined.
Ethnic background in particular has been identified as a possible predictor of complicated GERD. It is generally believed that Caucasian individuals with reflux are at higher risk for developing complications such as esophagitis and BE, while African Americans and Asians are less vulnerable[8–11]. However there are few studies that have addressed ethnic predisposition for manifestations of complicated GERD. The results that are available are conflicting.
In light of the limitations of available data on this subject, we designed a study to evaluate the belief that complicated GERD and BE are predominately diseases of the Caucasian population. By performing a retrospective study using the Clinical Outcomes Research Initiative (CORI), we sought to determine the frequency of complicated GERD and BE among racial groups of all patients undergoing diagnostic endoscopy as well as those who underwent endoscopy for GERD-related indications. Our aim was to identify associations between race and endoscopic finding that could subsequently be followed-up with further prospective studies.
Materials and Methods
Clinical Outcomes Research Initiative (CORI)
The CORI database was established in 1995 to study utilization and outcomes of endoscopy in diverse practice settings. All participating sites agree to use a standardized computerized report generator to create all endoscopic reports and comply with quality-control requirements. The sites’ data files are transmitted electronically to a central data repository—the National Endoscopic Database (NED). Patient and physician identifiers are removed from the data file prior to transmission to protect both patient and physician confidentiality. The data then undergoes computerized quality-control checks to identify missing fields. After the quality-control checks are completed, the data from all sites is merged into the NED for analysis.
Patients and Procedures
The database was queried between the dates of January 1, 2000 and December 31, 2005 for patients undergoing upper endoscopy. If patients had more than one exam during the time period, only the first was included. We excluded endoscopies that were performed for the indication of BE surveillance. Incomplete or duplicate reports and those of patients younger than 18 years old were also excluded. The remaining procedures were reviewed for patient racial group, age, gender, and specific endoscopic findings (see below). The racial groups in this study were defined as white non-Hispanic (WNH), black non-Hispanic (BNH), Hispanic, and Asian/Pacific Islander (API), and were assigned according to the designation entered by the CORI user/examiner. Other race/ethnicity groups were excluded from our analysis.
In addition to reviewing these cases collectively, we narrowed our procedures of interest to those performed for specific upper gastrointestinal symptoms of dyspepsia, reflux, and BE screening, symptoms that are frequently described by patients with GERD. Again, information on patient age, gender, and race were collected. These data were analyzed separately from the larger original cohort.
Endoscopic Findings
We examined the endoscopic diagnoses entered into the CORI database that were suggestive of GERD-related pathology such as esophagitis, suspected Barrett’s esophagus, esophageal stricture, or hiatal hernia. CORI also allows the provider to record Los Angeles (LA) grade when the finding of esophagitis is chosen, and these grades were noted when available (grade A, B, C, and D). Of note, a classification of “grade 0” is also an option on the database. This was interpreted as “no esophagitis,” and these cases were classified with those who did not have any finding of esophagitis on the procedure report.
Statistical Analyses
Contingency table analysis was used to compare categorical variables such as the prevalence of specific endoscopic findings among the four racial groups, as well as stratified by indication. Patients may have more than one endoscopic indication and diagnosis, thus results are not mutually exclusive. Pearson’s Chi-square test statistic or Fisher’s exact test were used for statistical comparisons as appropriate. Multivariate logistic regression was used to separately estimate the odds of endoscopic Barrett’s esophagus and esophagitis among the racial groups after adjusting for age and gender. The estimated probability of each outcome occurring given specified levels of predictors was obtained. The number needed to endoscope (NNE) is the reciprocal of the estimated probability and represents the number of endoscopies that must be done to detect one case. All analyses were performed using SAS software version 9.1 (SAS Institute, Inc., Cary, NC).
Results
From January 1, 2000 to December 31, 2005, 301,147 unique patients underwent upper endoscopy for any indication. Of this number, 8,714 were excluded for the indication of BE surveillance, and 8,438 were excluded for missing race/ethnicity. An additional 3,417 and 503 patients were of Native American non-Hispanic and multi-racial non-Hispanic origin, respectively. These latter numbers were felt to comprise an insignificant proportion of the overall number and therefore were also excluded. Thus, the final number of included upper endoscopies was 280,075. The majority of procedures were performed on WNH patients (82%, n = 229,725). BNH and Hispanic populations had similar relative frequencies (8% each, n = 21,415 and 23,136 respectively), while API patients represented the smallest proportion of procedures (2%, n = 5,799).
