Abstract
Failure to distinguish bipolar depression (BDd) from the unipolar depression of major depressive disorder (UDd) in adolescents has significant clinical consequences. We aimed to identify differential patterns of functional neural activity in BDd versus UDd and employed two (fearful and happy) facial expression/gender labeling functional magnetic resonance imaging (fMRI) experiments to study emotion processing in 10 BDd (8 females, mean age=15.1±1.1) compared to age- and gender-matched 10 UDd and 10 healthy control (HC) adolescents who were age- and gender-matched to the BDd group. BDd adolescents, relative to UDd, showed significantly lower activity to both intense happy (e.g., insula and temporal cortex) and intense fearful faces (e.g., frontal precentral cortex). Although the neural regions recruited in each group were not the same, both BDd and UDd adolescents, relative to HC, showed significantly lower neural activity to intense happy and mild happy faces, but elevated neural activity to mild fearful faces. Our results indicated that patterns of neural activity to intense positive and negative emotional stimuli can help differentiate BDd from UDd in adolescents.
Keywords: Bipolar depression, Major depressive disorder, Functional magnetic resonance imaging, (fMRI), Emotion processing, Adolescents
1. Introduction
Bipolar disorder (BD) in youth is now recognized as a significant public health problem that is often associated with significant morbidity and mortality (Birmaher et al., 2009; Diler, 2007). Depressive episodes are the first and most common manifestation of BD in youth (Birmaher et al., 2006; Geller et al., 2004); however, it is difficult to clinically differentiate the symptoms of BD depression (BDd) from those of the depression of major depressive disorder (UDd) (Chang, 2009). Furthermore, failure to differentiate BDd from UDd in adolescents has significant clinical consequences, and may result in inappropriate interventions for those youth with BDd misdiagnosed with UDd, such as antidepressant monotherapy.
Functional neuroimaging (e.g., functional magnetic resonance imaging) can help improve understanding of pathophysiologic processes by identifying abnormalities in neural systems implicated in core symptoms of BD. There is growing evidence from functional neuroimaging studies that limbic and prefrontal systems play an important role in emotion processing and regulation processes (Pavuluri et al., 2012; Phillips, 2003), and that functional abnormalities in these systems are commonly found in BD in youth and adults (Leibenluft and Rich, 2008; Phillips et al., 2008a). Recent neuroimaging studies have reported different types of abnormal activity in these neural systems in adults with BDd versus UDd, suggesting that examination of neural activity during processing of different emotional stimuli may help provide neural markers to distinguish BD from major depressive disorder during depression (Almeida et al., 2010, 2009; Bertocci et al., 2012; Lawrence et al., 2004). To date, however, no study has compared neural activity to emotional stimuli in BDd versus UDd youth. Identifying differential patterns of functional abnormalities in emotion processing in neural systems in BDd relative to UDd and healthy control (HC) adolescents may help differentiate BDd from UDd early in development, facilitate understanding of the depression-specific neural substrates of BD, and provide insight into the neurobiological and developmental etiology of BD in adolescents. The main goal of this preliminary cross-sectional study was thus to identify functional abnormalities in neural systems that may help differentiate BD from UDd in depressed adolescents (relative to age- and gender-ratio-matched healthy controls; HC). We aimed to study two separate experiments (e.g., happy and fearful faces) in a three conditions (intense emotion, mild emotion, and neutral) by three groups (BPd, UDd, and HC) design and hypothesized that neural activity to intense positive versus negative emotional stimuli during emotion processing can help differentiate BDd.
2. Methods
2.1. Subjects
We included right-handed adolescents aged 12 to 17 years who had reached puberty (with a score ≥3 on Tanner's Pubertal Development Scale (Marshall and Tanner, 1969)), and matched the three groups for age and sex ratio. Demographic and clinical variables of the study groups are shown in Table 1. There were 10 BDd adolescents (5 with BD type I, and 5 with BD type II; 8 females and 2 males, mean age=15.6±1.1 years old), 10 UDd adolescents (8 females and 2 males, mean age=15.9±1.1 years old), and 10 HC adolescents (8 females and 2 males, mean age=15.6±1.2 years old). One adolescent in the BDd group and two adolescents in the UDd group were in their first depressive episode; other adolescents in both depressed groups had history of past depressive episodes. The mean duration of current depressive episode, depression scores on the Children's Depression Rating Scale-Revised (CDRS-R) (Poznanski and Mokros, 1995), mania scores on the Young Mania Rating Scale (Young et al., 1978), anxiety scores on the child Screen for Child Anxiety Related Emotional Disorders (SCARED) (Birmaher et al., 1997), and Clinical Global Impression (CGI)-Severity scores (Spearing et al., 1997) were not statistically different (p < 0.05) between BDd and UDd adolescents (Table 1). In adolescents with comorbid attention deficit hyperactivity disorder (ADHD) (2 in BDd and 1 in UDd), we withdrew stimulant medications for at least 24 h before the neuroimaging assessment to minimize potential confounding effects of these medications. There were seven BDd adolescents and six UDd adolescents with comorbid anxiety disorders. We included unmedicated (2 BDd and 4 UDd adolescents) and medicated depressed adolescents (up to three medications): two adolescents with mood stabilizers (e.g., lithium or antiepileptic mood stabilizer) monotherapy; two with atypical antipsychotic plus antidepressant medication combinations; two with mood stabilizers plus atypical antipsychotic medication combinations; two with mood stabilizers plus atypical antipsychotic plus antidepressant medication combinations in the BDd group; and six with antidepressant monotherapy in the UDd group (Table 2).
Table 1.
Demographic information and clinical variables.
| BDd | UDd | HC | Overall significance* | Pair-wise comparisons (d.f.=18) | |
|---|---|---|---|---|---|
| Mean age (years) | 15.6 ± 0.9 | 15.9 ± 1.1 | 15.6 ± 1.2 | p = 0.6, F=0.4 | – |
| Gender (females) | 80% | 80% | 80% | p = 0.6, F=0 | – |
| Race (White) | 70% | 70% | 70% | p = 1, F=0.4 | – |
| Family history of BD (%) | 90% | 0% | 0% | – | – |
| BD subtypes | 5 BD I, 5 BD II | N/A | N/A | – | – |
| Comorbid ADHD | 30% | 20% | 0% | p = 0.2, F=1.7 | – |
| Comorbid anxiety disorders | 70% | 60% | 0% | p = 0.001, ×2 =8.6 | BDd > HC, ×2 = 10.8**UDd > HC, ×2 = 8.6** |
| Mean duration of the current depressive episode (weeks) | 8.1 ± 6.3 | 13.8 ± 6.2 | – | p = 0.06, F=4.2 | – |
| Children's Depression Rating Scale-Revised (CDRS-R) | 74.5 ± 12.8 | 65.8 ± 13.3 | 19.1 ± 1.8 | p = 0.0001, F=78.4 | BDd > HC, t=13.7**UDd > HC, t= 11.1** |
| Screen for Child Anxiety Related Emotional Disorders (SCARED) | 34.3 ± 17.8 | 28.1 ± 14.7 | 2.2 ± 1.4 | p = 0.0001, F=16.2 | BDd > HC, t=5.7**UDd > HC, t=5.5** |
| Young Mania Rating Scale (YMRS) | 2.5 ± 2.1 | 2.2 ± 1.1 | 0.8 ± 0.8 | p = 0.003, F = 7.5 | BDd > HC, t=3.8**UDd > HC, t=3.2** |
| Mean of the Clinical Global Impression (CGI)-Severity | 5.5 ± 0.5 | 5 ± 0.8 | 1 | p = 0.0001, F =190.2 | BDd > HC, t=26.9**UDd > HC, t=15.5** |
BDd: bipolar disorder in depressed state, UDd: unipolar major depressive disorder in depressed state, HC: healthy controls, N/A: not available, ADHD: attention deficit hyperactivity disorder.
