Abstract
In previous studies, exposure to live Borrelia burgdorferi was shown to induce inflammation and apoptosis of human oligodendrocytes. In this study we assessed the ability of non-viable bacteria (heat killed or sonicated) to induce inflammatory mediators and cell death. Both heat-killed and sonicated bacteria induced release of CCL2, IL-6, and CXCL8 from oligodendrocytes in a dose dependent manner. In addition, non-viable B. burgdorferi also induced cell death as evaluated by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and another cell viability assay. These results suggest that spirochetal residues left after bacterial demise, due to treatment or otherwise, may continue to be pathogenic to the central nervous system.
Keywords: B. burgdorferi, inflammation, apoptosis, oligodendrocytes, Lyme neuroborreliosis
1. Introduction
Lyme borreliosis (LB) is the leading vector borne-infectious disease in the US, Europe and parts of Asia [4]. Caused by the spirochete Borrelia burgdorferi sensu lato, initial manifestations of localized infection include erythema migrans, a rash with a typical “bulls eye” like appearance (although variations exist). This is followed by signs and symptoms of disseminated infection in other organs leading to conditions such as arthritis, carditis, uveitis, and neuroborreliosis. Signs and symptoms of neurological involvement may affect both the central and peripheral nervous systems (CNS and PNS) and include nerve palsies, radicular pain, meningitis, encephalomyelitis and others. Treatment of LB typically involves an antibiotic regimen that consists of cephalosporins (ceftriaxone, cefotaxime, and cefuroxime), penicillin G, amoxicillin, or doxycycline depending on the disease stage, organs involved, and age of the patient, and may either be an oral or a parenteral regimen [12]. However, even after proper treatment, in some patients, symptoms like musculoskeletal pain, cognitive difficulties, dysesthesia, and fatigue can persist for many months. These manifestations are termed post-treatment Lyme disease syndrome or PTLDS. Several theories have been put forward for PTLDS including persistence of infection, host-inflammatory response leading to autoimmunity, post-infective fatigue syndrome, as well as psychiatric disorders [1, 8]. In this report, we have analyzed the effect of non-viable B. burgdorferi, a state that can be achieved either naturally in vivo during infection or induced by bacteriostatic/cidal antibiotics [5], on human oligodendrocytes- a glial cell of the CNS. We find that inflammation and cell death can be induced in these cells in a dose dependent manner, even after bacterial killing.
2. Materials and Methods
2.1. Bacterial culture
B. burgdorferi strain B31 was cultured in Barbour-Stoenner-Kelly (BSK-H) medium (Sigma Aldrich, St. Louis-MO) supplemented with 0.25 mg/mL amphotericin, 193 mg/mL phosphomycin and 45.4 mg/mL rifampicin for about 5–7 days under microaerophilic conditions. Bacteria were counted under a dark field microscope and the concentration was determined. They were then centrifuged at 2095 × g for 30 minutes at room temperature with deceleration set at zero. The bacterial pellet was then resuspended in DMEM -high glucose (Invitrogen/Life Technologies, Inc., Grand Island-NY) containing 100 nM phorbol myristate acetate (PMA) (Sigma Aldrich, St. Louis-MO) to the same bacterial concentration prior to pelleting.
2.2. Cell culture
A human oligodendrocyte cell line MO3.13 (CELLutions Biosystems Inc., Ontario, Canada) was used to determine the effect of non-viable B. burgdorferi in eliciting inflammation and/or cell death in human brain cells. Cells were routinely cultured according to the manufacturer’s protocols, seeded at a density of 0.8 × 104/well for 6 well plates and 0.6 × 104/well for 2-well chamber slides and differentiated to mature oligodendrocytes as described elsewhere [10]. All of the experiments that were performed with mature MO3.13 oligodendrocytes were carried out in DMEM-high glucose with 100 nM PMA (Experimental Medium).