Table 1 shows the frequency of GERD-related endoscopic findings among all the endoscopies identified. Overall, esophagitis was seen most frequently in Hispanic patients. Esophagitis was further classified by LA Grade in <50% of cases, and these available data are dichotomized as LA Grade A/B and Grade C/D (Table 2). Again, those who had a finding of “grade 0 esophagitis” were classified as not having esophagitis and are not included in this analysis. Of those patients who had LA Grade recorded, there was more reported Grade A/B esophagitis compared to Grade C/D esophagitis among all racial groups. Among the racial groups, API were most likely to have Grade A/B esophagitis (86.2%), and BNH were most likely to have Grade C/D esophagitis (24.6%). These data indicate that there may be an ethnic association to severity of esophagitis, but the inconsistency of reported LA Grade classification weakens this interpretation.
Table 1.
Overall prevalence of findings and selected indications by race/ethnicity
| Total N (%) | White, NHa N (%) | Black, NHb N (%) | Hispanic N (%) | Asian/PI N (%) | P-value | |
|---|---|---|---|---|---|---|
| Totals | 280,075 | 229,725 | 21,415 | 23,136 | 5,799 | – |
| Esophagitis | 48,314(17.3) | 39,841(17.3) | 3,384(15.8) | 4,536(19.6) | 553(9.5) | <0.0001 |
| Suspected Barrett’s esophagus | 12,538(4.5) | 11,423(5.0) | 331(1.5) | 679(2.9) | 105(1.8) | <0.0001 |
| Esophageal structure | 26,678(9.5) | 24,525(10.7) | 1,330(6.2) | 719(3.1) | 104(1.8) | <0.0001 |
| Hiatal hernia | 91,905(32.8) | 76,761(33.4) | 6,245(6.2) | 7,843(33.9) | 1,056(18.2) | <0.0001 |
| Reflux symptoms | 81,549(29.1) | 69,650(30.3) | 4,563(21.3) | 6,217(26.9) | 1119(19.3) | <0.0001 |
| Dyspepsia | 29,696(10.6) | 21,879(9.5) | 1,951(9.1) | 4,377(18.9) | 1,489(25.7) | <0.0001 |
| Screening for BE | 4,042(1.4) | 3,623(1.6) | 173(0.8) | 197(0.9) | 49(0.8) | <0.0001 |
Indications and findings are not mutually exclusive
NH Non-Hispanic
Table 2.
Breakdown of LA grade among patients with documented classification by ethnic group
| Grade A/B N (%) | Grade C/D N (%) | P-value | |
|---|---|---|---|
| White, NH | 12,373 (79.5) | 3,184 (20.5) | <0.0001 |
| Black, NH | 740 (75.4) | 242 (24.6) | – |
| Hispanic | 1,489 (83.7) | 290 (16.3) | – |
| API | 162 (86.2) | 26 (13.8) | – |
Overall, suspected BE and esophageal stricture were found most often in WNH. The finding of hiatal hernia was common among all racial groups regardless of indication. However, WNH and Hispanic patients were more likely to have a hiatal hernia compared to BNH and API groups (Table 1).
Review of Endoscopies Performed for GERD-Related Indications
The frequencies of upper endoscopies performed for the specific indications of interest (dyspepsia, reflux symptoms, screening for BE) among each racial group are shown in Table 1. Among these indications, reflux was the most common for upper endoscopy for all racial groups except for API patients. In API, dyspepsia was performed most often among these indications of interest. Screening for BE was the least frequent indication and was most often performed for WNH patients at a frequency of 1.6%, approximately twice as often as the other racial groups.
The endoscopic findings of interest were examined for each GERD-related indication. Hispanics were again more likely to have a finding of esophagitis among all the racial groups for all specific indications (Figs. 1, 2, 3). Suspected BE was found most often in WNH at a frequency of 3.8 and 8.6% when the indication for upper endoscopy was for dyspepsia or for reflux symptoms, respectively (Figs. 1, 2). When the indication was BE screening, suspected BE was identified at a relatively higher frequency across all racial groups (Fig. 3). Interestingly, API had the highest likelihood of a suspected BE finding as compared to the other racial groups among these screening exams (API 20.4% vs. WNH 18.5% vs. Hispanic 16.9% vs. BNH 9.2%, P-value < 0.0001); however, the absolute numbers of API screened for BE during this 6-year period is very small (n = 49). When stratified by indication, esophageal stricture was detected most frequently in the WNH racial group. WNH and Hispanic patients were more likely to have a hiatal hernia compared to BNH and API groups among all specific indications of interest. API consistently had the lowest prevalence of hiatal hernia (Figs. 1, 2, 3).