Significance level was set at p < 0.05. Standard deviation is reported after ±. Statistical values, significance levels, and d.f. (degrees of freedom) are presented in the table. Group differences are shown in bold in the table and indicated by asterisks
for a significance level of p < 0.01.
Table 2.
Medication list of the depressed adolescents.
| Subject # | BDd | UDd |
|---|---|---|
| 1 | Risperidone, citalopram | No medication |
| 2 | No medication | Fluoxetine |
| 3 | Lamotrigine | Fluoxetine |
| 4 | Lithium, quetiapine | Paroxetine |
| 5 | Lamotrigine, aripiprazole | No medication |
| 6 | Lamotrigine, quetiapine, fluoxetine | No medication |
| 7 | No medication | Fluoxetine |
| 8 | Aripiprazole, fluoxetine | No medication |
| 9 | Lamotrigine, aripiprazole, fluoxetine | Citalopram |
| 10 | Lithium | Fluoxetine |
BDd: bipolar disorder in depressed state, UDd: unipolar major depressive disorder in depressed state.
2.2. Study design
This was a cross-sectional functional neuroimaging study. All adolescents and their parents gave informed consent before the study procedures. The University of Pittsburgh Institutional Review Board approved the study and consent forms. All depressed adolescents (BDd and UDd) met Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR) criteria for a diagnosis of major depressive episode (≥2 weeks), as determined by parent and adolescent interviews with the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (Kaufman et al., 1997). In addition, BDd adolescents met strict DSM-IV (APA, 2000) criteria (e.g., episodicity) for BD I or II. At the time of the scan, reviewing the past 2 weeks, a total score on the CDRS-R (Poznanski and Mokros, 1995) ≥40, and a total score on the Young Mania Rating Scale (Young et al., 1978) (YMRS) < 11, were required for depressed adolescents. In addition, we measured anxiety with a self-rated anxiety scale (SCARED) (Birmaher et al., 1997). Urine screening to exclude pregnancy and illicit substance abuse was performed before the scanning. No family history for BD (or personal history of medication-induced mania) was allowed for UDd adolescents and no personal or family psychiatric history was allowed for HC adolescents (Diler and Avci, 1999; Miklowitz and Chang, 2008; Pavuluri et al., 2005; Ross, 2006). Our exclusion criteria included any contraindications for functional magnetic resonance imaging (e.g., braces), psychosis, pervasive developmental disorders, eating disorders, learning disorders, BD Not Otherwise Specified, substance use disorders, and mental retardation.
2.3. Neuroimaging task
All adolescents performed an emotional facial expression gender labeling task: two separate 7-min-long event-related functional neuroimaging experiments were used to examine neural activity to positive (happy) and negative (fearful) emotional facial expressions (Surguladze et al., 2003). Here, in each experiment, participants viewed a total of 60 facial expressions comprising either intense (prototypical) happy or fearful (H100% or F100%), mild happy or fearful (H50% or F50%), and neutral (Hn or Fn) facial expressions. In both tasks, stimuli were gray-scale digitized photographs that were of fixed size (15 × 10.5 cm2), cropped, and morphed using software to depict emotional expressions ranging from neutral (0%) to mild (50%) to prototypical (100%) intensity of each emotion. Each stimulus was presented for 2 s with a mean inter-stimulus interval of 4.9 s during which a fixation cross was displayed. In each task, subjects viewed 20 neutral, 20 mild, and 20 prototypical faces. The order of which task would be presented first was counterbalanced across subjects. Participants were asked to judge whether each face was male or female by pressing the index or middle finger in a touch-sensitive glove. This task is a well-validated measure of implicit emotion processing, and has been associated with prefrontal cortical and limbic neural activity in healthy and psychiatric populations including BD (Hassel et al., 2008; Ladouceur et al., 2011; Surguladze et al., 2006, 2005, 2003).
2.4. Functional imaging data acquisition
All neuroimaging data were collected at the Magnetic Resonance Research Center (MRRC), University of Pittsburgh, on a Trio 3.0 T scanner (Siemens, AG). Anatomical images covering the entire brain were acquired using an axial 3D Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence, parallel to the anterior commissure-posterior commissure (ACPC) line (TE/TI/TR 3.29 ms/900 ms/2200 ms, flip angle=9, isotropic 1 mm3 voxel, 192 axial slices, matrix size=256 × 192). Blood Oxygen Level Dependent (BOLD) functional images were acquired with a gradient-echo EPI sequence (TR/TE=2000/25 ms, field of view=205 mm, matrix=64 × 64) and cover 34 axial slices (3 mm thick, 0 mm gap) encompassing the entire cerebrum and the majority of the cerebellum. Before collection of functional magnetic resonance imaging (fMRI) data for each subject, a reference EPI scan was acquired and inspected for artifacts (e.g., ghosting), as well as for good signal across the entire volume of acquisition, including the medial temporal lobes.
2.5. Data analysis
Using Statistical Parametric Mapping (SPM5; http://www.fil.ion.ucl.ac.uk/spm), data were pre-processed and analyzed. Data for each participant were first corrected for differences in acquisition time between slices; realigned and unwarped, co-registered, normalized and spatially smoothed. A first-level fixed-effect model was constructed to examine within-subject effects. Here, three emotion intensities (neutral, mild, intense) for each of the two facial gender labeling experiments (happy and fearful) were entered as separate conditions in an event-related design, with fixation cross as baseline in the design matrix. Movement was entered as a nuisance variable. Second-level random-effects group analyses were conducted on the t-contrast images generated in the previous single subject analyses in a 3 (group: BDd, UDd, HC) by 3 (condition: neutral, mild, intense emotion) repeated-measures analysis of variance (ANCOVA), covarying for age for each experiment (happy and fearful), given the potentially different relationships between age and neural activity in each group. A cluster-level false-positive detection rate of p < 0.05 was maintained for whole-brain activity surviving a voxel-wise threshold of p < 0.05, using a regional anatomic mask from the Wake Forest University Pick atlas for each whole-brain activity cluster ≥10 voxels, and a cluster (k) extent empirically determined by Monte Carlo simulation implemented in AlphaSim (Pan et al., 2011). Peak BOLD signal changes were extracted from regions showing a significant group-by-condition interaction in the 3 × 3 analysis for each experiment. Post hoc tests were then performed on these extracted BOLD signal values to examine the extent to which pairwise between-group differences in activity contributed to the significant group-by-condition interactions in these analyses, using independent t-tests and appropriate statistical thresholds (corrected p < 0.05/9=p < 0.006) to control for the three pair-wise between-group tests for each of the three emotional conditions in each experiment. In exploratory analyses, we used t-tests or correlational analyses (p < 0.05), as appropriate, to examine potential relationships among age, gender, severity of depression and anxiety symptoms, subtype of the mood disorders (BD I versus BD II, single versus recurrent depression), ADHD and anxiety comorbidity, and psychotropic medications upon patterns of abnormal neural activity in each depressed group.
