Abstract
The ovarian follicle (each contains a single oocyte) is the fundamental functional tissue unit of mammalian ovaries. In humans, it has been long held true that females are born with a maximum number of follicles (or oocytes) that are not only nonrenewable, but also undergoing degeneration with time with a sharply decreased oocyte quality after the age of ∼35. Therefore, it is of importance to isolate and bank ovarian follicles for in vitro culture to obtain fertilizable oocytes later, to preserve the fertility of professional women who may want to delay childbearing, young and unmarried women who may lose gonadal function because of exposure to environmental/occupational hazards or aggressive medical treatments, such as radiation and chemotherapy, and even endangered species and breeds. Although they contributed significantly to the understanding of follicle science and biology, most studies reported to date on this topic were done using the man-made, unnatural inbred animal species. It was found in this study that the conventional two-dimensional microliter drop and three-dimensional hanging drop (HD) methods, reported to be effective for in vitro culture of preantral follicles from inbred mice, are not directly transferrable to outbred deer mice. Therefore, a modified HD method was developed in this study to achieve a much higher (>5 times compared to the best conventional methods) percentage of developing early secondary preantral follicles from the outbred mice to the antral stage, for which, the use of an ovarian cell-conditioned medium and multiple follicles per HD were identified to be crucial. It was further found that the method for in vitro maturation of oocytes in antral follicles obtained by in vitro culture of preantral follicles could be very different from that for oocytes in antral follicles obtained by hormone stimulation in vivo. Therefore, this study should provide important guidance for establishing effective protocols of in vitro follicle culture to preserve the fertility of wildlife and humans outbred by nature.
Introduction
The ovarian follicle (each contains a single oocyte) is the fundamental functional tissue unit of mammalian ovaries. In humans, it has been long held true that females are born with a maximum number (∼1 million) of follicles or oocytes that are not only nonrenewable, but also undergoing degeneration with time1–5: less than ∼30% of the oocytes can survive to puberty and the percentage continues to decline throughout adulthood to the point of extinction at the age of ∼50 with the oocyte quality being sharply decreased after the age of ∼35. Therefore, banking (e.g., by cryopreservation) ovarian tissue containing healthy follicles has been proposed to preserve the future fertility of professional women who may want to delay childbearing, young and unmarried women who may lose gonadal function because of exposure to environmental/occupational hazards or aggressive medical treatments, such as radiation and chemotherapy, and even endangered species and breeds.6–11 Although orthotopic transplantation of the banked ovarian tissue has been shown to produce offspring in both mice and humans, it also contains the risk of reintroducing blood-borne (in ovarian tissue) malignant cells into the patients with cancer.12–15
An alternative way that is attracting more and more attention is to isolate preantral follicles from fresh or banked ovarian tissue for in vitro culture to obtain mature oocytes (the various stages of follicle and oocyte development are explained in Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea).16–20 The oocytes can be further banked and fertilized at the desired time for embryo transfer, a procedure that has been done routinely at clinical facilities for assisted reproduction for decades.21–25 Several methods have been developed for in vitro culture of ovarian follicles.26 For example, preantral follicles have been plated in a small drop of medium immersed in biocompatible oil on the two-dimensional (2D) flat surface of a culture dish.27 Preantral follicles have also been encapsulated in three-dimensional (3D) hydrogels of alginate or an interpenetrating network of alginate and fibrin.28–33 The latter has been shown to be a better system compared to pure alginate because the degradation of fibrin with time allows the expansion in volume of the growing preantral follicles during culture with minimal compression,30–33 which better mimics the physiologic condition in vivo, where the developing preantral follicles move from the more rigid peripheral cortical region to the softer inner cortical region adjacent to the ovarian medulla with profuse blood supply.34,35 Inspired by studies culturing embryonic stem cells to form an embryoid body, a recent study reported in vitro culture of preantral follicles from inbred mice in hanging drop (HD) and found it being better than the conventional 2D and even the 3D alginate hydrogel systems.36 This could be attributed partially to the nearly zero compressive stress on the growing follicles in the fluidic HD of the culture medium.
Moreover, the results from the past studies demonstrate the potential of in vitro preantral follicle culture for preserving the fertility of women as live birth of offspring has been reported using this approach for inbred laboratory mice.37–40 However, such success has been limited to inbred mice and further research needs to be done using other biomedical model systems, such as outbred animals, including outbred mice and nonhuman primates, so that the technology can be further optimized to eventually be applicable to human fertility preservation.19,33,41–49 This is because the widely used inbred animals for biomedical research are unnatural (or man-made) with their haplotype structure and genetic make-up not resembling that of humans or even wild mice.50–55
In this study, we reported for the first time, the isolation and further in vitro culture of early secondary preantral follicles from the genera Peromyscus (also called deer mice because their fur color resembles that of deer), outbred mice that are indigenous rodents in North America and have been proposed as a more appropriate animal model than inbred species for research on phylogeography, speciation, chromosomes, population genetics, aging, and evolution.53,56–60 It was found that the existing 2D microliter drop (MD) and 3D HD culture approaches developed for inbred mice are not directly transferrable to the outbred mice. Moreover, the use of the ovarian cell-conditioned medium (CM) was identified to be crucial in improving the outcome of in vitro culturing of the early secondary preantral follicles from deer mice using the 3D HD method. Therefore, this study should provide important guidance for establishing effective in vitro follicle culture protocols to preserve the fertility of wildlife and humans outbred by nature.
Materials and Methods
Animals and materials
Peromyscus maniculatus bairdii (BW stock) deer mice were purchased from the Peromyscus Genetic Stock Center at the University of South Carolina, Columbia, South Carolina and were maintained in a 16–8-h light–dark cycle. All procedures for animal use were approved by the Institutional Animal Care and Use Committee (IACUC) at the Ohio State University, and every effort was made to minimize animal suffering. Leibovitz L-15 and α-MEM–glutamax medium and fetal bovine serum (FBS) were purchased from Invitrogen and Hyclone, respectively. Unless otherwise specifically noted, all other chemicals were purchased from Sigma.
Isolation of preantral follicles
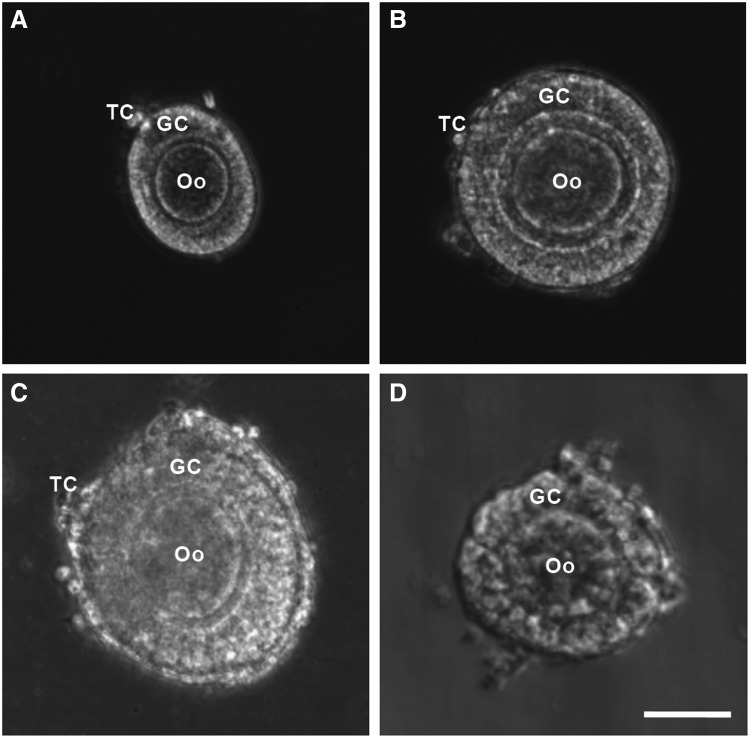
Preantral follicles were isolated using both mechanical and enzymatic methods from ovaries of female deer mice of 12–14 weeks old after administering PMSG and hCG (all at 5 IU as detailed elsewhere61) for superovulation. For the mechanical isolation of preantral follicles, the ovaries were placed in a 2 mL Leibovitz L-15 medium supplemented with 10% (v/v) heat-inactivated FBS and 1% (v/v) penicillin–streptomycin at 37°C in 5% CO2 air. Preantral follicles were retrieved by using two 30G needles to mechanically break the extracellular matrix between follicles in the ovarian tissue. For enzymatic retrieval, the collected ovaries were placed in the α-MEM–glutamax medium supplemented with 0.1% (v/w) type I collagenase (198 U/mg) and 0.03% (v/v) FBS for 1 h at 37°C in 5% CO2 air. To facilitate proteolytic digestion, the ovaries were mixed with the solution every 30 min by gentle pipetting. For both the methods, preantral follicles of various types (and sizes in diameter) were obtained, including primary (75–99 μm, Fig. 1A), early secondary preantral (100–125 μm, Fig. 1B), and late secondary preantral (126–180 μm, Fig. 1C) follicles.62 However, as will be shown in the Results and Discussion section, the early secondary preantral follicles dominated in the total number of follicles isolated using both the methods. Only the early secondary preantral follicles will be used for further investigations on in vitro culture of preantral follicles in this study.
