Abstract
A novel androgen receptor (AR) degradation enhancer ASC-J9® has displayed beneficial effects during the in vitro and in vivo studies for treatment of prostate cancer, liver cancer, bladder cancer and spinal and bulbar muscular atrophy (SBMA). It works mainly by inducing the degradation of AR with minimal side effects on the tested mice. Here we developed a fast, robust and more sensitive method for the quantification of ASC-J9® in 100 μL of mouse serum by using liquid chromatography tandem mass spectrometry (LC-MS/MS). The limit of quantification (LOQ) was found to be 5nM for ASCJ9®. This method was successfully applied to investigate the pharmacokinetics of ASC-J9® in mice serum samples and also the distribution of the drug in various mice organs after single dose injection with results showing that ASC-J9® could be quickly absorbed in vivo and had a relatively slow elimination half-life of 5.45 h. The ASC-J9® also exhibited a higher tendency to accumulate in organs such as liver, testes and prostate.
Keywords: ASC-J9®, androgen receptor, liquid chromatography tandem mass spectrometry, pharmacokinetics, distribution of drug
1. Introduction
Curcumin is a major constituent of the rhizome of the turmeric herb (Curcuma Longa Linn) [1–3]. Chemically, it is a bis-α,β-unsaturated β-diketone and exists predominantly in keto form in the acidic or neutral conditions but a stable enol form in the alkaline condition [1,2,4]. Curcumin has been suggested as a potential anti-cancer drug through perturbation of multiple molecular targets [5] and was safe even when applied at high doses during several clinical trials [3,7,8]. However, the viability as a drug with its low oral bioavailability and its rapid in vivo metabolism remains as a potential problem [1,3,5–7].
One suggested way of overcoming the shortcomings is to make structural modifications to curcumin [1,4–6,9]. In fact, several curcumin derivatives and analogues were developed that could inhibit tumour cell proliferation and invasion [4–9].
In our study, we have identified a curcumin derivative, ASC-J9 (5-hydroxy-1,7-bis(3,4-dimethoxyphenyl)-1,4,6-heptatrien-3-one) as an effective androgen receptor (AR) degradation [10,11]. It can suppress AR function via selective interruption between androgen receptors (ARs) and its selective co-activators (ARA55 or ARA70) that are expressed mainly in prostate stromal or epithelial luminal cells [11]. The consequences of such suppression of AR function may then lead to suppress the AR-mediated diseases, including prostate cancer (PCa) [10,11], liver cancer [12], bladder cancer [13] and spinal muscular atrophy (SBMA) [14]. Importantly, unlike currently used androgen deprivation therapy (ADT) with various anti-androgens that may develop the castration resistant tumours, ASC-J9® can continually suppress the PCa at the castration resistant stage [10,11,15,16], with little side efects on the sexual libido of the tested mice [14]. This suggested that this drug could be a better option to treat PCa or other AR related diseases, such as liver cancer, bladder cancer or SBMA. Therefore, it is important to quickly and accurately determine the ASC-J9® concentration in various biological samples.
In this study, we have developed and validated a fast and robust LC-MS/MS method to quantify ASCJ9® in mice serum and various organ tissue samples. The pharmacokinetic and distribution of ASC-J9® in mice sera and organs have been also investigated.
2. Experimental
2.1 Instrumentation
Chromatographic separation was carried out on an Agilent Technologies (Palo Alto, CA, USA) Model 1200 liquid chromatography system with a binary pump and an auto - sampler. Chromatographic data were recorded and processed by using the Analyst software version 1.4.2 (ABI-Sciex, Foster City, CA). A Phenomenex (Torrance, CA) Luna C18 (50 mm × 2.00 mm, 100Å) column attached to a security guard column with C18 (4 mm × 2.0 mm) filter cartridge was used for separation. The HPLC effluent was analyzed by an API 3200 triple quadrupole mass spectrometer (ABI-Sciex, Toronto, Ontario, Canada) equipped with a turbo ion spray source operated under positive multiple reaction monitoring (MRM) mode.
2.2 Chemicals
Formic acid and HPLC-grade solvents acetonitrile and ethyl acetate were purchased from Merck (Darmstadt, Germany). ASC-J9® was a kind gift from AndroScience Corporation (San Diego, CA, USA). Curcumin and bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Commercial mouse serum of innovative grade was purchased from Innovative Research Inc. (Peary court Novi, MI, USA). Phosphate buffer saline (PBS) was purchased from Vivantis Technologies Sdn Bhd (Selangor Darul Ehsan, Malaysia). Ultrapure water was prepared using a Milli-Q (Millipore, Bedford, MA, USA) water purification system.
