Abstract
It has been postulated that drugs of abuse act synergistically with HIV, leading to increased neurotoxicity and neurocognitive impairment. The CNS impacts of HIV and drug use converge on the mesocorticolimbic dopamine (DA) system, which contains two main receptor subtypes: dopamine receptor 1 and 2. (DRD1, DRD2). DRD1 and DRD2 have been linked to substance dependence; whether they predict HIV-associated neurocognitive disorder (HAND) is unclear. Using an advanced-stage HIV+ population, we sought to determine if drug dependence impacts the contribution of DA receptor polymorphisms on neurocognition. We observed that both DRD1 and DRD2 polymorphisms were associated with opiate and cocaine dependence (P<0.05) in Caucasian subjects, but not African-American individuals. Using linear regression analysis, we examined the polymorphisms for associations with neuropsychological performance in global and cognitive domain T-scores (Motor, Processing Speed, Verbal Fluency, Learning, Memory, Executive Functioning, Working Memory) while controlling for opiate and cocaine dependency. In the Motor domain, we observed an association for two DRD2 polymorphisms (P<0.05) in Caucasian subjects. The effects differed for substance dependence groups as the direction of the correlations with DRD2 were opposite to what was seen in subjects without these dependencies. In African-American subjects, associations were observed in nearly every domain and again, the direction of the correlation differed between substance dependent and independent groups. We conclude that studies to examine genetic risk for HAND must carefully account for substance dependence patterns when assaying dopaminergic systems, as the neurobiological substrates of cognition in HIV populations may vary with tonic alterations secondary to chronic substance exposures.
Keywords: HAND, cocaine, opiate, SNP
Introduction
Despite the widespread use of efficacious antiretroviral therapies, HIV-associated neurocognitive disorder (HAND) remains highly prevalent, and its dissociation from HIV replication makes it imperative to understand non-viral neurobiological factors related to its pathogenesis (Heaton et al, 2010; Heaton et al, 2011; McArthur, 2004; Sacktor et al, 2002). Dopamine (DA) dysregulation has been associated with HAND, and converging lines of evidence have implicated brain regions rich in DA (basal ganglia and related structures) as those highly susceptible to the effects of HIV. Decreased levels of DA have been reported in the basal ganglia of HIV+ brains and viral RNA levels are negatively correlated with DA levels (Kumar et al, 2009; Kumar et al, 2011). As HIV disease progresses, decreased concentrations of DA and homovanillic acid (HVA) have been found in the cerebrospinal fluid (Berger et al, 1994; di Rocco et al, 2000; Kumar et al, 2009; Kumar et al, 2011; Larsson et al, 1991; Obermann et al, 2009). In cognitively characterized subjects, imaging studies have revealed decreases in dopamine transporter in the putamen of those with HIV-associated dementia when contrasted with HIV-negative subjects (Chang et al, 2008). Recently, expression of DRD2 in dorsolateral prefrontal cortex has been correlated with the cognitive status of HIV-infected individuals (Gelman et al, 2012).
While there is evidence of association between polymorphisms for DA-related genes and neurocognitive functioning in HIV-negative populations (Frank and Fossella, 2011), studies of these associations in HAND have thus far been negative (Levine et al, 2012a; Levine et al, 2012b). HAND studies are limited in number; a greater number of genetic association studies in HAND have examined immunologic, metabolic and other non-dopaminergic genes (Bol et al, 2012; Levine et al, 2009; Pemberton et al, 2008; Spector et al, 2010). More recently, genome-wide association studies (GWAS) have focused on phenotypes such as HIV RNA viral load and disease progression (Aouizerat et al, 2011). Only one GWAS in HIV has utilized cognition as an endpoint, and it did not elucidate any dopaminergic associations (Levine et al, 2012a).
One limitation in current studies of dopaminergic polymorphisms and HAND is that there has not been a full accounting of substance dependence phenotypes. We are aware of only one study of HAND with a small percentage of substance users (2–5%) that included this variable at the analytic level (Levine et al, 2012a). It is well documented that altered dopaminergic function underlies many forms of drug addiction, and despite their varied mechanisms of action, drugs of dependence lead to increased DA release in the central nervous system (Di Chiara and Imperato, 1988). Thus, it is reasonable to postulate that addiction may significantly mediate the relationship between HAND and dopamine genetics.
Substance use is one of the most common comorbidities associated with HIV. Of individuals living with HIV in New York City (NYC) in 2010, 18.5% had injection drug use (IDU) as a risk factor for infection (NYC Dept of Mental Health and Hygiene, 2012). In NYC, nearly one million individuals reported using illicit substances in 2010 regardless of HIV status (NYC Dept of Mental Health and Hygiene, 2010). Among the substances of choice available for use, cocaine and opiate use have greatly increased in NYC in the past ten years; both have direct relevance to dopaminergic function. Chronic cocaine and heroin users have decreased DA receptor availability and expression (Jacobs et al, 2012; Volkow et al, 1993; Volkow et al, 2007). Furthermore, these two substances have been consistently linked to genetic associations with SNPs of both DRD1 and DRD2 (Jacobs et al, 2012; Lawford et al, 2000; Li et al, 2006; Moyer et al, 2011; Noble et al, 1993; Perez de los Cobos et al, 2007; Persico et al, 1996; Shahmoradgoli Najafabadi et al, 2005).
Given the overlap of HIV, HAND and substance use disorders, it was the goal of this pilot project to investigate whether genetic correlates of HAND are associated with substance dependence, utilizing a population with a high prevalence of well-characterized substance dependence and detailed neuropsychologic assessments. Our hypothesis was that individual polymorphisms of both DRD1 and DRD2 would be significantly and independently associated with cognitive functioning in HIV+ subjects, once their anticipated associations with cocaine and opiate dependency were accounted for.