FIG. 1.
Typical morphology of primary (75–99 μm, A), early secondary preantral (100–125 μm, B), and late secondary preantral (126–180 μm, C) follicles retrieved from ovaries of deer mice using the mechanical method together with that of early secondary preantral follicle (D) obtained using the enzymatic method: the follicles retrieved by the mechanical method have an intact outer membrane of TC and an intact layer of GC in the middle together with a primary Oo in the center. In contrast, the middle and particularly, the outer layer of the follicles retrieved by the enzymatic method were severely compromised. Scale bar: 50 μm. TC, theca cells; GC, granulosa cells; Oo, oocyte.
Preparation of ovarian cell-CM
To prepare ovarian cell-CM, the ovarian cells were first isolated by following a protocol reported elsewhere.63 Briefly, the ovaries of 12-week-old deer mice were collected and chopped after the removal of adherent tissues, such as fat pad. The specimens were incubated initially for 30 min in a dissociation medium consisting of a 50:50 (v:v) mixture of 0.25% (v/w) trypsin–ethylenediaminetetraacetic acid (EDTA) and Dulbecco's modified Eagle's medium (DMEM) supplemented with 750 units/mL type I collagenase and 0.03% (v/v) FBS at 37°C in 5% CO2 air. The dissociated cells were filtered through a 40-μm filter and subsequently centrifuged at 390g for 4 min. The collected cells were further cultured onto a 60-mm culture dish in 5 mL of the DMEM supplemented with 10% FBS and 1% penicillin–streptomycin. After 20 h of culture in the dish, the DMEM-based medium was removed and the cells were washed once using 1 × phosphate-buffered saline (PBS). A total of 5 mL (nonconditioned) of the α-MEM–glutamax medium supplemented with 10% (v/v) heat-inactivated FBS and a 1% (v/v) penicillin–streptomycin solution was then added into the dish. The cells were incubated with the nonconditioned medium at 37°C in 5% CO2 air for 2 days, and the resultant CM was collected and the procedure was repeated once to eventually make a total of 10 mL CM, which was supplemented with 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, and 100 mIU/mL recombinant human follicle-stimulating hormone (FSH) for further use.
In vitro culture of early secondary preantral follicles
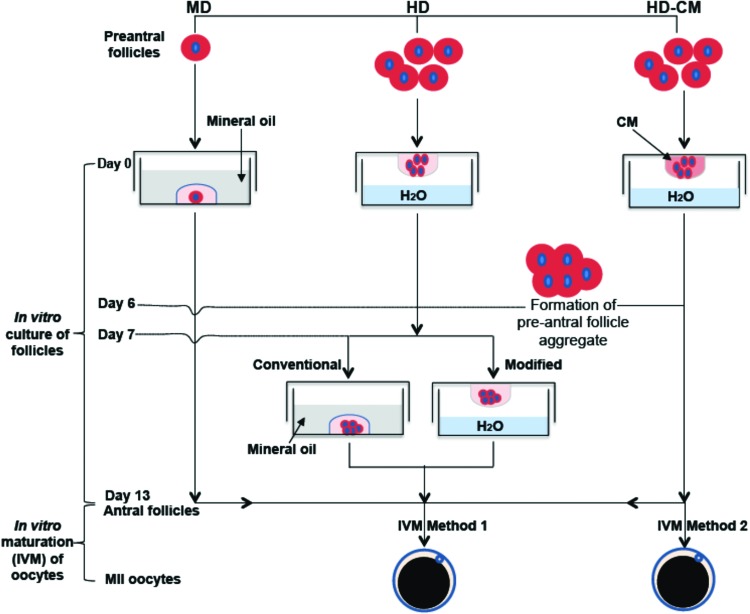
As illustrated in Figure 2, three different methods, including MD and HD of the nonconditioned medium and HD of ovarian cell-CM (HD-CM), were used to culture the early secondary preantral follicles in vitro. For the 2D MD method,27 the early secondary preantral follicles isolated mechanically or enzymatically were placed singly in 10 μL of drops of the nonconditioned culture medium overlaid with mineral oil in 60-mm culture dishes on day 0. The nonconditioned culture medium used was the α-MEM–glutamax medium, to which 1% (v/v) heat-inactivated FBS, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 100 mIU/mL recombinant human FSH, and 1% (v/v) penicillin and streptomycin were supplemented. On the following day, 10 μL of fresh medium was added to each drop, and starting from day 3, half the medium (10 μL) was replaced with the fresh medium every other day till day 13.
FIG. 2.
A schematic illustration of the various methods used for in vitro culture of the early secondary preantral follicles, including MD, HD with nonconditioned medium, and HD-CM: the early secondary preantral follicles were first cultured in vitro for 13 days to obtain antral follicles using the various methods, followed by IVM of oocytes in the antral follicles using two different methods detailed in the Materials and Methods section. MD, microliter drop; HD, hanging drop; HD-CM, HD of ovarian cell-conditioned medium; IVM, in vitro maturation. Color images available online at www.liebertpub.com/tea
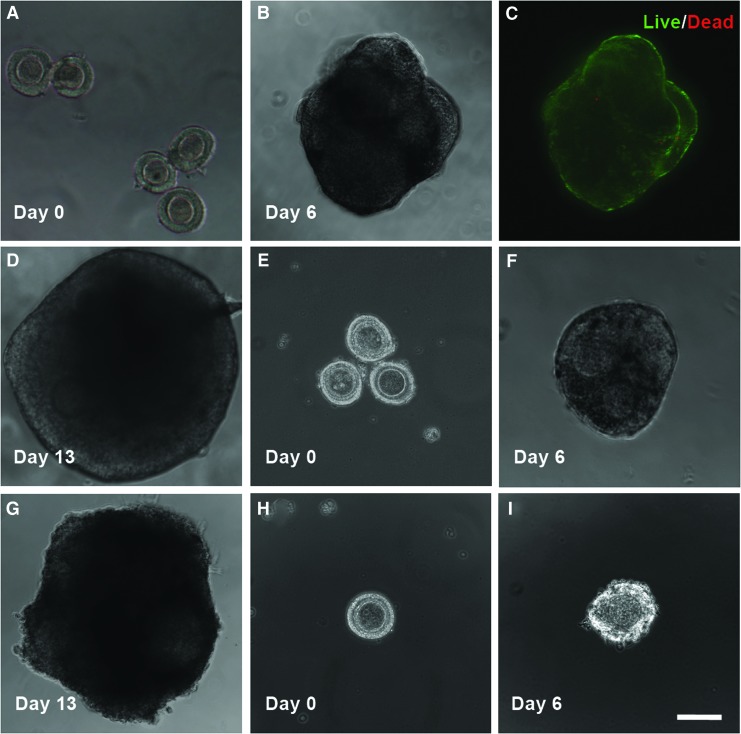
For the HD method, five mechanically isolated early secondary preantral follicles were placed in 25 μL HD of the nonconditioned culture medium and incubated at 37°C in 5% CO2 air on day 0. The five follicles were observed to form a single follicle aggregate on day 6 in each HD. Starting from day 7, the follicle aggregates were further cultured in both the conventional and a modified way (Fig. 2). The former is the same as that reported in the literature for inbred mice by transferring the aggregates into 30 μL drops (one aggregate per drop) of the nonconditioned culture medium covered with mineral oil.36 The modified method consisted of further culturing the aggregates in 30 μL HDs of the nonconditioned culture medium (also, one aggregate per HD) with half of the medium (15 μL) in each drop being replaced with the fresh medium every other day till day 13.