3. Results
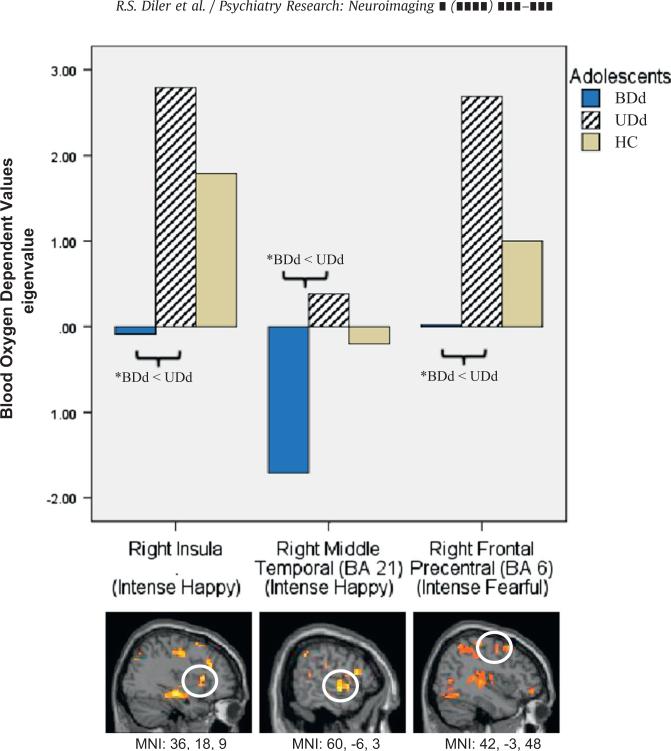
In the happy experiment there was a significant group by condition interaction in frontal precentral and superior prefrontal cortices (Brodmann's Area (BA) 6 and 8), anterior cingulate cortex (ACC, BA 24), insula, temporal cortex (BA 21 and 39), parahippocampus, parietal cortex (40), precuneus (BA 19), and occipital cortex (BA 19) (Table 3). Table 3 shows significant group differences at the level of p < 0.05 and the results of pair-wise analyses that remained significant (p < 0.006) after controlling for multiple comparisons are reported and discussed in the text. Pair-wise between-group comparisons showed that the majority of these group by condition interactions resulted mainly from both types of depressed (BDd and UDd) adolescents showing significantly lower activity than HC adolescents to intense and mild happy faces. In addition, UDd adolescents showed significantly elevated activity relative to HC to neutral faces in this experiment. Furthermore, BDd adolescents showed significantly lower activity relative to UDd adolescents in some of these regions to intense happy faces. Specifically, to intense happy faces BDd adolescents had significantly lower right insula and right middle temporal cortical (BA 21) activity relative to UDd adolescents (Fig. 1) and significantly lower left precentral cortical activity (BA6) relative to HC adolescents. To mild happy faces, BDd adolescents had significantly lower right parahippocampus activity relative to HC. Relative to HC, UDd adolescents had significantly lower left frontal precentral cortical (BA 8) and right superior temporal cortical (BA 39) activity to intense happy faces, lower right parahippocampus activity to mild happy faces, and higher left occipital cortical (BA 19) activity to neutral faces (Hn) presented in the happy experiment.
Table 3.
Neural regions showing significant group by emotion condition interactions and post-hoc pairwise between group comparisons for happy and fearful experiments.
| Brain region | Intensity | BA | MNI (x, y, z) | Voxels | F value | overall p value | Pair-wise comparisons (d.f.=18) |
||
|---|---|---|---|---|---|---|---|---|---|
| BDd vs. UDd | BDd vs. HC | UDd vs. HC | |||||||
| Happy experiment | |||||||||
| L Frontal Precentral | H100 | 6 | –15, –15,60 | 64 | 6.79 | 0.004 | BDdo Udd, t=3.0** | BDd < HCa, t=3.2** | –, t=0.85 |
| L Superior Prefrontal | H100 | 8 | –15,24,48 | 22 | 5.02 | 0.014 | –, t=0.77 | BDd < HC, t=2.1* | UDd < HCa, t=3.6** |
| R ACC | H100 | 24 | 3,36,3 | 76 | 4.06 | 0.03 | BDd < Udd, t=2.6* | BDd < HC, t=2.5* | –, t=0.1 |
| R Insula | H100 | 36,18,9 | 21 | 7.10 | 0.003 | BDd < UDda, t=4.0** | BDd < HC, t=2.5* | –, t=1.2 | |
| R Middle Temporal | H100 | 21 | 60, – 6,–3 | 50 | 6.74 | 0.004 | BDd < UDda, t=3.6** | BDd < HC, t=2.3* | –, t=1.1 |
| L Superior Temporal | H100 | 39 | – 45, – 60,30 | 96 | 7.50 | 0.003 | –, t=2.1 | – t=1.2 | UDd < HCa, t=4.1** |
| R Parahippocampus | H50 | 36 | 39,–21,–12 | 334 | 6.40 | 0.003 | –, t=1.0 | BDd < HCa, t=3.4** | UDd < HCa, t=4.8** |
| R Parahippocampus | Hn | 36 | 39,–21,–12 | 334 | 4.94 | 0.02 | –, t=2.0 | –, t=0.5 | –, t=1.1 |
| R Frontal Precentral | Hn | 6 | 66,–3,24 | 12 | 3.80 | 0.04 | –, t=2.0 | –, t=0.6 | –, t=1.1 |
| R Precuneus | Hn | 19 | 42, – 72,42 | 84 | 3.79 | 0.04 | –, t=0.3 | BDd < HC, t=2.6* | UDd < HC, t=2.2* |
| L Inferior Parietal | Hn | 40 | – 42,– 45,51 | 14 | 3.38 | 0.05 | –, t=0.1 | BDd < HC, t=2.3* | UDd > HC, t=2.9** |
| L Occipital | Hn | 19 | –9,–87,36 | 64 | 6.01 | 0.007 | –, t=0.7 | BDd > HC, t=2.5* | UDd > HCa, t=3.3** |
| Fearful experiment | |||||||||
| R Frontal Precentral | F100 | 6 | 42, – 3,48 | 15 | 9.27 | 0.001 | BDd < UDda, t=4.4** | –, t=1.6 | –, t=1.4 |
| R Insula | F100 | 27,27,6 | 159 | 3.36 | 0.05 | BDd < UDd, t=2.4* | –, t=1.3 | –, t=1.3 | |
| L Insula | F100 | – 39, – 9, 21 | 29 | 4.38 | 0.02 | –, t=1.9 | –, t=0.7 | UDd > HC, t=2.9** | |
| R Parahippocampus | F100 | 28 | 24,–15,–18 | 117 | 3.71 | 0.04 | –, t=2.0 | –, t=0.1 | –, t=2.0 |
| R Frontal Precentral | F50 | 4 | 39,–15,51 | 18 | 4.42 | 0.002 | BDd < Udd, t=2.2* | –, t=1.5 | UDd < HC, t=2.7* |
| L Middle Frontal | F50 | 6 | – 21, – 12,45 | 128 | 3.78 | 0.04 | –, t=0.2 | BDd > HC, t=2.8* | UDd < HC, t=2.2* |
| L Middle Frontal | F50 | 10 | – 36,54,– 3 | 78 | 3.78 | 0.04 | –, t=1.7 | –, t=1.3 | UDd < HC, t=2.3* |
| L VLPFC | F50 | 45 | – 45,24, 21 | 17 | 4.17 | 0.03 | –, t=0.3 | BDd > HCa, t=3.3** | –, t=2.0 |
| L DLPFC | F50 | 46 | – 48,45,9 | 25 | 4.18 | 0.03 | –, t=1.3 | –, t=1.5 | –, t=2.0 |
| L Insula | F50 | – 39, – 9, 21 | 29 | 6.62 | 0.005 | –, t=0.6 | BDd > HCa, t=3.9** | UDd > HC, t=2.6* | |
| L Insula | F50 | – 36,– 42,18 | 16 | 3.89 | 0.02 | –, t=1.7 | BDd > HCa, t=4.4** | –, t=0.7 | |
| R Middle Temporal | F50 | 20 | 54,–36,–12 | 12 | 4.42 | 0.02 | BDd < UDd, t=2.4* | –, t=0.7 | –, t=2.0 |
| R Superior Temporal | F50 | 22 | –57,–9, 6 | 93 | 4.15 | 0.03 | –, t=1.7 | –, t=1.0 | UDd > HC, t=2.9** |
| R Superior Temporal | F50 | 42 | 63,–30,12 | 22 | 5.96 | 0.007 | –, t=1.6 | BDd > HC, t=2.2* | UDd > HCa, t=3.2** |
| L PostCentral | F50 | 2 | – 60, – 27,45 | 66 | 8.64 | 0.001 | –, t=0.5 | BDd > HCa, t=3.7** | UDd > HCa, t=3.9** |
| L PostCentral | F50 | 3 | –18,–36,60 | 1853 | 4.97 | 0.02 | –, t=1.8 | –, t=1.6 | –, t=1.8 |
| R Occipital | F50 | 18 | 3,–87,18 | 1316 | 5.97 | 0.007 | –, t=1.9 | BDd > HC, t=2.2* | UDd > HCa, t=3.2** |