Materials and Methods
Patient population
The sample consisted of 250 participants in the Manhattan HIV Brain Bank (U01MH083501; U24MH100931). This study operates at the Icahn School of Medicine at Mount Sinai under IRB approval; subjects can opt in or out of genetic analyses. Individuals were included in the current study if they were HIV positive, assented to genetic analysis, and had available neuropsychologic and substance dependence data at study entry. At entry, approximately two-thirds of the MHBB cohort are on combination antiretroviral therapy (cART); of these, over 90% have had prior exposure to ARVs. Thus, this is a highly ARV experienced population. Of 267 individuals identified by substance/neuropsychologic criteria, 17 (approximately 6%) reported being of mixed racial ancestry, or having a racial identity other than White/Caucasian or Black/African American. As analyses were stratified by racial composition (see below), these individuals were not utilized in the study.
Subject characterization
Substance dependence characterization
DSM-IV diagnoses of opiate and/or cocaine dependence were obtained by administration of the Psychiatric Research Interview for Substance and Mental Disorders (Hasin et al, 1996; Morgello et al, 2001). Additionally, results of urine toxicologies for illicit substances, performed at 6-month intervals, were reviewed. Subjects who did not self-report dependence, but who displayed chronically positive urine toxicology for illicit opiates or cocaine over years of observation, were placed in the substance dependence category (n=8; all subjects were African-American). Subjects without cocaine and opiate dependence were individuals with no DSM-IV diagnoses of opiate or cocaine dependency with the PRISM, and consistent negative toxicology for illicit substances. We studied a total of 76 individuals without substance dependence (45 males, 31 females; average age 52.2 ± 9.5) and 174 substance dependent participants (95 males, 79 females; average age 51.5 ± 6.9). Race and ethnicity were determined by self-report; for first pass analyses in this study, Hispanics identifying as Black were characterized as African American, and those identifying as White were characterized as Caucasian. Additional analyses were then performed on the Hispanic subjects alone. Racially, 59% percent of the population was African American and 41% Caucasian. See Table 1A, Table 1B, and Table 1C for full population information.
Table 1A.
Demographics of the Caucasian study sample.
| Non-Substance Dependent | Substance Dependent | P-value | ||
|---|---|---|---|---|
|
|
||||
| N | 49 | 54 | ||
| Age (mean±SD) | 54.7 ± 9.3 | 51.2 ± 6.1 | 0.03 | |
| Gender | 0.99 | |||
| Male | 34 | 38 | ||
| Female | 15 | 16 | ||
Table 1B.
Demographics of the African-American study sample.
| Non-Substance Dependent | Substance Dependent | P-value | ||
|---|---|---|---|---|
|
|
||||
| N | 27 | 120 | ||
| Age (mean±SD) | 49.2 ± 9.0 | 52.0 ± 7.1 | 0.05 | |
| Gender | 0.39 | |||
| Male | 10 | 58 | ||
| Female | 17 | 62 | ||
Table 1C.
Demographics of the Hispanic subpopulation.
| Non-Substance Dependent | Substance Dependent | P-value | ||
|---|---|---|---|---|
|
|
||||
| N | 16 | 45 | ||
| Age (mean±SD) | 50.3 ± 7.6 | 50.1 ± 5.2 | 0.91 | |
| Gender | 0.56 | |||
| Male | 8 | 18 | ||
| Female | 8 | 27 | ||
Cognitive characterization
Subjects were cognitively characterized with a neuropsychological test battery as previously described (Woods et al, 2004). A total of 13 tests spanning 7 cognitive domains (Motor, Processing Speed, Executive Functioning, Learning, Memory, Verbal Fluency, Working Memory), with known sensitivity for the diagnosis of HAND, were administered. Raw scores were transformed to demographically adjusted T-scores to account for effects of age, gender, ethnicity and/or education as appropriate. T-scores were utilized for correlational analyses, as they are normally distributed. In addition to domain T-scores, a summary across all domains of the test battery, the Global T-score, was also examined. The battery performed upon study entry was used for analysis. For a list of the tests, domains, and normative data sources utilized, see Supplemental Table 1.
Genotyping
DNA was purified from peripheral blood lymphocytes with DNeasy columns (Qiagen, Valencia, CA). All SNP assays were obtained as Taqman Assays-On-Demand (Applied Biosystems, Foster City, CA) and genotyping was performed in triplicate according to the manufacturer's protocol using a LightCycler 480 (Roche Applied Science, Indianapolis, IN).
We selected a total of 10 genetic polymorphisms in the DRD1 (rs4532, rs686, rs265978, rs265975, rs265973) and DRD2 (rs12364283, rs2283265, rs1076560, rs6277, rs1800497) genes for analysis. All of the selected SNPs were either functional, in strong linkage disequilibrium with a functional polymorphism, or were previously associated with addiction-related phenotypes (For a review see: (Le Foll et al, 2009)). See Table 2 for a list of all SNPs genotyped, including SNP location, alleles and minor allele frequency with respect to the African American, Caucasian, and Hispanic non-substance dependent subjects in our population. Minor alleles and frequencies agree with that from reference populations for each ethnic group provided by the HapMap and NCBI dbSNP websites.
Table 2.
Description of polymorphisms tested.
| Gene | SNP | SNP Location | Alleles | Minor (AA Freq.) | Minor (Cauc Freq.) | Minor (Hisp Freq.) |
|---|---|---|---|---|---|---|
| DRD1 | rs4532 | Exon 1 (5'UTR) | C/T | C (0.19) | C (0.36) | C (0.27) |
| DRD1 | rs686 | Exon 4 (3'UTR) | A/G | A (0.37) | G (0.43) | G (0.41) |
| DRD1 | rs265978 | 3.7 kb downstream | C/T | C (0.37) | C (0.43) | C (0.38) |
| DRD1 | rs265975 | 5.5 kb downstream | C/T | C (0.46) | T (0.25) | T (0.35) |
| DRD1 | rs265973 | 7 kb downstream | C/T | T (0.42) | T (0.34) | T (0.30) |
| DRD2 | rs12364283 | Promoter (1kb upstream) | A/G | G (0.02) | G (0.10) | G (0.10) |
| DRD2 | rs2283265 | Intron 5 | A/C | A (0.12) | A (0.16) | A (0.22) |
| DRD2 | rs1076560 | Intron 6 | A/C | A (0.12) | A (0.18) | A (0.22) |
| DRD2 | rs6277 | Exon 7 (C957T) | A/G | A (0.14) | G (0.41) | G (0.44) |
| DRD2/ANKK1 | rs1800497 | 10kb downstream | A/G | A (0.31) | A (0.22) | A (0.25) |
Minor alleles and allele frequencies are with respect to the non-substance using population within each subset. Abbreviations: DRD1, dopamine D1 receptor; DRD2, dopamine D2 receptor; Freq: frequency; kb: kilobase; UTR: untranslated region.