For the method using HD-CM, the procedure was the same as the HD method with the follicles being continuously cultured in HD from day 0 to 13 except that (1) ovarian cell-CM was used in the HD and (2) the number of early secondary preantral follicles in each drop on day 0 could be 5, 3, or 1. The latter is for determining if it is important to use multiple follicles in each HD for better culturing and developing the follicles with the HD-CM method. To test the viability of cells in the aggregate of five preantral follicles formed on day 6, the aggregate was incubated in the culture medium with 5 μM calcein AM and 5 μM ethidium homodimer (Live/Dead assay kit from Invitrogen) for 10 min at 37°C in 5% CO2 air. Apotome structured illumination microscopy (SIM, confocal-like64,65) fluorescence images were taken using a Zeiss Axio Observer.Z1 microscope to monitor cell viability inside the aggregate.
In vitro maturation of oocytes in single and aggregated antral follicles
As illustrated in Figure 2, two different methods were used to induce final maturation of oocytes in single and aggregated antral follicles. For in vitro maturation (IVM) method 1,61 antral follicles (or their aggregates) obtained on day 13 were incubated for 18 h in the α-MEM–glutamax medium (containing ribonucleoside and deoxyribonucleoside) supplemented with 1% (v/v) heat-inactivated FBS, 5 μg/mL insulin, 5 μg/mL transferrin, 5 ng/mL selenium, 2.5 IU/mL hCG, and 5 ng/mL epidermal growth factor (EGF) at 37°C in 5% CO2 air.
For the IVM method 2,66 the aggregated antral follicles on day 13 were incubated for 48 h in 500 μL of the minimum essential medium-α containing Earle's salts and supplemented with 10 mg/mL streptomycin sulfate, 75 mg/mL penicillin G, and 5% (v/v) heat-inactivated FBS covered with 250 μL of mineral oil in a 4-well plate at 37°C in 5% CO2 air.
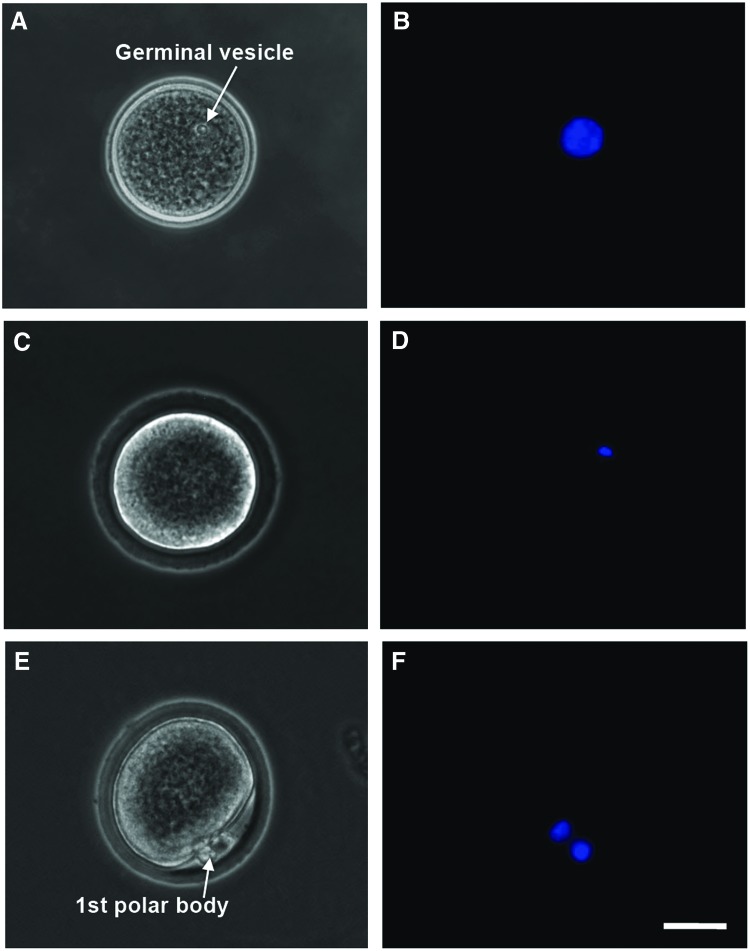
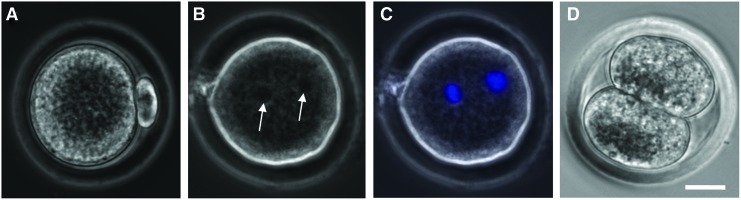
After maturation, the cumulus–oocyte complex (COC) in each antral follicle was isolated mechanically by follicular puncture as we did before.61 Retrieved oocytes were freed from cumulus cells by incubating in hyaluronidase (200 IU/mL) for 2 min. Oocytes at the germinal vesicle (GV) stage were judged by the presence of a clear GV (Fig. 3A, B) observed under a phase-contrast microscope; GV breakdown (GVBD) oocytes were judged by the disappearance of the clear GV (Fig. 3C, D); and metaphase II (MII) oocytes were judged by the disappearance of the clear GV and the appearance of a characteristic first polar body (Fig. 3E, F). A more detailed illustration of the anatomical structure, including the meiotic spindle of microtubules in MII oocytes, is given in Supplementary Figure S2. Nuclei of oocytes at the various stages were visualized by staining with Hoechst 33342 (1 μg/mL in 1×PBS) for 5 min at 37°C in 5% CO2 air and observing under a fluorescence microscope.
FIG. 3.
Typical phase-contrast and fluorescence images of primary (A–D) and secondary (E–F) oocytes showing their morphology and nuclei distribution, respectively: the primary oocyte includes both GV (A, B) and GVBD (C, D) oocyte, while the MII oocyte shown is secondary. A GV containing all nuclear materials can be clearly seen in the GV oocyte (A, B), the GVBD rendering the nuclear stain not as clearly identifiable in GVBD oocyte (C, D), and the nuclear materials separate and condense at two different locations with one being in the first polar body and the other in the cytoplasm of the MII oocyte (E, F). Scale bars: 25 μm. GV, germinal vesicle; GVBD, GV breakdown; MII, metaphase II. Color images available online at www.liebertpub.com/tea
In vitro fertilization and embryo culture
To obtain sperm for in vitro fertilization (IVF) of MII oocytes, male deer mice of 12–14 weeks old were euthanized by cervical dislocation and epididymides were collected by dissection. They were then placed in the center of an IVF dish with 1 mL of the TYH medium, a modified Krebs-Ringer bicarbonate solution containing 5.56 mM glucose, 1.0 mM sodium pyruvate, 4 mg/mL bovine serum albumin (BSA), and antibiotics. After making five to seven longitudinal cuts using syringe needle on each epididymis, the epididymides were incubated for 20 min at 37°C in 5% CO2 air to allow for sperm dispersion. The sperm suspensions were incubated for 2 h at 37°C in 5% CO2 air for capacitation. For IVF, four MII oocytes obtained by culturing the early secondary preantral follicles using the HD-CM method were inseminated with 1.6×104 sperms in a 160-μL drop of the TYH medium for 4.5 h. The fertilized oocytes were subsequently cultured in a drop of 50 μL of the Hoppe and Pitts medium modified by removing sodium lactate and increasing the sodium chloride concentration to 5.97 g/mL at 37°C in 5% CO2 air. The development of the fertilized oocytes was monitored using the phase-contrast and fluorescence microscope for the formation of two pronuclei and division into two cells. For the fluorescence microscopy, the two pronuclei in the fertilized oocyte were stained by incubating the cell with Hoechst 33342 (1 μg/mL in 1×PBS) for 5 min at 37°C in 5% CO2 air.