2.3 Preparation and extraction of calibration standards and quality controls
All the prepared stock and standard solutions of ASC-J9® and curcumin were stored at −20 °C and wrapped with aluminium foil. Two sets of calibration curves were established using different types of matrices including mouse sera and 1g/L of PBS/BSA solutions. The calibration standards were prepared by spiking 20 μL of each of the standard solution of ASC-J9® in acetonitrile into either 80 μL of blank mouse serum or 80 μL of PBS/BSA solution to make up a total of 100 μL of final sample solution. As such, the final calibration standards ranged from 0.005 to 5 μM in mouse sera and 0.005 to 10 μM in 1g/L of PBS/BSA matrix. 10 μL of 2.5 μM curcumin was spiked into each of the 100 μL sample as internal standards. Three quality controls with concentrations of 0.025 μM, 0.250 μM and 1 μM were also prepared in both matrices. These samples were extracted using liquid–liquid extraction with 0.5 mL of ethyl acetate and vortexed vigorously for 2 mins, followed by centrifuging at 13000 rpm for 3 mins at room temperature. The supernatants were transferred into clean microcentrifuge tubes. The extraction was repeated thrice and extracts were combined and dried under gentle stream of nitrogen gas. The dried residue was reconstituted with 100 μL of 60 % aqueous acetonitrile. 10 μL of the reconstituted solution was injected for the analysis.
2.4 Preparation of samples for stability studies
Triplicates of the 3 quality controls as mentioned in section 2.3 in both matrices were spiked with 10 μL of 2.5 μM internal standard, curcumin. These samples were stored at −80 °C and thawed at room temperature of 24 °C. They were subjected to three freeze-thaw cycles for determination of freeze-thaw stability. Another triplicate of the three quality controls were left at room temperature of 24 °C for 6, 8 and 24 hrs respectively before analysis to study the short term stability of ASC-J9® in mouse serum and PBS/BSA matrix under room temperature conditions. For both freeze-thaw and short term stability samples, the measurements were compared against measurements from triplicates of a reference set with the same concentrations of ASC-J9® but prepared freshly on the day of extraction and LC-MS/MS analysis.
2.5 Sample preparation for mouse serum and organ samples
2.5.1 Mouse serum samples
All the mice were administered with 75 mg/kg of ASC-J9® through intraperitoneal (i.p.) injection. Three serum samples were taken from three different mice per timepoint at several timepoints. After which, the blood samples were left standing for 20 to 30 mins before centrifuging at 1500 rpm for 10 mins. The supernatant was then transferred to a clean tube and immediately stored at −80 °C before extraction and LC-MS/MS analysis.
2.5.2 Mouse organ samples
Triplicates of organs were collected from six mice at 8 hrs and 24 hrs respectively after injection of ASC-J9®. After collection, the organs were immediately stored at −80 °C. Before extraction, the organs were thawed and rinsed with saline buffer and dried before weighing. Each organ sample was placed in a 2-ml microcentrifuge tube and homogenised. A 10 μL of 2.5 μM curcumin was spiked as internal standard into the homogenised sample. Similar extraction procedure was adhered to as mentioned in section 2.3 except that the tissues samples were subjected to ultrasonication for 10 mins after each addition of extraction solvent. They were then centrifuged at 6000 rpm for 10 mins before transferring the supernatants into clean microcentrifuge tubes.