Statistical Analysis
The PLINK 1.07 genetic association analysis program (Purcell et al, 2007) was used to verify SNP data quality, test for departure from Hardy-Weinberg equilibrium, test individual SNPs for statistical association using Fisher's exact test, perform multiple correction testing (Bonferroni), and perform linear regression analyses. SNPs in linkage disequilibrium with each other were analyzed as haploblocks. Analyses were performed within separate racial groups as both minor alleles and allele frequencies of the SNPs differed (see Table 2). Significance was set at P≤0.05 and trends considered for P≤0.10.
Results
Polymorphisms of DRD1 and DRD2 are associated with substance dependence
In this population, we genotyped ten SNPs within DRD1 and DRD2 (see Table 2 for a list of SNPs tested), and evaluated their association with opiate and cocaine dependence. In the Caucasian population (n=103, 49 not substance dependent and 54 substance dependent), we found significant associations for one DRD1 SNP (rs265975) and three DRD2 SNPs (rs6277, rs2283265, rs1076560) (Table 3A). No significant associations were observed in the African American sample (n=147, 27 not substance dependent and 120 substance dependent). In addition, a subset of Hispanic subjects were examined separately (n=61, 16 not substance dependent and 45 substance dependent) and a significant association was found for one DRD2 SNP (rs6277) (Table 3A). Thus, in this pilot sample, SNP associations with substance dependence appeared to be confined to Caucasian subjects, as the Hispanic subset is predominately comprised of Caucasian subjects (70%).
Table 3A.
SNPs significantly associated with substance dependence.
| Population | N (No SUD/SUD) | Gene | SNP | P |
|---|---|---|---|---|
| Caucasian | 103 (49/54) | DRD2 | rs6277 | 0.0002 |
| DRD2 | rs2283265 | 0.02 | ||
| DRD2 | rs1076560 | 0.05 | ||
| DRD1 | rs265975 | 0.05 | ||
|
| ||||
| African-American | 147 (27/120) | N/A | ||
|
| ||||
| Hispanic | 61 (16/45) | DRD2 | rs6277 | 0.00007 |
Haplotypes were determined from linkage disequilibrium blocks. In the Caucasian population, 2 haploblocks were found. Block 1 consisted of SNPs rs4532 and rs686 within the DRD1 gene and block 2 consisted of SNPs rs2283265, rs1076560, and rs6277 within the DRD2 gene. Association for each of these blocks was tested and significant association was found for each of the 3 possible genotypes within Block 2 of DRD2 (Table 3B). In the African-American population, 1 haploblock was found; SNPs rs2283265 and rs1076560 within the DRD2 gene make up this haploblock but neither of the possible genotypes within this haploblock were significantly associated with substance dependence. In the Hispanic population, 2 haploblocks were found. Block 1 consisted of SNPs rs4532, rs686, and rsrs265978 within the DRD1 gene and block 2 consisted of SNPs rs2283265 and rs1076560 within the DRD2 gene; neither of these were significantly associated with substance dependence in this population.
Table 3B.
Haplotype association tests
| Population | Gene | SNPs | Haplotype | P |
|---|---|---|---|---|
| Caucasian | DRD2 | rs6277/rs1076560/rs2283265 | ACC | 0.0002 |
| DRD2 | rs6277/rs1076560/rs2283265 | GAA | 0.02 | |
| DRD2 | rs6277/rs1076560/rs2283265 | GCC | 0.05 | |
|
| ||||
| African-American | N/A | |||
|
| ||||
| Hispanic | N/A | |||
Abbreviations: SUD, substance dependence; DRD1, dopamine D1 receptor; DRD2, dopamine D2 receptor; SNP, single nucleotide polymorphism. SNP in BOLD remains significant after Bonferroni correction for multiple comparisons. Hispanic population comprises a subset of the Caucasian and African American samples.
To account for increased risk of Type I error related to the multiple SNPs tested, Bonferroni corrections were performed. Following correction in the Caucasian sample, one DRD2 SNP remained significant (rs6277, P=0.002). This same SNP also remained significant in the Hispanic sample (P=0.0007).
Substance dependence has minimal effect on cognitive performance in the sample
To determine the potential effects of substance use on cognition, we examined the association of global and domain T-scores with substance dependence status. In the Caucasian population, substance dependent subjects performed slightly worse than non-substance dependent subjects in tasks of Learning and Memory (Table 4). However, for all other domains and the global scores, performance between non-substance dependent and substance dependent subjects was equivalent. In the African-American and Hispanic populations, there were no differences observed between non-substance dependent and substance dependent individuals in the global T-score or in any cognitive domain (Table 4).
Table 4.