Figure 1.
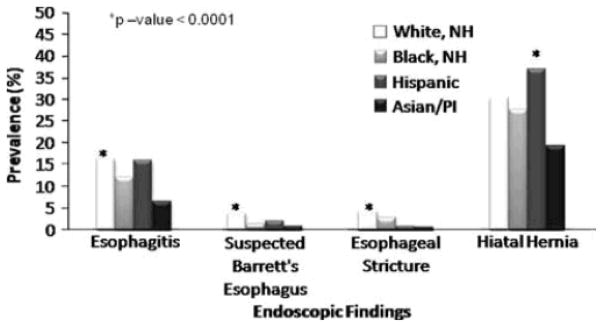
Prevalence of findings among patients undergoing upper endoscopy for dyspepsia
Figure 2.
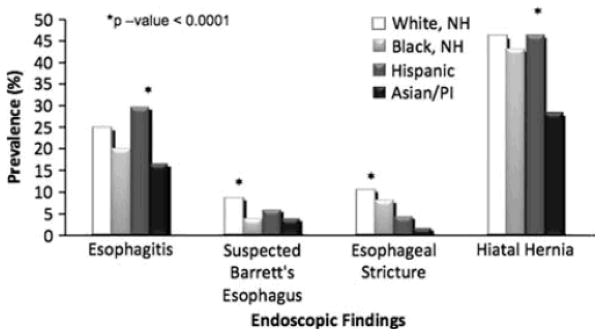
Prevalence of findings among patients undergoing upper endoscopy for reflux symptoms
Figure 3.
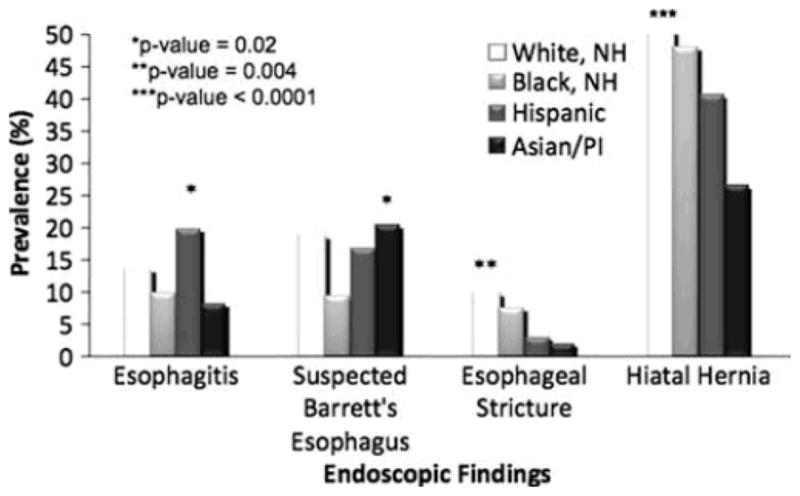
Prevalence of findings among patients undergoing upper endoscopy for BE screening
Adjusted Odds in Endoscopy Performed for Reflux and BE Screening Indications
We calculated the odds ratio of having esophagitis and BE for other races compared to white non-Hispanics in patients undergoing upper endoscopy for reflux symptoms or BE screening, adjusted for age and gender (Table 3). Using WNH as the reference group, Hispanics were more likely to have esophagitis detected (OR = 1.28, 95% CI 1.21–1.36), while BNH and API were less likely to have this finding. Meanwhile, compared to WNH all other racial groups were less likely to have suspected BE detected (Hispanic OR = 0.75, 95% CI 0.67–0.83; API OR = 0.48, 95% CI 0.36–0.64; BNH OR = 0.44, 95% CI 0.37–0.51, P-value < 0.0001).
Table 3.
Adjusted odds for finding of interest by race/ethnicity
| Odds ratio* | 95% CL | |
|---|---|---|
| Esophagitis | ||
| White, NH | 1.00 | – |
| Black, NH | 0.76 | 0.71–0.82 |
| Hispanic | 1.28 | 1.21–1.36 |
| Asian/PI | 0.59 | 0.50–0.69 |
| Suspected Barrette’s esophagus | ||
| White, NH | 1.00 | – |
| Black, NH | 0.44 | 0.37–0.51 |
| Hispanic | 0.75 | 0.67–0.83 |
| Asian/PI | 0.48 | 0.36–0.64 |
Adjusted for age and gender
Number Needed to Endoscope
The multivariate models were used to calculate the number of patients that would need to undergo endoscopy according to race, gender, age, and indication for exam to detect one case of esophagitis or suspected BE. Results are shown in Table 4; reflux symptoms and BE screening exams are compared to procedures performed for all other indications. In general, performing upper endoscopy for reflux symptoms or BE screening had a high yield for detecting esophagitis in WNH, BNH, and Hispanic patients. Of these groups, Hispanic males younger than 50 years of age who underwent endoscopy for reflux or BE screening required the lowest numbers of endoscopies to detect esophagitis. On the other hand, API who underwent endoscopy for other indications regardless of age and gender required the most examinations to find esophagitis.
