Abstract
Sufficient functional restoration of damaged peripheral nerves is a big clinical challenge. In this study, a nerve guide conduit (NGC) with selective permeability was prepared by rolling an asymmetrically porous polycaprolactone/Pluronic F127 membrane fabricated using a novel immersion precipitation method. Dual stimulation (nerve growth factor [NGF] as a biological stimulus and low-intensity pulse ultrasound [US] as a physical stimulus) was adapted to enhance nerve regeneration through an NGC. The animal study revealed that each stimulation (NGF or US) has a positive effect to promote the peripheral nerve regeneration through the NGC, however, the US-stimulated NGC group allowed more accelerated nerve regeneration compared with the NGF-stimulated group. The NGC group that received dual stimulation (NGF and US) showed more effective nerve regeneration behavior than the groups that received a single stimulation (NGF or US). The asymmetrically porous NGC with dual NGF and US stimulation may be a promising strategy for the clinical treatment of delayed and insufficient functional recovery of a peripheral nerve.
Introduction
Peripheral nerve damages resulting from transection injuries, burns, and degenerative conditions lead to the loss of sensory or motor functions as well as painful neuropathy.1–3 Direct reconnection between damaged nerve stumps and transplantation of autologous nerve graft have been commonly adapted for nerve regeneration. However, the limited application for only a slight or small defect (direct reconnection), an additional surgical procedure to obtain a donor nerve, and a permanent functional loss of the donor nerve (autologous nerve graft) remain as significant challenges in the clinical field.4 Recently, the artificial nerve guide conduit (NGC), which can provide a favorable microenvironment for nerve regeneration and properly guide the axonal sprouting from the proximal stump to the distal stump to reinnervate its original target,5–7 has been suggested as an alternative strategy for treating damaged nerves. A variety of biological tissues and natural or synthetic polymers have been adapted as NGCs,8 however, the relatively slow morphological and functional recovery and the limited regeneration distance of peripheral nerves through the NGC remain as critical drawbacks for clinical use. Some stimulation systems, including biological stimuli (growth factors, Schwann cells, etc.) and physical stimuli (laser, electricity, ultrasound [US], etc.) have been suggested to effectively compensate for these problems associated with NGCs,9–13 and they revealed somewhat encouraging outcomes, suggesting that the use of such stimuli can be a hopeful therapeutic technique to improve nerve regeneration through NGCs.
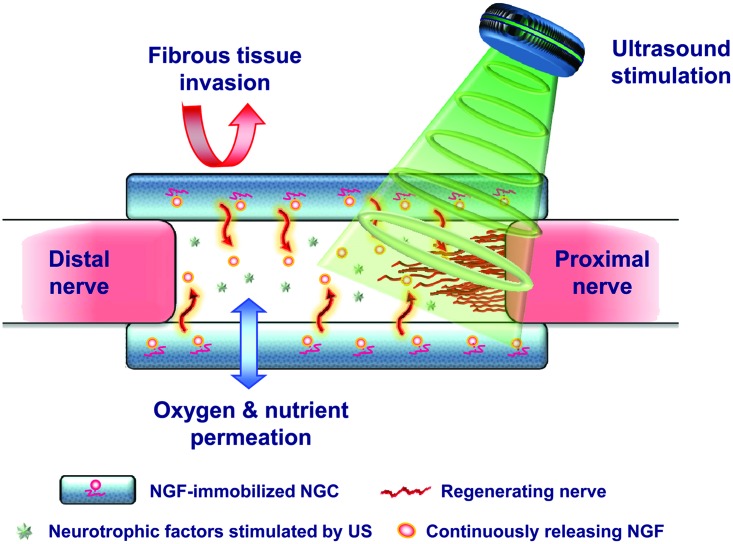
In a previous study, we developed an asymmetrically porous polycaprolactone (PCL)/Pluronic F127 membrane (nanopores on one side and micropores on the other side) with selective permeability (preventing fibrous scar tissue infiltration, but allowing permeation of nutrients/oxygen) and hydrophilicity (for effective nutrient permeation), which is an essential requirement of an NGC for effective nerve regeneration.14 The PCL/F127 NGCs with different surface pore structures were fabricated by rolling the opposite side of the asymmetrically porous membranes to investigate the effect of surface pores (nanoporous inner surface vs. microporous inner surface) on nerve regeneration through NGCs.15 We demonstrated that an NGC with a nanoporous inner surface allows much faster nerve regeneration compared to the NGC with a microporous one as well as nonporous silicone tube used clinically in an animal study using a rat model (sciatic nerve defect in rats).15,16 Based on these findings, we expected that if well-established stimuli for nerve regeneration were applied to the unique asymmetrically porous NGC with the nanoporous inner surface, it may be a promising strategy to accelerate peripheral nerve regeneration. Therefore, in this study, the nerve growth factor (NGF) as a source of biological stimulation to promote nerve regeneration was immobilized onto the pore surfaces of a PCL/F127 NGC17 by specific interactions between the Pluronic F127 and heparin (hydrogen bonding), and the following heparin and NGF (ionic interaction). We also applied low-intensity pulsed US as a simple and noninvasive physical stimulus source at the NGF-immobilized NGC-implanted site transcutaneously in rats to enhance nerve regeneration. US is described as an acoustic pressure wave, which can transfer mechanical energy into tissues and cause beneficial biochemical events at the cellular level to possibly promote tissue regeneration.14 The expected mechanism for enhanced nerve regeneration using the dual NGF and US-stimulated PCL/F127 NGC (NGF/US/NGC) system is shown in Figure 1. The nerve regeneration behaviors within the NGF/US/NGC were compared with the NGC without stimulation, NGF-stimulated NGC (NGF/NGC), and US-stimulated NGC (US/NGC) as well as normal nerve by immunohistochemical/histological observations, retrograde tracing, and electrophysiological evaluations.
FIG. 1.
Schematic diagram showing the possible mechanism for accelerated nerve regeneration by the NGF/US/NGC system. NGC, nerve guide conduit; NGF, nerve growth factor; US, ultrasound. Color images available online at www.liebertpub.com/tea
Materials and Methods
Materials
PCL (Mw 80,000; Aldrich) as an NGC substrate material, Pluronic F127 (Mw 12,500; BASF) as a hydrophilic additive, and tetraglycol (glycofurol; Sigma) as a cosolvent for PCL and Pluronic F127 were used to prepare asymmetrically porous NGCs. NGF as a source of biological stimulation was purchased from R&D Systems. All other chemicals were of analytical grade and were used as received. Ultrapure grade water (>18 mΩ) was purified using a Milli-Q purification system (Millipore).