| L Middle Frontal | Fn | 6 | – 21, – 12,45 | 128 | 3.63 | 0.04 | BDd > UDd, t – 2.2* | BDd > HC, t=2.9** | –, t=0.01 |
| L VLPFC | Fn | 45 | – 45,24, 21 | 17 | 3.63 | 0.04 | –, t=0.2 | BDd > HC, t=2.3* | UDd > HC, t=2.1* |
| R Insula | Fn | 30,– 15, 21 | 347 | 5.66 | 0.009 | BDd > UDd, t=2.9** | BDd > HC, t=2.6* | UDd < HC, t=2.2* | |
| L Insula | Fn | – 39, – 9, 21 | 29 | 3.36 | 0.05 | –, t=2.0 | BDd > HC, t=2.4* | –, t=0.6 | |
| L PostCentral | Fn | 2 | – 60, – 27,45 | 66 | 6.38 | 0.005 | –, t=2.0 | BDd > HC, t=2.3* | –, t=1.8 |
BA: Brodmann's Area, MNI: coordinates according to the Montreal Neurological Institute, d.f.: degrees of freedom, BDd: bipolar disorder in depressed state, UDd: unipolar major depressive disorder in depressed state, F: fear, H: happy, 100%: intense emotion, 50%: mild emotional intensity; Fn and Hn: neutral faces during the fear (F) and happy (H) emotional experiments, R=right, L=left, DLPFC: dorsolateral prefrontal cortex, VLPFC: ventrolateral prefrontal cortex, ACC: anterior cingulate cortex.
for a significance level of p <0.05.
for a significance level of p <0.01.
Pair-wise comparisons using t-tests for significant brain regions identified with ANCOVA analysis (group by condition interaction) and significance level was set at p < 0.006 (corrected for multiple comparisons) and illustrated in bold letters. Group differences are indicated by asterisks.
Fig. 1.
Significant neural activity to intense happy and fearful faces between adolescents with bipolar disorder in depressed state (BDd; N=10) and unipolar major depressive disorder in depressed state (UDd; N=10) relative to healthy controls (HC; N=10).
*Only significant pair-wise comparisons (after corrected for multiple comparisons (p≤0.006)) are illustrated.
In the fearful experiment, there was a significant group by condition interaction in frontal precentral, middle frontal, and lateral prefrontal cortices (BA 4, 6, 10, 45, and 46), temporal cortex (BA 20, 22, and 42), postcentral cortex (BA 2 and 3), occipital cortex (BA 18), insula, and parahippocampus (Table 3). Pair-wise between-group comparisons showed that the majority of these group by condition interactions resulted from both depressed groups showing significantly elevated activity relative to HC to mild fearful faces, while BDd adolescents showed significantly lower activity relative to UDd adolescents to intense fearful faces. Specifically, BDd adolescents had significantly lower right precentral cortical (BA 6) activity relative to UDd adolescents to intense fearful faces (Fig. 1), but significantly higher left ventrolateral prefrontal cortex (VLPFC, BA 45), left insula, and left postcentral cortical (BA 2) activity relative to HC to mild fearful faces. Relative to HC, UDd adolescents had significantly higher left postcentral cortical (BA 2), right superior temporal (BA 42), and right occipital cortical (BA 18) activity to mild fearful faces.
3.1. Exploratory analysis
There were no significant differences in neural activity between BD I versus BD II depressed adolescents in any regions showing functional abnormalities in BDd group relative to HC adolescents. In BDd adolescents, neural activity to the intense happy condition in the right middle temporal cortex (BA 21) was lower in males than in females (p=0.01, t=3.25, d.f. 8). Depression severity as assessed by the CDRS-R was inversely correlated in BDd adolescents with the right middle temporal cortex (BA 21) activity to the intense happy faces (r= –0.74, p=0.01) and with the left VLPFC (BA 45) activity to neutral faces presented in the fear experiment (r = –0.69, p=0.03). In UDd adolescents, depression severity on the CDRS-R was inversely correlated with the left dorsolateral prefrontal cortex (DLPFC, BA 46) to mild fearful faces (r= –0.72, p=0.02). There were no other significant relationships between depression severity and magnitude of activity in any of those neural regions showing functional abnormalities relative to HC adolescents in either BDd or UDd adolescents. Anxiety scores on the SCARED showed no significant correlation with neural activity to happy, fearful, and neutral faces in each study group. BDd and UDd adolescents with, versus without, ADHD and with, versus without, anxiety disorders did not differ in depression scores, duration of depression, and neural activity in any of those regions showing significant functional abnormalities relative to HC adolescents. There were no significant differences in activity in any of the above significant neural regions in each study group between those with (8 in BDd and 6 in UDd) versus those without psychotropic medication. When we analyzed association of neural activity and types of medications in BDd adolescents, left insula activity to the mild fearful experiment was lower in those who were on an atypical antipsychotic medication plus mood stabilizer combination (n=2) relative to those on no medication (n=2). In addition, the following analyses showed trend level differences: neural activity in right hippocampus (p=0.056, t=2.27, d.f.=8) and right insula (p=0.076, t=0.94, d.f.=8) to intense fearful faces was higher in BD type I versus type II, and depression severity on the CDRS-R was inversely correlated in BDd adolescents with left VLPFC (BA 45) activity to mild fearful faces (r= –0.60, p=0.06) and positively correlated in BDd adolescents with right parahippocampus activity to intense fearful faces (r=0.59, p=0.07).