2.6 LC-MS/MS Analysis
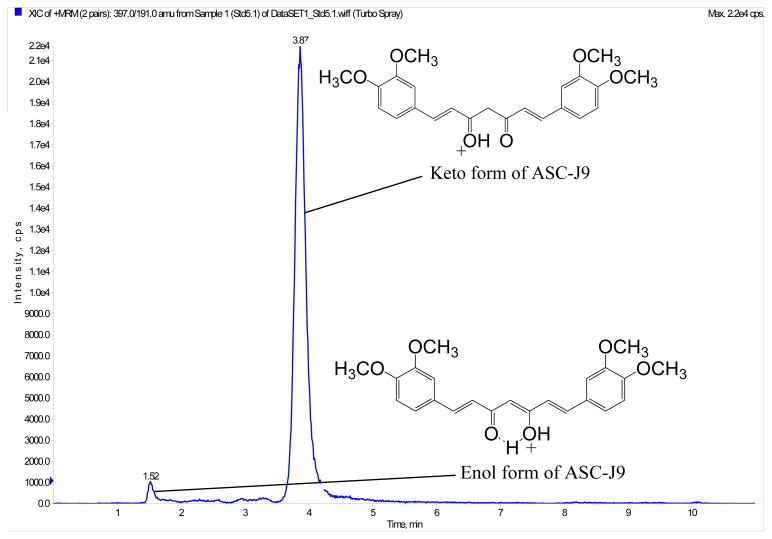
The mobile phase consisted of two eluents, solvent A (ultrapure water with 0.1 % formic acid) and solvent B (acetonitrile), delivered at a flow rate of 0.35 mL/min. The Luna C18 column was used as stationary phase for separation under isocratic mobile phase conditions of 40 % solvent A and 60 % solvent B. The temperature of the column compartment was kept at 40 °C. Figure 1 is the typical chromatogram of ASC-J9 where it contains two elution peaks. This is similar to the internal standard, curcumin. These two elution peaks represented the keto-enol isomers of each of the compounds [5,9]. For both ASC-J9® and curcumin, their latter peaks were their predominant peaks (with peak intensity ratio of approximately 1:22 for ASC-J9® and 1:11 for curcumin). Since the second peaks for both ASC-J9® and curcumin were much higher than their first peaks and the ratios of the two peaks were consistent, so only the second peaks for both ASC-J9® and curcumin were used for quantitation in this study.
Figure 1.
Chromatogram with identification of peaks of the keto-enol isomers of dimethoxycurcumin, ASC-J9®.
ASC-J9® and curcumin were determined using the positive ESI-MRM mode. Both of them showed similar fragmentation and the MRM transitions for monitoring ASC-J9® was at m/z 397 → 191 and for curcumin (internal standard), it was at m/z 369 → 177. All of the source and instrument parameters were optimized by flow injection analysis. The optimized conditions for ASC-J9® were as follows: declustering potential at 30 V, entrance potential at 8 V, collisional entrance potential at 18.7 V, collisional energy at 40 V, collisional cell exit potential at 4 V, curtain gas at 10, and collisional gas at 4. The ionspray was set at 5500 V with the turbo ion spray interface at 600 °C. The ion source drying gases 1 and 2 were set at 60 and 65 respectively. The concentration of ASC-J9® was quantified using an 8-point calibration curve of peak area ratio against the concentration ratio of the analyte to internal standard.
2.7 Method Validation
Interday (n = 9) and intraday (n= 6) assays were performed for each set of calibration standards in the two types of matrices, mouse serum and PBS/BSA solution, to determine the accuracy and the coefficient of variation (CV %) of the experimental method. The limit of quantification (LOQ) was based on signal-to-noise (S/N) ratio of 10:1. Matrix effects were also investigated by analyzing the blank mouse serum and blank PBS/BSA solution containing internal standard only.
3. Results and discussion
3.1 Validation of the LC-MS/MS method
Linear regression with 1/y2 was obtained for ASC-J9® in mouse serum from 0.005 to 5 μM, with regression coefficient, r2 of 0.99 and a typical calibration curve equation of y = 17 x + 0.0584. As for ASC-J9® in PBS/BSA matrix, quadratic regression with 1/y2 weighting was used to plot the calibration curves over the range of 0.005 to 10 μM, having regression coefficient, r2 of 0.99 as well and a typical calibration equation of y = −0.58 x2 + 15.1 x − 0.00286. Recoveries and matrix effect were determined using the quality controls of ASC-J9® prepared in both mouse serum and PBS/BSA solution as matrices. From the chromatograms of both types of blank matrices, there was no peaks with the same retention times as ASC-J9® at m/z 397 → 191. This shows that the method was selective and specific for detection of this curcuminoid. All the validated values for accuracies and coefficient of variation (CV%) fell within acceptable requirements based on the Guidance for Industry Bioanalytical Method Validation [17] as shown in Tables 1 to 2. The LOQ value of ASC-J9® was experimentally determined to be 0.005 μM in both matrices, having at least S/N ratio of at least 10:1 as calculated using the Analyst version 1.4.2 software. Compared to the reported method [9], our results indicated that our new method was more sensitive and robust and reproducible for the measurement of ASC-J9® in mouse serum and in PBS/BSA matrix and could easily be extended to other types of biological matrices.