Table 4.
Number of endoscopies needed to identify one patient with finding of interest
| Female
|
Male
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Reflux/screening indications | Other indicationsReflux/screening indications | Other indications | ||||||
|
|
|
|
|
|||||
| <50 years | ≥50 years | <50 years | ≥50 years | <50 years | ≥50 years | <50 years | ≥50 years | |
| Esophagitis | ||||||||
| White non-Hispanic | 5 | 5 | 8 | 9 | 4 | 4 | 6 | 7 |
| Black non-Hispanic | 5 | 5 | 8 | 9 | 4 | 4 | 6 | 7 |
| Hispanic | 4 | 5 | 7 | 8 | 3 | 4 | 5 | 6 |
| Asian/PI | 7 | 9 | 13 | 16 | 6 | 7 | 10 | 12 |
| Suspected Barrette’s esophagus | ||||||||
| White non-Hispanic | 23 | 17 | 64 | 48 | 10 | 8 | 27 | 20 |
| Black non-Hispanic | 62 | 47 | 181 | 136 | 26 | 20 | 75 | 56 |
| Hispanic | 34 | 26 | 97 | 73 | 15 | 11 | 41 | 31 |
| Asian/PI | 53 | 40 | 153 | 115 | 23 | 17 | 64 | 48 |
In general, the number of endoscopies needed to detect suspected BE was higher than that required to detect esophagitis. Endoscopies performed for GERD or BE screening yielded more cases of BE compared to procedures performed for other indications. The population that had the highest likelihood of having a finding of suspected BE was WNH males older than 50 years of age who were undergoing evaluation for reflux and screening, requiring only eight endoscopies to detect one case of suspected BE. On the other hand, the population least likely of having suspected BE was the BNH female younger than 50 years of age who undergoes endoscopy for other indications. According to our data, 153 patients from this group would have endoscopies to find one patient with BE. Overall, BNH and API subjects required more endoscopies to detect one case of suspected BE.
Discussion
Our study presents the results from a retrospective review of the prevalence of complicated GERD and BE among patients from different ethnic backgrounds evaluated at various academic, community, and VA/military institutions across the country. It is the first multi-center study performed in the U.S. to simultaneously examine complicated GERD in multiple racial groups, including Asian/Pacific Islanders. We found significant associations between racial groups and acid-related findings in this study. Of most interest is the frequent finding of esophagitis in the Hispanic population for all indications and for GERD-related indications as compared to other populations. To our knowledge, this association has not been previously reported in the literature. However, because of the limited LA class documentation in the database, we could not further characterize this relationship to esophagitis severity. This study also demonstrated that overall white patients are more likely to have suspected Barrett’s esophagus compared to the other ethnic groups of interest. This is not a novel observation as it is consistent with the prior evidence that BE is more common in Caucasians [9,12–14]. However, when endoscopy was performed specifically for BE screening, the highest prevalence of suspected BE was found in API, a group that had otherwise less-frequent complicated GERD in the study. The overall number of API patients undergoing BE screening was low (49), but ten (20.4%) of these patients did have suspected BE. This is a notable finding and should be followed-up with further studies. Finally, we determined that regardless of indication, esophageal stricture was most frequently found in WNH patients. Hiatal hernia was also found frequently among all racial groups, and although this was generally found more often in WNH and Hispanics, the difference is not thought to be clinically significant.
Thus far there is a paucity of data that describes an ethnic predilection of complicated GERD, and of the few studies available there are discordant findings. For instance, a retrospective study of a large population in the United Kingdom showed that White Caucasians were more likely than South Asians and Afro-Caribbeans to haves short-segment Barrett’s esophagus (SSBE), long-segment Barrett’s esophagus (LSBE), and esophagitis [13]. Another single center retrospective review of endoscopic reports performed by Spechler and colleagues indicated that complications of GERD as defined by the presence of BE, peptic esophageal ulcer, and peptic esophageal stricture were all more likely to be found in Whites as compared with Blacks, West Asians, and East Asians [14]. A prospective study performed by El-Serag et al. within a single Veteran’s Affairs (VA) center of 215 patients demonstrated that black patients may have a lower prevalence of esophagitis for the same frequency of GERD symptoms as compared with white subjects [12].