2.3. Co-culture assays
Assays were carried out using live, heat-killed or sonicated bacteria. Bacterial stocks resuspended in Experimental Medium were divided into equal volumes in tubes and either left undisturbed (in the incubator), heat killed at 55 °C (± 3 °C) for 30 minutes, or sonicated (5 pulses at amplitude 4 for 12–15 seconds each; probe sonicator, Heat Systems Ultrasonics, Inc., Model W-220F). As controls, similar volumes of medium alone were either “heat killed” or sonicated in the same fashion. From these stocks (viable and non-viable), appropriate volumes of bacteria were further diluted in Experimental Medium at the required multiplicity of infection (MOI) and added to the oligodendrocyte cell cultures along with the respective medium controls. The non- viability of the heat killed or sonicated bacteria was confirmed by allowing for regrowth, if any, in BSK medium under normal culture conditions (3–5 days) and concentrations determined. A live/dead bacterial viability kit (Invitrogen/Life Technologies Inc.) was also used to ascertain non-viability of the bacteria.
2.4. Enzyme-Linked Immuno Sorbent Assay (ELISA)
To evaluate the role of non-viable B. burgdorferi in inducing inflammation from human oligodendrocytes, supernatants were collected 48 h after exposure to live or non-viable bacteria, centrifuged at 2095 × g, 10 minutes at 4 °C and the clarified medium aliquoted and stored at −20°C until analysis by a multiplex ELISA for CCL2, IL-6, CXCL8, and additionally for CCL5 and CXCL (1,-2,-3) (EMD Millipore, Billerica- MA). The ELISA procedures were carried out at the Pathogen Detection and Quantification Core, Tulane National Primate Research Center according to the manufacturer’s protocols.
2.5. Cell viability assay
The effect of non-viable B. burgdorferi on oligodendrocyte cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)-based kit (Sigma Aldrich, St. Louis-MO). A 5 mg/mL concentration of MTT was added at a 10% volume to the medium in the wells and incubated at 37 °C, 5% CO2 for 2 h. Cells were solubilized with an equal volume of the solubilization solution provided and read spectrophotometrically at 570 nm. Percent change in absorbance was calculated by normalizing to medium only controls.
2.6. Apoptosis assay
The percentage of apoptotic cells obtained in response to various treatments was assessed by the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (ApopTag Fluorescein kit, Millipore) following staining of oligodendrocytes with anti-myelin basic protein (MBP), both carried out according to previously published protocols [10]. TUNEL-positive cells were visualized by a fluorescent microscope (Leica microsystems, Buffalo Grove-IL), imaged (Nuance Multispectral Imaging System; CRi, PerkinElmer, Waltham- MA) and the images assembled using Adobe® Photoshop. A semi-quantitative measurement was obtained by calculating percent of TUNEL positive cells in 500–1000 cells/well for each treatment.
2.7. Statistics
Statistical significance was determined using Student’s t-test. P values < 0.05 were considered significant.
3. Results
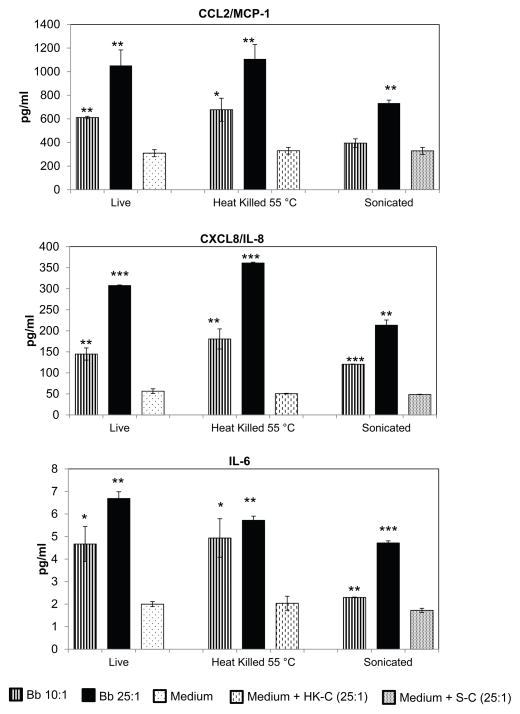