Fabrication of NGF-immobilized PCL/F127 NGC
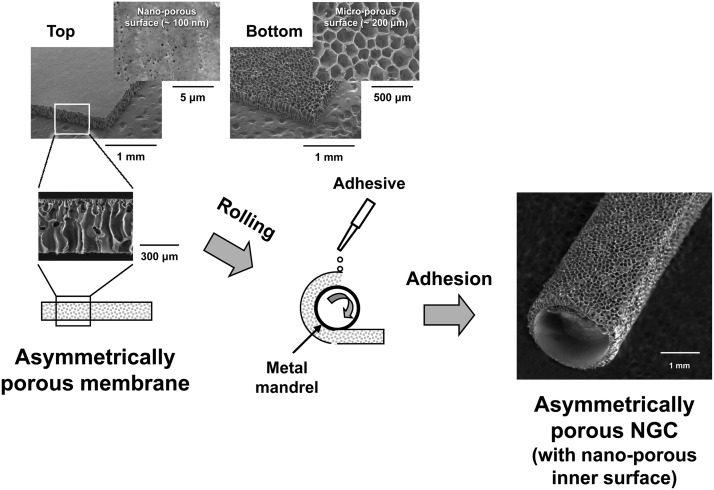
PCL/F127 NGC with selective permeability (nanoporous inner and microporous outer surfaces) was prepared by rolling an asymmetrically porous PCL/Pluronic F127 membrane fabricated using a novel immersion precipitation method.15 In brief, the PCL pellet was dissolved in hot tetraglycol (90°C, 12 wt%) and Pluronic F127 powder (5 wt%, PCL base) was added to the PCL solution. The hot polymer solution was cast in a mold (50×50×0.4 mm) and directly immersed in excess water for 1 h at room temperature. The asymmetrically porous PCL/F127 membrane was obtained after additional washing in excess water to remove residual solvent followed by vacuum drying. The asymmetrically porous membrane was rolled into a tube (NGC) using a metal mandrel (diameter, 1.5 mm) with the nanoporous surface of the membrane inside, and the overlapped edge of the membrane was adhered tightly using a tissue adhesive, N-butyl-2-cyanoacrylate (Histoacryl®; B. Braun) (Fig. 2). The prepared NGC had an ∼12-mm length, ∼0.4-mm wall thickness, and ∼1.5-mm inner diameter. The morphologies of the PCL/F127 membrane and NGC were observed by scanning electron microscopy (SEM, JSM-6335F; Jeol). The average pore sizes of the surfaces were determined using an image analysis program (i-solution; IMT) from the SEM images.
FIG. 2.
Schematic diagram showing the fabrication process of the NGC by rolling an asymmetrically porous PCL/Pluronic F127 membrane. PCL, polycaprolactone.
NGF to promote peripheral nerve regeneration after a peripheral nerve injury18,19 was incorporated onto the pore surfaces of the PCL/F127 NGC by heparin immobilization.15 To examine the NGF release profile, the NGF-immobilized NGCs were incubated in 1 mL phosphate-buffered saline (PBS) supplemented with 1% bovine serum albumin (BSA) at 37°C for up to 35 days under mild shaking (∼50 rpm). At preset time intervals, all media were collected and replaced with fresh ones. The amount of NGF released in the collected medium was determined using an enzyme-linked immunosorbent assay (ELISA) kit (Duoset®; R&D Systems).
Implantation of NGCs
Sprague-Dawley rats (weight, ∼250 g) were used to evaluate the dual biological (NGF) and physical (US) stimulation effect on peripheral nerve regeneration through the asymmetrically porous PCL/F127 NGC. Seventy-two rats were used for the analyses. The animals were divided into 4 groups (18 rats/group: NGC without stimulation [NGC], NGF-immobilized NGC [NGF/NGC], NGC with US stimulation [US/NGC], and NGF-immobilized NGC with US stimulation [NGF/US/NGC]). Each group was divided into six subgroups for the different analyses (three rats/subgroup): analyses for immunohistochemical evaluation (4 weeks); Toluidine blue staining, transmission electron microscopy (TEM), electrical physiological evaluation, and (gastrocnemius muscle) histological evaluation (4, 12, and 24 weeks); and FluoroGold (FG) retrograde tracing (12 and 24 weeks) (refer to evaluation methods below). This animal study was approved by the Animal Care Committee of the Hannam University in Korea, and all procedures were performed according to appropriate guidelines. Anesthesia was induced with an intramuscular injection of tiletamine/zolazepam (10 mg/kg, Zoletil 50®; Virbac Laboratories) and 2% xylazine hydrochloride (2 mg/kg, Rumpun®; Byely). The sciatic nerve on the left side was exposed, and a 10-mm segment of the nerve was removed from the mid-thigh level. Twelve-millimeter length NGCs were bridged between the proximal and distal stumps with two sutures (7-0 polydioxanone, Ethicon) at each junction. After implantation of the NGC, the muscle incision was closed using a 5-0 chromic catgut suture (Ethicon), and the skin was closed using a 5-0 nylon suture (Ethicon).
US treatment
The animals implanted with NGC and NGF/NGC received US stimulation using a portable US equipment currently being used in clinical practice (Omnisound® 3000; Physio Technology) with a small probe head (transducer diameter, 1.6 cm; area, 2 cm2). Pulsed US (2 ms ON and 8 ms OFF; 20%) with a frequency of 1 MHz and intensity of 0.4 W/cm2 was directly applied around the incision site for 2 min once per week for 24 weeks. In our preliminary study, we compared the nerve regeneration behavior among the different US treatments (2 min of daily treatment; 2 min of daily treatment for 1 week and following 2 min every week; and 2 min every week), and the optimum condition (2 min every week) was adapted in this study for enhanced nerve regeneration (data not shown). An aquasonic gel was used as a coupling medium on the incision site before US treatment. The first US irradiation was conducted at 24 h after surgery. The animal study for the NGC or NGC/NGF without the US treatment was also conducted for the purpose of comparison.
Immunohistochemical evaluation
Animals were sacrificed at predetermined periods for immunohistochemical analysis. The NGCs, including the regenerated nerve inside, were carefully dissected. After freezing immediately at −80°C, they were cut into transversal sections of 20 μm thickness (at 4 weeks; midportion of NGCs) to investigate nerve regeneration behavior from proximal to distal stumps through the NGCs. The specimens were mounted on positively charged slides (Fisher) and fixed with 4% paraformaldehyde and 4% sucrose in PBS for 45 min. Then, the specimens for anti-neurofilament staining were permeabilized with 0.5% Nonidet P-40 (Fluka) in PBS for 40 min at room temperature, and blocked with 2.5% horse serum and 2.5% BSA in PBS containing 10% Triton X-100 (PBST) for 12 h at 4°C. To visualize regenerated nerve fibers, the sections were subjected to immunofluorescence staining with primary antibodies against neurofilament-200 (NF-200, mouse monoclonal, 1:200; Sigma) and S100β (rabbit polyclonal, 1:400; Dako), respectively. Detection was accomplished by fluorescein-labeled goat anti-mouse IgG (for NF-200, 1:400; Invitrogen) or rhodamine-labeled goat anti-rabbit IgG (for S100β, 1:400; Invitrogen) secondary antibodies in 2.5% horse serum and 2.5% BSA in PBST for 1.5 h in a dark room. For cell nuclei detection, sections mounted on slides were stained with 2.5 μg/mL of Hoechst 33258 (bis-benzimide; Invitrogen) for 10 min before a final washing in 0.1% PBST. The stained sections were cover slipped with the aqueous mounting medium (Blomeda) and observed under a fluorescent microscope (Model BX51; Olympus).
Histological evaluation
Toluidine blue staining was conducted for the histological analysis, at 4, 12, and 24 weeks postimplantation. The regenerated nerve-containing NGCs were fixed with 2.5% glutaraldehyde in 0.1 M PBS for 2 h and postfixed in 1% osmium tetraoxide for 1.5 h. The specimens were dehydrated in a graded ethanol series, and embedded in Epon 812 resin (Polysciences). The midportions of the specimens were cut into thin cross sections with a 1-μm thickness using an ultramicrotome (MT-XL; RMC), stained with Toluidine blue, and observed under a light microscope. The diameter of myelinated axons, thickness of the myelin sheath, and total area of neural tissue in the regenerated nerves were evaluated using an image analysis program (i-solution) from the representative light microscope images of each animal (×1000 magnification). The specimens were also cut into ultrathin sections of 50–60 nm thickness to observe more detailed axon and myelin sheath regeneration through the NGCs. The sections were stained with lead citrate and uranyl acetate and then examined by TEM (Model H-7650; Hitachi).