Alternatively, other studies have suggested that Caucasians may not be the only ethnic group at risk for GERD complications. Bersentes et al. performed a retrospective review of Caucasians and Hispanics undergoing upper endoscopy for any indication at a single VA center. This study demonstrated that the two groups were similar in the prevalence of GERD symptoms and BE [15]. Furthermore, a Malaysian study has recently shown that the prevalence of BE among the diverse population of Malays, Chinese, and Indians is 6%, a figure that is similar to those of Caucasians [16]. Yeh et al. determined a prevalence of esophagitis and BE in Taiwan to be similar to those found in Western literature and higher than previous reports from the Far East [17].
Our intent was to determine if there is a racial proclivity in the U.S., specifically among Caucasians, to the development of complicated GERD by examining the populations represented in the CORI database. While this database represents a subset of gastroenterological procedures performed in the U.S., we feel that it accurately represents the racial distribution of those undergoing endoscopy. According to the U.S. Census in 2000, 75% of the nation is self-described as white and 87% of persons over 65 years old are white or Hispanic. These proportions are similar to those seen in our study. Furthermore, a recent study by Sonnenberg et al. showed that the racial distribution of endoscopic procedures among patients over 65 years old in CORI is very similar to the procedures among the U.S. Medicare population (87.9% white in CORI non-VA sites, vs. 86.6% white in Centers for Medicare and Medicaid Service (CMS) database) [18].
One important follow-up to this study will involve mechanistic analysis as to why certain complications are more prevalent in certain racial groups. When considering possible explanations, it is important to discuss other independent variables that are believed to affect the severity of reflux disease. For example, differences in body mass index, tobacco/alcohol/caffeine intake, and use of certain medications can compromise the lower esophageal sphincter tone and worsen reflux symptoms [2, 19–24]. Meanwhile, the use of anti-acid medications such as H2-blockers and proton pump inhibitors (PPI) can be protective. Although the theory remains controversial, Helicobacter pylori infection of the gastric body may be inversely related to the presence of GERD and its complications [25–27] by decreasing esophageal acid load. Finally, lifestyle patterns, dietary habits, and the use of allopathic medicine can vary among different ethnic groups in the U.S., depending on cultural beliefs and degree of assimilation into Western culture. These variables were not examined in our study due to limitations of the CORI database and should certainly be recognized as possible confounders.
Specifically, the use of PPI may play a very significant role in the presence or absence of complicated GERD. Since its advent in the early 1990s, it has become the mainstay of treatment for reflux symptoms in the U.S., with its annual expenditures increasing 80% from 1997 and 2002 [28]. It has been shown to be more effective than a placebo in treating esophagitis [29–33] and is also frequently recommended to patients who have known BE. However, the use of PPI among other cultures and racial groups may not be as prominent. In a questionnaire study in a Chinese population, it was shown that anti-reflux medications such as antacids, H2-blockers, and PPI were used in only 40% of symptomatic GERD patients within a year [34]. Meanwhile, a U.S. study using data from the 1996 Medical Expenditure Panel Survey (MEPS) demonstrated that overall, fewer blacks and Hispanics than whites received health care in physicians’ offices, outpatient clinics, and emergency departments [35]. As these are settings where GERD patients are frequently seen and treated, these trends can be used as surrogates to PPI access and use. Alternatively, no difference was observed in the use of H2-blockersor PPI between black and white patients in the study performed by El-Serag et al. [12]. These data suggest that PPI use may vary among racial groups, even within the U.S., and it is imperative that future study designs include this variable as a predictor of complicated GERD.
One possible theory to explain our findings is that Caucasians and Hispanics have a similar genetic predisposition towards developed GERD complications, but in the past, Hispanics have been protected by H. pylori infection. However, as minority groups living in the U.S. assimilate into the lifestyle and the prevalence of H. pylori decreases, the potential protective effects of the infection disappear and symptoms of acid reflux disease ensue. Subsequently, this increase in clinical GERD is not paralleled with a concurrent increase in PPI use, either due to lack of access to medical care or perhaps cultural beliefs about Western medicine. As a result, racial groups such as Hispanics are manifesting complications of GERD more often as esophagitis rather than BE, a condition which is frequently unmasked after mucosal healing with PPI.