Table 1.
| Table 1a: Summary of the Validation of Interday Calibration Curves in Serum Matrix
| |||||||
|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Interday Concentration (μM) | Interday Validation Summary | |||||
|
| |||||||
| Day 1 | Day 2 | Day 3 | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.005 | 0.0049 | 0.0052 | 0.0050 | 0.0050 | 0.0002 | 3.4 | 100.2 |
| 0.025 | 0.0265 | 0.0244 | 0.0273 | 0.0261 | 0.0015 | 5.7 | 104.2 |
| 0.05 | 0.0508 | 0.0502 | 0.0488 | 0.0499 | 0.0010 | 2.1 | 99.8 |
| 0.25 | 0.2720 | 0.2850 | 0.2537 | 0.2701 | 0.0157 | 5.8 | 108.1 |
| 0.5 | 0.5231 | 0.5235 | 0.5013 | 0.5160 | 0.0126 | 2.5 | 103.2 |
| 1 | 1.0911 | 0.9906 | 1.0915 | 1.0577 | 0.0581 | 5.5 | 105.8 |
| 2.5 | 2.4912 | 2.4363 | 2.3523 | 2.4266 | 0.0699 | 2.9 | 97.1 |
| 5 | 4.2408 | 4.7691 | 5.1412 | 4.7170 | 0.4524 | 9.6 | 94.3 |
| Summary of the Validation of Interday Quality Controls in Serum Matrix
| |||||||
|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Interday Concentration (μM) | Interday Validation Summary | |||||
|
| |||||||
| Day 1 | Day 2 | Day 3 | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.025 | 0.0224 | 0.0299 | 0.0224 | 0.0249 | 0.0043 | 17.2 | 99.7 |
| 0.25 | 0.2241 | 0.2828 | 0.2566 | 0.2545 | 0.0294 | 11.5 | 101.8 |
| 1 | 0.9706 | 1.2190 | 0.8866 | 1.0254 | 0.1729 | 16.9 | 102.5 |
| Table 1b: Summary of the Validation of Intraday Calibration Curve in Serum Matrix
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Intraday Concentration (μM) | Intraday Validation Summary | ||||||||
|
| ||||||||||
| 1st Run | 2nd Run | 3rd Run | 4th Run | 5th Run | 6th Run | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.005 | 0.0062 | 0.0062 | 0.0041 | 0.0045 | 0.0053 | 0.0041 | 0.0051 | 0.0010 | 19.5 | 101.5 |
| 0.025 | 0.0313 | 0.0297 | 0.0283 | 0.0293 | 0.0290 | 0.0276 | 0.0292 | 0.0013 | 4.4 | 116.8 |
| 0.05 | 0.0540 | 0.0495 | 0.0478 | 0.0483 | 0.0425 | 0.0542 | 0.0494 | 0.0044 | 8.8 | 98.7 |
| 0.25 | 0.2360 | 0.2430 | 0.2730 | 0.2540 | 0.2470 | 0.2350 | 0.2479 | 0.0142 | 5.7 | 99.2 |
| 0.5 | 0.4960 | 0.4620 | 0.5280 | 0.6190 | 0.5820 | 0.5700 | 0.5429 | 0.0582 | 10.7 | 108.6 |
| 1 | 0.8900 | 1.0900 | 0.8120 | 0.9090 | 1.100 | 0.9600 | 0.9597 | 0.1143 | 11.9 | 96.0 |
| 2.5 | 2.9200 | 2.6400 | 2.5200 | 2.6600 | 2.6500 | 2.7800 | 2.6940 | 0.1395 | 5.2 | 107.8 |
| 5 | 4.9500 | 4.5400 | 4.1400 | 5.4700 | 4.2100 | 4.6300 | 4.6575 | 0.4931 | 10.6 | 93.1 |
| Summary of the Validation of Intraday Quality Controls in Serum Matrix
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Intraday Concentration (μM) | Intraday Validation Summary | ||||||||
|
| ||||||||||
| 1st Run | 2nd Run | 3rd Run | 4th Run | 5th Run | 6th Run | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.025 | 0.0294 | 0.0272 | 0.0297 | 0.0220 | 0.0225 | 0.0238 | 0.0258 | 0.0034 | 13.4 | 103.0 |
| 0.25 | 0.2540 | 0.2440 | 0.2960 | 0.2130 | 0.2280 | 0.2470 | 0.2471 | 0.0283 | 11.4 | 98.8 |
| 1 | 1.2900 | 1.0700 | 1.0600 | 0.9950 | 1.0100 | 0.9670 | 1.0644 | 0.1159 | 10.9 | 106.4 |
Table 2.