The gastrocnemius muscles of NGC-implanted (left) and contralateral normal (right) sides were harvested and their wet weights were measured to evaluate the muscular atrophy caused by sciatic nerve denervation. Specimens were cut from the mid-belly of the gastrocnemius muscle and fixed with 4% formaldehyde in PBS. The specimens were embedded in paraffin wax and cut into 5-μm transverse sections. These sections were subjected to Masson's trichrome staining and observed under a light microscope. The diameter of muscle fibers and the percentage of collagen fiber area in the muscle sections were estimated using an image analysis program from the representative light microscope images of each animal (×200 magnification).
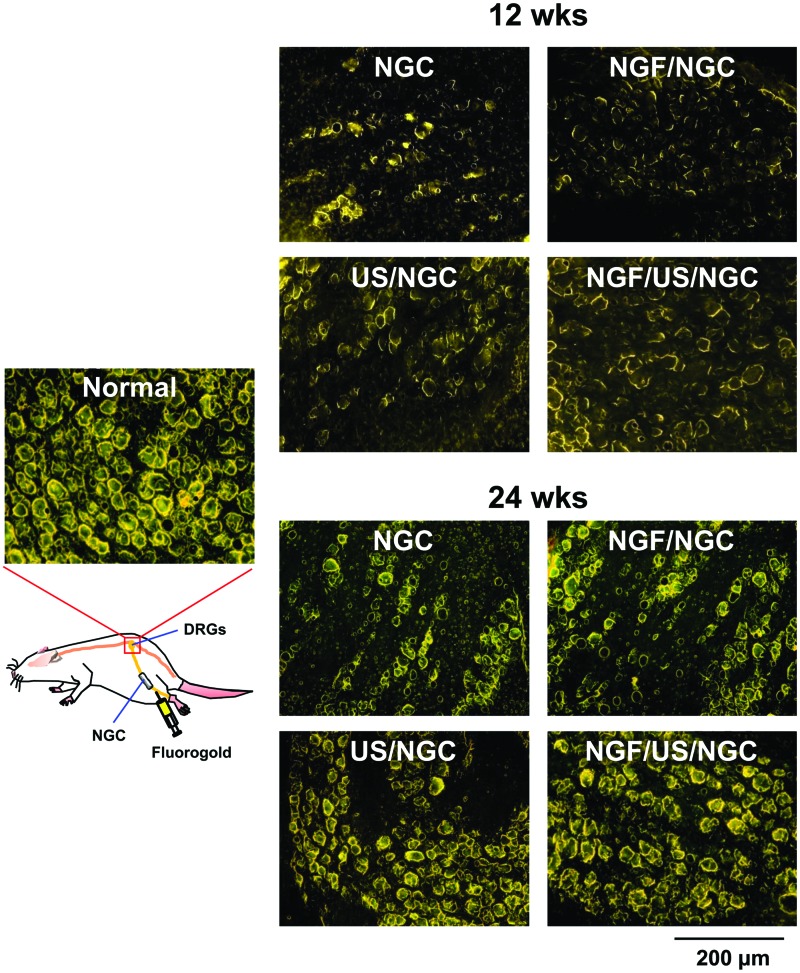
FG retrograde tracing
Retrograde tracing with FG was performed to examine the reinnervation between proximal and distal stumps at 12 and 24 weeks of NGC postimplantation. Thus, 10 μL of 5% FG in saline (Invitrogen) was injected into the sciatic nerve trunk positioned 10 mm from the distal end of the NGC. Animals were sacrificed 1 week after FG injection. Dorsal root ganglions (DRGs) at the lumbar five and six level of the spinal cord were dissected after exposing the spinal cord carefully. The specimens were frozen immediately at −80°C and were cut into transverse 20-μm-thick sections to investigate FG fluorescence of DRGs. The sections were mounted on positively charged slides and examined under a fluorescent microscope.
Electrophysiological evaluation
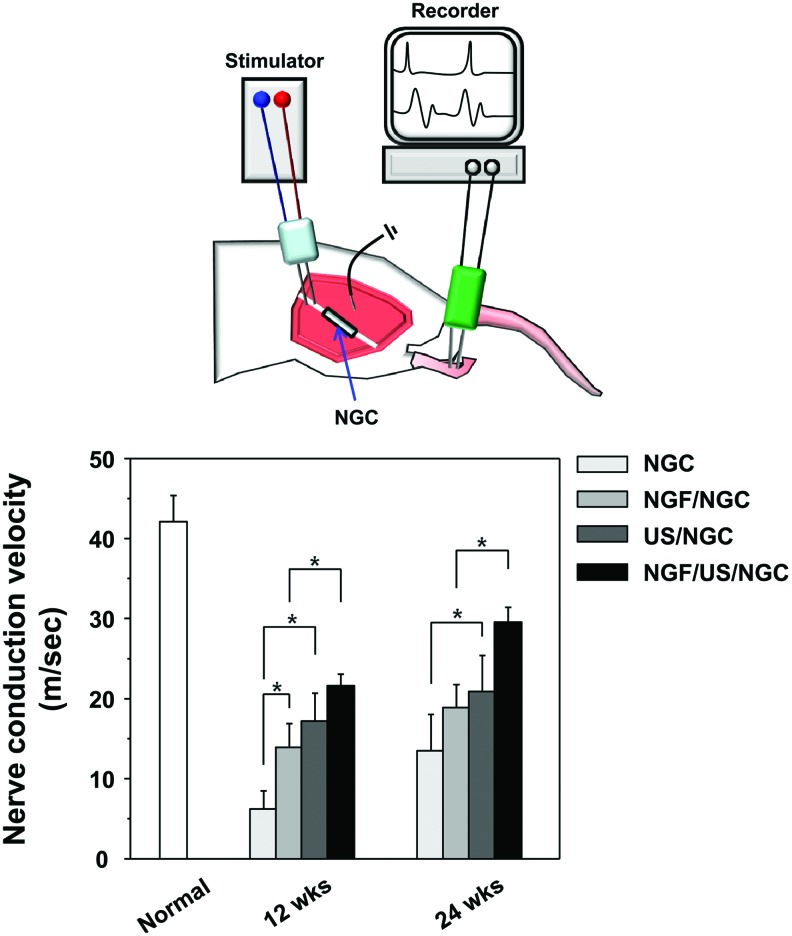
An electrophysiological evaluation was performed before animal sacrifice at 12 and 24 weeks postimplantation. Under anesthesia, the left sciatic nerve interposed by the NGC was carefully re-exposed and dissected from surrounding tissues. A recording needle electrode was placed in the anterior tibialis muscle and the sciatic nerve was stimulated by two stainless wire electrodes connected to a DC electrical stimulator (SI-10; Narco Bio-System). The nerve stimulation parameters used were 2 Hz pulses, 1 V strength, and 0.2 ms duration. A ground electrode was also placed in the surrounding muscle tissues to remove conduction of stimulation through muscle tissues. Nerve conduction velocity was obtained from the compound muscle action potentials recorded by an AxoScope system (Axon Instruments). The nerve conduction velocity was determined by measuring the difference in latency between known distances through the nerve.
Statistical analysis
The data obtained from each NGC group were averaged and expressed as the mean±standard deviation. The Student's t-test was used to determine differences between each NGC group. Differences were considered statistically significant at p<0.05.
Results
Characterization of NGF-immobilized PCL/F127 NGC
Figure 2 shows the gross appearance and detailed surface morphology of the PCL/F127 NGC with a nanoporous inner surface, which was prepared by rolling an asymmetrically porous PCL/Pluronic F127 membrane. The asymmetrically porous membrane was produced by placing the PCL/F127 mixture solution (in tetraglycol) in contact with water (nonsolvent for PCL) during the membrane fabrication process.15 The top surface (water contact side) of the membrane had nano-size pores (∼100 nm), whereas the opposite surface (mold contact side) had micro-size pores (∼200 μm), and both sides were connected by an asymmetric column-shape pore structure. The NGC can effectively prevent the infiltration of fibrous tissue (bigger than several micron sizes), but allow the permeation of nutrients (smaller than several nanometers) and retain neutrophic factors such as extracellular matrix molecules and Schwann cells, thus, providing an optimal environment for nerve regeneration.
