Abstract
In the liver, hepatocytes are exposed to a large array of stimuli that shape hepatic phenotype. This in vivo microenvironment is lost when hepatocytes are cultured in standard cell cultureware, making it challenging to maintain hepatocyte function in vitro. Our article focused on one of the least studied inducers of the hepatic phenotype—the mechanical properties of the underlying substrate. Gel layers comprised of thiolated heparin (Hep-SH) and diacrylated poly(ethylene glycol) (PEG-DA) were formed on glass substrates via a radical mediated thiol–ene coupling reaction. The substrate stiffness varied from 10 to 110 kPa by changing the concentration of the precursor solution. ELISA analysis revealed that after 5 days, hepatocytes cultured on a softer heparin gel were synthesizing five times higher levels of albumin compared to those on a stiffer heparin gel. Immunofluorescent staining for hepatic markers, albumin and E-cadherin, confirmed that softer gels promoted better maintenance of the hepatic phenotype. Our findings point to the importance of substrate mechanical properties on hepatocyte function.
Introduction
There is a rapidly expanding body of literature documenting the profound impact of the biophysical attributes of the extracellular matrix (ECM) on cellular behaviors, with substrate stiffness being recognized as a potent cue.1–5 Several studies have highlighted the effects of substrate stiffness on cellular function ranging from cell attachment and proliferation to stem cell differentiation.6–8 Therefore, an understanding of the substrate stiffness-cell function relationship is required to create in vitro environments that better mimic the in vivo condition.9–11
Maintaining functional hepatocytes in vitro is important for purposes of bioartificial liver development and drug screening.12 To promote hepatocyte attachment, cell cultureware is typically precoated with a molecular layer of ECM proteins, such as collagen I or IV, but even then, hepatocytes dedifferentiate rapidly over time, losing the ability to synthesize serum proteins and urea, and downregulating such markers of epithelial phenotype as E-cadherin.13 In contrast, some of the best maintenance of phenotype have been observed in hepatocytes cultured on top of or sandwiched between soft gel layers of fibrillar collagen or Matrigel.13–17 Importantly, liver cirrhosis—a disease associated with a loss of liver function—is accompanied by significant changes in mechanical properties at whole organ, regional, and cellular levels.18,19 Liver stiffness (modulus) values below 6 kPa are considered normal, but those of 20 kPa or higher are associated with fibrosis and cirrhosis.20–23 Therefore, it is reasonable to expect that matrix mechanical properties may play a role in the regulation of hepatic function.
Tuning mechanical properties of natural gels is somewhat challenging, and so, several studies have pursued the use of synthetic substrates of varying mechanical properties to examine hepatic phenotype expression.24,25 Chen et al. cultured hepatocytes on polyelectrolyte multilayers and found that hepatic albumin production decreased with increasing substrate stiffness.24 In another study, Semler et al. modified polyacrylamide gels with cell adhesive ligands to explore the effects of graded mechanical compliance on the function of primary hepatocytes.25 They demonstrated that increasing hydrogel compliance resulted in increased albumin secretion.
A number of laboratories, including our own, have been pursuing the development of hybrid, synthetic/natural gels, to retain bioactivity while introducing control over physicochemical properties of the material.26–30 One key component of ECM is heparin—a highly sulfated anionic polysaccharide, capable of noncovalent interactions with numerous growth factors and ECM proteins via heparin binding domains.31 To leverage bioactivity of heparin, we (Tae et al.) have developed heparin-based hydrogels based on thiolated heparin (Hep-SH) crosslinking with diacrylated poly(ethylene glycol) (PEG-DA) (Fig. 1)31–33 and used these biofunctional hydrogels for controlled release of growth factors and encapsulation of primary rat hepatocytes.34,35 In the present article, we sought to investigate the effects of heparin gel mechanical properties on the function of primary hepatocytes. Heparin hydrogels were formed by UV initiated thiol–ene reactions (Fig. 1A) with elastic moduli ranging from 11 to 110 kPa. Primary rat hepatocytes were then cultured on gels of varying stiffness to reveal that hepatic albumin production and E-cadherin expression were highest on soft gels with a modulus of ∼11 kPa. Our study is significant in that it offers a novel hepatocyte culture substrate that carries the bioactive component, heparin, and may be tuned to contain mechanical and biochemical cues most conducive to the maintenance of hepatic phenotypes.
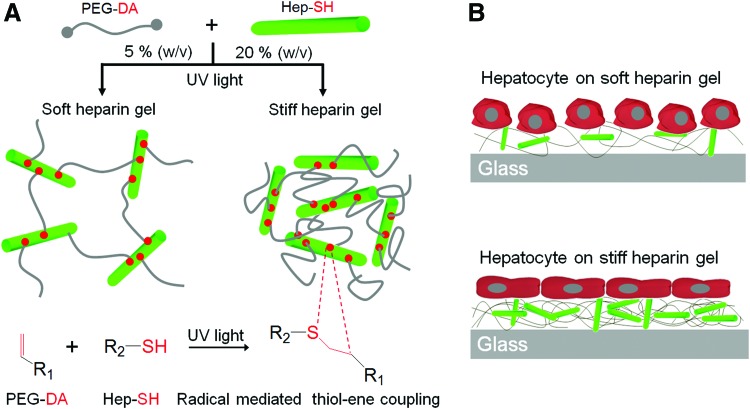
FIG. 1.