Cognitive Performance T-scores in Caucasian, African-American, and Hispanic subsets.
| Entire | Caucasian | African-American | Hispanic | ||||
|---|---|---|---|---|---|---|---|
| Domain | Population | No SUD | SUD | No SUD | SUD | No SUD | SUD |
| Global | 37.8 (7.9) | 39.9 (9.5) | 37.7 (8.1) | 37.5 (5.4) | 37.0 (7.4) | 35.0 (7.3) | 35.7 (7.8) |
| Motor | 32.9 (9.8) | 33.6 (11.1) | 35.0 (9.6) | 31.5 (9.5) | 31.8 (9.4) | 34.9 (11.7) | 35.1 (10.5) |
|
| |||||||
| Processing Speed | 40.6 (9.0) | 42.4 (10.4) | 41.1 (9.2) | 40.5 (7.0) | 39.7 (8.7) | 39.5 (7.9) | 39.5 (9.8) |
|
| |||||||
| Working Memory | 42.6 (9.3) | 43.1 (10.5) | 41.9 (10.5) | 40.9 (9.4) | 43.0 (8.1) | 37.8 (6.9) | 40.0 (8.8) |
|
| |||||||
| Executive Functioning | 39.4 (10.0) | 40.1 (11.0) | 40.6 (9.9) | 39.4 (10.1) | 38.2 (9.6) | 38.7 (9.6) | 38.1 (9.6) |
|
| |||||||
| Learning | 33.3 (10.5) | 36.6 (12.0) | 31.8 (10.7) | 33.7 (8.8) | 32.5 (9.9) | 28.8 (9.7) | 28.5 (7.8) |
|
| |||||||
| Memory | 34.1 (11.2) | 38.0 (12.9) | 33.0 (11.2) | 35.4 (10.3) | 32.7 (10.3) | 31.1 (11.2) | 30.8 (9.1) |
|
| |||||||
| Verbal Fluency | 47.1 (10.6) | 47.2 (12.8) | 44.8 (10.1) | 49.4 (8.7) | 47.6 (10.2) | 38.3 (10.5) | 42.4 (9.4) |
Abbreviation: Substance Dependent (SUD). Cognitive Performance T-scores represented as mean (SD) for the entire population as well as within the Caucasian, African-American, and Hispanic groups. Hispanics are a subset of the Caucasian and African-American individuals. Figures in BOLD are significantly different between the no SUD and SUD populations (P<0.05).
DRD1 and DRD2 associations with HAND in consideration of substance dependence
Using quantitative trait regression models incorporated into PLINK, we next examined our genotyped polymorphisms for their predictive value for cognitive functioning while controlling for opiate and cocaine dependence. We tested the association of DRD1 and DRD2 SNPs with global and domain T-scores while controlling for substance dependency. In Caucasian subjects, significant associations were found between the Motor domain T-scores and two DRD2 SNPs (rs2283265 and rs1076560, Table 5A). In African American subjects, several domains showed significant DRD1 and DRD2 associations, including Motor, Processing Speed, Working Memory and Memory T-scores (Table 5B). In Hispanic subjects, only the Memory domain showed a significant DRD1 association (Table 5C). Importantly, in both Caucasian and African American populations, but not Hispanic, for all but one association in the motor domain (rs265973 in African Americans), the direction of DRD1 and DRD2 effects was opposite in controls and substance dependents (had variably positive or negative beta coefficients). As the analyses were stratified by racial group and there were no gender composition differences between substance users and HIV non substance groups, this difference could not be accounted for by demographic composition of the HIV substance and non-substance populations.
Table 5A.
SNPs associated with cognitive performance T-scores controlling for substance dependence: Caucasian population
| Domain | N (No SUD/SUD) | Gene | SNP | β_No SUD | β_SUD | P |
|---|---|---|---|---|---|---|
| Global | 97 (45/52) | N/A | ||||
| Motor | 93 (43/50) | DRD2 | rs2283265 | 8.857 | −3.253 | 0.003 |
| DRD2 | rs1076560 | 7.107 | −3.253 | 0.004 | ||
|
| ||||||
| Processing Speed | 92 (42/52) | N/A | ||||
|
| ||||||
| Working Memory | 95 (45/50) | N/A | ||||
|
| ||||||
| Executive Functioning | 94 (42/52) | N/A | ||||
|
| ||||||
| Learning | 97 (45/52) | N/A | ||||
|
| ||||||
| Memory | 97 (45/52) | N/A | ||||
|
| ||||||
| Verbal Fluency | 96 (44/52) | N/A | ||||
Table 5B.
SNPs associated with cognitive performance T-scores controlling for substance dependence: African-American population
| Domain | N (No SUD/SUD) | Gene | SNP | β_No SUD | β_SUD | P |
|---|---|---|---|---|---|---|
| Global | 135 (23/112) | N/A | ||||
| Motor | 128 (23/105) | DRD1 | rs265973 | 10.51 | 2.186 | 0.022 |
| DRD1 | rs686 | −5.777 | 1.665 | 0.010 | ||
| DRD2 | rs12365283 | 23.59 | −1.815 | 0.007 | ||
|
| ||||||
| Processing Speed | 131 (23/108) | DRD1 | rs4532 | −3.124 | 2.917 | 0.038 |
|
| ||||||
| Working Memory | 132 (23/109) | DRD1 | rs265973 | −5.221 | 2.996 | 0.042 |
|
| ||||||
| Executive Functioning | 129 (22/107) | N/A | ||||
|
| ||||||
| Learning | 135 (23/112) | N/A | ||||
|
| ||||||
| Memory | 135 (23/112) | DRD2 | rs2283265* | −10.91 | 0.0869 | 0.039 |
| DRD2 | rs1076560* | −10.91 | 0.0869 | 0.039 | ||
|
| ||||||
| Verbal Fluency | 132 (20/112) | N/A | ||||
DRD2 SNPS rs2283265/rs1076560 form a haploblock within the AA subset, which accounts for the identical β and P values observed.
Table 5C.