The ability of non-viable B. burgdorferito induce inflammation and/or apoptosis in human oligodendrocytes was assessed by use of heat killed (HK), and sonicated (S) bacteria. The non-viability of bacteria by these treatments was ensured by assessing for regrowth in permissive medium as well as by a live/dead viability kit, as described in Materials and Methods. Heat killing resulted in ~ 60–70% of the bacteria appearing as non-fragmented, while sonication fully fragmented the bacteria. Both treatments were found to be effective in rendering the bacteria non-viable (data not shown). We then assessed the ability of these non-viable bacteria to elicit inflammation in oligodendrocytes. As shown in Fig. 1, HK bacteria were able to induce significant amounts of inflammatory mediators such as CCL2, CXCL8, and IL-6 at 48h, at both MOI tested (10:1 and 25:1), as did the sonicated bacteria with the exception of CCL2 at the MOI of 10:1. Heat killing or sonication of medium alone did not affect inflammatory mediator release from the cells in comparison to untreated medium (Fig. 1). This indicated that effects seen are through bacterial products alone and not artifacts of medium treatments. In addition to CCL2, CXCL8 and IL-6, significant upregulation of CCL5/RANTES and CXCL(1,-2,-3)/GRO was seen in response to both forms of non-viable bacteria. At the MOI of 10:1, HK bacteria induced a 1.3 fold and a 1.91 fold upregulation of CCL5 and CXCL (1,-2,-3) respectively, in comparison to cells exposed to medium alone. Sonicated bacteria at the same MOI induced a 2.83 and a 1.73 fold of CCL5 and CXCL (1,-2,-3) respectively, with all the indicated fold increases being statistically significant (data not shown).
Fig. 1.
Non-viable B. burgdorferi induce release of inflammatory mediators from mature MO3.13 cells. Bacteria were either heat killed at 55 °C for 30 minutes or sonicated and added to MO3.13 cells for 48 h at the indicated MOI. As controls, medium alone without bacteria was similarly heat-treated or sonicated and added to regular medium at a volume equal to that which contained the heat-treated or sonicated bacteria at the highest MOI (HK-C; Heat killed component or S-C-Sonicated component). A representative experiment of 2–3 experiments for each treatment is shown, with bars representing standard deviation. P values were calculated over respective medium controls. * p < 0.05; ** p < 0.01; *** p < 0.001.
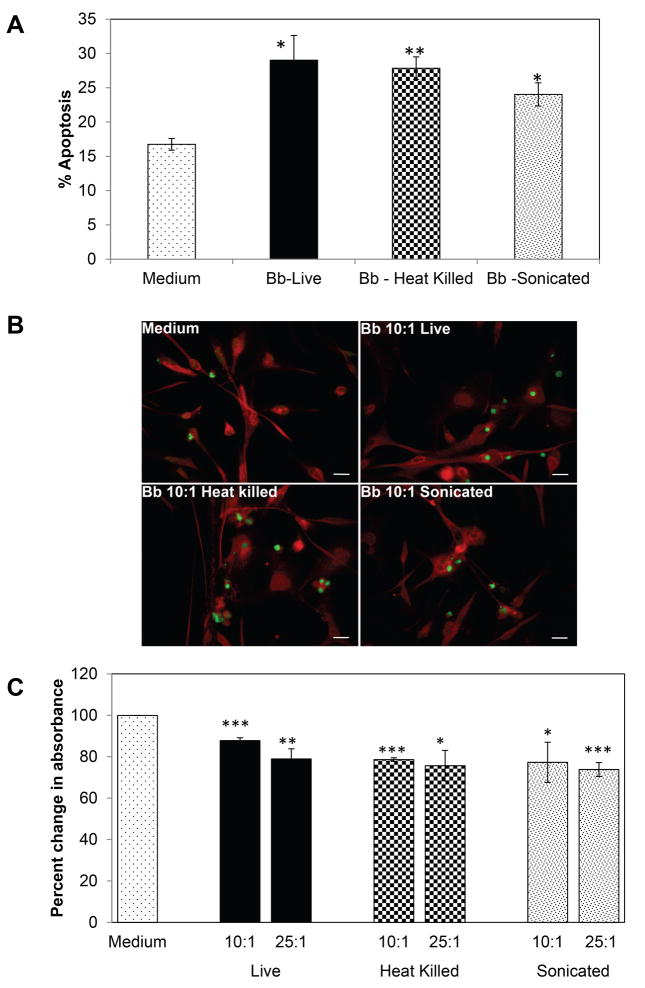
Data from previous studies have shown that live B. burgdorferi can induce apoptosis in human oligodendrocytes [10]. We therefore assessed the role of non-viable bacteria in inducing cell death. All the forms of bacteria tested were able to induce cell death as determined by both TUNEL (Fig. 2A and 2B) and MTT assays (Fig. 2C). There was a statistically significant increase in TUNEL-positive oligodendrocytes when cells were exposed to either HK or sonicated (S) bacteria (equivalent to 10:1 MOI) for 48h compared to cells with medium alone (Fig. 2A and 2B). Accordingly, there was a significant reduction in cell viability of oligodendrocytes as measured by the MTT assay at 72h for both the MOI tested (Fig. 2C). When used at the MOI of 10:1, the viability decreased to 88 (live), 79 (HK), 77 (S) % when compared to medium only controls (100%), and further dropped to 79 (live), 76 (HK) and 74 (S) % at the higher MOI of 25:1. Cells exposed to medium containing equivalent “HK” or S medium fractions without bacteria did not differ significantly in viability when compared to medium only cells (data not shown).