| Table 2a: Summary of the Validation of Interday Calibration Curves in 1g/L PBS/BSA Matrix
| |||||||
|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Interday Concentration (μM) | Interday Validation Summary | |||||
|
| |||||||
| Day 1 | Day 2 | Day 3 | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.005 | 0.0049 | 0.0050 | 0.0052 | 0.0050 | 0.0001 | 2.2 | 100.4 |
| 0.025 | 0.0271 | 0.0294 | 0.0251 | 0.0272 | 0.0021 | 7.8 | 108.7 |
| 0.05 | 0.0553 | 0.0438 | 0.0449 | 0.0479 | 0.0063 | 13.2 | 96.0 |
| 0.25 | 0.2749 | 0.2318 | 0.2405 | 0.2491 | 0.0228 | 9.2 | 99.6 |
| 0.5 | 0.4939 | 0.5645 | 0.5420 | 0.5334 | 0.0361 | 6.8 | 106.7 |
| 1 | 0.9766 | 0.9309 | 1.1541 | 1.0206 | 0.1179 | 11.6 | 102.1 |
| 2.5 | 2.2628 | 2.6803 | 2.6992 | 2.5474 | 0.2467 | 9.7 | 101.9 |
| 5 | 4.6319 | 5.7530 | 4.9839 | 5.1229 | 0.5733 | 11.2 | 102.5 |
| 10 | 10.8912 | 9.2838 | 9.7128 | 9.9626 | 0.8322 | 8.4 | 99.7 |
| Summary of the Validation of Interday Quality Controls in 1g/L PBS/BSA Matrix
| |||||||
|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Interday Concentration (μM) | Interday Validation Summary | |||||
|
| |||||||
| Day 1 | Day 2 | Day 3 | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.025 | 0.0237 | 0.0208 | 0.0268 | 0.0238 | 0.0030 | 12.7 | 95.0 |
| 0.25 | 0.2506 | 0.2346 | 0.2768 | 0.2540 | 0.0213 | 8.4 | 101.6 |
| 1 | 0.9306 | 0.9332 | 0.8691 | 0.9110 | 0.0363 | 4.0 | 91.1 |
| Table 2b: Summary of the Validation of Intraday Calibration Curve in 1g/L PBS/BSA Matrix
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Intraday Concentration (μM) | Intraday Validation Summary | ||||||||
|
| ||||||||||
| 1st Run | 2nd Run | 3rd Run | 4th Run | 5th Run | 6th Run | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.005 | 0.0052 | 0.0054 | 0.0053 | 0.0052 | 0.0044 | 0.0055 | 0.0052 | 0.0004 | 7.8 | 103.1 |
| 0.025 | 0.0257 | 0.0255 | 0.0251 | 0.0238 | 0.0242 | 0.0236 | 0.0247 | 0.0009 | 3.7 | 98.6 |
| 0.05 | 0.0426 | 0.0428 | 0.0523 | 0.0520 | 0.0399 | 0.0399 | 0.0449 | 0.0057 | 12.8 | 89.8 |
| 0.25 | 0.2320 | 0.2290 | 0.2520 | 0.2480 | 0.2410 | 0.2350 | 0.2395 | 0.0091 | 3.8 | 95.8 |
| 0.5 | 0.4440 | 0.4420 | 0.6170 | 0.6030 | 0.6080 | 0.6060 | 0.5533 | 0.0856 | 15.5 | 110.7 |
| 1 | 1.1700 | 1.1600 | 1.1600 | 1.1700 | 1.1400 | 1.1500 | 1.1583 | 0.0117 | 1.0 | 115.8 |
| 2.5 | 2.7000 | 2.6700 | 2.7200 | 2.7600 | 2.7100 | 2.6900 | 2.7083 | 0.0306 | 1.1 | 108.3 |
| 5 | 4.9500 | 5.0500 | 4.9200 | 4.9800 | 5.0800 | 5.0700 | 5.0083 | 0.0674 | 1.3 | 100.2 |
| 10 | 9.5700 | 9.6500 | 9.5300 | 9.8600 | 9.7500 | 9.7200 | 9.6800 | 0.1220 | 1.3 | 96.8 |
| Summary of the Validation of Intraday Quality Controls in 1g/L PBS/BSA Matrix
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nominal Concentration (μM) | Estimated Intraday Concentration (μM) | Intraday Validation Summary | ||||||||
|
| ||||||||||
| 1st Run | 2nd Run | 3rd Run | 4th Run | 5th Run | 6th Run | Mean Conc. (μM) | Stdev | CV % | Accuracy % | |