4. Discussion
Accurately diagnosing BD in adolescents presenting with depression is a key goal to improving the long-term prognosis of this debilitating illness. Studies in adults suggest that patterns of neural activity may be different in BDd and UDd (Surguladze et al., 2005), and that examining neural activity to different emotional stimuli may help provide neural markers to differentiate the two disorders (Almeida et al., 2010, 2009; Bertocci et al., 2012; Lawrence et al., 2004). In parallel, there is growing evidence from functional neuroimaging studies that suggests a disturbance in affective neurocircuitry in pediatric BD (Leibenluft and Rich, 2008; Mayanil et al., 2011; Pavuluri and Passarotti, 2008; Rosen and Rich, 2010); however, there were no data in adolescents regarding the extent to which neural activity to emotional stimuli may differentiate BDd from UDd or HC.
4.1. Common findings in BDd versus UDd
In our study, several of the neural regions involved in the pathophysiology of BD were significant (e.g., prefrontal, anterior cingulate, insula, parahippocampus, temporal, parietal, and occipital) in our group by emotion analysis. Pair-wise comparisons showed that BDd adolescents, relative to UDd adolescents, showed significantly lower activity to both intense happy and intense fearful faces. Relative to HC, both BDd and UDd adolescents showed significantly lower activity to intense and mild happy faces. Furthermore, UDd adolescents showed significantly elevated activity to neutral faces in the happy experiment. In contrast to the happy experiment with mild intensity of emotional faces, depressed adolescents, particularly those with BDd, showed significantly elevated activity to mild fearful faces relative to HC. It is important to underscore that adolescents with BDd and UDd had different patterns of neural activity to mild emotional faces relative to HC, but significant differences between these two depressed groups were identified specifically in neural activity to intense emotional stimuli. In addition, depression severity was inversely correlated with neural activity to the intense happy faces and to neutral faces presented in the fear experiment in BDd adolescents and to mild fearful faces in UDd adolescents. Our study provides the first findings that BDd adolescents may be distinguished from UDd adolescents by patterns of neural activity to positive and negative emotional stimuli, similar to the few findings from studies of adults with BDd and UDd (Almeida et al., 2009; Lawrence et al., 2004; Surguladze et al., 2005).
The present study in depressed adolescents, using an implicit emotion processing task, identified abnormal functioning in neural regions implicated in face-responsive visual processing circuitry and affective circuitry that are hypothesized to be involved in the pathophysiology of mood disorders (Phillips et al., 2008a). The neural circuitry is involves in perceptual processing of facial features and emotion and includes neural regions such as the occipital cortex, fusiform gyrus, parahippocampus, amygdala, middle temporal and parietal cortices (Passarotti et al., 2007; Pavuluri et al., 2008). The affective circuitry, also called the Affect Evaluation and Regulation Network (Passarotti et al., 2012), includes evaluative affective circuitry (e.g., higher cortical evaluation of emotional and behavioral control in the ventral prefrontal, emotion modulation in the medial prefrontal, and executive function of emotion modulation in the DLPFC, and the complex interface of affective and cognitive processing in the ACC) and reactive affective circuitry (e.g., occipital cortex-amygdala activity in response to incidental emotion processing and medial prefrontal cortex activity to moderate impulsive automatic response tendencies) (Pavuluri et al., 2012, 2008; Pavuluri and Sweeney, 2008). Patterns of abnormal neural activity in this study were significantly different in the happy and fearful experiments between BDd and UDd adolescents relative to HC. After controlling for multiple comparisons, our results suggested that BDd adolescents were distinguished from UDd adolescents by lower neural activity to intense emotional (both happy and fearful) faces; however, both depressed groups showed similar patterns of neural activities that differentiated them from HC: lower activity to intense and mild happy faces but elevated activity to mild fearful faces. These patterns of abnormal activity in both types of depression relative to HC (e.g., reduced processing of intense happy stimuli (positive emotion) and greater processing of milder, more subtle/ambiguous fear (negative emotion)) are consistent with Beck's theory of cognitive (e.g., attention, memory) bias toward negative and away from positive emotional stimuli in depression (Disner et al., 2011; Surguladze et al., 2005). In parallel with the reports of impaired attention to emotional faces and the difficulty in labeling emotions correctly when the emotion was not intense in euthymic adolescents with BD relative to HC (Leibenluft and Rich, 2008; Rich et al., 2008b), our findings that BDd adolescents were differentiated from UDd adolescents by lower neural activity to intense faces of both emotions may suggest impaired attention to prototypical facial emotion of any type in BDd relative to UDd. In addition, the differences between the two depressed groups in neural activity to intense positive and negative emotional stimuli may be associated with depressive symptoms of anhedonia (e.g., impaired positive cognitive processing) and hopelessness (e.g., impaired negative cognitive processing) that were reported as more common in BDd than in UDd youth (Wozniak et al., 2004).
4.2. Positive emotional stimuli in BDd versus UDd
The few studies that examined the valance of emotional stimuli in depressed adults suggested that neural activity to happy but not fearful faces was helpful in differentiating BDd adults from those with UDd (Almeida et al., 2010, 2009; Lawrence et al., 2004). The only fMRI study to date in BDd adolescents was a treatment study in bipolar spectrum disorder (BD I, II, and NOS) and did not examine neural activity to positive emotional stimuli and did not include a control group (HC or UDd) (Chang et al., 2008). Our study provided the first findings that BDd adolescents can be differentiated from UDd adolescents by lower neural activity to intense happy faces in right insula and right middle temporal (BA 21), both of which play various roles in emotion processing and are implicated in the pathophysiology of BD in adults and adolescents (Lagopoulos et al., 2007; Mayanil et al., 2011; Pavuluri et al., 2007; Pavuluri and Passarotti, 2008). In addition, severity of depression was correlated with lower activity to intense happy faces in the right middle temporal cortex in BDd, not in UDd, adolescents. The temporal cortex is associated with visual memory and interacts with prefrontal and subcortical limbic regions for attentional control and processing, respectively, of emotionally valent visual information (Lagopoulos et al., 2007). On the other hand, the insula has a wide range of connections with cortical and sub-cortical regions providing an interface between external information and internal motivational states (Nieuwenhuys, 2012). Lower Insula activity to the positive stimuli, which was also reported in euthymic BD adolescents (Pavuluri et al., 2008) may be associated with disruption in identification (and generation) of positive emotions and pessimistic bias (Herwig et al., 2007) in BDd adolescents.
4.3. Positive emotional stimuli in depression (BDd and UDd)
Our results also indicated that BDd and UDd adolescents shared some abnormalities in neural activity to the positive stimuli that may help differentiate these two depressed groups from HC. There are few published fMRI studies (using emotion processing (Chang et al., 2004; Pavuluri et al., 2007; Pavuluri and Passarotti, 2008) and affective working memory tasks (Passarotti et al., 2010b)) that examined neural activity to happy faces in BD relative to HC youth, but these studies included non-depressed BD youth and reported increased (Chang et al., 2004; Passarotti et al., 2010b, 2011) and decreased (Pavuluri et al., 2007; Pavuluri and Passarotti, 2008) prefrontal, but increased subcortical activity (Chang et al., 2004; Passarotti et al., 2010b, 2011; Pavuluri et al., 2007). In our study, both BDd and UDd adolescents were differentiated from HC by lower neural activity to intense happy faces in left prefrontal cortex and to mild happy faces in the right parahippocampus. In contrast to previous reports (Pavuluri et al., 2007; Pavuluri and Passarotti, 2008) of increased bilateral hippocampus activity to happy faces in euthymic BD adolescents relative to HC, our results were consistent with studies in depressed adults (Fu et al., 2007; Savitz and Drevets, 2009) suggesting biased memory processing (e.g., impairment in encoding and retrieval of emotional facial expressions) to positive emotional stimuli in depression of adolescents with BDd and UDd. In addition, UDd adolescents were also differentiated from HC by lower left superior temporal (BA 39) activity (e.g., possibly reflecting lower attentional allocation) to intense happy but increased left occipital (BA 19) activity (e.g., possible reflecting increased visual processing) to neutral faces in the happy experiment that are consistent with the theory of cognitive bias away from positive stimuli in depression (Disner et al., 2011; Surguladze et al., 2005). A few reports that examined happy stimuli in UDd relative to HC adolescents reported no difference (Beesdo et al., 2009) and increased amygdala and hippocampus (Yang et al., 2010) activity, but they did not study the intensity of emotional stimuli. Despite some discrepant reports, our findings emphasized the importance of examining neural activity to the positive stimuli; because it may help differentiate BDd from UDd in adolescents and identify elements of neural circuitry of positive emotion processing that is disturbed in depression in both disorders relative to HC.