Fabricating heparin gels of varying stiffness by changing the concentration of gel precursor solutions and culturing primary hepatocytes on soft and stiff heparin gels. (A) Schematic illustration of UV-initiated thio–ene reaction between acrylated poly(ethylene glycol) (PEG) and thiolated heparin to form heparin gels. (B) Schematic illustration of culturing hepatocytes on soft versus stiff heparin gels. Hepatocytes cultured on soft heparin gels retained a distinctive cuboidal shape, whereas hepatocytes on stiff gels became more elongated and fibroblastic. PEG-DA, diacrylated poly(ethylene glycol); HEP-SH, thiolated heparin. Color images available online at www.liebertpub.com/tea
Experimental Section
Chemicals and materials
Glass slides (75×25 mm2) were purchased from Fisher Scientific (Pittsburg, PA). 3-acryloxypropyl trichlorosilane was purchased from Gelest (Morrisville, PA). Heparin (sodium salt, from porcine intestinal mucosa, MW 12 kDa) was purchased from Celsus Ins. (Cincinnati, IA). Hep-SH was synthesized with the modification of carboxylic groups of heparin using carbodiimide chemistry, as previously reported.31 PEG-DA (MW 6 kDa, 98% degree of substitution) and tetra-functional PEG sulfhydryl (PEG-SH4, MW 10 kDa) were purchased from SunBio, Inc. (Anyang, Korea). 4-(2-hydroxyethoxy) phenyl-(2-hydroxy-2-propyl) ketone (Irgacure 2959) was purchased from Ciba Specialty Chemicals, Inc. (Basel, Switzerland). Sulfuric acid, ethanol, bovine serum albumin, and toluidine blue O were purchased from Sigma Aldrich (St. Louis, MO). Glucagon and recombinant human insulin were obtained from Eli Lilly (Indianapolis, IN), and hydrocortisone sodium succinate was obtained from Pfizer, Inc. (Ann Arbor, MI). Phosphate-buffered saline (PBS) was purchased from Gibco (Grand Island, NY). Dulbecco's modified Eagle's medium (DMEM), sodium pyruvate, fetal bovine serum (FBS), penicillin/streptomycin were purchased from Invitrogen (Carlsbad, CA). Rat albumin ELISA kit was obtained from Bethyl Laboratories (Montgomery, TX). Paraformaldehyde was purchased from Election Microscopy Sciences (Hatfield, PA). Sheep anti-albumin and FITC anti-sheep IgG were obtained from Bethyl Labs and Santa Cruz Biotechnologies, Inc. (Santa Cruz, CA). Mouse anti-E-cadherin and Alexafluor 546 anti-mouse were purchased from BD Science and Invitrogen. Mounting medium with DAPI was purchased from Vector Laboratories, Inc. (Burlingame, CA).
Surface modification of glass substrates
Silane modification was used to anchor gel structures to glass. Glass slides were cleaned by sonication for 15 min in a 70% ethanol solution. Glass slides were then thoroughly rinsed with deionized (DI) water, and then dried at 100°C. For silane modification, the glass slides were treated in an oxygen plasma chamber (YES-R3, San Jose, CA) at 300 W for 5 min, and then placed in a 2 mM solution of 3-acryloxypropyl trichlorosilane in anhydrous toluene for 1 h. The reaction was performed in a glove bag under nitrogen purge to eliminate atmospheric moisture. After modification, the slides were rinsed with fresh toluene, dried under nitrogen, and cured at 100°C for 2 h. Silane modified slides were placed in a desiccator until further use.
Preparation of heparin hydrogels of varying stiffness
Heparin-based hydrogels were prepared by UV-initiated thio–ene polymerization of Hep-SH and PEG-DA, as reported previously (Fig. 1).35 Hep-SH and 6 kDa PEG-DA (1:1 molar ratio of thiol to acrylate group) were mixed in PBS containing 1% w/v photoinitiator (Irgacure 2959), which was dissolved in 70% v/v ethanol, to achieve 5%, 10%, and 20% w/v of gel precursor solutions. These precursor solutions were applied onto the silane-treated glass surface (12×12 mm) containing terminal acrylate functional groups and then were covered with a cover slip (25×25×0.13 mm) to create a uniform prepolymer layer sandwiched between two glass substrates. It was exposed to 365 nm, 18 W/cm2 UV light using an OmniCure series 1000 light source (EXFO; Vanier, Quebec, Canada). The regions exposed to UV light underwent polymerization and became crosslinked heparin gel. The elastic modulus of heparin gels (5%, 10%, and 20%) was measured by the contact mechanics using an atomic force microscopy (AFM, Fig. 2). Specifics of measuring heparin gel stiffness are discussed later in this section.
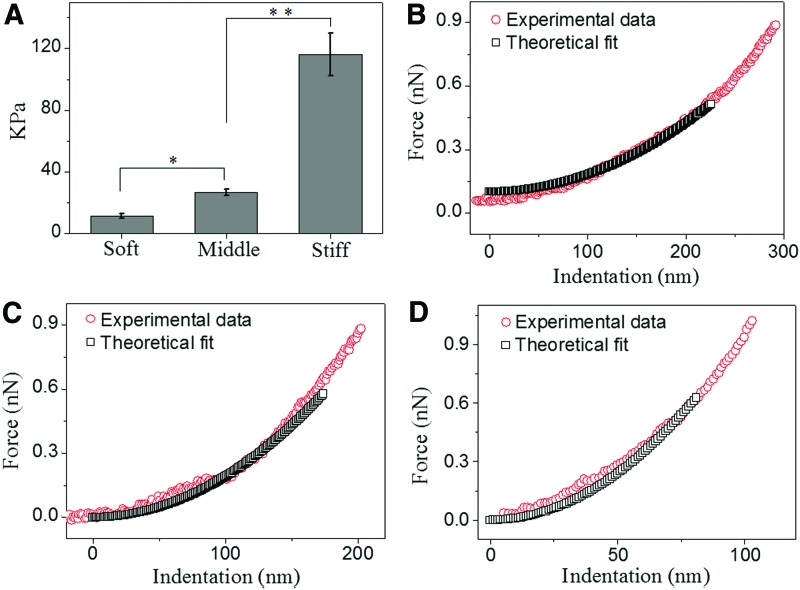
FIG. 2.
