Abstract
Given into the brain, melanin-concentrating hormone (MCH) increases alcohol consumption, but the mechanism and physiological relevance of this effect are unclear. We hypothesized that endogenous MCH will enhance alcohol drinking and that MCH increases alcohol’s reinforcing properties. An MCH receptor 1 (MCHR1) antagonist, or saline was administered centrally alone, or preceding MCH or saline to rats trained to drink 10% alcohol using sucrose fading. Blocking MCHR1 neither reduced alcohol intake (saline = 0.4 ± 0.1g, 30 μg MCHR1 antagonist = 0.4 ± 0.1 g/kg alcohol), nor attenuated MCH-induced alcohol drinking (MCHR1 antagonist/saline = 0.7 ± 0.1 g/kg, MCHR1 antagonist/MCH = 0.9 ± 0.1 g/kg alcohol). Another cohort of rats was trained to lever press for alcohol on a progressive ratio schedule. MCH or saline was administered centrally and lever presses were measured. MCH had no effect prior to the break point, but increased total responding during the session (saline = 87.2 ± 32.0, MCH = 315.4 ± 61.0 presses). In conclusion, these data suggest that MCH augments alcohol drinking partly by enhancing the drug’s reinforcing value. Further, endogenous MCH does not seem to regulate alcohol drinking, however because the antagonist failed to attenuate MCH-induced alcohol intake this conclusion is tentative.
INTRODUCTION
Melanin-concentrating hormone (MCH) is a cyclic peptide first isolated in the salmon pituitary and originally named for its ability to lighten the skin of teleost fish by inducing aggregation of melanocytes (Kawauchi et al., 1983). Although there is no indication that MCH performs this function in mammals, the peptide is present and its structure is highly conserved, with only a slight difference between the salmon and human form of MCH. In the mammal, MCH is produced by magnocellular neurons in the lateral hypothalamus and zona incerta (Pissios and Maratos-Flier, 2003). Two G-coupled protein receptors have been described that carry out the functions of MCH, MCH receptor 1 and 2 (MCHR1 and MCHR2). MCHR1 is conserved among the mammalian species, while MCHR2 expression is limited to primates and some carnivores (e.g., dogs) (Tan et al., 2002). The broad expression pattern of MCHR1 throughout the central nervous system (CNS), as well as the extensive monosynaptic projections of MCH neurons, foreshadowed the many functions for which the neuropeptide is believed to be involved.
The best known action of MCH is in energy homeostasis. MCH is a signal of negative energy balance in the brain and its administration leads to increased energy consumption (Qu et al., 1996). MCH also participates in the regulation of fluid balance. Changes in osmotic pressure activate MCH-producing neurons in multiple species (i.e., frog, teleost fish and rodents) (Presse and Nahon, 1993; Francis and Baker, 1995; Francis et al., 1997), and central infusion of MCH increases water consumption independently of food intake in rats (Clegg et al., 2003). MCH had also been proposed to influence reward because of the dense expression of MCH receptors in the shell of the nucleus accumbens (NAc). (DiLeone et al., 2003). Interestingly, injection of MCH directly into the shell of the NAc increases food intake in rats (Georgescu et al., 2005), and mice lacking MCHR1 have increased D1 and D2 binding in the NAc (Smith et al., 2005).
More recently, we identified a potential role for MCH to increase alcohol intake. When administered into the 3rd-cerebral ventricle, MCH increased alcohol intake in rats (Duncan et al., 2005). In those experiments, MCH increased the consumption of two solutions, 10% alcohol and a calorically equivalent, 17.75% sucrose solution, as well as increasing food intake (Duncan et al., 2005). While one interpretation is that MCH increases the reward value of alcohol, alternatively it may increase the consumption these ingestants through its impact on energy balance, and/or fluid balance. Because MCH increased alcohol intake in the presence of water, fluid balance is an unlikely explanation. Thus, the two remaining possibilities are that MCH augments alcohol intake by regulating energy balance, and/or that it increases the reward value of alcohol.
The purpose of the present study was two-fold. First, in order to determine whether the impact of MCH on alcohol intake is physiologically relevant, or merely a pharmacological consequence of exogenous MCH administration, we assessed alcohol consumption following the administration of an MCHR1 antagonist to rats that were voluntarily consuming alcohol. Second, we tested the hypothesis that one mechanism by which MCH acts to augment alcohol intake is by increasing the rewarding properties of alcohol. In order to isolate the role of reward from energy need we utilized free-feeding animals that were trained to lever press for alcohol under a progressive ratio schedule, a validated measure of reward value (Hodos, 1961).