4.4. Negative emotional stimuli in BDd versus UDd
Similar to our findings with the positive stimuli, and consistent with previous studies that reported prefrontal dysfunction to the negative stimuli in depressed (Chang et al., 2008) and euthymic BD youth (Pavuluri et al., 2007, 2009), BDd adolescents in our study were differentiated from UDd adolescents by lower neural activity to intense negative stimuli in the prefrontal cortex (e.g., premotor cortex (BA 6); implicated in planning of complex coordinated movements). On the other hand, the majority of studies reported that the VLPFC in BD adults (Foland-Ross et al., 2012; Phillips et al., 2008a; Savitz and Drevets, 2009) and adolescents (DelBello et al., 2006; Fleck et al., 2010; Passarotti et al., 2010a; Pavuluri et al., 2011) and DLPFC in UDd adults (Savitz and Drevets, 2009) and adolescents (Croarkin et al., 2010; Killgore et al., 2007) were the significant prefrontal regions particularly implicated in the pathophysiology of these mood disorders. Similarly, the depression severity in our study was correlated with left VLPFC (BA 45) activity (to neutral faces presented in the fear experiment) in BDd adolescents and with left DLPFC (BA 46) activity (to mild fearful faces) in UDd adolescents.
4.5. Negative emotional stimuli in depression (BDd and UDd)
Previous studies in euthymic (Chang et al., 2004; Pavuluri et al., 2009) and manic (Passarotti et al., 2011) BD adolescents relative to HC reported higher (Chang et al., 2004) and lower (Passarotti et al., 2011; Pavuluri et al., 2009) prefrontal activity to the negative stimuli. In our study, in contrast to the happy experiment with mild and intense emotional stimuli, BDd adolescents relative to HC had overall higher neural activity to the fearful experiment, particularly to mild fearful faces (e.g., left VLPFC (BA 45), right and left insula, and left postcentral (BA 2)) that suggested preserved ability to increase recruitment of these regions to process the negative emotions in BDd when the intensity of the emotion was mild. Similarly, increased left insula activity to negative relative to neutral stimuli (e.g., words) was identified in euthymic youth with BD (Pavuluri et al., 2008) and greater engagement of left insula during response inhibition was shown in manic youth with BD (relative to controls and after treatment with risperidone) (Pavuluri et al., 2012). However, in contrast to the majority of BD studies with adults (Chen et al., 2006) and youth (Mayanil et al., 2011; Passarotti et al., 2011; Pavuluri et al., 2008), we did not find amygdala activation to the negative stimuli in BDd adolescents. It is possible that the type of emotional stimuli we used in this study (e.g., fearful faces) could be responsible for the negative findings in subcortical activity elicited in this study versus other previous reported other fMRI studies in youth with BD that mainly used angry faces (Singh et al., 2012) or employed paradigms to interpret fear in neutral faces (Rich et al., 2008a). It was also reported that amygdala activity in youth with BD might show delayed activation to fearful faces and fMRI paradigms with slow event-related designs might be needed to elicit its abnormal hemodynamic response (Wegbreit et al., 2013). Similar to our study, a few other studies in euthymic BD adolescents (Brotman et al., 2010; Chang et al., 2004) and in BDd adults (Altshuler et al., 2008; Chen et al., 2006) as well as a recent meta-analysis in BDd adults (Van der Schot et al., 2010) did not report amygdala activation to the negative stimuli suggesting that the amygdala might habituate quickly and therefore show no increased activity in BDd (Chen et al., 2011; Van der Schot et al., 2010) or might show increased activation in response to sad or angry but not fearful faces (Almeida et al., 2010; Brotman et al., 2010; Dickstein et al., 2007). Despite advanced neural models of depression in adults, we know very little about the neural regions involved in adolescents with UDd relative to HC. The few available fMRI studies with UDd adolescents relative to HC reported lower prefrontal (Yang et al., 2009) and increased (Beesdo et al., 2009; Lau et al., 2009; Yang et al., 2009; Yang et al., 2010) or decreased (Thomas et al., 2001) amygdala activity to the negative stimuli. In our study, UDd adolescents were not differentiated from HC by frontal or subcortical activity to the intense negative stimuli. Similar to BDd, UDd adolescents, relative to HC, showed significantly higher neural activity in regions involved in emotional face and visual processing (e.g., postcentral, temporal, occipital) when the negative emotional stimuli were not intense, suggesting a cognitive bias for processing the mild negative stimuli in UDd adolescents.
4.6. Limitations
Limitations of this study should be taken into account before generalizing our findings and drawing any conclusion. This is a cross-sectional study with a small sample size and subjects were not matched for IQ. We recruited predominantly female adolescents who had low ADHD comorbidity (10–20%) relative to some pediatric BD studies (Axelson et al., 2006) and they were allowed to be on (non-stimulant) psychotropic medications during scanning. Available studies, mostly in adults and relatively few in adolescents, indicated that medication may have normalizing rather than confounding effects upon abnormal neural activity in adolescents with BD (Almeida et al., 2010, 2009; Hassel et al., 2008; Leibenluft et al., 2007; Nelson et al., 2007; Phillips et al., 2008b; Rich et al., 2008a). Moreover, including only unmedicated individuals with BD who can tolerate medication withdrawal may restrict study participants to those with milder forms of the illness and limit the ability to identify biomarkers reflecting pathophysiologic processes generalizable to the majority of individuals with BD (Phillips et al., 2008b). In our study, there were no significant differences in activity in any of the above significant neural regions in each study group between those with versus without psychotropic medication. However, we found lower left insula activity to the mild fearful experiment in BDd adolescents in those who were on a combination of typical antipsychotic medication plus moodstabilizers relative to those BDd adolescents on no medication suggesting the possibility of a type II (e.g., the normalization effect of this medication combination on neural abnormality in BDd versus HC) rather than a type I error.
4.7. Implications
Considering the protracted development of the prefrontal cortex coupled with relatively matured but dysfunctional limbic regions (Fleck et al., 2010; Luna, 2009) in adolescents with BD, there is a window of opportunity for early identification of BD in depressed adolescents than can lead to appropriate interventions and possibly help halt the progression of the BD. Thus, early identification of BD in youth, especially in depression, is very critical. Our study provided the first neuroimaging data demonstrating that examining patterns of neural activity to positive and negative stimuli can help differentiate BDd from UDd and HC adolescents. Furthermore, our preliminary findings in both depressed groups, particularly in BDd, were consistent with the cognitive model of depression suggesting bias toward the negative stimuli (e.g., specifically with milder emotional stimuli where the ability to engage the neural regions was possibly still preserved) and away from the positive stimuli (e.g., both intense and mild) when processing emotions. Larger longitudinal studies are needed to better understand clinical correlates of disease-specific versus depressed mood-specific neural activity to varying intensities of emotional stimuli in BDd versus UDd adolescents relative to HC.