Statistical analysis
All data are presented as mean±standard deviation. A generalized linear model (PROC-GLM) and one-way ANOVA in Statistical Analysis System (SAS) program were employed for statistical analysis to determine the p-value between various treatments. The difference was taken as significant when the p-value was less than 0.05.
Results and Discussion
Isolation of primary and preantral follicles: mechanical versus enzymatic methods
Typical morphology of the primary, early secondary preantral, and late secondary preantral follicles isolated using the mechanical method is shown in Figure 1A–C, respectively. Although they all consist of theca cells in a thin outer layer, granulosa cells in the middle, and a follicular oocyte in the center, they are different in terms of size. This difference is a result of the proliferation of granulosa cells in the middle as follicles grow. Unlike the mechanical method for which the isolated follicles at all stages could retain their intact morphology, the architecture of all follicles isolated using the enzymatic method was compromised as shown in Figure 1D for an early secondary preantral follicle isolated using the enzymatic method. Similar observations of the breakdown of basement membranes, detachment of granulosa cells, and compromised morphology of the follicular oocytes in enzymatically isolated follicles have been reported elsewhere.41,67,68 This is not surprising because the preantral follicle is a functional tissue unit with the extracellular matrix and basement membrane, which are mainly made of collagen (types I and IV).34 They are certainly vulnerable to the collagenase used for the enzymatic method to digest the interfollicular matrix of collagen I in ovarian tissue.
The number of preantral follicles in total and at the three different stages retrieved per animal (or per ovary) is given in Table 1. Although the enzymatic method can be used to retrieve significantly more follicles at all the three different stages than the mechanical method (p=0.0385, 0.0051, 0.0208, and 0.0009 for primary, early secondary preantral, late secondary preantral follicles, and total follicles retrieved, respectively), the follicles were severely damaged and underwent degeneration during further in vitro culture (to be discussed more later). Moreover, the number of early secondary preantral follicles dominated that of the primary and late secondary preantral follicles for both the methods. In this study, only the early secondary preantral follicles were used for further investigations of in vitro follicle culture.
Table 1.
A Summary of the Number of Total, Primary, Early Secondary Preantral, and Late Secondary Preantral Follicles Retrieved Per Animal by Either the Mechanical or Enzymatic Method from Ovaries of 12–14-Week-old Deer Mice (n=7)
| |
|
Follicles retrieved by type |
||
|---|---|---|---|---|
| Isolation method | Total no. of follicles retrieved | Primary | Early secondary preantral | Late secondary preantral |
| Mechanical | 40.2±8.6 | 6.0±2.6 | 29.7±6.4 | 4.6±1.6 |
| Enzymatic | 57.0±5.3 | 9.0±2.2 | 40.4±5.3 | 7.6±2.5 |
In vitro follicle culture: the conventional MD method
We first cultured the early secondary preantral follicles in vitro using the conventional MD method by plating the follicles in drops (one follicle per drop) of the nonconditioned medium immersed in mineral oil on the 2D surface of the culture dish. As shown in Table 2, all the 101 early secondary preantral follicles isolated using the enzymatic method degenerated by day 6 (Supplementary Fig. S3A) and they did not develop to the antral stage. As a result, no viable oocyte was obtained. For the mechanically isolated early secondary preantral follicles, a total of 15 of the 188 (8%) follicles developed to the antral stage on day 13, although most follicles together with their oocytes were degenerated (Supplementary Fig. S3B). Figure 4 presents typical images of an early secondary preantral follicle isolated using the mechanical method at day 0 (Fig. 4A), at the diffused stage (Fig. 4B), on day 6, with theca and granulosa cells being proliferated, differentiated, and nonphysiologically diffused away from the follicles, and at the antral stage (Fig. 4C), on day 13, with a characteristic antral cavity (or space) of fluid. In addition, shown in the figure is the COC (Fig. 4D) released after in-follicle maturation of oocyte using the IVM method 1. After removing the cumulus cells with hyaluronidase, a total of 12 (6.4%), 6 (3.2%), and 5 (2.7%) of the 188 follicular oocytes were observed to develop to the GV, GVBD, and MII stage, respectively. These results indicate that the mechanical method is better than the enzymatic approach for isolating the early secondary preantral follicles from the outbred deer mice ovary. However, the percentage (2.7%) of the follicular oocytes eventually developed to MII stage is very low, which could be a result of the nonphysiologic 2D culture condition that caused the degeneration of most of the follicles. In addition, the reduced oxygen transport to the follicles due to the oil barrier overlaying on the MD could contribute to the degeneration of follicles. Therefore, further studies were performed to culture the early secondary preantral follicles isolated using the mechanical method under the 3D culture condition of HD in vitro without the use of oil.
Table 2.
Development of Early Secondary Preantral Follicles of Deer Mice Obtained by Mechanical and Enzymatic Methods Under In Vitro Culture in MD of Nonconditioned Medium
| |
|
|
No. (%) of oocyte at stage |
||
|---|---|---|---|---|---|
| Isolation method | On day 0, no. of preantral F/Oo | On day 13, no. (%) of antral F/Oo | GV | GVBD | MII |
| Enzymatic | 101 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Mechanical | 188 | 15 (8.0) | 12 (6.4) | 6 (3.2) | 5 (2.7) |
MD, microliter drop; F/Oo, follicle or oocyte; GV, germinal vesicle; GVBD, GV breakdown; MII, metaphase II.
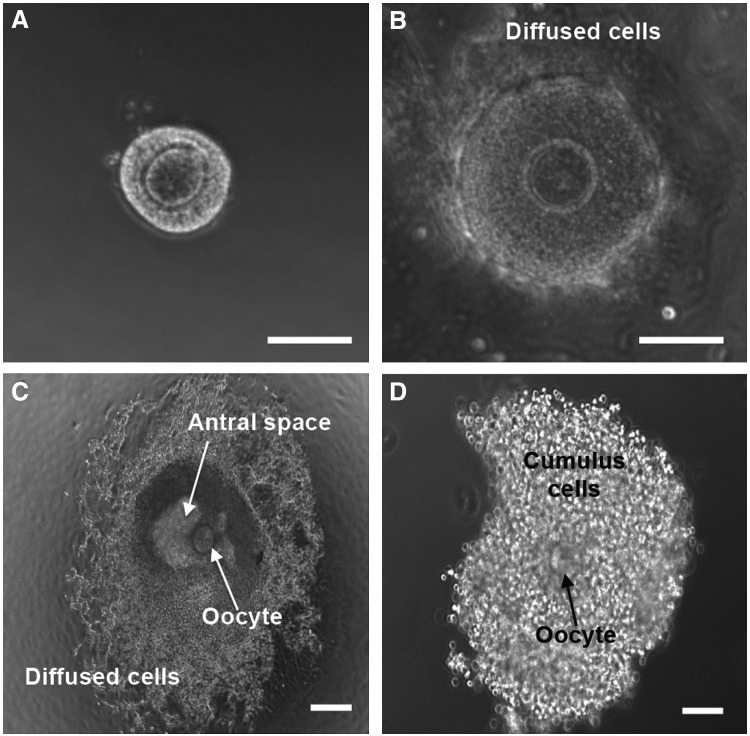
FIG. 4.
Typical micrographs showing the development of the early secondary preantral follicle retrieved mechanically from deer mouse ovaries during in vitro culture in MD of the nonconditioned medium: ∼8% of the early secondary preantral follicle (A) could develop through the nonphysiologically diffused stage (B) to antral stage (C). After further IVM, COC (D) was obtained from the antral follicle by follicular puncture. Cumulus cells in the COC were removed using hyaluronidase to obtain clean oocytes, shown in Figure 3. Only 2.7% of the primary oocytes in the early secondary preantral follicle developed to the MII stage using this conventional MD method. Scale bars: 100 μm. COC, cumulus–oocyte complex.