MATERIALS and METHODS
Subjects
Male Long-Evans rats (n = 88, Harlan, Indianapolis, IN) weighing 250–300 g at the start of each experiment were housed individually in plastic tubs, and maintained on a 12:12-hr light/dark cycle in a room controlled for temperature (21 °C) and humidity (40–60%). Pelleted rat chow (Harlan Teklad, Indianapolis, IN) and tap water were provided ad libitum, except when otherwise noted. Experiments commenced 1 week following the arrival of the rats to the animal housing facility and were always carried out during the light. Research was conducted in AAALAC-approved facilities conforming to NIH and USDA regulations, with the approval of the University of Cincinnati Institutional Animal Care and Use Committee.
Experimental Procedures
Experiment 1
Intake training procedure
Experimentally naïve rats (n = 42) were trained to voluntarily consume alcohol or sucrose using a sucrose-fading procedure adapted from Grant and Samson (Grant and Samson, 1985). A limited access procedure was used such that rats had daily 2-hr access to solutions in their home cage in the middle of the light cycle for the duration of the study. For the first 10 days all rats were given 10% sucrose (w/v). Every 4 days thereafter for 32 days the rats were presented with a novel solution. Half of the rats (ALC group) were transitioned from 10% sucrose to 10% alcohol by incrementally adding alcohol to 10% sucrose (alcohol (w/v) = 0 to 2.5% to 5% to 7.5% to 10% alcohol), and then gradually reducing the sucrose concentration (i.e., 10% to 7.5% to 5% to 2.5% to 0 sucrose). After the 32-day fading process the rats were given 10 additional days of 2-hour access to 10% alcohol to establish stable drinking. As a caloric control, the remaining 21 rats (SUC group) received an amount of sucrose equal in caloric density to the solution presented to the ALC group on any given day during the 2-hour drinking session. In addition, the sucrose solution contained a small amount of quinine (25 μM – 750 μM) to limit sucrose consumption to a caloric level similar to the amount of alcohol calories consumed by the ALC rats. This amount of quinine does not affect food intake or body weight (Kratz et al., 1978; Tolliver et al., 1988; Sclafani and Ackroff, 1994). The final solution presented to the SUC group was 17.75% sucrose (w/v) containing 750 μM quinine. This solution was equal in caloric content to 10% alcohol (i.e., 0.71 kilocalories/ 1 ml).
Intracerebroventricular cannulation and injection procedure
Following training, rats were anesthetized using a mixture of intraperitoneal ketamine (86 mg/kg) and xylazine (12.9 mg/kg). Stainless steel 23-gauge cannulas were implanted into the 3rd-cerebral ventricle using a stereotaxic procedure (coordinates: anteroposterior: −2.2 mm from bregma and dorsoventral: −7.5 mm from the dura) (Paxinos, 1998). Following surgery animals were given 7 days to recover with continuing daily 2-hr access to their accustomed solutions. Intracerebroventricular (i3vt) infusions were administered manually in a 1-μl volume using a 25-μl Hamilton syringe (Hamilton, Reno, NV). Cannula placement was verified by an i3vt infusion of angiotensin II (7.5 ng). Animals that consumed at least 5 ml water within 1 hr following this infusion were used in the study.
Effect of the MCHR1 antagonist on ingestion
To reestablish stable drinking levels, rats were allowed to recover from the surgery for two weeks. On the final 3 days of the recovery period rats were handled immediately before their routine drinking period. On Day 15 after the surgery rats were randomly assigned to receive i3vt infusions of one of three doses of the MCHR1 antagonist known as Compound B (Merck Research Laboratories, Rahway, NJ; 10, 20, or 30 μg in saline) (Bednarek et al., 2002)) or saline (1-μl volume) immediately before the presentation of solutions on the cage (n = 5/6 per treatment group). Ingestive behavior (i.e., alcohol or sucrose/quinine and chow intake) was measured after 2 hr and the alcohol and sucrose/quinine bottles were taken off the cages. Chow intake was then monitored at 4, 6, 12, and 24 hr post-infusion.
Experiment 2
Effect of the MCHR1 antagonist on MCH-induced ingestive behavior
An experimentally naïve cohort of animals (n = 21) was trained to drink alcohol as described above and implanted with i3vt cannulas. There was no SUC group in this experiment. Following surgery animals were given 2 weeks to recover. Rats were handled daily during the latter 7 days of the recovery period, immediately prior to receiving alcohol. On the 15th day after surgery, 10 μg of the MCHR1 antagonist, Compound B, or its saline vehicle, were administered via i3vt infusion 15 min before an i3vt infusion of 10 μg MCH or saline. This dose of compound B is reported to have no effect on food intake when administered into the lateral cerebral ventricle of Sprague-Dawley rats (Shearman et al., 2003). Rats were randomly assigned to receive one of the following infusion combinations: saline-saline (n = 5), saline-MCH (n = 6), Compound B- saline (n = 5), Compound B – MCH (n = 5). Immediately following the second infusion alcohol bottles were presented to the animals. Alcohol, chow and water intake were measured 2 hr post-infusion, while simultaneously the alcohol bottles were removed from the cages. Chow and water intake were measured again at 6 and 24 hr post-injection.