Our investigation was a retrospective review of the CORI database, so it is important to consider the biases that are inherent in retrospective studies. The most important implication of this design is the susceptibility for misclassification bias. We classified our racial groups according to the assignments entered by the provider, not the patient. This raises the possibility that the distribution of our racial groups may be inaccurate. As mentioned above, the recent results presented by Sonnenberg et al. suggest that the CORI database is similar in racial distribution as the CMS database, however, this is not direct evidence that it represents the true distribution of all endoscopies performed in the U.S. Moreover, the CORI database provides only a limited number of racial classifications, which may not allow patients of other races or of mixed heritage to be assigned and may have contributed to the large number of excluded cases with missing racial/ ethnic data. Despite these potential confounding variables, our data indicate some interesting trends of racial proclivity to esophageal reflux disease that may have implications for patient care and, thus, merit further investigation.
In addition, the database is utilized by providers across the country who are not primed to our endpoints of interest and therefore, data may have been missing due to incomplete reporting. This was the case with our data collection of LA grade classification for esophagitis. Likewise, we could only report on findings of “suspected BE” because pathology results indicating the presence or absence of intestinal metaplasia on esophageal biopsy are not consistently recorded in the database. As noted above, variables such as PPI use and H. pylori status could not be extrapolated from the database, which may be confounders of our results.
In conclusion, our study demonstrates that performing endoscopy in WNH and Hispanics yields more findings of esophagitis, esophageal stricture, and suspected BE compared to other racial groups. As the presence of these conditions justifies more aggressive management for GERD and has implications on surveillance, lowering the threshold for endoscopy in these populations could be considered. However, further studies that evaluate the role of the many independent variables that can affect reflux disease are still necessary for accurate risk stratification among racial groups.
Acknowledgments
This project was supported with funding from NIDDK UO1 CA 89389-01 and R33-DK61778-01. In addition, the practice network (CORI) has received support from the following entities to support the infrastructure of the practice-based network: AstraZeneca, Bard International, Pentax USA, ProVation, Endosoft, GIVEN Imaging, and Ethicon. The commercial entities had no involvement in this research. DL is the executive director of CORI and GE is the executive co-director of CORI, a nonprofit organization that receives funding from federal and industry sources. This potential conflict of interest has been reviewed and managed by the Oregon Health and Science University (OHSU) Conflict of Interest in Research Committee. This research was conducted with support from the Investigator-Sponsored Study Program of AstraZeneca.
Abbreviations
- API
Asian/Pacific Islander
- ACG
American College of Gastroenterology
- BE
Barrett’s esophagus
- BNH
Black non-Hispanic
- CMS
Centers for Medicare and Medicaid Service
- CORI
Clinical Outcomes Research Initiative
- GERD
Gastroesophageal reflux disease
- LA
Los Angeles
- LSBE
Long-segment Barrett’s esophagus
- NED
National Endoscopic Database
- NNE
Number needed to endoscope
- PPI
Proton pump inhibitors
- SSBE
Short-segment Barrett’s esophagus
- VA
Veteran’s Affairs
- WNH
White non-Hispanic
References
- 1.Locke GR, III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Prevalence and clinical spectrum of gastroesophageal reflux: a population-based study in Olmsted County, Minnesota. Gastroenterology. 1997;112:1448–1456. doi: 10.1016/s0016-5085(97)70025-8. 10.1016/S0016-5085(97) 70025-8. [DOI] [PubMed] [Google Scholar]