SNPs associated with cognitive performance T-scores controlling for substance dependence: Hispanic subset of above populations
| Domain | N (No SUD/SUD) | Gene | SNP | β_No SUD | β_SUD | P |
|---|---|---|---|---|---|---|
| Global | 58 (16/42) | N/A | ||||
|
| ||||||
| Motor | 54 (14/40) | N/A | ||||
|
| ||||||
| Processing Speed | 56 (14/42) | N/A | ||||
|
| ||||||
| Working Memory | 56 (16/40) | N/A | ||||
|
| ||||||
| Executive Functioning | 56 (14/42) | N/A | ||||
|
| ||||||
| Learning | 58 (16/42) | N/A | ||||
|
| ||||||
| Memory | 58 (16/42) | DRD1 | rs4532 | −11.33 | −2.344 | 0.046 |
|
| ||||||
| Verbal Fluency | 57 (15/42) | N/A | ||||
Discussion
Considering the commonalties in fronto-striatal neurocircuitry that underlie both HAND and drug dependence, it is reasonable to postulate that genetic polymorphisms within select genes of the dopaminergic system contribute to both disorders. Dopamine rich regions in the brain, particularly the striatum, are sensitive to the effects of both HIV and substance use. Neuroanatomically, both disorders converge on the mesocorticolimbic DA system, which contains DRD1 and DRD2. The prior literature supports an association between DA receptor polymorphisms and opiate and cocaine dependence; it is unclear whether relationships exist between these same polymorphisms and HAND (Jacobs et al, 2012; Lawford et al, 2000; Li et al, 2006; Moyer et al, 2011; Noble et al, 1993; Perez de los Cobos et al, 2007; Persico et al, 1996; Shahmoradgoli Najafabadi et al, 2005). As substance dependence is a common feature of HIV populations, and may contribute to HAND through a variety of synergistic neurotoxicities, apportioning genetic risk is a complex problem. Our aim was to determine whether polymorphisms of DRD1 and DRD2 would predict cognitive impairment once substance dependence and its genetic associations were accounted for. Furthermore, since allele frequencies differed between racial groups (as would be expected), we undertook the analysis within racially segregated samples (analysis of the racially mixed Hispanic subset was undertaken only as a secondary analysis). Importantly, this strategy assured that race-dependent normative scoring in neuropsychological tests could not be responsible for genetic associations with cognitive performance.
As would be expected from the prior literature, the well-characterized substance dependencies in our HIV population had associations with DRD1 and DRD2 SNPs, and the effects differed within the segregated racial groups (Table 3). Effects were limited to the Caucasian and Hispanic (predominantly Caucasian) populations; we found significant associations for one DRD1 SNP (rs265975) and three DRD2 SNPs (rs6277, rs2283265, rs1076560) in Caucasian subjects and one DRD2 SNP (rs6277) in Hispanic subjects. Of particular interest is that these DRD2 SNPs are also part of a haploblock in Caucasian subjects, and all possible genotypes within this haploblock were also significantly associated with substance dependence in the Caucasian population. In the prior literature examining SNP associations with substance dependence, studies that examine DRD1 are limited. One study found associations with DRD1 variants in opiate abuse in three separate populations, entailing Caucasian, African-American, and Hispanic subjects (Jacobs et al, 2012). Another study found an association with a single polymorphism in African-American heroin addicts. To date, no study has examined DRD1 variants in cocaine users. Studies of DRD2 variants are much more numerous and several studies have linked polymorphisms to heavy stimulant use in Caucasian subjects (Comings et al, 1999; Persico et al, 1996), but not racially mixed Europeans or African-American populations (Gelernter et al, 1999; Lohoff et al, 2010; Moyer et al, 2011). In addition, several studies have linked DRD2 polymorphisms to heroin use in Caucasian populations (Lawford et al, 2000; Xu et al, 2004), but to date, no studies have examined these polymorphisms in African-American or Hispanic populations.
Chronic substance dependence and its genetic associations are likely to have direct relevance to neurocognition and HAND. With chronic substance dependence, adaptive brain changes occur, most notably reduced tonic dopamine levels and increased phasic dopamine neurotransmission following drug dependence (Nestler, 2005). In addition, chronic drug use produces reduced brain metabolism in the frontal cortex that correlates with reductions in DRD2, leading to impairments in working memory and attention (Volkow et al, 1993; Volkow et al, 2007). In HIV infected subjects, decreased tonic dopamine levels have also been shown with viral replication and advancing disease, both by neuroimaging and direct study of brain tissue. Thus, the environmental effect of long-term substance use should be factored in when studying the relationship between dopaminergic polymorphisms and behavioral outcomes in HIV populations that contain significant numbers of long-term substance dependent individuals. When we employed a strategy to account for gene × environment effects (looking for genetic associations with cognition while controlling for substance dependence), several domains were significantly associated with genotyped polymorphisms, most notably in the African-American population; thus, we detected associations that might not have been evident when simple genetic correlations with cognition without consideration of substance dependence patterns were done (Table 5). This may, in part, account for why our pilot study detected these associations, and prior studies of HIV cognition have not resulted in similar findings. In one, substance users were excluded and only COMT, DAT and BDNF were examined (Levine et al, 2012b). In another, a GWAS study that included both HAND and substance use phenotypes in the analysis (Levine et al, 2012a), the population had a very low frequency (2–5%) of psychostimulants only (cocaine and methamphetamine), contrasting with our population in which substance dependence had much higher prevalence (69%).
In our population, when controlled for substance dependence status, two SNPs in DRD2 (rs2283265/rs1076560) were significantly associated with the Motor domain T-score in the Caucasian population. These two SNPs play a role in modulating alternative splicing of DRD2 to yield long (DRD2L, expressed mainly postsynaptically) and short (DRD2S, expressed mainly presynaptically) isoforms of the receptor (Khan et al, 1998; Usiello et al, 2000). While both isoforms regulate presynaptic inhibition of GABAergic neurotransmission in the striatum, DRD2S is preferentially involved in inhibition of glutamate release (Centonze et al, 2003; Centonze et al, 2004). Recent studies in cognitively normal subjects have revealed roles for these SNPs in a test of working memory and attentional control (N-back working memory task), such that individuals carrying the low expression of DRD2L isoform demonstrated better performance (Zhang et al, 2007). Congruently, reduced expression of DRD2L mRNA in dorsolateral prefrontal cortex has been shown in HIV infected subjects with intact cognitive status relative to those with neurocognitive impairment (Gelman et al, 2012). When individual neuropsychological test domains were examined in a National NeuroAIDS Tissue Consortium (NNTC) population, significant negative correlations with DRD2L mRNA expression and performance were found for the Verbal Fluency, Attention and Working Memory and Processing Speed Domain T-scores, suggesting that stronger performance was linked to decreased DRD2L expression (Gelman et al, 2012). Incidentally, in this study, there were no significant differences in DRD2L mRNA expression in subjects with and without substance use (Gelman et al, 2012). Thus, these prior studies together with the current data point to a cognitive impact of DRD2 genetics and expression in HIV and non-infected populations.