Experiment 3
Animals and apparatus
A naïve cohort of animals (n = 25) was trained to self-administer 10% alcohol or 45-mg sucrose pellets (Formula F, PJ Noyes Co, Inc, Lancaster, NH). The experiment utilized 4 operant chambers composed of polycarbonate walls and stainless steel rod floors (Lafayette Instrument, Lafayette, IN). Two stainless steel levers, a pellet dispenser and a liquid dispenser were incorporated into one wall of the chamber. The chambers were interfaced with an IBM-compatible computer that recorded data (i.e., lever presses and rewards) using the Animal Behavior Environment Test (ABET) software (Lafayette Instrument, Lafayette, IN).
Operant training for sucrose on a progressive ratio (PR) schedule
Rats (n = 10) were restricted to 85% of their normal daily food intake (assessed by a 3-day mean of 24-hr chow intake) to initiate lever pressing for 2 days prior to the beginning of training. Training sessions lasted for 1 hr and occurred once daily. For the first 2–4 days of training the animals were rewarded with 1 sucrose pellet for every lever press (i.e., fixed ratio 1:1, FR1). In the event that the animal did not press the lever for a 5-min period, the pellet dispenser would automatically drop 1 pellet into the reservoir (fixed interval (FI) 5 min). After an animal had established reliable lever-pressing for sucrose on this schedule, it was returned to ad libitum food intake and put through 2 FR1 training sessions. Subsequently, animals were trained to lever-press for sucrose on the following schedules for 2 sessions each: FR2, FR3 and FR4. Once all rats were lever-pressing for sucrose on the FR4 schedule they were placed on a progressive ratio (PR) schedule in which the response requirement for 1 sucrose pellet increased arithmetically by 2 lever presses following each reward (i.e., 1, 3, 5, 7, 9, 11, 13, etc…). The break point was defined as the number of reinforcements received prior to an interval of 20 min without responding. After reaching the break point the animals remained in the operant chamber and were able to respond over a total of 4 hr per session. The response requirement was not reset following the break-point and continued from that point over the remainder of the 4 hr session. Every rat had completed the PR schedule (i.e., stopped responding) within the 4-hr period. Once all animals had established a stable baseline (< 20% mean variation per subject) of responding on the PR schedule, surgery was performed to implant i3vt cannulas (as described in Experiment 1).
Operant training for alcohol on a PR schedule
To initiate lever pressing rats (n = 15) were water restricted overnight (16 hr) prior to the first training session. All training sessions lasted for 1 hr and each animal had 1 session per day. To start, rats were trained to lever press for 100 μl of sucrose (10% w/v) in a combined FR1, FI 5-min schedule as describe above. Water access was restricted to 3 hr per day (following training sessions) until reliable lever pressing was established. This required no more than 3 days for any rat. Subsequently the FR schedule for 10% sucrose was increased every two days as follows: FR1, FR2, FR3 and FR4. Once on the FR4 schedule rats were trained to self-administer alcohol (10% w/v) by using a sucrose fading procedure (Samson, 1986). The fading process was similar to the procedure described above for home cage alcohol training except that solutions were made available for 1 hr in the operant chamber on an FR4 schedule. After sucrose fading, the rats were allowed to self-administer 10% alcohol on an FR4 schedule for 10 days. On the 7th day blood samples were collected from the tip of the tail 15 min after the beginning of the session to validate alcohol consumption (see below section on blood alcohol measurement). Finally, animals were trained to respond for 10% alcohol on the PR schedule described above. After approximately 2 weeks animals had established a stable baseline of responding on the PR schedule and were implanted with i3vt cannulas as described above.
Blood alcohol measurement
Headspace gas chromatography was used to analyze blood alcohol levels using a Carbopack B/5% gas chromatography column, Shimadzu GC-17A gas chromatograph (GC) with Shimadzu Class VP 4.3 software. Immediately following blood collection, 20 μl of whole blood was mixed with 80 μl of an internal standard, N-propanol. The vials containing the samples were sealed and kept on ice until analysis on the same day. Prior to analysis, the vial was incubated (40 °C for 15 min) and a portion of the headspace gas was injected into the gas chromatograph fitted with a RTX-BAC1 column. The resolved components were detected and quantified with a flame ionization detector by comparison with retention times and response of standard solutions.
Impact of MCH on progressive ratio responding for alcohol or sucrose
Rats were given 1 week to recover from surgery and were then allowed 4-hr daily access to sucrose or 10% alcohol in the operant chambers for 2 weeks to reestablish responding on the PR schedule. Rats were randomly assigned to receive an i3vt infusion of MCH (5 or 10 μg) or saline. The treatment groups had equivalent average baseline responding rates on the PR schedule. Infusions were administered immediately prior to placing the animal in the operant chamber. The number of lever presses an animal made prior to reaching the break point and total presses over the 4-hr session were recorded as the dependent variables.