Characterizing the heparin gel stiffness based on contact mechanics with atomic force microscopy (AFM). (A) The elastic modulus of heparin gels measured by AFM. (B–D) Representative data illustrate indentation force versus indentation depth of various heparin gels obtained by AFM with an overlay of the theoretical force–indentation curves estimated using Equation 1. *p<0.01, **p<0.001, n=4. Color images available online at www.liebertpub.com/tea
Toluidine blue O staining was performed to confirm the presence of heparin and to quantify the amount of heparin in hydrogels, as reported previously.36,37 Staining of negatively charged heparin molecules by this dye (purple color) was visualized using optical microscopy (Zeiss Axiovert 40; Carl Zeiss, Cape May, NJ). Heparin gel micropatterns were fabricated to clearly visualize and compare the heparin content in the gels of varying stiffness as shown in Figure 3B–E. To fabricate the heparin gel micropatterns, a photomask was placed on top of the liquid prepolymer layer and the regions exposed to UV light underwent polymerization and became crosslinked, while unexposed regions were dissolved after immersion in DI water. Toluidine blue O staining was employed on four types of hydrogel micropatterns: (1–3) different elastic hydrogels created by UV-initiated thiol–ene reaction of 5%, 10%, and 20% w/v gel precursor solutions, (4) hydrogel created by UV-initiated thiol–ene reaction of tetrafunctional PEG-SH4 (10 kDa), and 3.4 kDa PEG-DA (1:1 molar ratio of thiol group and acrylate group). The amount of heparin in the gels of varying stiffness was determined using a toluidine assay whereby heparin gels of varying stiffness were first immersed in the toluidine blue solution (3 mL). Subsequently, an aqueous phase with toluidine blue was extracted by immersion in hexane, separated, and analyzed by UV-Vis spectroscopy.
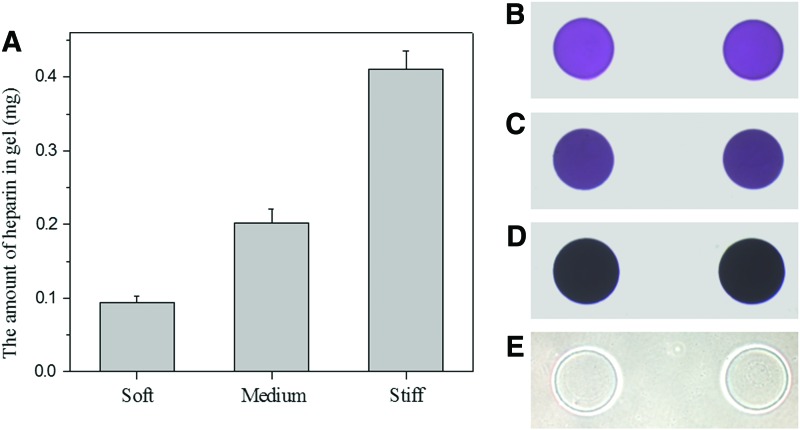
FIG. 3.
Heparin content in gels of varying stiffness. (A) The amount of heparin in heparin gels of different mechanical properties. (B–E) Optical microscopic images of (B) soft heparin hydrogel (5%), (C) medium heparin hydrogel (10%), and (D) stiff heparin hydrogel (20%), and (E) PEG-SH and PEG-DA stained by toluidine blue O. Color images available online at www.liebertpub.com/tea
Cell culture on heparin gels of varying stiffness
Primary hepatocytes were isolated from adult female Lewis rats (Charles River Laboratories, Boston, MA) weighing 125–200 g, using a two-step collagenase perfusion procedure as described previously.38 Typically, 100–200 million hepatocytes were obtained with viability >90% as determined by trypan blue dye exclusion. Primary hepatocytes were maintained in the DMEM supplemented with 10% FBS, 200 U/mL penicillin, 200 mg/mL streptomycin, 7.5 mg/mL hydrocortisone sodium succinate, 20 ng/mL EGF, 14 ng/mL glucagon, and 0.5 U/mL recombinant human insulin at 37°C in a humidified 5% CO2 atmosphere.
Before cell seeding, heparin hydrogels were formed on silane-coated glass substrates using the protocol described earlier. The substrates were then incubated with the collagen I solution (0.05 mg/mL) for 1 h, washed in the PBS solution, rinsed in DI water, and dried using nitrogen. The surfaces were placed into a 12-well plate and seeded with 1×106 rat primary hepatocytes suspended in 2 mL of culture medium. After 1 h of incubation at 37°C, the samples were washed twice in PBS to remove unattached hepatocytes and a fresh medium added to the sample wells.35,39,40
Measurement of heparin gel stiffness based on contact mechanics
The contact mechanics of the gels were determined with an AFM (MFP-3D-BIO; Asylum Research, Santa Barbara, CA). Silicon nitride cantilevers with a square pyramid tip incorporated at the free end (k=0.06 N/m, PNO-TR-50; Nano and More, Lady's Island, SC) were used in these studies. Spring constants of the cantilevers were measured independently for each experiment. Two separate experiments were performed for each substrate, with five force-versus-indentation curves measured on each substrate from five different locations; this amounts to 25 force curves for every substrate in each experiment. Contact between the AFM tip and the substrate was defined by the point when cantilever deflection deviated from a linear extrapolation of zero force. The elastic modulus was determined by fitting each force-versus-indentation curve using a theoretical model for a rigid cone indenter,41–43 according to Equation 1. The values of the elastic modulus determined by fitting the data for every force curve for each substrate were averaged and are reported here.