In vitro follicle culture: the conventional and modified HD method with nonconditioned medium
The results of follicle growth and oocyte maturation using the HD method with the nonconditioned medium are shown in Table 3 and Figure 5A–D for both the conventional and modified versions of the method. The only difference between the two versions is from day 7 to 13 when MDs and HDs were used for the conventional and modified methods, respectively (Fig. 2). The five (per drop) early secondary preantral follicles (Fig. 5A) cultured in all HDs formed a single aggregate (Fig. 5B) in each drop on day 6 with high cell viability (green) throughout the aggregates (Fig. 5C). The latter could be because the five follicles in the aggregates were packed in a way to allow all the follicles having a direct contact with the medium in the HD, as shown in Figure 5B. However, when the aggregated follicles were further cultured using the conventional way in MDs on the 2D surface of the culture dishes, all follicles in the aggregates became degenerated with diffused theca and granulosa cells attached on the dish (Supplementary Fig. S4) and they could not develop to the antral stage. Again, the nonphysiologic 2D culture condition and the reduced oxygen transport to the aggregated follicles resulting from the oil barrier could be responsible for the degeneration of the follicles.
Table 3.
Development of Early Secondary Preantral Follicles (Obtained from Deer Mice Using the Mechanical Method) in HD of Nonconditioned Medium Under In Vitro Culture into Follicle Aggregates from Day 0 to 6, Followed by Further Culturing the Aggregates from Day 7 to 13 in Either MD or HD of Nonconditioned Medium
| |
|
|
|
|
No. (%a) of oocytes at stage |
||
|---|---|---|---|---|---|---|---|
| On day 0, no. of HD (total no. of F/Oo) | Culture method | On day 6, no. of preantral FA (F/Oo) | Culture method | On day 13, no. of antral FA (F/Oo) | GV | GVBD | MII |
| 8 (40) | HD | 8 (40) | MD | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| 8 (40) | HD | 8 (40) | HD | 1 (5) | 3 (7.5) | 2 (5) | 0 (0) |
Out of the 40 follicular oocytes used on day 0.
HD, hanging drop; FA, follicle aggregate.
FIG. 5.
Typical micrographs showing the development of the early secondary preantral follicles obtained mechanically from the ovaries of deer mice under in vitro culture in HD: with five follicles per drop on day 0 (A), the follicles could form a single aggregate on day 6 in the HD of either the nonconditioned or ovarian cell-CM (B) with high cell viability (C) and the aggregated follicles could further develop to the antral stage (D) if continuously cultured in the HD of the nonconditioned (12.5%) or ovarian cell-conditioned (65%) medium from day 7 to 13. With three follicles per drop on day 0 (E), the follicles could form a single aggregate on day 6 in each HD (F) and the aggregated follicles could further develop to the antral stage (G) if continuously cultured in HD-CM (66.7%). However, with only one follicle per HD of CM on day 0 (H), the follicle degenerated on day 6 (I). Scale bar: 100 μm. Color images available online at www.liebertpub.com/tea
Although this conventional HD method works effectively for the in vitro culture of the secondary preantral follicles of inbred mice,36 it is apparently not directly transferrable to outbred deer mice. Therefore, we modified the conventional method by continuously culturing the aggregated follicles in HD on day 7–13. As a result, one of the eight aggregates of the five early secondary preantral follicles further developed to an aggregate of antral stage follicles (Fig. 5D). This result (together with the data shown in Table 2, Fig. 4B, C, and Supplementary Fig. S4) suggests that a 3D culture environment mimicking the ovarian tissue architecture in vivo to maintain the anatomical integrity of follicles as well as a close and tight granulosa–oocyte interaction is more important for culturing and developing the aggregated than single early secondary preantral follicles in vitro. However, the percentage (12.5%, 1/8) of the early secondary preantral follicle aggregates developed to the stage of antral follicle aggregates is still low. Moreover, none of the five follicular oocytes in the antral follicle aggregate developed to the MII stage, although three and two of them did develop to the GV and GVBD stage, respectively.
In vitro follicle culture: the modified HD-CM
As mentioned above, it is surprising that the 3D HD culture method was observed to be worse than the 2D MD method in terms of the production of MII oocytes, even though the 3D culture could maintain the follicular architecture much better than the 2D culture method (Figs. 5B, D vs. 4B, C). Therefore, we further modified the HD method by using ovarian cell-CM for in vitro culture of the early secondary preantral follicles because the culture medium developed for inbred mice may not completely recapitulate the soluble milieu in the ovary of outbred deer mice. The results of culturing the early secondary preantral follicles in 20 HDs of the deer mice ovarian cell-CM are given in Table 4. As expected, the five preantral follicles formed a single preantral follicle aggregate in all the 20 HDs on day 6. Moreover, 13 of the 20 (or 65%) of the preantral follicle aggregates further developed to aggregates of antral follicles, which is more than five times higher compared to both the 3D HD (≤1/8 or 12.5%, Table 3) and 2D MD (8%, Table 2) culture methods using the nonconditioned medium.
Table 4.
Development of Early Secondary Preantral Follicles (Obtained Using the Mechanical Method) Cultured In Vitro in HD-CM into Preantral and Antral Follicle Aggregates and the Effect of Methods for In-Follicle Maturation of Oocytes on the Development of Oocytes in the Aggregated Antral Follicles
| |
|
|
|
|
No. (%a) of oocytes at stage |
||
|---|---|---|---|---|---|---|---|
| On day 0, no. of F/Oo per drop (in total) | On day 6, no. of preantral FA (F/Oo) | On day 13, no. of antral FA (F/Oo) | No. of FA (F/Oo) used for IVM | Method for IVM of oocytes | GV | GVBD | MII |
| 5 (100) | 20 (100) | 13 (65) | 6 (30) | Method 1 | 13 (28.2) | 3 (6.5) | 0 (0) |
| 7 (35) | Method 2 | 18 (33.4) | 10 (18.6) | 7 (13) | |||
| 3 (36) | 12 (36) | 8 (24) | 8 (24) | Method 2 | 12 (33.3) | 5 (13.4) | 3 (8.3) |
| 1 (30) | 0 (0) | 0 (0) | – | – | – | – | – |
Out of the total number of follicular oocytes on day 0. For the condition of five follicles per drop, it was calculated as the product of the percentage (i.e., 65%) of the development from the preantral to antral stage and the ratio of the number of GV, GVBD, and MII oocytes to the total number of oocytes in the antral follicles used for IVM.
HD-CM, HD of ovarian cell-conditioned medium; IVM, in vitro maturation.
Since the ovarian cell-CM was made in the absence of follicles in this study, the above-mentioned results are consistent with recent studies reporting that the paracrine effect of nonfollicular stromal cells in the ovary on follicle growth is largely unidirectional (i.e., from the stromal cells to follicular cells).69,70 Moreover, it was also reported that these paracrine communications between follicular cells and nonfollicular stromal cells are very complex and could not be recapitulated by simply adding a few identifiable major cytokines or chemokines into the culture medium.69,70 This is not surprising because the ovary consists of a diverse mix of cell types and adhesion molecules, including theca–interstitial cells, granulosa cells, cumulus cells, immune cells (mainly macrophages), endothelial cells of the blood vessels, smooth muscle cells, and several types of extracellular matrix proteins.69 These cells all have their specific functions that are well coordinated either locally in the ovary or systemically by the central nervous system (e.g., hypothalamus and pituitary gland) via endocrine hormones, including the gonadotropin release hormone (GnRH), FSH, and luteinizing hormone (LH).71,72 For example, granulosa cells release sex hormones (e.g., progesterone and estradiol) and can aromatize androgens secreted by theca cells into estrogens. Androgens have been shown to have an important impact on follicle growth in mice, cows, and nonhuman primates.73,74 Cumulus cells provide energy substrates for the oocyte meiotic resumption.75 Macrophages secrete a variety of cytokines and growth factors, including interleukin-1, 6, 10, and 12, interferon-α, tumor necrosis factor-α, granulocyte–macrophage colony-stimulating factor, basic fibroblast growth factor, EGF, and insulin-like growth factor. These cytokines and growth factors and their concentrations (that could be different for inbred vs. outbred animals) all play important roles in directing the follicle growth and differentiation.71,72,76–78 Although it would be challenging and beyond the scope of this work, further studies are warranted to potentially identify one or more factors in the CM that are crucial for the development of the aggregated preantral follicles into antral ones.