Statistical Analysis
The data were analyzed with ANOVAs using the appropriate between- and within-subjects factors followed by post hoc (Tukey HSD) tests. Significance was set at p < 0.05.
RESULTS
Experiment 1
Effect of the MCHR1 antagonist on ingestion
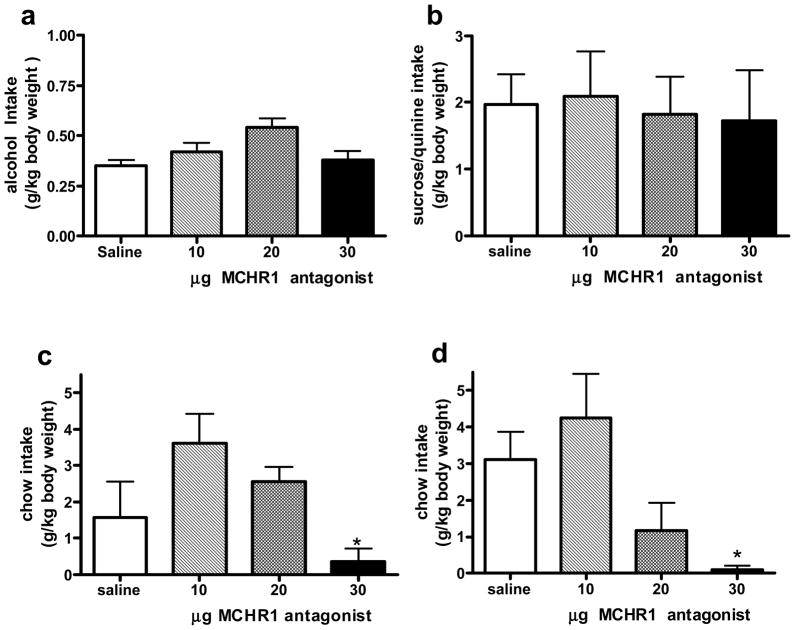
A 1-way ANOVA found no significant effect of treatment on alcohol (Fig 1a) or sucrose (Fig 1b) consumption during the 2-hr drinking period. Although Compound B had no effect on the intake of these calorie-rich solutions, the ANOVA revealed a significant impact of the MCHR1 antagonist on 2-hr chow intake in both groups. The 30 μg dose decreased 2-hr chow intake (0.3 ± 0.4 g/kg body weight) compared to saline (1.6 ± 0.9 g/kg body weight) in the alcohol drinking group (F (3, 17) = 3.8, p = 0.05, Fig 1c), as well as in the sucrose/quinine group (saline = 0.2 ± 0.1 g/kg body weight, 30 μg MCHR1 antagonist = 3.1 ± 1.0 g/kg body weight, F (3, 17) = 3.95, p < 0.05). Neither the 10 nor the 20 μg dose affected chow intake at 2 hr (Fig 1c and 1d). At the later time points, there was no significant effect of Compound B compared to saline, although there was a trend at the 6-hr measurement in the alcohol group (F (3, 17) = 3.04, p = 0.06, Table 1).
Figure 1.
Mean (± SEM) alcohol intake (a), sucrose/quinine intake (b), chow intake for the alcohol group (c) and chow intake for the sucrose/quinine group (d), in g/kg body weight 2 hr following i3vt administration of saline, 10, 20, or 30 μg of the MCHR1 antagonist, Compound B. Significantly different from saline, * p < 0.05.
Table 1.
Impact of MCHR1 antagonism on chow intake.
| alcohol group | sucrose group | |||||||
|---|---|---|---|---|---|---|---|---|
| treatment | chow g/kg 4-hr | chow g/kg 6-hr | chow g/kg 12-hr | chow g/kg 24-hr | chow g/kg 4-hr | chow g/kg 6-hr | chow g/kg 12-hr | chow g/kg 24-hr |
| saline | 2.8 ± 0.9 | 10.0 ± 1.8 | 25.1 ± 1.4 | 47.7 ± .47 | 7.3 ± 1.3 | 10.3 ± 2.6 | 25.0 ± 5.0 | 43.5 ± 7.4 |
| 10 μg Comp B | 4.4 ± 0.6 | 9.7 ± 0.9 | 30.0 ± 1.6 | 52.8 ± 1.1 | 6.2 ± 2.5 | 8.9 ± 2.8 | 27.1 ± 1.8 | 46.2 ± 1.5 |
| 20 μg Comp B | 4.8 ± 1.0 | 7.4 ± 1.4 | 21.8 ± 4.1 | 38.4 ± 8.2 | 3.3 ± 1.3 | 7.0 ± 2.1 | 20.0 ± 4.2 | 38.1 ± 6.0 |
| 30 μg Comp B | 1.4 ± 0.57 | 5.3 ± 0.8* | 20.0 ± 1.9 | 38.8 ± 5.8 | 3.3 ± .86 | 8.0 ± 1.5 | 19.1 ± 2.8 | 35.1 ± 6.7 |
Trend towards difference from saline,
p = 0.06.