NGF immobilization on the NGC and its release profile were quantitatively assessed by ELISA.15 The loading amount of NGF on the PCL/F127 NGC was estimated to be 324±65 ng. NGF was continuously released from the NGC up to ∼80% of the initial loading (∼260 ng) over 35 days.
Nerve regeneration evaluation
The sciatic nerve defect model in SD rats was used to evaluate the effect of dual biological (NGF) and physical (US) stimulation on peripheral nerve regeneration through the asymmetrically porous PCL/F127 NGC. All rats remained in good health condition and did not show any wound complications during the experiment. At explantation, any inflammatory signs or adverse tissue reactions were not seen.
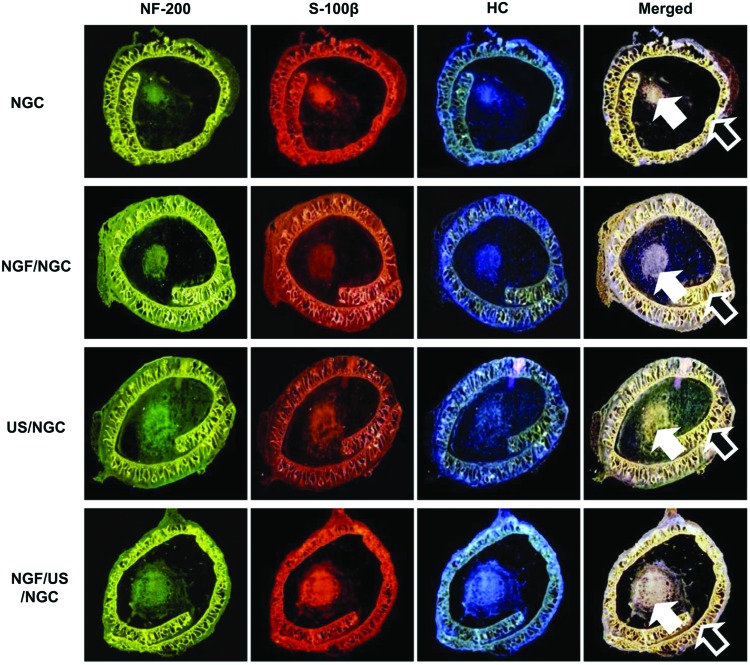
At 4 weeks postimplantation, the nerve regeneration behavior through the NGCs without and with different stimulation (single or dual stimulations of NGF and US) was observed using a fluorescent microscopy after immunofluorescence staining (NF-200 for axon fiber, S100β for myelin, and Hoechst for cell nuclei) using transversal sections at the mid-portion of the NGCs (Fig. 3). Each type of stimulation showed a positive effect on peripheral nerve regeneration through the NGC (thicker neural tissues than the NGC without stimulation), as expected. The US-stimulated group allowed more accelerated nerve regeneration behavior than the NGF-stimulated group. The group with dual NGF and US stimulation (NGF/US/NGC) showed the most effective nerve regeneration behavior compared to that in the other groups in our system. The fluorescent signals of the NGC (outer circles) are understood by autofluorescence of polymeric biomaterials.20
FIG. 3.
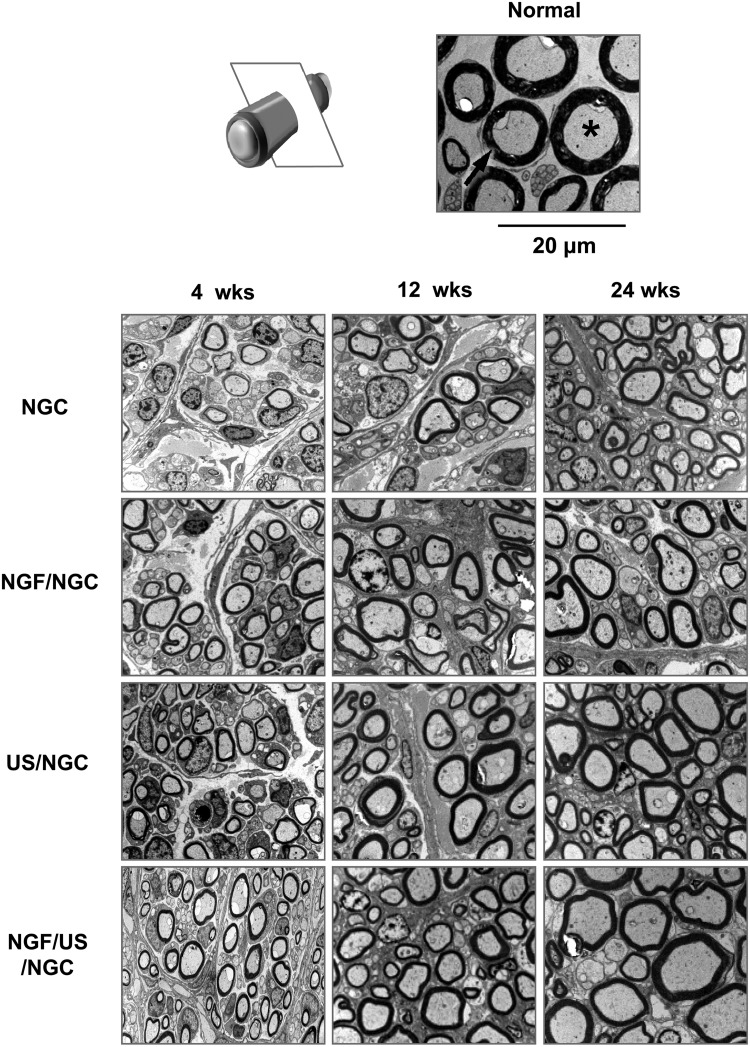
Transverse sections of the regenerated nerve through the asymmetrically porous PCL/F127 NGCs with different stimulations (4 weeks after implantation; mid-portion of NGCs, ×4; white arrow, neural tissues; black arrow, NGC wall). Each stimulation group showed a positive effect for peripheral nerve regeneration through the NGC. The US-stimulated group allowed more accelerated nerve regeneration behavior than that in the NGC groups without stimulation and with NGF stimulation. The group with dual stimulation (NGF/US/NGC) showed the most effective nerve regeneration behavior compared to that in the other groups. Color images available online at www.liebertpub.com/tea
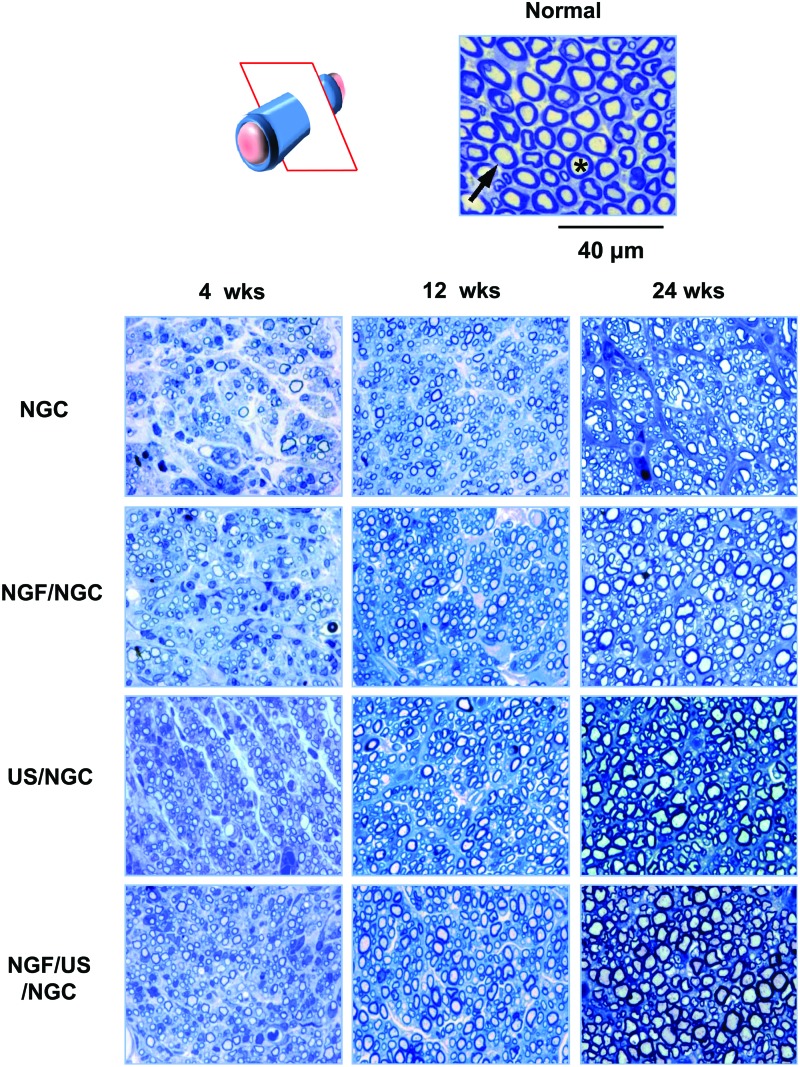
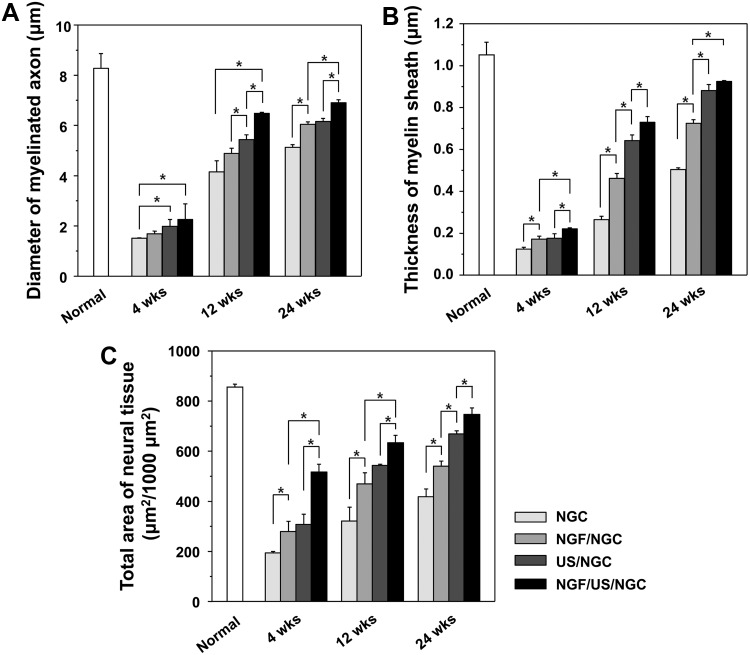