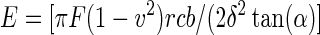 |
(Eq.1), |
where F is the force exerted by the substrate on the cantilever, α is the half-angle opening of the square pyramid, δ is indentation depth, ν is the Poisson's ratio (0.5), and E is the elastic modulus.
Characterizing phenotype of hepatocytes
Quantification of hepatocyte adhesion on heparin gels was carried out by counting cells in microscopic images of six 20×magnification fields per each condition at 1 day after cell seeding. For quantitative evaluation of cell morphological changes depending on heparin gel stiffness, more than 20 single cells were randomly chosen to measure the cell surface area. The analysis of the cell surface area was performed using ImageJ software. The albumin production of hepatocytes was assessed using standard ELISA protocols.38 To detect intracellular albumin and E-cadherin, hydrogel substrates were fixed in 4% paraformaldehyde and were washed in the PBS solution. The substrates were then incubated with primary antibodies for albumin (1:100 dilution in PBS) and for E-cadherin (1:50 dilution in PBS). After overnight incubation in 4°C, substrates were washed in the PBS solution, and then stained with secondary antibodies for albumin (1:200 dilution in PBS) and for E-cadherin (1:750 dilution in PBS). After 1 h of incubation at room temperature, the substrates were washed in PBS and were mounted using a mounting medium containing DAPI to determine the location of nuclei. Stained cells were visualized and imaged using a laser scanning confocal microscope (LSM700; Carl Zeiss, Jena, Germany).
Statistical analysis
The results are expressed as the mean±standard deviation. Student's t-test analysis was used for statistical analysis. Differences were considered to be statistically significant at p<0.05.
Results and Discussion
This article explores how heparin gel stiffness affects the phenotype of cultured hepatocytes. Primary hepatocytes cultured on softer heparin gels with a modulus of 11 kPa were more functional than cells on stiffer substrates as judged by albumin synthesis and E-cadherin expression. This work supports the notion that mechanical properties represent an important inducer of phenotypes in primary hepatocytes (Fig. 1B).
Measuring modulus of heparin gels
In this study, the elastic modulus (E) of heparin gels was tuned by controlling the concentration of the precursor solution. The modulus was determined by fitting the Hertz model to force versus indentation data obtained from nanoindentation measurements with AFM. Figure 1A illustrates the impact of precursor concentrations on the resulting elastic moduli of heparin gels.
A 5% (w/v) heparin gel was the softest with an elastic modulus of 11.38±1.48 kPa, whereas the 20% (w/v) heparin gel was the stiffest with an elastic modulus of 115.93±13.81 kPa.
The modulus of 10% (w/v) heparin gel was 26.89±2.01 kPa (Fig. 2A). Therefore, 10% heparin hydrogel exhibited ∼2-fold higher stiffness and 20% heparin hydrogel showed ∼10-fold higher stiffness compared to 5% gel.
Indentation versus depth profiles for the three heparin gels with an overlay of the theoretical force–indentation curves, used to determine the elastic modulus of the substrates applying the Hertz model (Eq. 1), is illustrated in Figure 2B (soft), Figure 2C (medium), and Figure 2D (stiff), respectively. The appropriate indentation depth over which the fit was applied was determined from a plot of E versus δ (data not shown), where the gel behaved as an elastic solid.
Characterizing of heparin content in hydrogels of varying stiffness
As seen in Figure 3A, the stiff gel contained approximately four times more heparin than the soft gel. To clearly visualize the difference in the heparin content, we created gel microstructures of varying composition and stained with toluidine blue O.
As seen in Figure 3B–E, stiff heparin gel microstructures exhibited a much stronger toluidine blue O staining (darker purple color) compared to the soft one, indicating that a larger amount of heparin was present in the stiff heparin gel. On the other hand, the toluidine blue O staining of purple color was not observed in the gel comprising PEG-SH4 and PEG-DA serving as a negative control.
Cultivating primary rat hepatocytes on heparin gels of varying stiffness
Upon characterizing gel mechanical properties and heparin content, we proceeded to culture hepatocytes and monitor phenotype expression on gel surfaces to investigate the effect of mechanical stiffness on primary hepatocytes. Collagen-coated glass substrates (E ∼ 109 Pa) served as tissue culture control.