Fig. 2.
(A) Percent of TUNEL-positive cells in response to treatment with B. burgdorferi for 48 hr at 10:1 MOI. Values represent mean of 2- 3 experiments each with bars representing standard error of the mean (SEM). (B) representative fluorescent microscopy photographs of TUNEL-positive cells (green) for various treatments on MO3.13 cells stained red with anti-MBP antibody. Bar represents 50 μm. (C) Mature MO3.13 human oligodendrocytes treated with either live, heat killed (55 °C, 30 minutes) or sonicated B. burgdorferi at an MOI of 10:1 or 25:1 for 72 h. Cell viability of MO3.13 cells resulting from treatment with viable and non-viable bacteria was determined using an MTT-based assay. Percent change in absorbance was calculated over medium only control. Values represent averages from two independent and identical experiments with bars representing SEM. * p < 0.05; ** p < 0.01; *** p < 0.001.
4. Discussion
Lyme neuroborreliosis is a morbid form of Lyme disease with pleotropic effects. Although early treatment with antibiotics is usually successful, in 10 to 20% of patients, particularly those that are diagnosed and treated later in the course of infection, long-term disabilities persist [1, 2, 12]. Here we show that, as with live B. burgdorferi, non-viable bacteria in the form of HK or sonicated organisms, are still able to induce inflammatory mediators in human oligodendrocytes in a dose dependent manner (Fig. 1) and cause the death of these cells (Fig. 2), even at a low MOI of 10:1. This is similar to the results from previous studies [3], where sonicated bacteria (MOI of 10:1) were able to induce inflammatory mediators such as IL-6, CXCL8, CCL3, and CCL4 in microglia and astrocytes. Together, these results show that major glial cells in the CNS are able to produce significant amounts of inflammatory mediators in response to spirochete residues. In addition to inducing inflammation in oligodendrocytes, these bacterial fragments were able to induce cell death in these CNS glial cells (Fig. 2). These results resemble those published for other bacteria that infect the CNS, such as Brucella abortusan d Streptococcus pneumoniae. HK B. abortus were able to induce IL-6, TNF, CCL2, and other mediators in primary glial cells, and cause astrocyte apoptosis [7]; pneumococcal cell-wall components were shown to be inflammatory [11] and induce apoptosis in hippocampal neurons [9]. While heat-killing at 55°C would destabilize a majority of proteins, it would presumably leave more stable proteins like flagellin intact and not affect lipids, the lipid moiety of bacterial lipoproteins, or lipo-oligosaccharides. Sonication would disintegrate the cell wall and fully release cytoplasmic contents, making additional antigens available. Thus while HK or sonication ensures no further propagation of the bacteria, the processes do not eliminate some possible antigens and in fact may add to the antigenic repertoire encountered by the host, especially in case of sonication. Antibiotics may leave similar antigenic residues. In fact, evidence obtained with animal models of Lyme disease shows that spirochetal fragments are left in the tissues after antibiotic treatment [5, 6] and may continue to cause inflammation both in the heart [6] and in the joints [5]. The authors have argued that this phenomenon could contribute to explain treatment-resistant Lyme arthritis [5]. Similarly, PTLDS that affects the CNS could also be due to responses to residual spirochetal antigens. Our demonstration, albeit in vitro, that such antigens can induce inflammatory mediators and death in oligodendrocytes, which are cells that play a major role in neuronal homeostasis in the CNS, further supports this contention.