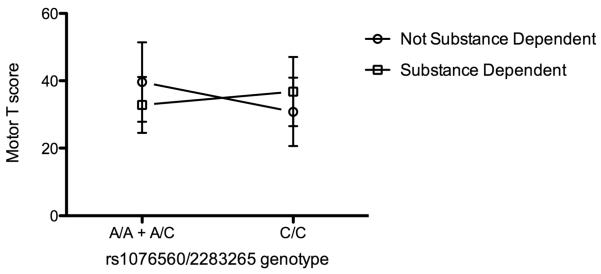
Another feature of our analysis was that for all cognitive domains displaying significant genetic associations, the direction of the DRD1 and DRD2 effects in racially segregated populations were, with only one exception, opposite among non-substance using and substance dependent individuals (in the racially heterogeneous Hispanic group, this did not pertain). This supports the hypothesis that the altered tonic dopaminergic milieu of the addicted brain may predicate an altered dopaminergic neurobiology of cognition; elevated levels of transcripts in one setting may be cognitively deleterious, whereas in another may be cognitively beneficial. For example, in the case of the Motor Domain in Caucasians, the preponderance of alleles favoring the DRD2L isoform in substance users (MAF=0.29) compared to non-users (MAF=0.16) in our population suggested that substance users would have greater expression of DRD2L in brain regions relevant to cognitive function (striatum, pre-frontal cortex) in addition to their decreased performance in cognitive tasks (Figure 1). Recently, in a study of healthy controls, increased expression of DRD2L has been linked to greater activity (fMRI BOLD responses) in striatal regions during a visually paced motor task, suggesting individuals with increased DRD2L expression recruit additional neuronal resources during motor tasks, possibly due to less efficient brain activity (Fazio et al, 2011). Future studies should confirm DRD2L mRNA expression as well as brain activity changes.
Figure 1. Cognitive performance in the Motor domain is differentially affected by DRD2 genotype.
Caucasian substance dependent subjects with the minor alleles at DRD2 rs1076560 and rs2283265 have decreased performance on tasks within the Motor domain as compared with non-substance using subjects of the same race and genotype.
Another aspect of this study was the differences seen between our Caucasian and African American subjects (Table 5). In the cognition × substance dependence analyses, we observed a greater number of significant associations in our African American subjects than in the Caucasian subset. This is unusual; in genetic association studies, significant results are more often observed in Caucasian populations that display a greater “genetic bottleneck” than heterogeneous African American populations (Tishkoff and Williams, 2002). Indeed, in our simpler analysis of SNP associations with substance dependency, significant associations were present only in the Caucasian sample. One explanation for the reverse phenomenon in our cognition × substance use analysis might be the inclusion of Hispanics, who had greater representation in our Caucasian than in our African-American subset, and indeed when we examined the Hispanic population separately we only observed single associations for substance dependence as well as cognitive performance. This admixture would be anticipated to decrease our sensitivity by increasing genetic variability in the Caucasian subset. In addition, with cognitive testing (the endpoint of our analyses) it is well known that Hispanic individuals do not perform as well as would be expected by normative standards or in comparison to non-Hispanic whites (Rivera Mindt et al, 2010). Again, inclusion of this group within our Caucasian subset may have worked to diminish effects in an analysis of cognition, but not substance dependency. On the other hand, normative neuropsychological data is also problematic for African American populations; they would be less impacted by inclusion of Hispanics, and their segregation may have eliminated potential test biases that worked to mask results in the entire population, particularly in light of their greater representation in the substance dependent group.
In summary, this study contributes to our understanding of the genetic associations between dopaminergic genes and HAND, importantly showing that substance dependence characteristics must be carefully accounted for when evaluating HIV populations. The distinct and opposing patterns of association displayed by substance users and non-substance users suggest that in an HIV-positive population, neurobiologic processes leading to cognitive impairment may be different, despite the phenotypic similarities of HAND in both groups. Further studies with segregation by substance dependence patterns, to examine brain transcript expression and increase the number of subjects under observation, are warranted.
Supplementary Material
Acknowledgements
This work was funded by grants from the National Institutes on Drug Abuse, T32DA007135 (MMJ) and R01DA015446 (YLH), the National Institute of Mental Health, R25MH080663 (MMJ, SM) and U01MH083501 (SM), and the NIH UL1RR029887 (MSSM CTSA).
Footnotes
Conflict of Interest The authors declare no conflict of interest.