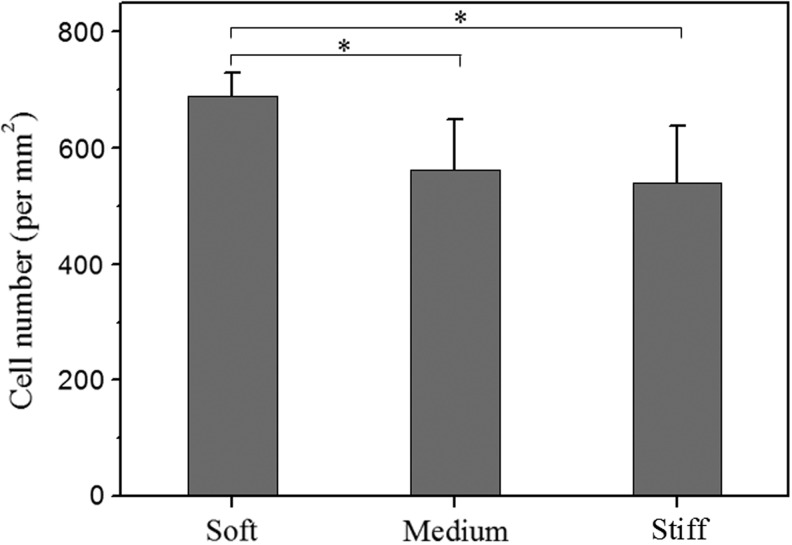
Most synthetic hydrogels such as PEG are known to prevent protein adsorption and cellular adhesion because of PEG-induced steric hindrance. In contrast, a hybrid natural/synthetic gel containing heparin proved to be an excellent matrix for incorporation of proteins and cells, including hepatocytes.34,35,44,45 As shown in Figure 4, cell density on soft and stiff heparin gels was 690 cells/mm2 and 540 cells/mm2, respectively, whereas on collagen-coated glass substrates it was 480 cells/mm2. Therefore, better attachment of hepatocytes was observed on softer substrates.
FIG. 4.
Quantification of cell adhesion on collagen-coated heparin gels of varying stiffness. Number of cells attached on collagen-coated glass control was 480/mm2. *p<0.05, n=4.
Figure 5A–D shows representative images of hepatocytes cultured on heparin gels of different mechanical properties and a glass substrate at day 1 and 4. As seen from these images, hepatocytes cultured on soft heparin gels retained a distinctive cuboidal shape and distinct cell–cell boundaries at day 4. Hepatocytes cultured on soft and medium stiffness heparin gels for 4 days displayed a projected surface area of 703±210 and 886±302 μm2/cell, respectively. On the other hand, hepatocytes on stiff heparin gels acquired a fibroblastic shape—more planar and extended morphology with less distinct nuclei. On glass substrates, hepatocytes spread out and became less cuboidal—a sign of dedifferentiation and loss of epithelial phenotypes.13,15,18 Hepatocytes cultured on stiff heparin gels and glass control exhibited projected surface areas of 1638±485 and 2092±707 μm2/cell.
FIG. 5.
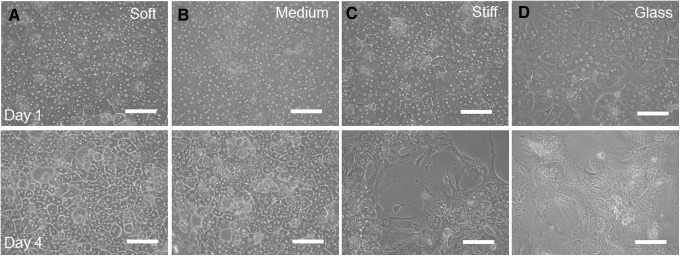
Morphology of primary rat hepatocytes on heparin gels of varying stiffness. Brightfield microscopic images of hepatocytes cultured on (A) soft, (B) middle, (C) stiff heparin gels, and (D) rigid glass substrate at day 1 and 4. Scale bar=100 μm.
Given that a normal liver has an elastic modulus (E) below 6000 Pa,20–23 the soft heparin gels may have more closely approximated the mechanical properties of the normal liver compared to stiffer heparin gels.
This may explain the improved cell morphology and hepatocyte function observed for cells cultured on soft heparin gels. As shown in Figure 6A–D, hepatocytes cultured on the softest heparin gels had significantly higher albumin compared to cells cultured on stiffer substrates.
FIG. 6.
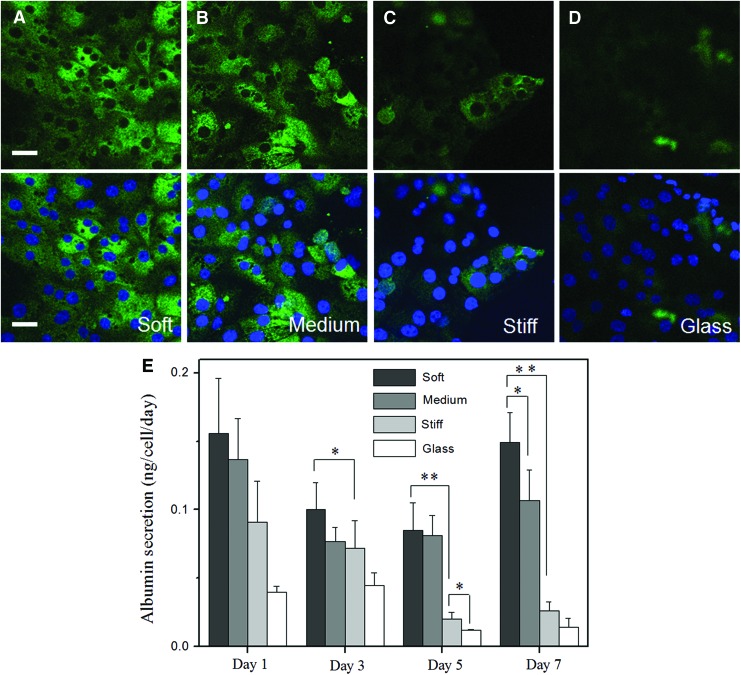
Hepatic albumin expression as a function of gel stiffness. (A–D) Intracellular albumin in hepatocytes cultured on heparin gels of varying stiffness at day 5 in culture. (A) 5% (soft, 11 kPa), (B) 10% (intermediate, 27 kPa), (C) 20% (stiff, 116 kPa) heparin gels, and (D) glass substrate. Green fluorescence is intracellular albumin, whereas blue fluorescence is DAPI staining of nuclei. Scale bar=100 μm. (E) ELISA analysis of albumin secretion by primary hepatocytes cultured on heparin gels of varying stiffness and glass substrate. Error bars indicate standard deviation of the mean for n=3 samples. *p<0.05, **p<0.0005. Color images available online at www.liebertpub.com/tea
Albumin ELISA results in Figure 6E corroborated immunofluorescent staining, with the highest level of albumin observed for hepatocytes cultured on the softest substrates. As seen from these data, albumin production by hepatocytes cultured on soft heparin gels was more than five times higher at day 7 compared to hepatocytes cultured on the stiffest heparin gels. The levels of albumin secretion of hepatocytes cultured on soft heparin gels were comparable to results (0.03–0.1 ng/cell/day) reported for hepatocytes cultivated in a collagen gel sandwich, which is one of the standards for maintenance of functional hepatocytes.15,46,47 It is important to note that synthesis of this hepatic biomarker was dependent on the stiffness of the underlying heparin gel.