- 2.Locke GR, III, Talley NJ, Fett SL, Zinsmeister AR, Melton LJ., III Risk factors associated with symptoms of gastroesophageal reflux. Am J Med. 1999;106:642–649. doi: 10.1016/S0002-9343(99)00121-7.. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Zinsmeister AR, Schleck CD, Melton LJ., III Dyspepsia and dyspepsia subgroups: a population-based study. Gastroenterology. 1992;102:1259–1268. [PubMed] [Google Scholar]
- 4.DeVault KR, Castell DO. Updated guidelines for the diagnosis and treatment of gastroesophageal reflux disease. Am J Gastroenterol. 2005;100:190–200. doi: 10.1111/j.1572-0241.2005.41217.x. [DOI] [PubMed] [Google Scholar]
- 5.Nandurkar S, Locke GR, III, Murray JA, et al. Rates of endoscopy and endoscopic findings among people with frequent symptoms of gastroesophageal reflux in the community. Am J Gastroenterol. 2005;100:1459–1465. doi: 10.1111/j.1572-0241.2005.41115.x.. [DOI] [PubMed] [Google Scholar]
- 6.Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–1895. doi: 10.1111/j.1572-0241.2002.05910.x.. [DOI] [PubMed] [Google Scholar]
- 7.Wang KK, Sampliner RE. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2008;103:788–797. doi: 10.1111/j.1572-0241.2008.01835.x. 10.1111/j.1572-0241.2008. 01835.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong WM, Lam SK, Hui WM, et al. Long-term prospective follow-up of endoscopic oesophagitis in southern Chinese—Prevalence and spectrum of the disease. Aliment Pharmacol Ther. 2002;16:2037–2042. doi: 10.1046/j.1365-2036.2002.01373.x.. [DOI] [PubMed] [Google Scholar]
- 9.Spechler SJ, Goyal RK. Barrett’s esophagus. N Engl J Med. 1986;315:362–371. doi: 10.1056/NEJM198608073150605. [DOI] [PubMed] [Google Scholar]
- 10.Lee JI, Park H, Jung HY, Rhee PL, Song CW, Choi MG. Prevalence of Barrett’s esophagus in an urban Norean population: a multicenter study. J Gastroenterol. 2003;38:23–27. doi: 10.1007/s005350300002. 10.1007/ s005350300002. [DOI] [PubMed] [Google Scholar]
- 11.Sonnenberg A, Massey BT, Jacobsen SJ. Hospital discharges resulting from esophagitis among Medicare beneficiaries. Dig Dis Sci. 1994;39:183–188. doi: 10.1007/BF02090080.. [DOI] [PubMed] [Google Scholar]
- 12.El-Serag HB, Petersen NJ, Carter J, et al. Gastroesophageal reflux among different racial groups in the United States. Gastroenterology. 2004;126:1692–1699. doi: 10.1053/j.gastro.2004.03.077.. [DOI] [PubMed] [Google Scholar]
- 13.Ford AC, Forman D, Reynolds PD, Cooper BT, Moayyedi P. Ethnicity, gender, and socioeconomic status as risk factors for esophagitis and Barrett’s esophagus. Am J Epidemiol. 2005;162:454–460. doi: 10.1093/aje/kwi218.. [DOI] [PubMed] [Google Scholar]
- 14.Spechler SJ, Jain SK, Tendler DA, Parker RA. Racial differences in the frequency of symptoms and complications of gastrooesophageal reflux disease. Aliment Pharmacol Ther. 2002;16:1795–1800. doi: 10.1046/j.1365-2036.2002.01351.x.. [DOI] [PubMed] [Google Scholar]
- 15.Bersentes K, Fass R, Padda S, Johnson C, Sampliner RE. Prevalence of Barrett’s esophagus in Hispanics is similar to Caucasians. Dig Dis Sci. 1998;43:1038–1041. doi: 10.1023/a:1018834902694. 10.1023/A:101 8834902694. [DOI] [PubMed] [Google Scholar]
- 16.Rajendra S, Kutty K, Karim N. Ethnic differences in the prevalence of endoscopic esophagitis and Barrett’s esophagus: the long and short of it all. Dig Dis Sci. 2004;49:237–242. doi: 10.1023/B:DDAS.0000017444.30792.94.. [DOI] [PubMed] [Google Scholar]
- 17.Yeh C, Hsu CT, Ho AS, Sampliner RE, Fass R. Erosive esophagitis and Barrett’s esophagus in Taiwan: a higher frequency than expected. Dig Dis Sci. 1997;42:702–706. doi: 10.1023/a:1018835324210. 10.1023/A:101883 5324210. [DOI] [PubMed] [Google Scholar]
- 18.Sonnenberg A, Amorosi SL, Lacey MJ, Lieberman DA. Patterns of endoscopy in the United States: analysis of data from the centers for Medicare and Medicaid services and the National Endoscopic Database. Gastrointest Endosc. 2008;67:489–496. doi: 10.1016/j.gie.2007.08.041. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: the NHANES I Epidemiologic Follow-up Study. First national health and nutrition examination survey. Ann Epidemiol. 1999;9:424–435. doi: 10.1016/S1047-2797(99)00020-4.. [DOI] [PubMed] [Google Scholar]