Experiment 2
Effect of the MCHR1 antagonist on MCH-induced ingestive behavior
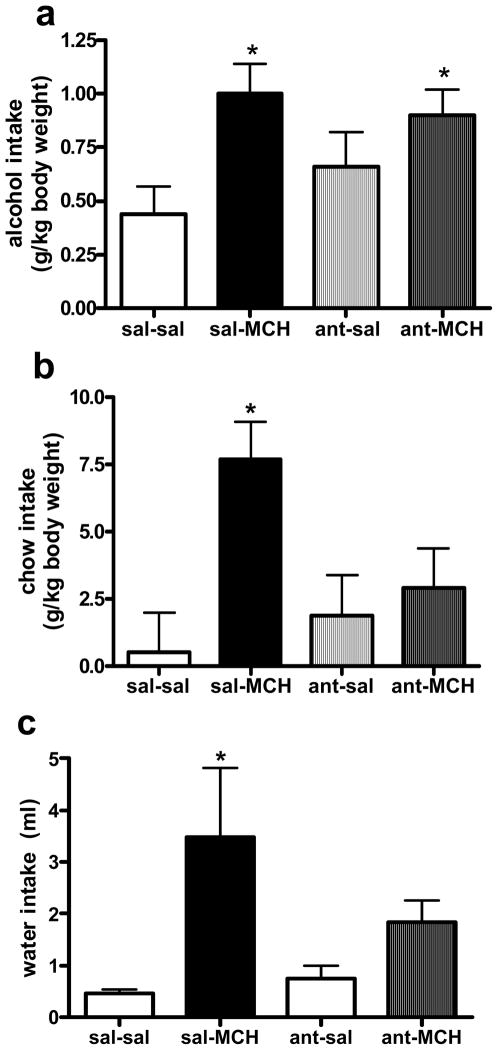
A 2-way ANOVA yielded a significant overall effect of MCH on alcohol drinking (F (1, 17) = 8.4, p = 0.01). Consistent with previous reports (Duncan et al., 2005), the post-hoc analysis revealed that animals which received MCH drank significantly more alcohol than saline control animals (p < 0.01, Fig 2a). The 2-way ANOVA revealed no effect of the MCHR1 antagonist treatment and no interaction between the two treatments. Therefore, blocking the MCHR1 did not attenuate MCH-induced alcohol intake.
Figure 2.
Mean (± SEM) alcohol intake (a) chow intake (b) and water intake (c) intake 2 hr following a double infusion of saline-saline (sal-sal), saline-MCH (sal-MCH), the MCHR1 antagonist-saline (ant-sal), or the MCHR1 antagonist-MCH (ant-MCH). Significantly different from sal-sal, * p < 0.05.
Although the MCHR1 antagonist did not block MCH-induced alcohol consumption, its ability to attenuate other MCH-induced ingestive behaviors was apparent. A dose of Compound B (10 μg) that had no impact on chow intake by itself significantly attenuated MCH-induced chow intake 2 hr following administration. That is, the 2-way ANOVA revealed a significant effect of MCH to increase 2-hr chow intake (F (1, 17) = 7.4, p = 0.01) and there was a significant interaction between the two treatments (F (1, 17) = 4.3, p < 0.05, Fig 2b). Chow intake remained significantly elevated by MCH 6 hr after administration (F (1, 17) = 7.9, p = 0.01), while the impact of the antagonist was no longer present, Table 2. No significant effects were revealed between treatment groups in the 24-hr chow intake (Table 2).
Table 2.
Impact of MCHR1 antagonism on MCH-induced chow and water intake.
| treatment | chow (g/kg) 6-hr | water (ml) 6-hr | chow (g/kg) 24-hr | water (ml) 24-hr |
|---|---|---|---|---|
| saline – saline | 7.4 ± 3.1 | 2.8 ± 1.1 | 43.9 ± 8.9 | 15.5 ± 3.8 |
| saline – MCH | 21.2 ± 2.9*,# | 6.8 ± 1.0*,# | 63.0 ± 8.1 | 20.8 ± 3.5 |
| 10 μg Comp B - saline | 12.1 ± 3.1 | 2.7 ± 1.1 | 51.0 ± 8.9 | 16.9 ± 3.8 |
| 10 μg Comp B - MCH | 15.6 ± 3.1* | 6.0 ± 1.1# | 63.2 ± 8.9 | 23.4 ± 3.8 |
Significantly different from saline-saline,
p < 0.05,
p < 0.01 or from 10 μg Comp B – saline
p < 0.05.
Similar to its impact on MCH-induced chow consumption, this dose of Compound B significantly attenuated MCH-induced water intake 2 hr after administration. The 2-way ANOVA revealed a significant effect of MCH on 2-hr water intake (F (1, 17) = 6.9, p < 0.05, Fig 2c), and a significant interaction between treatments (F (1, 17) = 4.3, p < 0.05). The effect of MCH on water intake was still present 6 hr after treatment (F (1, 17) = 10.9, p < 0.01), but the ability of Compound B to attenuate this effect had dissipated (Table 3). There was no effect of treatment on 24-hr water intake (Table 2).