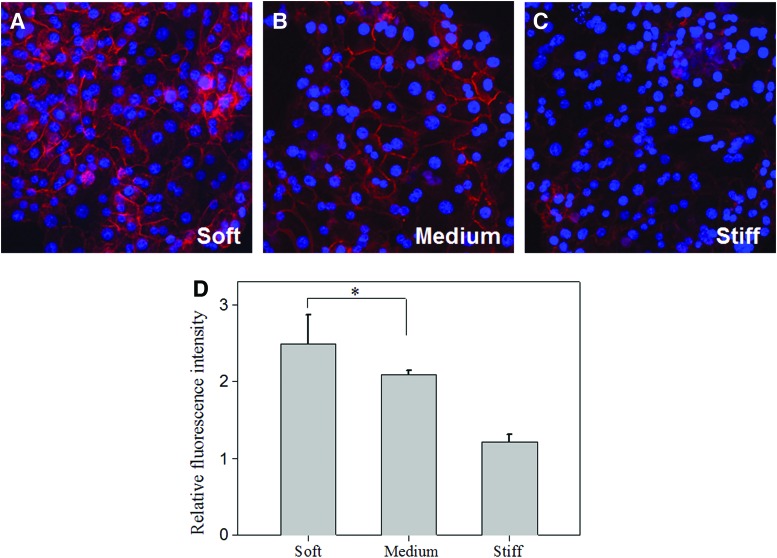
To further analyze hepatic function on heparin gels, we investigated the expression of E-cadherin—an epithelial marker expressed by differentiated hepatocytes. As shown in Figure 7, E-cadherin expression was higher on soft (5%) heparin gels compared to stiffer gels. In addition, this protein was expressed at cell–cell boundaries on soft gels, whereas on stiffer substrates, it appeared in the cytoplasm. Quantitation of fluorescence intensity (Fig. 7D) confirmed significant differences in E-cadherin expression as a function of gel stiffness. Overall, analysis of albumin synthesis and E-cadherin expression clearly indicated that the hepatic phenotype was maintained better on the soft heparin gels. This is despite the fact that the heparin content of soft gels was four times lower compared with stiff gels. Future studies will attempt to decouple the heparin content and substrate compliance to examine relative contributions of these factors to hepatic phenotype expression and to optimize substrates for maintenance of functional hepatocytes.
FIG. 7.
Expression of E-cadherin in hepatocytes cultured on heparin gels. Confocal images of E-cadherin of hepatocytes cultured on (A) soft (11 kPa), (B) medium (27 kPa), and (C) stiff (116 kPa) heparin gels after 7 days of culture. Red color was E-cadherin staining and blue color was DAPI staining of nuclei. (D) Expression of E-cadherin at cell–cell contacts was quantified by measuring fluorescence intensity on immunofluorescence images. *p<0.05, n=5. Color images available online at www.liebertpub.com/tea
Conclusion
In this study, we explored the function of primary hepatocytes cultured on heparin hydrogels of varying stiffness. Our experiments documented that hepatocytes expressed higher levels of liver markers, albumin and E-cadherin, on the softer heparin gel. Our findings pointing to the importance of substrate mechanical properties on hepatocyte function inform the development of improved scaffolds for hepatocyte culture and stem cell differentiation.