We further investigated how the total number of early secondary preantral follicles per HD would affect the outcome of their culture in vitro using the HD-CM method, and the results are shown in Table 4 and Figure 5. With three early secondary preantral follicles per HD on day 0 (Fig. 5E), the follicles formed a single aggregate (Fig. 5F) in each drop efficiently (100%) and further developed to the antral stage (Fig. 5G) with a similar percentage (66.7%) to that of using five follicles per HD. However, when only one early secondary preantral follicle per HD was used on day 0 (Fig. 5H), all of the 30 early secondary preantral follicles in 30 HD-CM degenerated on day 6 (Fig. 5I). These data suggest that using multiple early secondary preantral follicles per drop is necessary to ensure successful culture and development of the follicles to the antral stage using the hanging method. This might be because some interfollicular interactions exist that are important to the survival and development of the early secondary preantral follicles cultured in the 3D HD environment, which certainly warrants further investigation in a future study.
IVM and IVF of oocytes obtained by in vitro follicle culture using the HD-CM method
Although a high percentage (∼65%) of antral follicle aggregates could be attained with the HD-CM culture method, we found that the method (i.e., IVM method 1) that was reported61 to be effective for IVM of oocytes in antral follicles of deer mice obtained by superovulation did not work for IVM of oocytes in the aggregated antral follicles. As shown in Table 4, no MII oocyte could be obtained from the 30 follicular oocytes in 6 of the antral follicle aggregates attained using the 5 early secondary preantral follicles per HD on day 0, although 13 and 3 of the 30 antral follicular oocytes did develop to the GV and GVBD stage. Therefore, we tried the IVM method 266 with a different medium and a much longer time (48 vs. 18 h for IVM method 1) for the maturation of oocytes in the aggregated antral follicles. As shown in Table 4 (using five early secondary preantral follicles per drop on day 0), a total of 7, 10, and 18 of the 35 antral follicular oocytes developed to MII, GVBD, and GV stages, respectively. The percentages are all higher compared with the IVM method 1, and the difference is significant for GVBD (p=0.0377) and MII (p=0.0057) oocytes. When using the three early secondary preantral follicles in each HD-CM, the follicular oocytes could develop to the MII stage with a lower percentage (8.3%, Table 4).
To ascertain the quality of the MII oocytes obtained using the HD-CM method for follicle culture and the IVM method 2 for oocyte maturation, further IVF study was performed using four of the MII oocytes (Fig. 6A). A total of three (75%) of the fertilized oocytes developed to the two-pronuclei stage, and one (25%) of them further developed to the two-cell stage. The latter is similar to that (23%) reported in the literature for deer mice oocytes obtained by IVM of COCs in antral follicles directly isolated from the ovary and higher compared with (8%) deer mice oocytes obtained directly by superovulation.61 These results suggest that the HD-CM method for culturing the early secondary preantral follicles together with IVM method 2 for oocyte maturation is a much better approach for obtaining MII oocytes from the outbred deer mice than the conventional MD and HD methods.
FIG. 6.
Typical micrographs showing the development of the MII oocyte (A) after IVF to the two-pronuclei (B, C) and two-cell (D) stage: the two pronuclei visible in the phase-contrast image (arrows in B) were further confirmed using fluorescence staining of the pronuclei (C, blue stains). The MII oocyte was obtained using the HD-CM method for the culture of the early secondary preantral follicle and the IVM method 2 for the oocyte maturation. Scale bar: 20 μm. IVF, in vitro fertilization. Color images available online at www.liebertpub.com/tea
In summary, a large number of early secondary preantral follicles can be mechanically isolated from the ovaries of outbred deer mice. However, the efficiency of developing the early secondary preantral follicles to the antral stage is very low when cultured in vitro using the conventional 2D MD and 3D HD methods developed for inbred mice. It was demonstrated that ovarian cell-CM could be used to significantly improve (by more than five times compared to the conventional methods) the efficiency of developing the early secondary preantral follicles to the antral stage by culturing them continuously in HD of the CM for 14 days (day 0–13). In addition, it was found that the optimal method for IVM of oocytes in the antral follicles obtained by in vitro culture of early secondary preantral follicles could be different from that obtained directly from antral follicles developed in vivo by hormone stimulation. Taken together, the results reported in this study should provide important guidance for developing effective technologies of in vitro culturing the early secondary preantral follicles from outbred species and even humans to preserve their future fertility.
Supplementary Material
Acknowledgments
This work was supported by a grant from NIH (R01EB012108). We thank Dr. Gabor Szalai for his technical support to prepare the deer mice used in this study at the University of South Carolina Peromyscus Stock Center.
Disclosure Statement
No competing financial interest exists.
References
- 1.Woodruff T.K. Making eggs: is it now or later? Nat Med. 2008;14:1190. doi: 10.1038/nm1108-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett K.R. Schilling C. Greenfeld C.R. Tomic D. Flaws J.A. Ovarian follicle development and transgenic mouse models. Hum Reprod Update. 2006;12:537. doi: 10.1093/humupd/dml022. [DOI] [PubMed] [Google Scholar]
- 3.Broekmans F.J. Soules M.R. Fauser B.C. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30:465. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 4.Hassold T. Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet. 2001;2:280. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- 5.Malizia B.A. Hacker M.R. Penzias A.S. Cumulative live-birth rates after in vitro fertilization. N Engl J Med. 2009;360:236. doi: 10.1056/NEJMoa0803072. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J. Dolmans M.M. Cryopreservation and transplantation of ovarian tissue. Clin Obstet Gynecol. 2010;53:787. doi: 10.1097/GRF.0b013e3181f97a55. [DOI] [PubMed] [Google Scholar]
- 7.Andersen C.Y. Kristensen S.G. Greve T. Schmidt K.T. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595. doi: 10.2217/fon.12.47. [DOI] [PubMed] [Google Scholar]
- 8.Jeruss J.S. Woodruff T.K. Preservation of fertility in patients with cancer. N Engl J Med. 2009;360:902. doi: 10.1056/NEJMra0801454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin M. Yu Y. Huang H. An update on primary ovarian insufficiency. Sci China Life Sci. 2012;55:677. doi: 10.1007/s11427-012-4355-2. [DOI] [PubMed] [Google Scholar]
- 10.Goswami D. Conway G.S. Premature ovarian failure. Hum Reprod Update. 2005;11:391. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- 11.Santos R.R. Amorim C. Cecconi S. Fassbender M. Imhof M. Lornage J., et al. Cryopreservation of ovarian tissue: an emerging technology for female germline preservation of endangered species and breeds. Anim Reprod Sci. 2010;122:151. doi: 10.1016/j.anireprosci.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Grynberg M. Poulain M. Sebag-Peyrelevade S. le Parco S. Fanchin R. Frydman N. Ovarian tissue and follicle transplantation as an option for fertility preservation. Fertil Steril. 2012;97:1260. doi: 10.1016/j.fertnstert.2012.04.042. [DOI] [PubMed] [Google Scholar]