Experiment 3
Blood alcohol levels (BAL)
The BALs of animals responding on an FR4 schedule for 10% alcohol were significantly correlated with both the number of rewards collected during the 15-min period (r2 = 0.70) and the amount of alcohol consumed (r2 = 0.84). BALs ranged from 0 – 48.4 mg/dl (mean = 12.5, median = 14.2 mg/dl), while alcohol intake ranged from 0 – 0.77 g/kg body weight (mean = 0.32, median = 0.33 g/kg body weight) during the first 15 min of responding.
Effect of MCH on progressive ratio responding for alcohol or sucrose
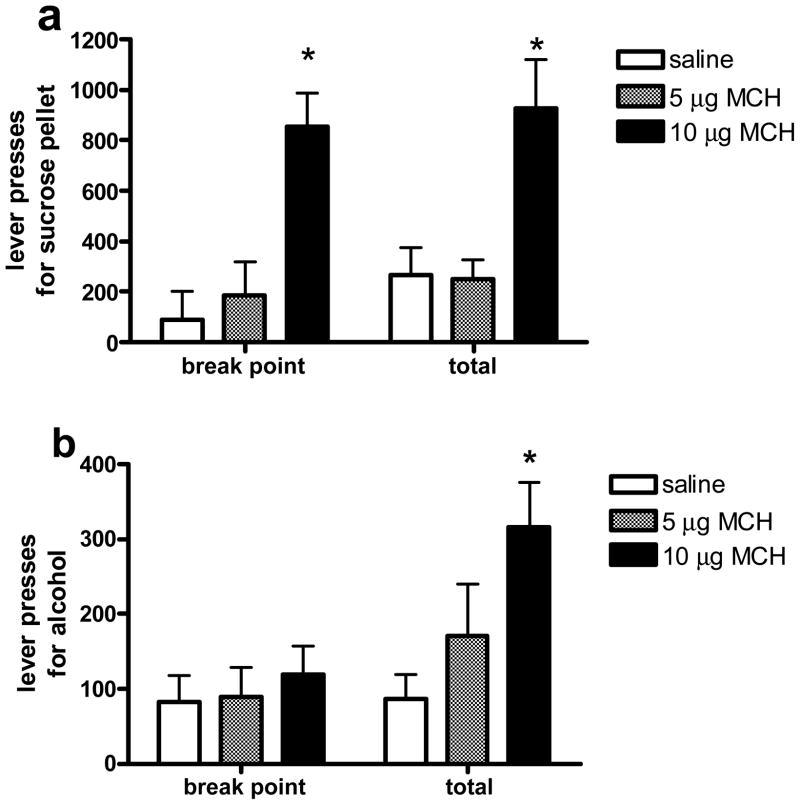
ANOVA yielded a significant effect of MCH on lever pressing for sucrose pellets prior to reaching the break point (F (2, 7) = 11.0, p < 0.01). Lever pressing following the highest dose of MCH (10 μg) was significantly elevated compared to responding following saline, p = 0.01), and the lower dose of the peptide (5 μg), p < 0.05. Responding increased for all groups during the time following the break point (F (1, 7) = 7.9, p < .05, Fig 3a). In contrast to the effect of MCH on responding for sucrose, MCH did not increase responding for alcohol prior to the break point. However, there was a significant effect of the peptide on responding for 10% alcohol over the entire 4-hr period (F (2, 12) = 4.2, p < 0.05), with the 10 μg dose of MCH significantly increasing responses compared to saline, p < 0.05. There was a significant interaction between treatment and time for lever pressing for alcohol (F (2, 12) = 5.9, p < 0.05). Animals that received the highest dose of MCH responded significantly more after reaching the break point when compared to the other two groups (p < 0.01, Fig 3b).
Figure 3.
Mean (± SEM) lever press responses for a 45 mg sucrose pellet (a) or 100 μl of 10% alcohol (b) on a PR schedule following the administration of saline, 5 μg MCH, or 10 μg MCH, either prior to the break point (break point), or over the entire 4-hr session (total). Significantly different from saline, * p < 0.05.
DISCUSSION
One goal of these experiments was to test the hypothesis that endogenous MCH signaling enhances alcohol drinking. The results were not consistent with this hypothesis. While administration of a selective MCHR1 antagonist effectively reduced 2-hr chow intake, it had no impact on 10% alcohol intake, or on the consumption of a sucrose solution equal in calories to 10% alcohol. Further, it is not clear that the MCHR1 antagonist was able to attenuate MCH-induced alcohol intake. Consistent with previous reports (Shearman et al., 2003; Morens et al., 2005), a dose of the MCHR1 antagonist that had no effect on ingestive behavior alone significantly blocked MCH-induced chow and water intake. However, administration of the MCHR1 antagonist prior to administration of MCH resulted in a level of alcohol intake that did not significantly differ from the appropriate control condition (i.e., animals that received the antagonist followed by saline). In light of this finding it seems plausible that the impact of MCH on alcohol drinking may not be specific to the MCHR1. However, there is no evidence for an additional MCH receptor in rodents.