| 0.025 | 0.0278 | 0.0246 | 0.0247 | 0.0274 | 0.0278 | 0.0250 | 0.0262 | 0.0016 | 6.1 | 104.9 |
| 0.25 | 0.3240 | 0.2290 | 0.2480 | 0.2440 | 0.2620 | 0.2510 | 0.2597 | 0.0333 | 12.8 | 103.9 |
| 1 | 0.8430 | 0.9670 | 0.8780 | 0.9120 | 0.8990 | 0.9580 | 0.9095 | 0.0473 | 5.2 | 91.0 |
3.2 Stability of ASC-J9® in serum and PBS/BSA matrix
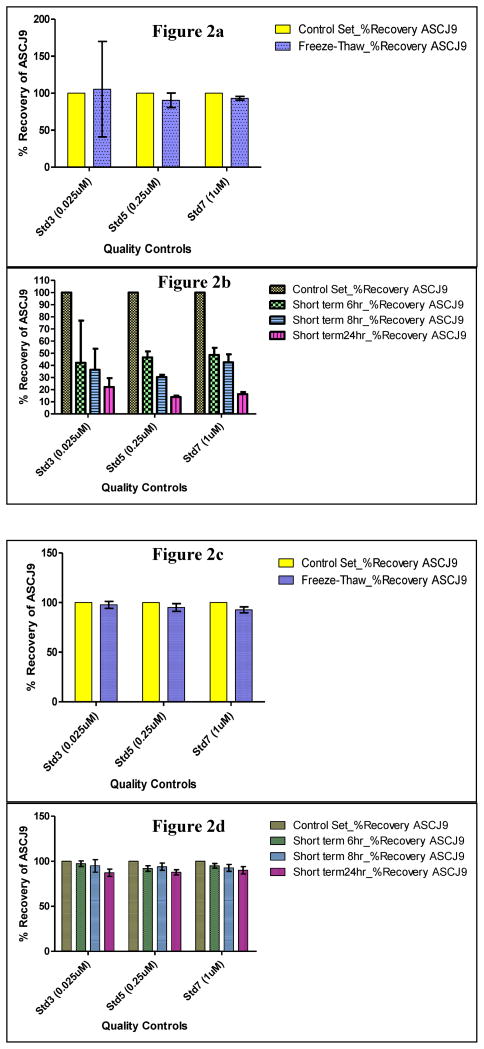
It is very important to study the stability of ASC-J9® in the different matrices in order to ensure that ASC-J9® does not undergo degradation during the storage and sample preparation period. It could also give indications of metabolism for ASC-J9® when compared to curcumin. In this study, freeze-thaw and short term stability studies were conducted. For the samples prepared in serum matrix, the mean recoveries were from 90 to 105 % for ASC-J9® samples with three freeze-thaw cycles when compared to the same concentrations of the freshly-spiked ASC-J9® samples (Figure 2a). As for the samples for the short term stability tests which were left at room temperature of 24 °C for 6, 8 and 24 hrs respectively, much lower recoveries for ASC-J9® were obtained. Recoveries were 42 to 48 % for 6 hrs, 30 to 42 % for 8 hrs, and 13 to 22 % for 24 hrs (as shown in Figure 2b). This implied that significant degradation of ASC-J9® in serum occurred at room temperature. On the other hand, there was a relatively much lesser extent of degradation for ASC-J9® in PBS/BSA matrix than in serum for both the freeze thaw (recoveries were 93% to 98 % as shown in Figure 2c) and short term stability samples (recoveries were 92 to 97 % for 6 hrs, 93 to 95 % for 8hrs, and 87 to 90 % for 24 hrs as shown in Figure 2d) conducted under the test same conditions..
Figure 2. Recovery data of ASC-J9® in various processing ways.
(a) Recovery data of ASC-J9® in serum from freeze-thaw stability samples subjected to 3 freeze-thaw cycles and compared against control set freshly spiked with ASC-J9® on day of extraction. (b) Recovery data of ASC-J9® in serum from short term stability samples left under room temperature for 6, 8 and 24 hrs respectively compared against control set freshly spiked with ASC-J9® on day of extraction. (c) Recovery data of ASC-J9® in 1g/L of PBS/BSA from freeze-thaw stability samples subjected to 3 freeze-thaw cycles and compared against control set freshly spiked with ASC-J9® on day of extraction. (d) Recovery data of ASC-J9® in 1g/L of PBS/BSA from short term stability samples left under room temperature for 6, 8 and 24 hrs respectively compared against control set freshly spiked with ASC-J9® on day of extraction.
When compared to ASC-J9® in serum or in PBS/BSA, curcumin degraded much faster. Under the exact conditions, curcumin was observed to undergo more significant degradation for both the freeze-thaw and short term stability samples (data not shown). As such, to ensure that curcumin can be used as internal standard for quantification here, it was freshly prepared and spiked into the samples just before extraction.