Further histological evaluations on nerve regeneration behaviors were performed among the NGCs without and with different stimulations (single or dual stimulations of NGF and US) at 4, 12, and 24 weeks postimplantation. The regenerated nerve in the NGCs was observed at the middle section under a light microscope after Toluidine blue staining (Fig. 4) and by TEM to provide a more detailed view of the myelinated axons in the NGCs (Fig. 5). The NGC group stimulated with both NGF and US showed a larger axon diameter and a thicker myelin sheath than those in the other groups, indicating better nerve regeneration. Figure 6 compares the diameter of myelinated axons, thickness of the myelin sheath, and area occupied by neural tissue in the NGCs with those of normal rat nerves. It was observed that neural tissues, including myelinated axons and myelin sheath were continuously grown in all NGCs with time. The NGF/US/NGC group showed a significantly larger neural tissue area than that in the other groups. The area occupied by neural tissue in NGF/US/NGC after 24 weeks implantation was ∼87% (747.1±25.9 μm2/1000 μm2) relative to the normal nerve (855.9±11.9 μm2/1000 μm2). The NGC, NGF/NGC, and US/NGC groups showed less neural tissue area compared with NGF/US/NGC; ∼49% (418.4±31.3 μm2/1000 μm2), ∼63% (539.9±20.5 μm2/1000 μm2), and ∼78% (668.8±12.2 μm2/1000 μm2), respectively, relative to the normal nerve values.
FIG. 4.
Light micrographs of semithin sections showing myelinated axons at the mid-portion of the NGC (12 and 24 weeks after implantation; Toluidine blue staining; ×1000; asterisk, axon; arrow, myelin). Color images available online at www.liebertpub.com/tea
FIG. 5.
Transmission electron microscopy images of ultrathin sections showing myelinated axons at the mid-portion of the NGCs (12 and 24 weeks after implantation; ×3000; asterisk, axon; arrow, myelin). The myelinated axons and myelin sheath were continuously grown in all NGCs with time. The NGF/US/NGC group showed the larger axon diameter and a thicker myelin sheath than that in the NGC, NGF/NGC, and US/NGC groups, indicating better nerve regeneration.
FIG. 6.
Comparison of (A) the diameter of the myelinated axon, (B) thickness of myelin sheath, and (C) area occupied by neural tissue in the NGC at the mid-portion at 4, 12, and 24 weeks after implantation (n=3; *p<0.05). Neural tissues, including myelinated axons and myelin sheath grew continuously in all NGCs with time. The NGF/US/NGC group showed significantly larger neural tissue areas than those in the other groups.