Whether or not exogenous MCH acts specifically on MCHR1 is a mute point if MCH signaling is not physiologically relevant in the regulation of alcohol intake. In fact, the finding that the blockade of MCHR1 did not reduce alcohol or sucrose intake indicates that endogenous MCH signaling does not regulate the consumption of rewarding solutions (i.e., alcohol or sucrose) in general. In contrast, others report that MCHR1 antagonists reduce the consumption of sweetened condensed milk (Borowsky et al., 2002; Morens et al., 2005). However, those studies are difficult to compare to the present experiments as sweetened condensed milk is highly palatable, while sucrose adulterated with quinine and 10% alcohol are generally avoided by the rat unless a sucrose-fading technique or other method is utilized. It is possible that in the present study animals were not consuming enough solution in the baseline condition to observe a measurable decrease. However, we think that this is an unlikely caveat because we have accurately measured lower levels of alcohol intake in a 15 min alcohol drinking session in a previous study (based on a positive correlation with BAL levels) (Duncan et al., 2006).
Another explanation for the failure of blockade of MCHR1 signaling to affect alcohol and sucrose intake, at least when the antagonist was given alone, is activation of a counteracting learned anticipatory response. The experience of receiving access to alcohol and sucrose in a routine manner, daily for several months, is analogous to the phenomenon of meal-feeding. Meal-fed rats, or rats given their entire daily food ration in a restricted but predictable time each day, acquire specific anticipatory responses (e.g., insulin secretion (Woods et al., 1970; Woods et al., 1977), changes in blood glucose levels (Woods and Kulkosky, 1976) and ghrelin secretion (Drazen et al., 2006)) through learning, allowing them to consume a larger amount of calories during a single meal (Woods, 1991; Woods and Ramsay, 2000). In addition, rats fed ad libitum, but given limited access to a palatable source of calories, also display entrained changes in brain activation and behavior (Mistlberger and Rusak, 1987; Mendoza et al., 2005; Mendoza et al., 2005). In a perhaps analogous example, meal-feeding reduced the ability of an anorexic agent (a melanocortin agonist) to decrease food intake (Benoit et al., 2003). Hence, the animals trained to consume alcohol and sucrose at the same time each day may have learned specific anticipatory responses which facilitate the consumption and processing of these solutions, and these anticipatory responses may interfere with or otherwise stifle the effect of an MCHR1 antagonist. This could explain why the antagonist was effective at reducing the consumption of chow, an ad libitum source of calories, but not sucrose or alcohol. It might require a larger dose to reduce consumption of these anticipated fluids.
The second aim of these studies was to test the hypothesis that one way by which MCH augments alcohol intake is to increase alcohol’s rewarding properties. The initial analysis of the data revealed that MCH augmented responding for sucrose, but not for alcohol, on the progressive ratio schedule prior to reaching the defined break point (i.e., 20 min without responding). At first glance, these data suggest that reward is not involved in MCH-mediated alcohol intake. However, analysis of the entire 4-hr operant session revealed that MCH in fact did increase total responding for alcohol. That is, unlike control animals, animals given 10 μg MCH began responding again after they had reached break point. This phenomenon was not observed in the sucrose animals; the highest dose of MCH increased lever-pressing for sucrose prior to the break point, but no further elevation in responding occurred during the 4-hr session.
These findings suggest that there is a fundamental difference between how MCH motivates the intake of alcohol vs. sucrose. The differences between the two macronutrients are substantial. While both are rewarding substances, alcohol has many properties that are not inherent to sucrose, such as the ability to induce locomotor sedation. This is an important distinction to make in light of the present data because the sedating properties of alcohol might provide a logical explanation for the delayed effect of MCH on lever pressing for alcohol under the progressive ratio schedule. However, the average dose of alcohol self-administered prior to the break point in this experiment was very low (0.2 g/kg alcohol, range 0.02 – 0.3 g/kg alcohol). If anything, this low dose of alcohol would be expected to increase locomotor activity (Read et al., 1960; Moore et al., 1993; Paivarinta and Korpi, 1993; Colombo et al., 1998). Therefore, we believe it is unlikely that alcohol-induced sedation is responsible for MCH’s relatively sluggish impact to increase lever pressing for alcohol, compared to sucrose.