- 20.Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003;290:66–72. doi: 10.1001/jama.290.1.66.. [DOI] [PubMed] [Google Scholar]
- 21.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am J Gastroenterol. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x.. [DOI] [PubMed] [Google Scholar]
- 22.Nocon M, Labenz J, Willich SN. Lifestyle factors and symptoms of gastro-oesophageal reflux—a population-based study. Aliment Pharmacol Ther. 2006;23:169–174. doi: 10.1111/j.1365-2036.2006.02727.x.. [DOI] [PubMed] [Google Scholar]
- 23.Veugelers PJ, Porter GA, Guernsey DL, Casson AG. Obesity and lifestyle risk factors for gastroesophageal reflux disease, Barrett esophagus and esophageal adenocarcinoma. Dis Esophagus. 2006;19:321–328. doi: 10.1111/j.1442-2050.2006.00602.x.. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed I, Nightingale P, Trudgill NJ. Risk factors for gastrooesophageal reflux disease symptoms: a community study. Aliment Pharmacol Ther. 2005;21:821–827. doi: 10.1111/j.1365-2036.2005.02426.x.. [DOI] [PubMed] [Google Scholar]
- 25.Graham DY, Yamaoka Y. H. pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145–151. doi: 10.1046/j.1523-5378.1998.08031.x.. [DOI] [PubMed] [Google Scholar]
- 26.Loffeld RJ, Werdmuller BF, Kuster JG, Perez-Perez GI, Blaser MJ, Kuipers EJ. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett’s esophagus. Digestion. 2000;62:95–99. doi: 10.1159/000007801.. [DOI] [PubMed] [Google Scholar]
- 27.Varanasi RV, Fantry GT, Wilson KT. Decreased prevalence of Helicobacter pylori infection in gastroesophageal reflux disease. Helicobacter. 1998;3:188–194. doi: 10.1046/j.1523-5378.1998.08001.x.. [DOI] [PubMed] [Google Scholar]
- 28.Banthin JS, Miller GE. Trends in prescription drug expenditures by Medicaid enrollees. Med Care. 2006;44:I27–I35. doi: 10.1097/01.mlr.0000208132.36055.84.. [DOI] [PubMed] [Google Scholar]
- 29.Cloud ML, Enas N, Humphries TJ, Bassion S. Rabeprazole in treatment of acid peptic diseases: results of three placebo-con-trolled dose-response clinical trials in duodenal ulcer, gastric ulcer, and gastroesophageal reflux disease (GERD). The Rabeprazole Study Group. Dig Dis Sci. 1998;43:993–1000. doi: 10.1023/A:1018822532736.. [DOI] [PubMed] [Google Scholar]
- 30.Earnest DL, Dorsch E, Jones J, Jennings DE, Greski-Rose PA. Aplacebo-controlled dose-ranging study of lansoprazole in the management of reflux esophagitis. Am J Gastroenterol. 1998;93:238–243. doi: 10.1111/j.1572-0241.1998.00238.x.. [DOI] [PubMed] [Google Scholar]
- 31.Hetzel DJ, Dent J, Reed WD, et al. Healing and relapse of severe peptic esophagitis after treatment with omeprazole. Gastroenterology. 1988;95:903–912. doi: 10.1016/0016-5085(88)90162-x. [DOI] [PubMed] [Google Scholar]
- 32.Richter JE, Bochenek W. Oral pantoprazole for erosive esophagitis: a placebo-controlled, randomized clinical trial. Pantoprazole US GERD Study Group. Am J Gastroenterol. 2000;95:3071–3080. doi: 10.1111/j.1572-0241.2000.03254.x.. [DOI] [PubMed] [Google Scholar]
- 33.Sontag SJ, Hirschowitz BI, Holt S, et al. Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: the U.S. Multicenter Study. Gastroenterology. 1992;102:109–118. doi: 10.1016/0016-5085(92)91790-b. [DOI] [PubMed] [Google Scholar]
- 34.Wong WM, Lai KC, Lam KF, et al. Prevalence, clinical spectrum and health care utilization of gastro-oesophageal reflux disease in a Chinese population: a population-based study. Aliment Pharmacol Ther. 2003;18:595–604. doi: 10.1046/j.1365-2036.2003.01737.x. 10.1046/j.1365-2036.2003. 01737.x. [DOI] [PubMed] [Google Scholar]
- 35.Bliss EB, Meyers DS, Phillips RL, Jr, Fryer GE, Dovey SM, Green LA. Variation in participation in health care settings associated with race and ethnicity. J Gen Intern Med. 2004;19:931–936. doi: 10.1007/s11606-004-0008-x.. [DOI] [PMC free article] [PubMed] [Google Scholar]