Acknowledgment
This study was supported by the American Academy of Child and Adolescent Psychiatry (AACAP) Ryan Licht Sang Foundation Quest for the Test for Bipolar Disorder Award.
Dr. Ladouceur (K01 MH083001) received funding from NIMH.
References
- Almeida JR, Versace A, Hassel S, Kupfer DJ, Phillips ML. Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry. 2010;67:414–421. doi: 10.1016/j.biopsych.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JR, Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML, Almeida J.R.C.d., Versace A, Mechelli A, Hassel S, Quevedo K, Kupfer DJ, Phillips ML. Abnormal amygdala-prefrontal effective connectivity to happy faces differentiates bipolar from major depression. Biological Psychiatry. 2009;66:451–459. doi: 10.1016/j.biopsych.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disorders. 2008;10:708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and Statistical Manual of Mental Disorders, Disorders. 4th ed. American Psychiatric Association; Washington, DC: 2000. Text Revision (DSM-IV-TR) [Google Scholar]
- Axelson D, Birmaher B, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Bridge J, Keller M. Phenomenology of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63:1139–1148. doi: 10.1001/archpsyc.63.10.1139. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Lau JY, Guyer AE, McClure-Tone EB, Monk CS, Nelson EE, Fromm SJ, Goldwin MA, Wittchen HU, Leibenluft E, Ernst M, Pine DS. Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Archives of General Psychiatry. 2009;66:275–285. doi: 10.1001/archgenpsychiatry.2008.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR, Phillips ML. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychological Medicine. 2012;42(7):1417–1428. doi: 10.1017/S003329171100242X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Strober M, Gill MK, Hunt J, Houck P, Ha W, Iyengar S, Kim E, Yen S, Hower H, Esposito-Smythers C, Goldstein T, Ryan N, Keller M. Four-year longitudinal course of children and adolescents with bipolar spectrum disorders: the Course and Outcome of Bipolar Youth (COBY) study. American Journal of Psychiatry. 2009;166:795–804. doi: 10.1176/appi.ajp.2009.08101569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Strober M, Gill MK, Valeri S, Chiappetta L, Ryan N, Leonard H, Hunt J, Iyengar S, Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Archives of General Psychiatry. 2006;63:175–183. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Brotman MA, Rich BA, Guyer AE, Lunsford JR, Horsey SE, Reising MM, Thomas LA, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. American Journal of Psychiatry. 2010;167:61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. Challenges in the diagnosis and treatment of pediatric bipolar depression. Dialogues in Clinical Neuroscience. 2009;11:73–80. doi: 10.31887/DCNS.2009.11.1/kchang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A. Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Archives of General Psychiatry. 2004;61:781–792. doi: 10.1001/archpsyc.61.8.781. [DOI] [PubMed] [Google Scholar]
- Chang KD, Wagner C, Garrett A, Howe M, Reiss A. A preliminary functional magnetic resonance imaging study of prefrontal-amygdalar activation changes in adolescents with bipolar depression treated with lamotrigine. Bipolar Disorders. 2008;10:426–431. doi: 10.1111/j.1399-5618.2007.00576.x. [DOI] [PubMed] [Google Scholar]
- Chen CH, Lennox B, Jacob R, Calder A, Lupson V, Bisbrown-Chippendale R, Suckling J, Bullmore E. Explicit and implicit facial affect recognition in manic and depressed states of bipolar disorder: a functional magnetic resonance imaging study. Biological Psychiatry. 2006;59:31–39. doi: 10.1016/j.biopsych.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- Croarkin PE, Wall CA, McClintock SM, Kozel FA, Husain MM, Sampson SM. The emerging role for repetitive transcranial magnetic stimulation in optimizing the treatment of adolescent depression. Journal of ECT. 2010;26:323–329. doi: 10.1097/YCT.0b013e3181dd17eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectrums. 2006;11:298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- Dickstein DP, Rich BA, Roberson-Nay R, Berghorst L, Vinton D, Pine DS, Leibenluft E. Neural activation during encoding of emotional faces in pediatric bipolar disorder. Bipolar Disorders. 2007;9:679–692. doi: 10.1111/j.1399-5618.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diler RS, Avci A. SSRI-induced mania in obsessive-compulsive disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 1999;38:6–7. doi: 10.1097/00004583-199901000-00010. [DOI] [PubMed] [Google Scholar]
- Diler R.S.e. Pediatric Bipolar Disorder: A Global Perspective. Nova Science Publishers, Inc.; New York: 2007. [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Fleck DE, Cerullo MA, Nandagopal J, Adler CM, Patel NC, Strakowski SM, Delbello M. Neurodevelopment in bipolar disorder: a neuroimaging perspective. In: Miklowitz DJ, Cicchetti D, editors. Understanding Bipolar Disorder: A Developmental Psychopathology Perspective. The Guilford Press; New York: 2010. pp. 259–281. [Google Scholar]
- Foland-Ross LC, Bookheimer SY, Lieberman MD, Sugar CA, Townsend JD, Fischer J, Torrisi S, Penfold C, Madsen SK, Thompson PM, Altshuler LL. Normal amygdala activation but deficient ventrolateral prefrontal activation in adults with bipolar disorder during euthymia. Neuroimage. 2012;59:738–744. doi: 10.1016/j.neuroimage.2011.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Brammer MJ, Suckling J, Kim J, Cleare AJ, Walsh ND, Mitterschiffthaler MT, Andrew CM, Pich EM, Bullmore ET. Neural responses to happy facial expressions in major depression following antidepressant treatment. American Journal of Psychiatry. 2007;164:599–607. doi: 10.1176/ajp.2007.164.4.599. [DOI] [PubMed] [Google Scholar]
- Geller B, Tillman R, Craney JL, Bolhofner K. Four-year prospective outcome and natural history of mania in children with a prepubertal and early adolescent bipolar disorder phenotype. Archives of General Psychiatry. 2004;61:459–467. doi: 10.1001/archpsyc.61.5.459. [see comment] [DOI] [PubMed] [Google Scholar]
- Hassel S, Almeida JR, Kerr N, Nau S, Ladouceur CD, Fissell K, Kupfer DJ, Phillips ML. Elevated striatal and decreased dorsolateral prefrontal cortical activity in response to emotional stimuli in euthymic bipolar disorder: no associations with psychotropic medication load. Bipolar Disorders. 2008;10:916–927. doi: 10.1111/j.1399-5618.2008.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herwig U, Kaffenberger T, Baumgartner T, Jancke L. Neural correlates of a ‘pessimistic’ attitude when anticipating events of unknown emotional valence. Neuroimage. 2007;34:848–858. doi: 10.1016/j.neuroimage.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data [see comments]. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Gruber SA, Yurgelun-Todd DA. Depressed mood and lateralized prefrontal activity during a Stroop task in adolescent children. Neuroscience Letters. 2007;416:43–48. doi: 10.1016/j.neulet.2007.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Farchione T, Diwadkar V, Pruitt P, Radwan J, Axelson DA, Birmaher B, Phillips ML. Differential patterns of abnormal activity and connectivity in the amygdala-prefrontal circuitry in bipolar-I and bipolar-NOS youth. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:1275–1289. doi: 10.1016/j.jaac.2011.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagopoulos J, Ivanovski B, Malhi GS. An event-related functional MRI study of working memory in euthymic bipolar disorder. Journal of Psychiatry and Neuroscience. 2007;32:174–184. [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Goldman D, Buzas B, Fromm SJ, Guyer AE, Hodgkinson C, Monk CS, Nelson EE, Shen PH, Pine DS, Ernst M. Amygdala function and 5-HTT gene variants in adolescent anxiety and major depressive disorder. Biological Psychiatry. 2009;65:349–355. doi: 10.1016/j.biopsych.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA. Pediatric bipolar disorder. Annual Review of Clinical Psychology. 2008;4:163–187. doi: 10.1146/annurev.clinpsy.4.022007.141216. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Rich BA, Vinton DT, Nelson EE, Fromm SJ, Berghorst LH, Joshi P, Robb A, Schachar RJ, Dickstein DP, McClure EB, Pine DS. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. American Journal of Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Advances in Child Development and Behavior. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood. 1969;44:291–303. doi: 10.1136/adc.44.235.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayanil T, Wegbreit E, Fitzgerald J, Pavuluri M. Emerging biosignature of brain function and intervention in pediatric bipolar disorder. Minerva Pediatrica. 2011;63:183–200. [PubMed] [Google Scholar]