Acknowledgments
We would like to thank Profs. Marcu and Louie for use of their microscopy facilities. Financial support for this work was provided by the NIH grants DK073901 (awarded to A.R.) and EY01613404 (awarded to C.J.M.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Kumachev A. Greener J. Tumarkin E. Eiser E. Zandstra P.W. Kumacheva E. High-throughput generation of hydrogel microbeads with varying elasticity for cell encapsulation. Biomaterials. 2011;32:1477. doi: 10.1016/j.biomaterials.2010.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Lanniel M. Huq E. Allen S. Buttery L. Williams P.M. Alexander M.R. Substrate induced differentiation of human mesenchymal stem cells on hydrogels with modified surface chemistry and controlled modulus. Soft Matter. 2011;7:6501. [Google Scholar]
- 3.Trappmann B. Gautrot J.E. Connelly J.T. Strange D.G.T. Li Y. Oyen M.L., et al. Extracellular-matrix tethering regulates stem-cell fate. Nat Mater. 2012;11:642. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 4.Wang L.S. Boulaire J. Chan P.P. Chung J.E. Kurisawa M. The role of stiffness of gelatin-hydroxyphenylpropionic acid hydrogels formed by enzyme-mediated crosslinking on the differentiation of human mesenchymal stem cell. Biomaterials. 2010;31:8608. doi: 10.1016/j.biomaterials.2010.07.075. [DOI] [PubMed] [Google Scholar]
- 5.Liliensiek S.J. Wood J.A. Yong J. Auerbach R. Nealey P.F. Murphy C.J. Modulation of human vascular endothelial cell behaviors by nanotopographic cues. Biomaterials. 2010;31:5418. doi: 10.1016/j.biomaterials.2010.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartalena G. Loosli Y. Zambelli T. Snedeker J.G. Biomaterial surface modifications can dominate cell-substrate mechanics: the impact of PDMS plasma treatment on a quantitative assay of cell stiffness. Soft Matter. 2012;8:673. [Google Scholar]
- 7.Rehfeldt F. Brown A.E. Raab M. Cai S. Zajac A.L. Zemel A., et al. Hyaluronic acid matrices show matrix stiffness in 2D and 3D dictates cytoskeletal order and myosin-II phosphorylation within stem cells. Integr Biol: quantitative biosciences from nano to macro. 2012;4:422. doi: 10.1039/c2ib00150k. [DOI] [PubMed] [Google Scholar]
- 8.Shi X. Qin L. Zhang X. He K. Xiong C. Fang J., et al. Elasticity of cardiac cells on the polymer substrates with different stiffness: an atomic force microscopy study. Physical chemistry chemical physics: PCCP. 2011;13:7540. doi: 10.1039/c1cp20154a. [DOI] [PubMed] [Google Scholar]
- 9.Discher D.E. Mooney D.J. Zandstra P.W. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ni Y. Chiang M.Y.M. Cell morphology and migration linked to substrate rigidity. Soft Matter. 2007;3:1285. doi: 10.1039/b703376a. [DOI] [PubMed] [Google Scholar]
- 11.Cortese B. Gigli G. Riehle M. Mechanical gradient cues for guided cell motility and control of cell behavior on uniform substrates. Adv Funct Mater. 2009;19:2961. [Google Scholar]
- 12.Hewitt N.J. Lechon M.J.G. Houston J.B. Hallifax D. Brown H.S. Maurel P., et al. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab Rev. 2007;39:159. doi: 10.1080/03602530601093489. [DOI] [PubMed] [Google Scholar]
- 13.Moghe P.V. Berthiaume F. Ezzell R.M. Toner M. Tompkins R.G. Yarmush M.L. Culture matrix configuration and composition in the maintenance of hepatocyte polarity and function. Biomaterials. 1996;17:373. doi: 10.1016/0142-9612(96)85576-1. [DOI] [PubMed] [Google Scholar]
- 14.Dunn J.C.Y. Tompkins R.G. Yarmush M.L. Hepatocytes in collagen sandwich: evidence for transcriptional and translational regulation. J Cell Biol. 1992;4:1043. doi: 10.1083/jcb.116.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunn J.C.Y. Yarmush M.L. Koebe H.G. Tompkins R.G. Hepatocyte function and extracellular matrix geometry: long-term culture in a sandwich configuration. FASEB J. 1989;3:174. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 16.Hansen L.K. Wilhelm J. Fassett J.T. Regulation of hepatocyte cell cycle progression and differentiation by type I collagen structure. Curr Top Dev Biol. 2006;72:205. doi: 10.1016/S0070-2153(05)72004-4. [DOI] [PubMed] [Google Scholar]
- 17.Fassett J. Tobolt D. Hansen L.K. Type I collagen structure regulates cell morphology and EGF signaling in primary rat hepatocytes through cAMP-dependent protein kinase A. Molecular biology of the cell. 2006;17:345. doi: 10.1091/mbc.E05-09-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godoy P. Hengstler J.G. Ilkavets I. Meyer C. Bachmann A. Muller A., et al. Extracellular matrix modulates sensitivity of hepatocytes to fibroblastoid dedifferentiation and transforming growth factor beta-induced apoptosis. Hepatology. 2009;49:2031. doi: 10.1002/hep.22880. [DOI] [PubMed] [Google Scholar]
- 19.Wells R.G. The role of matrix stiffness in regulating cell behavior. Hepatology. 2008;47:1394. doi: 10.1002/hep.22193. [DOI] [PubMed] [Google Scholar]
- 20.Mederacke I. Wursthorn K. Kirschner J. Rifai K. Manns M.P. Wedemeyer H., et al. Food intake increases liver stiffness in patients with chronic or resolved hepatitis C virus infection. Liver Int. 2009;29:1500. doi: 10.1111/j.1478-3231.2009.02100.x. [DOI] [PubMed] [Google Scholar]