- 13.Donnez J. Jadoul P. Squifflet J. Van Langendonckt A. Donnez O. Van Eyck A.S., et al. Ovarian tissue cryopreservation and transplantation in cancer patients. Best Pract Res Clin Obstet Gynaecol. 2010;24:87. doi: 10.1016/j.bpobgyn.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Meirow D. Hardan I. Dor J. Fridman E. Elizur S. Ra'anani H., et al. Searching for evidence of disease and malignant cell contamination in ovarian tissue stored from hematologic cancer patients. Hum Reprod. 2008;23:1007. doi: 10.1093/humrep/den055. [DOI] [PubMed] [Google Scholar]
- 15.Shaw J.M. Bowles J. Koopman P. Wood E.C. Trounson A.O. Fresh and cryopreserved ovarian tissue samples from donors with lymphoma transmit the cancer to graft recipients. Hum Reprod. 1996;11:1668. doi: 10.1093/oxfordjournals.humrep.a019467. [DOI] [PubMed] [Google Scholar]
- 16.Eppig J. O'Brien M. Development in vitro of mouse oocytes from primordial follicles. Biol Reprod. 1996;54:197. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- 17.Gosden R.G. Mullan J. Picton H.M. Yin H. Tan S.L. Current perspective on primordial follicle cryopreservation and culture for reproductive medicine. Hum Reprod Update. 2002;8:105. doi: 10.1093/humupd/8.2.105. [DOI] [PubMed] [Google Scholar]
- 18.Varghese A.C. du Plessis S.S. Falcone T. Agarwal A. Cryopreservation/transplantation of ovarian tissue and in vitro maturation of follicles and oocytes: challenges for fertility preservation. Reprod Biol Endocrinol. 2008;6:47. doi: 10.1186/1477-7827-6-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smitz J. Dolmans M.M. Donnez J. Fortune J.E. Hovatta O. Jewgenow K., et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cortvrindt R.G. Smitz J.E. Follicle culture in reproductive toxicology: a tool for in-vitro testing of ovarian function? Hum Reprod Update. 2002;8:243. doi: 10.1093/humupd/8.3.243. [DOI] [PubMed] [Google Scholar]
- 21.Trounson A. Mohr L. Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature. 1983;305:707. doi: 10.1038/305707a0. [DOI] [PubMed] [Google Scholar]
- 22.Steptoe P.C. Edwards R.G. Birth after the reimplantation of a human embryo. Lancet. 1978;2:366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 23.Mandelbaum J. Belaisch-Allart J. Junca A.M. Antoine J.M. Plachot M. Alvarez S., et al. Cryopreservation in human assisted reproduction is now routine for embryos but remains a research procedure for oocytes. Hum Reprod. 1998;13(Suppl 3):161. doi: 10.1093/humrep/13.suppl_3.161. ; discussion 75–77. [DOI] [PubMed] [Google Scholar]
- 24.Deepinder F. Agarwal A. Technical and ethical challenges of fertility preservation in young cancer patients. Reprod Biomed Online. 2008;16:784. doi: 10.1016/s1472-6483(10)60143-5. [DOI] [PubMed] [Google Scholar]
- 25.Roque M. Lattes K. Serra S. Sola I. Geber S. Carreras R., et al. Fresh embryo transfer versus frozen embryo transfer in in vitro fertilization cycles: a systematic review and meta-analysis. Fertil Steril. 2013;99:156. doi: 10.1016/j.fertnstert.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Desai N. Alex A. AbdelHafez F. Calabro A. Goldfarb J. Fleischman A., et al. Three-dimensional in vitro follicle growth: overview of culture models, biomaterials, design parameters and future directions. Reprod Biol Endocrinol. 2010;8:119. doi: 10.1186/1477-7827-8-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim I.W. Gong S.P. Yoo C.R. Choi J.H. Kim D.Y. Lim J.M. Derivation of developmentally competent oocytes by the culture of preantral follicles retrieved from adult ovaries: maturation, blastocyst formation, and embryonic stem cell transformation. Fertil Steril. 2009;92:1716. doi: 10.1016/j.fertnstert.2008.08.084. [DOI] [PubMed] [Google Scholar]
- 28.Xu M. Banc A. Woodruff T.K. Shea L.D. Secondary follicle growth and oocyte maturation by culture in alginate hydrogel following cryopreservation of the ovary or individual follicles. Biotechnol Bioeng. 2009;103:378. doi: 10.1002/bit.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu M. West-Farrell E.R. Stouffer R.L. Shea L.D. Woodruff T.K. Zelinski M.B. Encapsulated three-dimensional culture supports development of nonhuman primate secondary follicles. Biol Reprod. 2009;81:587. doi: 10.1095/biolreprod.108.074732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shikanov A. Xu M. Woodruff T.K. Shea L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials. 2009;30:5476. doi: 10.1016/j.biomaterials.2009.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikanov A. Xu M. Woodruff T. Shea L. A method for ovarian follicle encapsulation and culture in a proteolytically degradable 3 dimensional system. J Vis Exp. 2011:e2695. doi: 10.3791/2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin S.Y. Lei L. Shikanov A. Shea L.D. Woodruff T.K. A novel two-step strategy for in vitro culture of early-stage ovarian follicles in the mouse. Fertil Steril. 2010;93:2633. doi: 10.1016/j.fertnstert.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu M. Fazleabas A.T. Shikanov A. Jackson E. Barrett S.L. Hirshfeld-Cytron J., et al. In vitro oocyte maturation and preantral follicle culture from the luteal-phase baboon ovary produce mature oocytes. Biol Reprod. 2011;84:689. doi: 10.1095/biolreprod.110.088674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woodruff T.K. Shea L.D. The role of the extracellular matrix in ovarian follicle development. Reprod Sci. 2007;14:6. doi: 10.1177/1933719107309818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woodruff T.K. Shea L.D. A new hypothesis regarding ovarian follicle development: ovarian rigidity as a regulator of selection and health. J Assist Reprod Genet. 2011;28:3. doi: 10.1007/s10815-010-9478-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W. Tang Y. Ni L. Jongwutiwes T. Liu H. Rosenwaks Z. A modified protocol for in vitro maturation of mouse oocytes from secondary preantral follicles. Adv Biosci Biotechnol. 2012;3:57. [Google Scholar]
- 37.Liu J. Van der Elst J. Van den Broecke R. Dhont M. Live offspring by in vitro fertilization of oocytes from cryopreserved primordial mouse follicles after sequential in vivo transplantation and in vitro maturation. Biol Reprod. 2001;64:171. doi: 10.1095/biolreprod64.1.171. [DOI] [PubMed] [Google Scholar]
- 38.Xu M. Kreeger P.K. Shea L.D. Woodruff T.K. Tissue-engineered follicles produce live, fertile offspring. Tissue Eng. 2006;12:2739. doi: 10.1089/ten.2006.12.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eppig J.J. Schroeder A.C. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in-vitro. Biol Reprod. 1989;41:268. doi: 10.1095/biolreprod41.2.268. [DOI] [PubMed] [Google Scholar]
- 40.Nayudu P.L. Osborn S.M. Factors influencing the rate of preantral and antral growth of mouse ovarian follicles in vitro. J Reprod Fertil. 1992;95:349. doi: 10.1530/jrf.0.0950349. [DOI] [PubMed] [Google Scholar]
- 41.Roy S.K. Treacy B.J. Isolation and long-term culture of human preantral follicles. Fertil Steril. 1993;59:783. [PubMed] [Google Scholar]
- 42.Oktay K. Nugent D. Newton H. Salha O. Chatterjee P. Gosden R.G. Isolation and characterization of primordial follicles from fresh and cryopreserved human ovarian tissue. Fertil Steril. 1997;67:481. doi: 10.1016/s0015-0282(97)80073-8. [DOI] [PubMed] [Google Scholar]
- 43.Abir R. Fisch B. Nitke S. Okon E. Raz A. Ben Rafael Z. Morphological study of fully and partially isolated early human follicles. Fertil Steril. 2001;75:141. doi: 10.1016/s0015-0282(00)01668-x. [DOI] [PubMed] [Google Scholar]