3.3 Pharmacokinetic study of ASC-J9® in mouse serum
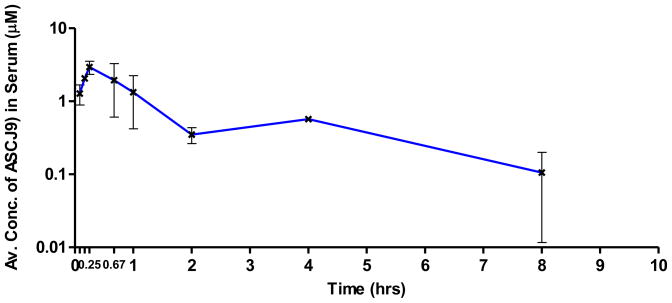
This validated LC–MS/MS method was used to determine the concentration of ASC-J9 in mouse serum samples collected at different timepoints for a simple pharmacokinetic study after intraperitoneal (i.p.) injection of ASC-J9 at 75 mg/kg. The pharmacokinetics estimates of ASC-J9® were calculated based on a two-compartment model using the Phoenix WinNonlin software version 6.0. The peak concentration of ASC-J9® after administration, Cmax was found to be 2.91 μmol/L, which occurred at 0.34 hr. The concentration of ASC-J9® in serum decreased very rapidly but remained detectable up to 8 hrs (Figure 3). These results are similar to a previously reported pharmacokinetic study on ASC-J9® [9]. Among the other parameters obtained, the absorption half-life, k01_t½ is 0.19 hrs, which indicated the rate at which the drug enters the systemic circulation upon intraperitoneal injection. Distribution half-life, α_t½ is estimated to be 0.24 hrs, which indicated that ASC-J9® can be rapidly distributed in the tissues and caused the steep decrease of the concentration of drug in serum. In addition, the elimination rate constant, β is estimated to be 0.13 hr−1, which resulted in an elimination half-life, β_t1/2 of 5.45 hrs. This is much longer than the 0.4 hrs of elimination half life reported for curcumin [5]. This could imply that ASC-J9® could potentially exhibit higher bioavailability than curcumin in vivo which required further confirmation.
Figure 3. Pharmacokinetic diagram of ASC-J9®.
(Log plot of concentration of ASC-J9® in mouse serum against time in hrs) Serum samples were taken at different time points after intraperitoneal injection of 75 mg ASC-J9®/kg mouse body weight.
Note: Log plot was used in this case. And negligible ASC-J9 was detected at 24 hrs and thus not reflected on the plot.
3.4 Distribution of ASC-J9® in mouse organs at 8 and 24 hrs after single dose injection
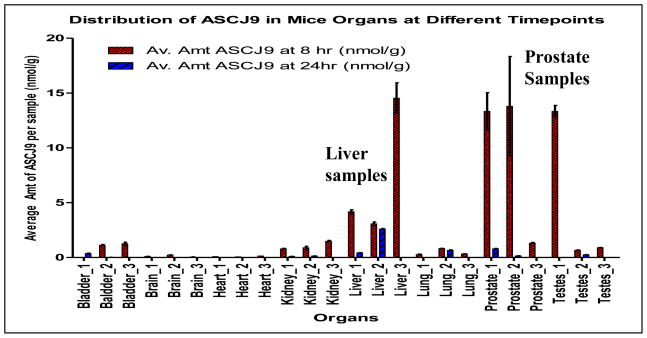
At 8 hr after the single injection, the concentrations of ASC-J9® remained relatively high in the prostate and the liver. This correlates to the pharmacokinetic results as discussed earlier, where the concentration of ASC-J9® in serum decreased rapidly but was quickly distributed in the organs as indicated by the short distribution half-life, α_t½. However, there was minimal or negligible amount of ASC-J9® left in almost all the organs up till 24 hrs, as shown in Figure 4. ASC-J9® seemed to be more likely to remain in the liver, testes and especially in prostate with higher concentrations. However, more studies will have to be carried out to further investigate whether the accumulation of ASC-J9® in these organs after prolonged treatment could have beneficial or detrimental effects since there is no toxicology information of this drug on the organs as yet.
Figure 4. Distribution of ASC-J9® in mouse organs.
Mouse organs were harvested at 8 and 24 hrs after intraperitoneal injection of 75 mg ASC-J9®/kg mouse body weight.
4. Conclusions
A simple, fast, robust and more sensitive LC-MS/MS method has been successfully developed and validated to accurately quantify the ASC-J9® concentration in mouse serum and tissue samples. This validated method has been successfully applied to investigate the pharmacokinetics of ASC-J9® in mouse serum and the distribution of the drug in various mice organs. ASC-J9® was found to be quickly absorbed in vivo and had a relatively slow elimination half life of 5.45 hrs when compared to curcumin. The ASC-J9® has exhibited a higher tendency to accumulate in organs such as liver, testes and especially at prostate. However, more work has to be done to explore the potential of this compound as a potent anti-cancer drug.