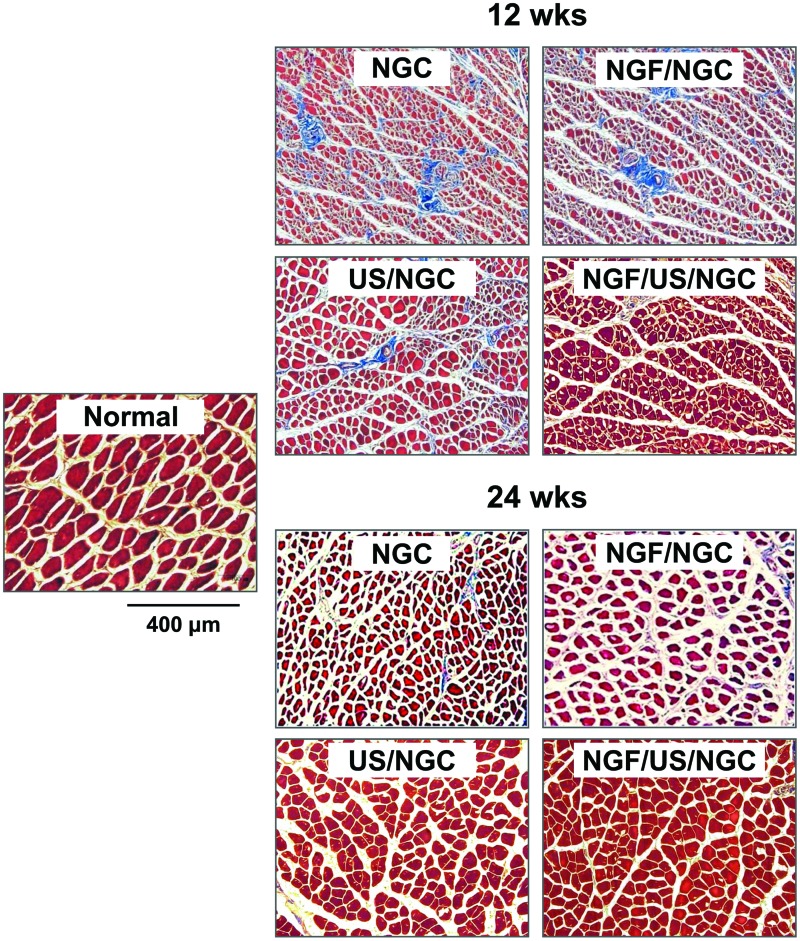
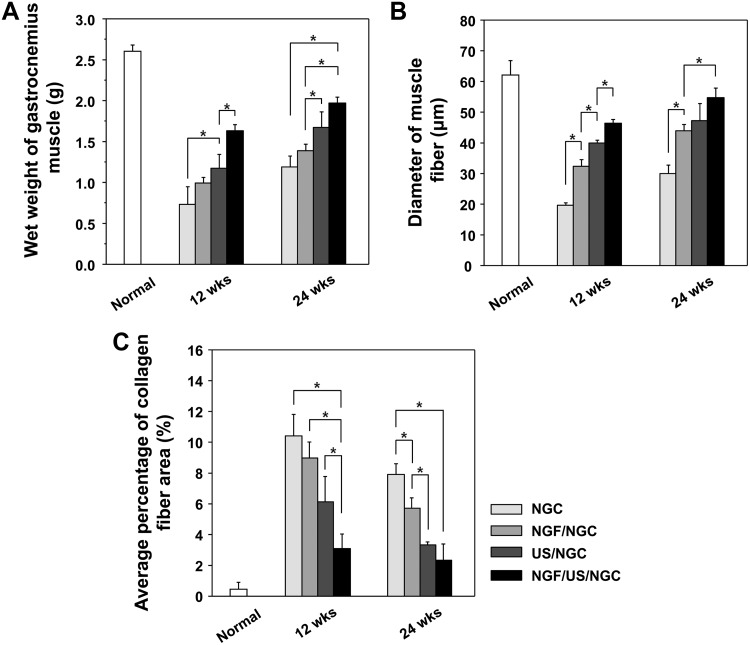
To evaluate the extent of reinnervation of target muscle, the wet weight, diameter of muscle fibers, and collagen fiber area in the gastrocnemius muscle were compared among the NGCs without and with the different stimulation treatments (single or dual stimulations of NGF and US). All NGC groups showed reduced wet weight of muscle, diminished muscle fibers, and increased collagen fibers when compared to those in the normal group, suggesting that muscle atrophy was caused by the severe nerve defect (Figs. 7 and 8). However, the muscles recovered gradually from atrophy with time (an increased wet weight of muscle and diameter of muscle fiber, but a decreased collagen fiber area), regardless of stimulation, suggesting that the NGCs may provide a good environment for nerve regeneration and thus allow appropriate muscle reinnervation. The NGF/US/NGC group showed significantly faster recovery from muscle atrophy compared with the other groups with time (a greater wet weight of muscle, a thicker diameter of muscle fiber, and a smaller collagen fiber area), indicating better nerve regeneration.
FIG. 7.
Light micrographs of gastrocnemius muscles of NGC-implanted and contralateral normal sides (Masson's trichrome staining; ×100). The muscles recovered gradually from the atrophy with time (increased diameter of muscle fiber and decreased collagen fiber area). The NGF/US/NGC group showed faster recovery from the muscle atrophy than that in the other groups, indicating better nerve regeneration. Color images available online at www.liebertpub.com/tea
FIG. 8.
Comparison of (A) wet weight of gastrocnemius muscle, (B) diameter of the muscle fibers, and (C) percentage of collagen fiber area at 12 and 24 weeks after NGC implantation (n=3; *p<0.05). The NGF/US/NGC group showed faster recovery from muscle atrophy compared with the other groups.
To determine whether the reconnection between proximal and distal stumps was induced through the NGCs or not, FG retrograde tracing was performed. FG-labeled neuron cell bodies (gold color) were observed in the DRGs of all NGCs. As shown in Figure 9, the number of cells increased gradually with time, indicating that the reconnection between defect nerves occurred continuously. The NGF/US/NGC group contained more FG-labeled neuron cells compared to those in the other groups, indicating that greater nerve fibers connecting the defect stumps were present. The nerve reconnection rates through NGCs were as follows:
FIG. 9.
Fluorescent micrographs following FG retrograde tracing in the DRGs of the NGC-implanted groups at 1 week after FG injection (FG injection was conducted at 12 and 24 weeks of postimplantation; ×100). The number of FG-labeled neuron cell bodies (gold color) increased gradually with time, indicating that the reconnection between defective nerves was occurring continuously. The NGF/US/NGC group showed more FG-labeled neuron cells compared to the other groups, indicating that the greater nerve fibers connecting the defect stumps were present. FG, FluoroGold; DRG, dorsal root ganglion. Color images available online at www.liebertpub.com/tea
NGF/US/NGC>US/NGC>NGF/NGC>NGC.
An electrophysiological analysis was performed to determine whether functional reinnervation occurred through the NGCs. The nerve conduction velocities of the NGCs treated with different simulations after 12 and 24 weeks of implantation were compared with that of the normal nerve shown in Figure 10. The nerve conduction velocities of all the NGC groups increased continuously with time. In particular, the NGF/US/NGC group showed significantly faster nerve conduction velocity than that in the NGC group, which received no or single stimulation, indicating greater functional reinnervation of the damaged nerve. The nerve conduction velocity of the NGF/US/NGC (29.6±1.8 m/s) after 24 weeks of implantation was about 69% of the normal nerve (42.1±3.3 m/s). The difference in the nerve conduction velocity between the NGC groups that received a single stimulation (NGF/NGC and US/NGC) after 24 weeks of implantation was not significant.
FIG. 10.