- 44.Gorman K.B. Flint P.L. Esler D. Williams T.D. Ovarian follicle dynamics of female Greater Scaup during egg production. J Field Ornithol. 2007;78:64. [Google Scholar]
- 45.Guimaraes D.A. de Garcia S.C. Ferreira M.A. da Silva Sdo S. de Albuquerque N.I. Le Pendu Y. Ovarian folliculogenesis in collared peccary, Pecari tajacu (Artiodactyla: Tayassuidae) Rev Biol Trop. 2012;60:437. [PubMed] [Google Scholar]
- 46.Ting A.Y. Yeoman R.R. Lawson M.S. Zelinski M.B. In vitro development of secondary follicles from cryopreserved rhesus macaque ovarian tissue after slow-rate freeze or vitrification. Hum Reprod. 2011;26:2461. doi: 10.1093/humrep/der196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Telfer E.E. McLaughlin M. In vitro development of ovarian follicles. Sem Reprod Med. 2011;29:15. doi: 10.1055/s-0030-1268700. [DOI] [PubMed] [Google Scholar]
- 48.Hovatta O. Wright C. Krausz T. Hardy K. Winston RML. Human primordial, primary and secondary ovarian follicles in long-term culture: effect of partial isolation. Hum Reprod. 1999;14:2519. doi: 10.1093/humrep/14.10.2519. [DOI] [PubMed] [Google Scholar]
- 49.Hreinsson J.G. Scott J.E. Rasmussen C. Swahn M.L. Hsueh AJW. Hovatta O. Growth differentiation factor-9 promotes the growth, development, and survival of human ovarian follicles in organ culture. J Clin Endocr Metab. 2002;87:316. doi: 10.1210/jcem.87.1.8185. [DOI] [PubMed] [Google Scholar]
- 50.Niu Y. Liang S. Genetic differentiation within the inbred C57BL/6J mouse strain. J Zool. 2009;278:42. [Google Scholar]
- 51.Mott R. A haplotype map for the laboratory mouse. Nat Genet. 2007;39:1054. doi: 10.1038/ng0907-1054. [DOI] [PubMed] [Google Scholar]
- 52.Wade C.M. Kulbokas E.J., 3rd Kirby A.W. Zody M.C. Mullikin J.C. Lander E.S., et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 53.Shorter K.R. Crossland J.P. Webb D. Szalai G. Felder M.R. Vrana P.B. Peromyscus as a mammalian epigenetic model. Genet Res Int. 2012;2012:179. doi: 10.1155/2012/179159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salcedo T. Geraldes A. Nachman M.W. Nucleotide variation in wild and inbred mice. Genetics. 2007;177:2277. doi: 10.1534/genetics.107.079988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klein M.S. Markert C.L. Production of Chimeras in the deer mouse Peromyscus-Maniculatus-Bairdi. J Exp Zool. 1981;218:183. [Google Scholar]
- 56.Joyner C. Myrick L. Crossland J. Dawson W. Deer mice as laboratory animals. ILAR J. 1998;39:322. doi: 10.1093/ilar.39.4.322. [DOI] [PubMed] [Google Scholar]
- 57.Veres M. Duselis A.R. Graft A. Pryor W. Crossland J. Vrana P.B., et al. The biology and methodology of assisted reproduction in deer mice (Peromyscus maniculatus) Theriogenology. 2012;77:311. doi: 10.1016/j.theriogenology.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Duselis A.R. Vrana P.B. Aberrant growth and pattern formation in Peromyscus hybrid placental development. Biol Reprod. 2010;83:988. doi: 10.1095/biolreprod.110.085654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dewey M.J. Dawson W.D. Deer mice: “The Drosophila of North American mammalogy.”. Genesis. 2001;29:105. doi: 10.1002/gene.1011. [DOI] [PubMed] [Google Scholar]
- 60.Chappell M.A. Rezende E.L. Hammond K.A. Age and aerobic performance in deer mice. J Exp Biol. 2003;206:1221. doi: 10.1242/jeb.00255. [DOI] [PubMed] [Google Scholar]
- 61.Choi J.K. He X. In vitro maturation of cumulus-oocyte complexes for efficient isolation of oocytes from outbred deer mice. PLoS One. 2013;8:e56158. doi: 10.1371/journal.pone.0056158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee S.T. Choi M.H. Gong S.P. Han J.Y. Lim J.M. Establishment of a basic method for manipulating preantral follicles: effects of retrieval method on in vitro growth of preantral follicles and intrafollicular oocytes. Zygote. 2007;15:109. doi: 10.1017/S0967199407004121. [DOI] [PubMed] [Google Scholar]
- 63.Gong S.P. Lee S.T. Lee E.J. Kim D.Y. Lee G. Chi S.G., et al. Embryonic stem cell-like cells established by culture of adult ovarian cells in mice. Fertil Steril. 2010;93:2594. doi: 10.1016/j.fertnstert.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weigel A. Schild D. Zeug A. Resolution in the ApoTome and the confocal laser scanning microscope: comparison. J Biomed Opt. 2009;14:014022. doi: 10.1117/1.3083439. [DOI] [PubMed] [Google Scholar]
- 65.Gines T.B. Davidson M.W. Structured illumination: ZEISS ApoTome. http://zeiss-campus.magnet.fsu.edu/tutorials/opticalsectioning/apotome/index.html. [Mar 6;2013 ]. http://zeiss-campus.magnet.fsu.edu/tutorials/opticalsectioning/apotome/index.html
- 66.Martin-Coello J. Gonzalez R. Crespo C. Gomendio M. Roldan E.R. Superovulation and in vitro oocyte maturation in three species of mice (Mus musculus, Mus spretus and Mus spicilegus) Theriogenology. 2008;70:1004. doi: 10.1016/j.theriogenology.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 67.Demeestere I. Delbaere A. Gervy C. Van Den Bergh M. Devreker F. Englert Y. Effect of preantral follicle isolation technique on in-vitro follicular growth, oocyte maturation and embryo development in mice. Hum Reprod. 2002;17:2152. doi: 10.1093/humrep/17.8.2152. [DOI] [PubMed] [Google Scholar]
- 68.Liu J. Van Der Elst J. Van Den Broecke R. Dumortier F. Dhont M. Maturation of mouse primordial follicles by combination of grafting and in vitro culture. Biol Reprod. 2000;62:1218. doi: 10.1095/biolreprod62.5.1218. [DOI] [PubMed] [Google Scholar]
- 69.Tingen C.M. Kiesewetter S.E. Jozefik J. Thomas C. Tagler D. Shea L., et al. A macrophage and theca cell-enriched stromal cell population influences growth and survival of immature murine follicles in vitro. Reproduction. 2011;141:809. doi: 10.1530/REP-10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tagler D. Tu T. Smith R.M. Anderson N.R. Tingen C.M. Woodruff T.K., et al. Embryonic fibroblasts enable the culture of primary ovarian follicles within alginate hydrogels. Tissue Eng Part A. 2012;18:1229. doi: 10.1089/ten.tea.2011.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gilchrist R. Mottershead D. Thompson J. Oocyte maturation and ovulation—an orchestral symphony of signalling. Aust Biochemist. 2011;42:8. [Google Scholar]
- 72.Scaramuzzi R.J. Baird D.T. Campbell B.K. Driancourt M.A. Dupont J. Fortune J.E., et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod Fertil Dev. 2011;23:444. doi: 10.1071/RD09161. [DOI] [PubMed] [Google Scholar]
- 73.Murray A.A. Gosden R.G. Allison V. Spears N. Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil. 1998;113:27. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- 74.Vendola K.A. Zhou J. Adesanya O.O. Weil S.J. Bondy C.A. Androgens stimulate early stages of follicular growth in the primate ovary. J Clin Invest. 1998;101:2622. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fauser B.C. Diedrich K. Bouchard P. Dominguez F. Matzuk M. Franks S., et al. Contemporary genetic technologies and female reproduction. Hum Reprod Update. 2011;17:829. doi: 10.1093/humupd/dmr033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brannstrom M. Norman R.J. Involvement of leukocytes and cytokines in the ovulatory process and corpus luteum function. Hum Reprod. 1993;8:1762. doi: 10.1093/oxfordjournals.humrep.a137929. [DOI] [PubMed] [Google Scholar]
- 77.Terranova P.F. Rice V.M. Review: cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 78.Wu R. Van der Hoek K.H. Ryan N.K. Norman R.J. Robker R.L. Macrophage contributions to ovarian function. Hum Reprod Update. 2004;10:119. doi: 10.1093/humupd/dmh011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.