- 21.Georges P.C. Hui J.J. Gombos Z. McCormick M.E. Wang A.Y. Uemura M., et al. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1147. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 22.Rouviere O. Yin M. Dresner M.A. Rossman P.J. Burgart L.J. Fidler J.L., et al. MR elastography of the liver: preliminary results. Radiology. 2006;240:440. doi: 10.1148/radiol.2402050606. [DOI] [PubMed] [Google Scholar]
- 23.Bastard C. Bosisio M.R. Chabert M. Kalopissis A.D. Mahrouf-Yorgov M. Gilgenkrantz H., et al. Transient micro-elastography: a novel non-invasive approach to measure liver stiffness in mice. World J Gastroenterol. 2011;17:968. doi: 10.3748/wjg.v17.i8.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen A.A. Khetani S.R. Lee S. Bhatia S.N. Van Vliet K.J. Modulation of hepatocyte phenotype in vitro via chemomechanical tuning of polyelectrolyte multilayers. Biomaterials. 2009;30:1113. doi: 10.1016/j.biomaterials.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Semler E.J. Lancin P.A. Dasgupta A. Moghe P.V. Engineering hepatocellular morphogenesis and function via ligand-presenting hydrogels with graded mechanical compliance. Biotechnol Bioeng. 2005;89:296. doi: 10.1002/bit.20328. [DOI] [PubMed] [Google Scholar]
- 26.Lutolf M.P. Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 27.Wang X.Z. Wang H.L. Brown H.R. Jellyfish gel and its hybrid hydrogels with high mechanical strength. Soft Matter. 2011;7:211. [Google Scholar]
- 28.DeForest C.A. Anseth K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat Chem. 2011;3:925. doi: 10.1038/nchem.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeForest C.A. Anseth K.S. Photoreversible patterning of biomolecules within click-based hydrogels. Angew Chem Int Edit. 2012;51:816. doi: 10.1002/anie.201106463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsurkan M.V. Levental K.R. Freudenberg U. Werner C. Enzymatically degradable heparin-polyethylene glycol gels with controlled mechanical properties. Chem Commun. 2010;46:1141. doi: 10.1039/b921616b. [DOI] [PubMed] [Google Scholar]
- 31.Tae G. Kim Y.J. Choi W.I. Kim M. Stayton P.S. Hoffman A.S. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 32.Kim M. Lee J.Y. Shah S.S. Tae G. Revzin A. On-cue detachment of hydrogels and cells from optically transparent electrodes. Chem Commun. 2009;39:5865. doi: 10.1039/b909169f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tae G. Scatena M. Stayton P.S. Hoffman A.S. PEG-cross-linked heparin is an affinity hydrogel for sustained release of vascular endothelial growth factor. J Biomat Sci Polym Ed. 2006;17:187. doi: 10.1163/156856206774879090. [DOI] [PubMed] [Google Scholar]
- 34.Kim M. Lee J.Y. Jones C.N. Revzin A. Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah S.S. Kim M. Cahill-Thompson K. Tae G. Revzin A. Micropatterning of bioactive heparin-based hydrogels. Soft Matter. 2011;7:3133. [Google Scholar]
- 36.Gosey L.L. Howard R.M. Witebsky F.G. Ognibene F.P. Wu T.C. Gill V.J., et al. Advantages of a modified toluidine blue-O stain and bronchoalveolar lavage for the diagnosis of pneumocystis-carinii pneumonia. J Clin Microbiol. 1985;22:803. doi: 10.1128/jcm.22.5.803-807.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi N. Kiick K.L. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dunn J.C.Y. Yarmush M.L. Koebe H.G. Tompkins R.G. Hepatocyte function and extracellular-matrix geometry—long-term culture in a sandwich configuration. FASEB J. 1989;3:174. doi: 10.1096/fasebj.3.2.2914628. [DOI] [PubMed] [Google Scholar]
- 39.Jones C.N. Tuleuova N. Lee J.Y. Ramanculov E. Reddi A.H. Zern M.A. Revzin A. Cultivating hepatocytes on printed arrays of HGF and BMP7 to characterize protective effects of these growth factors during in vitro alcohol injury. Biomaterials. 2010;23:5936. doi: 10.1016/j.biomaterials.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones C.N. Tuleuova N. Lee J.Y. Ramanculov E. Reddi A.H. Zern M.A. Revzin A. Cultivating liver cells on printed arrays of hepatocyte growth factor. Biomaterials. 2009;30:3733. doi: 10.1016/j.biomaterials.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 41.Love A.E.H. Boussinesq's problem for a rigid cone. Q J Math. 1939;10:161. [Google Scholar]
- 42.Harding J.W. Sneddon I.N. The elastic stresses produced by the indentation of the plane surface of a semi-infinite elastic solid by a rigid punch. Math Proc Cambridge Philos Soc. 1945;41:16. [Google Scholar]
- 43.McKee C.T. Raghunathan V.K. Russell P. Murphy C.J. Probing effect of biophysical cues on cell behavior with atomic force microscopy. Microsc Anal. 2011;25:25. [Google Scholar]
- 44.Kim M. Shin Y. Hong B.H. Kim Y.J. Chun J.S. Tae G., et al. In vitro chondrocyte culture in a heparin-based hydrogel for cartilage regeneration. Tissue Eng Part C Methods. 2010;16:1. doi: 10.1089/ten.TEC.2008.0548. [DOI] [PubMed] [Google Scholar]
- 45.Kim M. Kim Y.-J. Gwon K. Tae G. Modulation of cell adhesion of heparin-based hydrogel by efficient physisorption of adhesive proteins. Macromol Res. 2012;20:271. [Google Scholar]
- 46.Wang S. Nagrath D. Chen P.C. Berthiaume F. Yarmush M.L. Three-dimensional primary hepatocyte culture in synthetic self-assembling peptide hydrogel. Tissue Eng Part A. 2008;14:227. doi: 10.1089/tea.2007.0143. [DOI] [PubMed] [Google Scholar]
- 47.Kidambi S. Sheng L. Yarmush M.L. Toner M. Lee I. Chan C. Patterned co-culture of primary hepatocytes and fibroblasts using polyelectrolyte multilayer templates. Macromol Biosci. 2007;7:344. doi: 10.1002/mabi.200600205. [DOI] [PubMed] [Google Scholar]