References
- Aouizerat BE, Pearce CL, Miaskowski C. The search for host genetic factors of HIV/AIDS pathogenesis in the post-genome era: progress to date and new avenues for discovery. Curr HIV/AIDS Rep. 2011;8:38–44. doi: 10.1007/s11904-010-0065-1. doi: 10.1007/s11904-010-0065-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B. Cerebrospinal fluid dopamine in HIV-1 infection. Aids. 1994;8:67–71. doi: 10.1097/00002030-199401000-00010. doi: 10.1097/00002030-199401000-00010. [DOI] [PubMed] [Google Scholar]
- Bol SM, Booiman T, van Manen D, Bunnik EM, van Sighem AI, Sieberer M, Boeser-Nunnink B, de Wolf F, Schuitemaker H, Portegies P, Kootstra NA, van 't Wout AB. Single nucleotide polymorphism in gene encoding transcription factor Prep1 is associated with HIV-1-associated dementia. PLoS One. 2012;7:e30990. doi: 10.1371/journal.pone.0030990. doi: 10.1371/journal.pone.0030990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Grande C, Usiello A, Gubellini P, Erbs E, Martin AB, Pisani A, Tognazzi N, Bernardi G, Moratalla R, Borrelli E, Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J Neurosci. 2003;23:6245–54. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centonze D, Gubellini P, Usiello A, Rossi S, Tscherter A, Bracci E, Erbs E, Tognazzi N, Bernardi G, Pisani A, Calabresi P, Borrelli E. Differential contribution of dopamine D2S and D2L receptors in the modulation of glutamate and GABA transmission in the striatum. Neuroscience. 2004;129:157–66. doi: 10.1016/j.neuroscience.2004.07.043. doi: 10.1016/j.neuroscience.2004.07.043. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. Neuroimage. 2008;42:869–78. doi: 10.1016/j.neuroimage.2008.05.011. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comings DE, Gonzalez N, Wu S, Saucier G, Johnson P, Verde R, MacMurray JP. Homozygosity at the dopamine DRD3 receptor gene in cocaine dependence. Mol Psychiatry. 1999;4:484–7. doi: 10.1038/sj.mp.4000542. doi: 10.1038/sj.mp.4000542. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A. 1988;85:5274–8. doi: 10.1073/pnas.85.14.5274. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–4. doi: 10.1097/00002826-200007000-00004. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Fazio L, Blasi G, Taurisano P, Papazacharias A, Romano R, Gelao B, Ursini G, Quarto T, Lo Bianco L, Di Giorgio A, Mancini M, Popolizio T, Rubini G, Bertolino A. D2 receptor genotype and striatal dopamine signaling predict motor cortical activity and behavior in humans. Neuroimage. 2011;54:2915–21. doi: 10.1016/j.neuroimage.2010.11.034. doi: 10.1016/j.neuroimage.2010.11.034. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Fossella JA. Neurogenetics and pharmacology of learning, motivation, and cognition. Neuropsychopharmacology. 2011;36:133–52. doi: 10.1038/npp.2010.96. doi: 10.1038/npp.2010.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Satel SL. No association between D2 dopamine receptor (DRD2) alleles or haplotypes and cocaine dependence or severity of cocaine dependence in European-and African-Americans. Biol Psychiatry. 1999;45:340–5. doi: 10.1016/s0006-3223(97)00537-4. doi: 10.1016/S0006-3223(97)00537-4. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal Dopaminergic and Enkephalinergic Synaptic Accommodation in HIV-associated Neurocognitive Disorders and Encephalitis. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9345-4. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric Research Interview for Substance and Mental Disorders (PRISM): reliability for substance abusers. Am J Psychiatry. 1996;153:1195–201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs MM, Okvist A, Horvath M, Keller E, Bannon MJ, Morgello S, Hurd YL. Dopamine receptor D1 and postsynaptic density gene variants associate with opiate abuse and striatal expression levels. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.140. doi: 10.1038/mp.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan ZU, Mrzljak L, Gutierrez A, de la Calle A, Goldman-Rakic PS. Prominence of the dopamine D2 short isoform in dopaminergic pathways. Proc Natl Acad Sci U S A. 1998;95:7731–6. doi: 10.1073/pnas.95.13.7731. doi: 10.1073/pnas.95.13.7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Fernandez JB, Singer EJ, Commins D, Waldrop-Valverde D, Ownby RL, Kumar M. Human immunodeficiency virus type 1 in the central nervous system leads to decreased dopamine in different regions of postmortem human brains. J Neurovirol. 2009;15:257–74. doi: 10.1080/13550280902973952. doi: 10.1080/13550280902973952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Larsson M, Hagberg L, Forsman A, Norkrans G. Cerebrospinal fluid catecholamine metabolites in HIV-infected patients. J Neurosci Res. 1991;28:406–9. doi: 10.1002/jnr.490280313. doi: 10.1002/jnr.490280313. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young RM, Noble EP, Sargent J, Rowell J, Shadforth S, Zhang X, Ritchie T. The D(2) dopamine receptor A(1) allele and opioid dependence: association with heroin use and response to methadone treatment. Am J Med Genet. 2000;96:592–8. doi: 10.1002/1096-8628(20001009)96:5<592::aid-ajmg3>3.0.co;2-y. doi: 10.1002/1096-8628(20001009)96:5<592::AID-AJMG3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P. Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol. 2009;20:1–17. doi: 10.1097/FBP.0b013e3283242f05. doi: 10.1097/FBP.0b013e3283242f05. [DOI] [PubMed] [Google Scholar]