Highlights.
A simple LC-MS/MS method for the quantification of ASC-J9 in mouse serum was developed.
Stability tests in different matrices were conducted to validate the types of samples to be analysed.
Method was applied to study the pharmacokinetics of ASC-J9 in mouse serum samples.
Method was also used to find the distribution of drug in mice organs.
Acknowledgments
These works were supported by the Ministry of Education of Singapore, National University of Singapore (Grant No.: R-174-000-13-112), Singapore National Medical Research Council (Grant No.: R-174-000-137-275) and to NIH Grants (CA127300 and CA156700) and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004 (China Medical University, Taichung, Taiwan). Disclosure summary: ASC-J9® was patented by the University of Rochester, the University of North Carolina, and AndroScience, and then licensed to AndroScience. Both the University of Rochester and C.C. own royalties and equity in AndroScience. And special thanks to Dr Feng Sun from National University of Singapore, Department of Obstetrics and Gynaecology for his help in analyzing the pharmacokinetic data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Mol Pharmaceutics. 2007;4(6):807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB. Cancer Letters. 2008;267:133–164. doi: 10.1016/j.canlet.2008.03.025. [DOI] [PubMed] [Google Scholar]
- 3.Vareed SK, Kakarala M, Ruffin MT, Crowell JA, Normolle DP, Djuric Z, Brenner DE. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams BK, Ferstl EM, Davis MC, Herold M, Kurtkaya S, Camalier RF, Hollingshead MG, Kaur G, Sausville EA, Rickles FR, Snyder JP, Liotta DC, Shojia M. Bioorganic & Medicinal Chemistry. 2004;12:3871–3883. doi: 10.1016/j.bmc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 5.Saradhi UVRV, Ling Y, Wang J, Chiu M, Schwartz EB, Fuchs JR, Chan KK, Liu Z. Journal of Chromatography B Analyt Technol Biomed Life Sci. 2010 Nov 15;878(30):3045–3051. doi: 10.1016/j.jchromb.2010.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei X, Du ZY, Zheng X, Cui XX, Conney AH, Zhang K. European Journal of Medicinal Chemistry. 2012;53:235–245. doi: 10.1016/j.ejmech.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 7.Singh SP, Wahajuddin, Jain GK. J Bioanal Biomed. 2011;2:079–084. [Google Scholar]
- 8.Li R, Qiao X, Li Q, He R, Ye M, Xiang C, Lin X, Guo D. Journal of Chromatography B. 2011;879:2751–2758. doi: 10.1016/j.jchromb.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 9.Tamvakopoulos C, Dimas K, Sofianos ZD, Hatziantoniou S, Han Z, Liu ZL, Wyche JH, Pantazis P. Clin Cancer Res. 2007;13:1269–1277. doi: 10.1158/1078-0432.CCR-06-1839. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita S, Lai KP, Chuang KL, Xu D, Miyamoto H, Tochigi T, Pang ST, Li L, Arai Y, Kung HJ, Yeh S, Chang C. Neoplasia. 2012;14(1):74–83. doi: 10.1593/neo.111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai KP, Huang CK, Chang YJ, Chung CY, Yamashita S, Li L, Lee SO, Yeh S, Chang C. American Journal of Pathology. 2013 Feb;182(2):460–473. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu MH, Ma WJ, Hsu CL, Chen YL, Ou JH, Ryan CK, Hung YC, Yeh S, Chang C. Sci Trans Med. 2010;2:32–35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto H, Yang Z, Chen YT, Ishiguro H, Uemura H, Kubota Y, Hu YC, Tsai MY, Yeh S, Messing EM, Chang C. J Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 14.Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. Nature Medicine. 2007 Mar;13(3):348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- 15.Lin TH, Izumi K, Lee SO, Lin WJ, Yeh S, Chang C. Cell Death disease. 4:e764. doi: 10.1038/cddis.2013.270. doi:1038/ccdis/2013.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin TH, Niu Y, Lee SO, Xu L, Liang L, Li L, Yeh SD, Fujimoto N, Yeh S, Chang C. J Biol chem. 2013;288:19359–19369. doi: 10.1074/jbc.M113.477216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guidance for Industry Bioanalytical Method Validation, available from website. <URL> http://www.fda.gov/cder/guidance/4252fnl.pdf.