In conclusion, results from this study suggest that B. burgdorferi may continue to be pathogenic in the CNS even when rendered non-viable. Therefore, other therapies might be needed in addition to the traditional antibiotic therapies as treatment regimen.
Highlights.
Non-viable B. burgdorferi can induce inflammatory mediators from oligodendrocytes
Non- viable B. burgdorferi can cause oligodendrocyte cell death
B. burgdorferi antigens continue to be pathogenic in the CNS
Acknowledgments
This work was supported by the National Center for Research Resources/Office of Research Infrastructure Programs of the National Institutes of Health grant P51RR000164/P51OD011104; and by the National Institute of Neurologic Disorders and Stroke grant NS048952. We would like to thank the Tulane National Primate Research Center Pathogen Detection and Quantification Core Laboratory for help with the multiplex ELISA assays.
Abbreviations
- ELISA
Enzyme-Linked Immuno Sorbent Assay
- LB
Lyme Borreliosis
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PTLDS
post-treatment Lyme disease syndrome
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
- PMA
phorbol myristate acetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aucott JN, Rebman AW, Crowder LA, Kortte KB. Post-treatment Lyme disease syndrome symptomatology and the impact on life functioning: is there something here? Quality of life research: an international journal of quality of life aspects of treatment, care and rehabilitation. 2013;22:75–84. doi: 10.1007/s11136-012-0126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aucott JN, Seifter A, Rebman AW. Probable late lyme disease: a variant manifestation of untreated Borrelia burgdorferi infection. BMC infectious diseases. 2012;12:173. doi: 10.1186/1471-2334-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infection and immunity. 2008;76:4385–4395. doi: 10.1128/IAI.00394-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhate C, Schwartz RA. Lyme disease: Part I. Advances and perspectives. Journal of the American Academy of Dermatology. 2011;64:619–636. doi: 10.1016/j.jaad.2010.03.046. quiz 637–618. [DOI] [PubMed] [Google Scholar]
- 5.Bockenstedt LK, Gonzalez DG, Haberman AM, Belperron AA. Spirochete antigens persist near cartilage after murine Lyme borreliosis therapy. The Journal of clinical investigation. 2012;122:2652–2660. doi: 10.1172/JCI58813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PloS one. 2012;7:e29914. doi: 10.1371/journal.pone.0029914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia Samartino C, Delpino MV, Pott Godoy C, Di Genaro MS, Pasquevich KA, Zwerdling A, Barrionuevo P, Mathieu P, Cassataro J, Pitossi F, Giambartolomei GH. Brucella abortus induces the secretion of proinflammatory mediators from glial cells leading to astrocyte apoptosis. The American journal of pathology. 2010;176:1323–1338. doi: 10.2353/ajpath.2010.090503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ljostad U, Mygland A. Chronic Lyme; diagnostic and therapeutic challenges. Acta neurologica Scandinavica Supplementum. 2013:38–47. doi: 10.1111/ane.12048. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell L, Smith SH, Braun JS, Herzog KH, Weber JR, Tuomanen EI. Dual phases of apoptosis in pneumococcal meningitis. The Journal of infectious diseases. 2004;190:2039–2046. doi: 10.1086/425520. [DOI] [PubMed] [Google Scholar]
- 10.Ramesh G, Benge S, Pahar B, Philipp MT. A possible role for inflammation in mediating apoptosis of oligodendrocytes as induced by the Lyme disease spirochete Borrelia burgdorferi. Journal of neuroinflammation. 2012;9:72. doi: 10.1186/1742-2094-9-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. The Journal of infectious diseases. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 12.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, Krause PJ, Bakken JS, Strle F, Stanek G, Bockenstedt L, Fish D, Dumler JS, Nadelman RB. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2006;43:1089–1134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]