- Levine AJ, Singer EJ, Sinsheimer JS, Hinkin CH, Papp J, Dandekar S, Giovanelli A, Shapshak P. CCL3 genotype and current depression increase risk of HIV-associated dementia. Neurobehav HIV Med. 2009;1:1–7. doi: 10.2147/nbhiv.s6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Service S, Miller EN, Reynolds SM, Singer EJ, Shapshak P, Martin EM, Sacktor N, Becker JT, Jacobson LP, Thompson P, Freimer N. Genome-wide association study of neurocognitive impairment and dementia in HIV-infected adults. Am J Med Genet B Neuropsychiatr Genet. 2012a doi: 10.1002/ajmg.b.32071. doi: 10.1002/ajmg.b.32071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ, Sinsheimer JS, Bilder R, Shapshak P, Singer EJ. Functional polymorphisms in dopamine-related genes: effect on neurocognitive functioning in HIV+ adults. J Clin Exp Neuropsychol. 2012b;34:78–91. doi: 10.1080/13803395.2011.623118. doi: 10.1080/13803395.2011.623118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Shao C, Zhang D, Zhao M, Lin L, Yan P, Xie Y, Jiang K, Jin L. The effect of dopamine D2, D5 receptor and transporter (SLC6A3) polymorphisms on the cue-elicited heroin craving in Chinese. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:269–73. doi: 10.1002/ajmg.b.30264. doi: 10.1002/ajmg.b.30264. [DOI] [PubMed] [Google Scholar]
- Lohoff FW, Bloch PJ, Hodge R, Nall AH, Ferraro TN, Kampman KM, Dackis CA, O'Brien CP, Pettinati HM, Oslin DW. Association analysis between polymorphisms in the dopamine D2 receptor (DRD2) and dopamine transporter (DAT1) genes with cocaine dependence. Neurosci Lett. 2010;473:87–91. doi: 10.1016/j.neulet.2010.02.021. doi: 10.1016/j.neulet.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Morgello S, Gelman BB, Kozlowski PB, Vinters HV, Masliah E, Cornford M, Cavert W, Marra C, Grant I, Singer EJ. The National NeuroAIDS Tissue Consortium: a new paradigm in brain banking with an emphasis on infectious disease. Neuropathol Appl Neurobiol. 2001;27:326–35. doi: 10.1046/j.0305-1846.2001.00334.x. doi: 10.1046/j.0305-1846.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Moyer RA, Wang D, Papp AC, Smith RM, Duque L, Mash DC, Sadee W. Intronic polymorphisms affecting alternative splicing of human dopamine D2 receptor are associated with cocaine abuse. Neuropsychopharmacology. 2011;36:753–62. doi: 10.1038/npp.2010.208. doi: 10.1038/npp.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–9. doi: 10.1038/nn1578. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- Noble EP, Blum K, Khalsa ME, Ritchie T, Montgomery A, Wood RC, Fitch RJ, Ozkaragoz T, Sheridan PJ, Anglin MD, et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend. 1993;33:271–85. doi: 10.1016/0376-8716(93)90113-5. doi: 10.1016/0376-8716(93)90113-5. [DOI] [PubMed] [Google Scholar]
- Obermann M, Kuper M, Kastrup O, Yaldizli O, Esser S, Thiermann J, Koutsilieri E, Arendt G, Diener HC, Maschke M. Substantia nigra hyperechogenicity and CSF dopamine depletion in HIV. J Neurol. 2009;256:948–53. doi: 10.1007/s00415-009-5052-3. doi: 10.1007/s00415-009-5052-3. [DOI] [PubMed] [Google Scholar]
- Pemberton LA, Stone E, Price P, van Bockxmeer F, Brew BJ. The relationship between ApoE, TNFA, IL1a, IL1b and IL12b genes and HIV-1-associated dementia. HIV Med. 2008;9:677–80. doi: 10.1111/j.1468-1293.2008.00614.x. doi: 10.1111/j.1468-1293.2008.00614.x. [DOI] [PubMed] [Google Scholar]
- Perez de los Cobos J, Baiget M, Trujols J, Sinol N, Volpini V, Banuls E, Calafell F, Luquero E, del Rio E, Alvarez E. Allelic and genotypic associations of DRD2 TaqI A polymorphism with heroin dependence in Spanish subjects: a case control study. Behav Brain Funct. 2007;3:25. doi: 10.1186/1744-9081-3-25. doi: 10.1186/1744-9081-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Bird G, Gabbay FH, Uhl GR. D2 dopamine receptor gene TaqI A1 and B1 restriction fragment length polymorphisms: enhanced frequencies in psychostimulant-preferring polysubstance abusers. Biol Psychiatry. 1996;40:776–84. doi: 10.1016/0006-3223(95)00483-1. doi: 10.1016/0006-3223(95)00483-1. [DOI] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera Mindt M, Byrd D, Saez P, Manly J. Increasing culturally competent neuropsychological services for ethnic minority populations: a call to action. Clin Neuropsychol. 2010;24:429–53. doi: 10.1080/13854040903058960. doi: 10.1080/13854040903058960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–42. doi: 10.1080/13550280290049615. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Shahmoradgoli Najafabadi M, Ohadi M, Joghataie MT, Valaie F, Riazalhosseini Y, Mostafavi H, Mohammadbeigi F, Najmabadi H. Association between the DRD2 A1 allele and opium addiction in the Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:39–41. doi: 10.1002/ajmg.b.30117. doi: 10.1002/ajmg.b.30117. [DOI] [PubMed] [Google Scholar]
- Spector SA, Singh KK, Gupta S, Cystique LA, Jin H, Letendre S, Schrier R, Wu Z, Hong KX, Yu X, Shi C, Heaton RK. APOE epsilon4 and MBL-2 O/O genotypes are associated with neurocognitive impairment in HIV-infected plasma donors. Aids. 2010;24:1471–9. doi: 10.1097/QAD.0b013e328339e25c. doi: 10.1097/QAD.0b013e328339e25c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tishkoff SA, Williams SM. Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet. 2002;3:611–21. doi: 10.1038/nrg865. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rouge-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM, Telang F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch Neurol. 2007;64:1575–9. doi: 10.1001/archneur.64.11.1575. doi: 10.1001/archneur.64.11.1575. [DOI] [PubMed] [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Hinkin CH, Lazzaretto D, Cherner M, Marcotte TD, Gelman BB, Morgello S, Singer EJ, Grant I, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. J Clin Exp Neuropsychol. 2004;26:759–78. doi: 10.1080/13803390490509565. doi: 10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Xu K, Lichtermann D, Lipsky RH, Franke P, Liu X, Hu Y, Cao L, Schwab SG, Wildenauer DB, Bau CH, Ferro E, Astor W, Finch T, Terry J, Taubman J, Maier W, Goldman D. Association of specific haplotypes of D2 dopamine receptor gene with vulnerability to heroin dependence in 2 distinct populations. Arch Gen Psychiatry. 2004;61:597–606. doi: 10.1001/archpsyc.61.6.597. doi: 10.1001/archpsyc.61.6.597. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadee W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–7. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.