Comparison of the nerve conduction velocity at 12 and 24 weeks after NGC implantation (n=3; *p<0.05). The nerve conduction velocities of all NGC groups increased gradually with time. In particular, the NGF/US/NGC group showed significantly faster nerve conduction velocity than that in the other groups. The difference in nerve conduction velocity between the NGC groups that received single stimulation (NGFNGC and US/NGC) after 24 weeks of implantation was not significant. Color images available online at www.liebertpub.com/tea
Discussion
It is well established that a distal nerve stump after injury, such as a cut through the nerve fiber, separates from its cell body and starts to degenerate in a series of steps called Wallerian degeneration.17 Nerve regeneration is initiated from the proximal stump to distal stump through proliferating Schwann cells in the basal lamina, such as the Büngner's band.19,21 Although autologous nerve grafts have been used to bridge the nerve defects and have been a gold standard in the clinical field for nerve regeneration over the last 50 years, it's inevitable limitations, including the low success rate on treated patients (<50%), the need for a second surgical procedure to extract the donor nerve, a permanent loss of the donor nerve function, a limited supply of available grafts, and a mismatch in the axonal size/distribution/alignment between the defective nerves (usually pure motor [tibial] or motor/sensor mixed nerves [sciatic]) and graft nerve (commonly sensory nerves), still remain.22–25 Thus, an artificial NGC has been commonly accepted as an alternative technique that can correctly guide the growth of nerve fibers and provide an appropriate environment for nerve regeneration.6,7 A variety of tissues and natural/synthetic polymers have been utilized as NGCs; however, NGCs also have intrinsic drawbacks to apply for clinical use, including relatively slow morphological and functional recovery and limited nerve regeneration through NGC, because they cannot stimulate Schwann cell migration and provide an appropriate substrate to support neurite outgrowth.1,26,27
In this study, NGF (as a biological stimulus) was incorporated onto the pore surfaces of an asymmetrically porous PCL/F127 NGC with a nanoporous inner surface (Fig. 2) through heparin immobilization, and the low-intensity pulsed US (as a simple and noninvasive physical stimulus) was additionally applied at the NGF-immobilized NGC-implanted site transcutaneously in rats to investigate the feasibility of a dual stimulation system for accelerated nerve regeneration through the NGC and to evaluate its potential use as a clinically useful therapeutic system. The NGF is a well recognized growth factor that promotes peripheral nerve regeneration and prevents nerve cell loss after peripheral nerve damage.28,29
The NGF immobilized on the NGC (∼324 ng) released gradually from the NGC up to ∼80% of the initial loading for 35 days15 to promote continuous nerve regeneration (cumulative amount of NGF, ∼260 ng after 35 days). NGF loading amounts in NGCs applied for peripheral nerve regeneration appeared to be diverse in the literatures; Wang et al.30 incorporated NGF into aligned core shell nanofiber NGCs (similar size NGC used in this study) with the loading amount of NGF, ∼200 ng/NGC and showed the continuous release of NGF from the NGC, up to ∼65% of the initial loading for 30 days with a sharp initial burst during the first 3 days, while Dodla et al.31 reported the cumulative amount of NGF released over 18 days, ∼60 ng/NGC (double-size length of NGC used in this study) without a sharp initial burst. Low-intensity pulsed US has been widely utilized for therapeutic purposes in various soft tissue systems,32,33 however, its effects on damaged peripheral nerve are still unclear. It has been hypothesized that US, which is mainly compression-type acoustic waves, may generate a deforming environment in the path as the low-intensity US passes through soft tissue, inducing a certain level of stress to the affected area and thus producing some stimulatory effects at the cellular level.34
The animal study showed that NGF or US stimulation has a positive effect to promote the peripheral nerve regeneration through the NGC; however, the US-stimulated group allowed more accelerated nerve regeneration than the NGF-stimulated group. The group with both NGF and US stimulation had the most effective nerve regeneration behavior compared to that in the other groups in our system. The stimulatory effect of NGF on nerve regeneration is understood by its inherent biological function, which can lead to neuronal survival and axonal growth, and thus allow functional reinnervation of injured peripheral nerves.35 A more detailed mechanism of neutrophic factors, including NGF for nerve repair, is described well by Bell.36 Some investigators have demonstrated that a NGF solution-containing silicone chamber shows more mature peripheral nerve regeneration (more myelinated axons, greater thickness of myelin sheaths, etc.) and an earlier recovery of sensory function compared to a control solution-containing chamber.37,38 We expected that the sustained release of NGF (up to 35 days) from the NGC in our system would maximize its biological effectiveness for nerve regeneration, allowing for prolonged bioactivity of the growth factor.39 Although the mechanism of the therapeutic effect of US is still unclear, it is believed that low-intensity US stimulates Schwann cells, which aid in myelinating axons, directionally guiding neurons, and playing a predominant role in peripheral nerve regeneration.40,41 Madison et al.42 demonstrated that Schwann cells are the richest source of neurotrophic factors for peripheral nerve regeneration. It was also revealed that low-intensity US activates Schwann cells to produce neurotrophic proteins.43 Continuous and long-term secretion of neurotrophic factors from US-stimulated Schwann cells may be the reason why the US/NGC group allowed more promoted nerve regeneration than the NGF/NGC group (NGF was released from the NGC up to 5 weeks of postimplantation in our system) (Figs. 3–10). The NGF/US/NGC group, which received dual stimulation showed increased nerve regeneration behavior compared to that in the single stimulation groups (NGF/NGC and US/NGC), expecting the synergistic or at least additive effect on both biological and physical stimulations. This phenomenon may be explained by the sustained release of NGF from the NGC, which can prolong bioactivity of the growth factor16 (although the NGF is released during the relatively short period compared to that of nerve regeneration) and the continuous production of neurotrophic factors from Schwann cells activated by US. Alrashdan et al.44 also reported that low-intensity electricity (physical) stimulation or recombinant adenoviral vector-mediated brain-derived neurotrophic factor (biological) stimulation improved peripheral nerve regeneration when a single stimulation was applied; however, their combined stimulation did not show any further positive effect compared to the single stimulation. Based on our findings, we suggest that the NGF/US/NGC system could be a simple and effective therapeutic technique for peripheral nerve regeneration, and thus may be a very helpful strategy to apply for long-gap nerves to accelerate the reinnervation rate and provide sufficient functional recovery of peripheral nerves, which are pivotal criteria for clinical applications.
Acknowledgment
This study was supported by the Midcareer Research Program through a grant from the National Research Foundation (NRF) of Korea (No. 2011-0000244).
Disclosure Statement
No competing financial interests exist.
References
- 1.Mukhatyar V. Karumbaiah L. Yeh J. Bellamkonda R. Tissue engineering strategies designed to facilitate the endogenous regenerative potential of peripheral nerves. Adv Mater. 2009;21:4670. [Google Scholar]
- 2.Noble J. Munro C.A. Prasad V.S. Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a multi-trauma population. J Trauma. 1998;45:116. doi: 10.1097/00005373-199807000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Kingham P.J. Terenghi G. Bioengineered nerve regeneration and muscle reinnervation. J Anat. 2006;209:511. doi: 10.1111/j.1469-7580.2006.00623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S. Cai Q. Hou J. Bei J. Zhang T. Yang J., et al. Acceleration effect of basic fibroblast growth factor on the regeneration of peripheral nerve through a 15-mm gap. J Biomed Mater Res. 2003;66:522. doi: 10.1002/jbm.a.10008. [DOI] [PubMed] [Google Scholar]