- Miklowitz DJ, Chang KD. Prevention of bipolar disorder in at-risk children: theoretical assumptions and empirical foundations. Development and Psycho-pathology. 2008;20:881–897. doi: 10.1017/S0954579408000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Vinton DT, Berghorst L, Towbin KE, Hommer RE, Dickstein DP, Rich BA, Brotman MA, Pine DS, Leibenluft E. Brain systems underlying response flexibility in healthy and bipolar adolescents: an event-related fMRI study. Bipolar Disorders. 2007;9:810–819. doi: 10.1111/j.1399-5618.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R. The insular cortex—a review. Progress in Brain Research. 2012;195:123–163. doi: 10.1016/B978-0-444-53860-4.00007-6. [DOI] [PubMed] [Google Scholar]
- Pan LA, Batezati-Alves SC, Almeida JR, Segreti A, Akkal D, Hassel S, Lakdawala S, Brent DA, Phillips ML. Dissociable patterns of neural activity during response inhibition in depressed adolescents with and without suicidal behavior. Journal of the American Academy of Child and Adolescent Psychiatry. 2011;50:602–611. e603. doi: 10.1016/j.jaac.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Ellis J, Wegbreit E, Stevens MC, Pavuluri MN. Reduced functional connectivity of prefrontal regions and amygdala within affect and working memory networks in pediatric bipolar disorder. Brain Connectivity. 2012;2:320–334. doi: 10.1089/brain.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Smith J, DeLano M, Huang J. Developmental differences in the neural bases of the face inversion effect show progressive tuning of face-selective regions to the upright orientation. Neuroimage. 2007;34:1708–1722. doi: 10.1016/j.neuroimage.2006.07.045. [DOI] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 2010a;16:106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention-deficit/ hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010b;49:1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology. 2011;216:485–499. doi: 10.1007/s00213-011-2243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Birmaher B, Naylor MW. Pediatric bipolar disorder: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Ellis JA, Wegbreit E, Passarotti AM, Stevens MC. Pharmacotherapy impacts functional connectivity among affective circuits during response inhibition in pediatric mania. Behavioural Brain Research. 2012;226:493–503. doi: 10.1016/j.bbr.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral E, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biological Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Research: Neuroimaging. 2008;162:244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti A. Neural bases of emotional processing in pediatric bipolar disorder. Expert Review of Neurotherapeutics. 2008;8:1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Research: Neuroimaging. 2011;193:28–37. doi: 10.1016/j.pscychresns.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavuluri MN, Sweeney JA. Integrating functional brain neuroimaging and developmental cognitive neuroscience in child psychiatry research. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47:1273–1288. doi: 10.1097/CHI.0b013e318185d2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML. Understanding the neurobiology of emotion perception: implications for psychiatry. British Journal of Psychiatry. 2003;182:190–192. doi: 10.1192/bjp.182.3.190. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry. 2008a;13:833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008b;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poznanski E, Mokros H. Children's Depression Rating Scale-Revised. Western Psychological Services; Los Angeles: 1995. [Google Scholar]
- Rich BA, Fromm SJ, Berghorst LH, Dickstein DP, Brotman MA, Pine DS, Leibenluft E. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2008a;49:88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich BA, Grimley ME, Schmajuk M, Blair KS, Blair RJ, Leibenluft E. Face emotion labeling deficits in children with bipolar disorder and severe mood dysregulation. Development and Psychopathology. 2008b;20:529–546. doi: 10.1017/S0954579408000266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen HR, Rich BA. Neurocognitive correlates of emotional stimulus processing in pediatric bipolar disorder: a review. Postgraduate Medical Journal. 2010;122:94–104. doi: 10.3810/pgm.2010.07.2177. [DOI] [PubMed] [Google Scholar]
- Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. American Journal of Psychiatry. 2006;163:1149–1152. doi: 10.1176/ajp.2006.163.7.1149. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neuroscience and Biobehavioral Reviews. 2009;33:699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, DelBello MP, Chang KD. Functional brain imaging in bipolar disorder. In: Strakowski SM, editor. The Bipolar Brain. Oxford University Press; New York: 2012. pp. 103–123. [Google Scholar]
- Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Research. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biological Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Russell T, Kucharska-Pietura K, Travis MJ, Giampietro V, David AS, Phillips ML. A reversal of the normal pattern of parahippocampal response to neutral and fearful faces is associated with reality distortion in schizophrenia. Biological Psychiatry. 2006;60:423–431. doi: 10.1016/j.biopsych.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Brammer MJ, Young AW, Andrew C, Travis MJ, Williams SC, Phillips ML. A preferential increase in the extrastriate response to signals of danger. Neuroimage. 2003;19:1317–1328. doi: 10.1016/s1053-8119(03)00085-5. [DOI] [PubMed] [Google Scholar]
- Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Archives of General Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- Van der Schot A, Kahn R, Ramsey N, Nolen W, Vink M. Trait and state dependent functional impairments in bipolar disorder. Psychiatry Research: Neuroimaging. 2010;184:135–142. doi: 10.1016/j.pscychresns.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Wegbreit E, Passarotti AM, Ellis JA, Wu M, Witowski N, Fitzgerald JM, Stevens MC, Pavuluri MN. Where, when, how high, and how long? The hemodynamics of emotional response in psychotropic-naive patients with adolescent bipolar disorder. Journal of Affective Disorders. 2013;147:304–311. doi: 10.1016/j.jad.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Wozniak J, Spencer T, Biederman J, Kwon A, Monuteaux M, Rettew J, Lail K. The clinical characteristics of unipolar vs. bipolar major depression in ADHD youth. Journal of Affective Disorders. 2004;82(Suppl 1):S59–69. doi: 10.1016/j.jad.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Bischoff-Grethe A, Lansing AE, Wu J, Brown GG, Paulus MP. Depressed adolescents demonstrate greater subgenual anterior cingulate activity. Neuroreport. 2009;20:440–444. doi: 10.1097/WNR.0b013e3283262e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang TT, Simmons AN, Matthews SC, Tapert SF, Frank GK, Max JE, Bischoff-Grethe A, Lansing AE, Brown G, Strigo IA, Wu J, Paulus MP. Adolescents with major depression demonstrate increased amygdala activation. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:42–51. doi: 10.1097/00004583-201001000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]