- 5.Gluck T. Ueber neuroplastik aut dem wege der transplantation. Arch Klin Chir. 1880;25:606. [Google Scholar]
- 6.Maquet V. Martin D. Malgrange B. Franzen R. Schoenen J. Moonen G., et al. Peripheral nerve regeneration using bioresorbable macroporous polylactide scaffolds. J Biomed Mater Res. 2000;52:639. doi: 10.1002/1097-4636(20001215)52:4<639::aid-jbm8>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Heath C.A. Rutkowski G.E. The development of bioartificial nerve grafts for peripheral-nerve regeneration. Trends Biotechnol. 1998;16:163. doi: 10.1016/s0167-7799(97)01165-7. [DOI] [PubMed] [Google Scholar]
- 8.Oh S.H. Lee J.H. Fabrication and characterization of hydrophilized porous PLGA nerve guide conduits by a modified immersion precipitation method. J Biomed Mater Res. 2007;80A:530. doi: 10.1002/jbm.a.30937. [DOI] [PubMed] [Google Scholar]
- 9.Fine E.G. Decosterd I. Papaloizos M. Zurn A.D. Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 10.Tessa H. Cathryn S. Daniel H. Mack C. Joseph P. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh Y.L. Chou L.W. Chang P.L. Yang C.C. Kao M.J. Hong C.Z. Low-level laser therapy alleviates neuropathic pain and promotes function recovery in rats with chronic constriction injury: possible involvements in hypoxia-inducible factor 1α (HIF-1α) J Comp Neurol. 2012;520:2903. doi: 10.1002/cne.23072. [DOI] [PubMed] [Google Scholar]
- 12.Pomeranz B. Mullen M. Markus H. Effect of applied electrical fields on sprouting of intact saphenous nerve in adult rat. Brain Res. 1984;303:331. doi: 10.1016/0006-8993(84)91219-8. [DOI] [PubMed] [Google Scholar]
- 13.Crisci A.R. Ferreira A.L. Low-intensity pulsed ultrasound accelerates the regeneration of the sciatic nerve after neurotomy in rats. Ultrasound Med Biol. 2002;28:1335. doi: 10.1016/s0301-5629(02)00576-8. [DOI] [PubMed] [Google Scholar]
- 14.Oh S.H. Kim T.H. Chun S.Y. Park E.K. Lee J.H. Enhanced guided bone regeneration by asymmetrically porous PCL/Pluronic F127 membrane and ultrasound stimulation. J Biomater Sci. 2012;23:1673. doi: 10.1163/092050611X589518. [DOI] [PubMed] [Google Scholar]
- 15.Oh S.H. Kim J.R. Kwon G.B. Namgung U. Song K.S. Lee J.H. Effect of surface pore structure of nerve guide conduit on peripheral nerve regeneration. Tissue Eng Part C Methods. 2013;19:233. doi: 10.1089/ten.tec.2012.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh S.H. Kim J.H. Song K.S. Jeon B.H. Yoon J.H. Seo T.B., et al. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials. 2008;29:1601. doi: 10.1016/j.biomaterials.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Park S.C. Oh S.H. Lee J.H. Fabrication and characterization of nerve growth factor-immobilized asymmetrically porous PDOCL/Pluronic F127 nerve guide conduit. Tissue Eng Regen Med. 2011;8:192. [Google Scholar]
- 18.Pan Y.A. Misgeld T. Lichtman J.W. Sanes J.R. Effects of neurotoxic and neuroprotective agents on peripheral nerve regeneration assayed by time-lapse imaging in vivo. J Neurosci. 2003;23:11479. doi: 10.1523/JNEUROSCI.23-36-11479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salzer J.L. Bunge R.P. Studies of Schwann cell proliferation: I. An analysis in tissue culture of proliferation during development, Wallerian degeneration, and direct injury. J Cell Biol. 1980;84:739. doi: 10.1083/jcb.84.3.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaafar I.H. LeBlon C.E. Wei M.T. Ou-Yang D. Coulter J.P. Jedlicka S.S. Improving fluorescence imaging of biological cells on biomedical polymers. Acta Biomater. 2011;7:1588. doi: 10.1016/j.actbio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Clark R.K. Anatomy and Physiology: Understanding the Human Body. Sundbury: Jones and Bartlett Publishers; 2005. [Google Scholar]
- 22.Lee S.K. Wolfe S.W. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Pabari A. Yang S.Y. Seifalian A.M. Mosahebi A. Modern surgical management of peripheral nerve gap. J Plast Reconstr Aesthet Surg. 2010;63:1941. doi: 10.1016/j.bjps.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 24.Nichols C.M. Brenner M.J. Fox I.K. Tung T.H. Hunter D.A. Rickman S.R. Mackinnon S.E. Effects of motor versus sensory nerve grafts on peripheral nerve regeneration. Exp Neurol. 2004;190:347. doi: 10.1016/j.expneurol.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Brenner M.J. Hess J.R. Myckatyn T.M. Hayashi A. Hunter D.A. Mackinnon S.E. Repair of motor nerve gaps with sensory nerve inhibits regeneration in rats. Laryngoscope. 2006;116:1685. doi: 10.1097/01.mlg.0000229469.31749.91. [DOI] [PubMed] [Google Scholar]
- 26.Kehoe S. Zhang X.F. Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 27.de Ruiter G.C.W. Malessy M.J.A. Yaszemski M.J. Windebank A.J. Spinner R.J. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26:E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun W. Sun C. Lin H. Zhao H. Wang J. Ma H. Chen B. Xiao Z. Dai J. The effect of collagen-binding NGF-beta on the promotion of sciatic nerve regeneration in a rat sciatic nerve crush injury model. Biomaterials. 2009;30:4649. doi: 10.1016/j.biomaterials.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Otto D. Unsicker K. Grothe C. Pharmacological effects of nerve growth factor and fibroblast growth factor applied to the transectioned sciatic nerve on neuron death in adult rat dorsal root ganglia. Neurosci Lett. 1987;83:156. doi: 10.1016/0304-3940(87)90233-3. [DOI] [PubMed] [Google Scholar]
- 30.Wang C.Y. Liu J.J. Fan C.Y. Mo X.M. Ruan H.J. Li F.F. The effect of aligned core-shell nanofibers delivering NGF on the promotion of sciatic nerve regeneration. J Biomater Sci Polym Edn. 2012;23:167. doi: 10.1163/092050610X545805. [DOI] [PubMed] [Google Scholar]
- 31.Dodla M.C. Bellamkonda R.V. Differences between the effect of anisotropic and isotropic laminin and nerve growth factor presenting scaffolds on nerve regeneration across long peripheral nerve gaps. Biomaterials. 2008;29:33. doi: 10.1016/j.biomaterials.2007.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young S.R. Dyson M. The effect of therapeutic ultrasound on angiogenesis. Ultrasound Med Biol. 1990;16:261. doi: 10.1016/0301-5629(90)90005-w. [DOI] [PubMed] [Google Scholar]
- 33.Dyson M. Suckling J. Stimulation of tissue repair by ultrasound: a survey of the mechanisms involved. Physiotherapy. 1978;64:105. [PubMed] [Google Scholar]
- 34.Chi J.H. Park K. Park S.R. Min B.H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: an in vitro study. Tissue Eng. 2006;12:75. doi: 10.1089/ten.2006.12.75. [DOI] [PubMed] [Google Scholar]
- 35.Lewin G.R. Barde Y.A. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 36.Bell C. Chemical Factors in Neural Growth, Degeneration and Repair. Amsterdam: Elsevier; 1996. [Google Scholar]
- 37.Rich R. Alexander T. Pryor J. Hollowell J.P. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105:162. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 38.Derby A. Engleman V.W. Frierdich G.E. Neises G. Rapp S.R. Roufa D.G. Nerve growth factor facilitates regeneration across nerve gaps: morphological and behavioral studies in rat sciatic nerve. Exp Neurol. 1993;119:176. doi: 10.1006/exnr.1993.1019. [DOI] [PubMed] [Google Scholar]
- 39.Xu X. Yu H. Gao S. Ma H.Q. Leong K.W. Wang S. Polyphosphoester microspheres for sustained release of biologically active nerve growth factor. Biomaterials. 2002;23:3765. doi: 10.1016/s0142-9612(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 40.Rath E.M. Kelly D. Bouldin T.W. Popko B. Impaired peripheral nerve regeneration in a mutant strain of mice (Enr) with a Schwann cell defect. J Neurosci. 1995;15:7226. doi: 10.1523/JNEUROSCI.15-11-07226.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhatheja K. Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Madison R.D. Zormorodi A. Robinson G.A. Netrin-1 and peripheral nerve regeneration in the adult rat. Exp Neurol. 2000;161:563. doi: 10.1006/exnr.1999.7292. [DOI] [PubMed] [Google Scholar]
- 43.Zhang H. Lin X. Wan H. Li J.H. Li J.M. Effect of low-intensity pulsed ultrasound on the expression of neurotrophin-3 and brain-derived neurotrophic factor in cultured Schwann cells. Microsurgery. 2009;29:479. doi: 10.1002/micr.20644. [DOI] [PubMed] [Google Scholar]
- 44.Alrashdan M.S. Sung M.A. Kim Kwon Y.H. Chung H.J. Kim S.J. Lee J.H. Effects of combining electrical stimulation with BDNF gene transfer on the regeneration of crushed rat sciatic nerve. Acta Neurochir (Wien) 2011;153:2021. doi: 10.1007/s00701-011-1054-x. [DOI] [PubMed] [Google Scholar]