Another important difference between these two rewards is the amount of calories they offer. The standard 45-mg sucrose pellet contains 3.6 Kcal/g (i.e., 0.16 Kcal/pellet), while the alcohol reward contains 7.1 Kcal/g (i.e., 0.07 Kcal/ 0.1 ml 10% alcohol, w/v). Thus if it were the case that the effect of MCH on responding for these rewards was purely energy-motivated, one would expect equivalent amounts of calories to be received. The lever pressing for 10% alcohol was slightly lower than for the sucrose pellet following saline administration, but there was no significant difference. However, following MCH administration, lever pressing for the sucrose pellet increased by around 800% compared to saline, while responding for the lesser-calorie source, alcohol, only increased by 300%. Further, augmentation in lever pressing for alcohol occurred only after responding had stopped for more than 20 minutes. Hence, caloric density does not seem to be a factor determining the effect of MCH on responding for these two macronutrients.
Finally, the most obvious difference between the alcohol and sucrose rewards is palatability. Perhaps MCH stimulated the animals to work harder for sucrose than for alcohol because of its greater palatability. However, other studies have suggested that MCH does not discriminate based on the palatability of the diet offered. MCH administration stimulated the consumption of a high-fat diet equally to that of low-fat diet. Further, the effect of MCH on food intake was found to be independent of the opioid system, which is thought to be involved in signaling the relative palatability of food (Clegg et al., 2002).
In summary, we investigated the role of reward in the effect of MCH on alcohol drinking by utilizing a progressive ratio operant self-administration model. MCH increased responding for both sucrose and alcohol during the 4-hr session. However, the motivational effect of MCH on alcohol intake seemed to be delayed in comparison to that for sucrose. The effect of MCH on lever pressing did not seem to be related to the caloric properties of the reward.
In addition we found that pharmacological antagonism of the MCHR1 was unable to reduce alcohol or sucrose/quinine consumption at a dose that effectively reduced chow intake. These findings suggest that endogenous MCH signaling is not involved in alcohol drinking. at least in brain regions affected by the i3vt route of peptide administration. The implication of these findings is MCH signaling is not physiologically relevant to the regulation of alcohol drinking and hence the utilization of the MCHR1 as a target for the development of pharmacological treatments for alcohol dependence is questionable. Finally, because the MCHR1 antagonist did not block MCH-induced alcohol intake, we cannot rule out the possibility that the impact of exogenous MCH on alcohol ingestion is not specific to the peptide’s activity at the MCHR1.
Acknowledgments
The authors are grateful to Karine Proulx for expert technical assistance and to Alison Strack at Merck for providing the MCHR1 antagonist. This research was supported by NIH grants DK17844 and T32 DK59803, and NIAAA grant F31 AA015819-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bednarek MA, Hreniuk DL, Tan C, Palyha OC, MacNeil DJ, Van der Ploeg LH, Howard AD, Feighner SD. Synthesis and biological evaluation in vitro of selective, high affinity peptide antagonists of human melanin-concentrating hormone action at human melanin-concentrating hormone receptor 1. Biochemistry. 2002;41:6383–6390. doi: 10.1021/bi0200514. [DOI] [PubMed] [Google Scholar]
- Benoit SC, Clegg DJ, Barrera JG, Seeley RJ, Woods SC. Learned meal initiation attenuates the anorexic effects of the melanocortin agonist MTII. Diabetes. 2003;52:2684–2688. doi: 10.2337/diabetes.52.11.2684. [DOI] [PubMed] [Google Scholar]
- Borowsky B, Durkin MM, Ogozalek K, Marzabadi MR, DeLeon J, Lagu B, Heurich R, Lichtblau H, Shaposhnik Z, Daniewska I, Blackburn TP, Branchek TA, Gerald C, Vaysse PJ, Forray C. Antidepressant, anxiolytic and anorectic effects of a melanin-concentrating hormone-1 receptor antagonist. Nat Med. 2002;8:825–830. doi: 10.1038/nm741. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18:7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Hirota-Okuno S, Nishiguchi M, Shimazaki T, Iijima M, Grottick AJ, Kanuma K, Omodera K, Sekiguchi Y, Okuyama S, Tran TA, Semple G, Thomsen W. Anxiolytic- and antidepressant-like profile of ATC0065 and ATC0175: nonpeptidic and orally active melanin-concentrating hormone receptor 1 antagonists. J Pharmacol Exp Ther. 2005;313:831–839. doi: 10.1124/jpet.104.081711. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Benoit SC, Sakai RS, Seeley RJ, Woods SC. Intraventricular melanin-concentrating hormone stimulates water intake independent of food intake. Am J Physiol Regul Integr Comp Physiol. 2003;284:R494–499. doi: 10.1152/ajpregu.00399.2002. [DOI] [PubMed] [Google Scholar]
- Clegg DJ, Air EL, Woods SC, Seeley RJ. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Vacca G, Gessa GL. Stimulation of locomotor activity by voluntarily consumed ethanol in Sardinian alcohol-preferring rats. Eur J Pharmacol. 1998;357:109–113. doi: 10.1016/s0014-2999(98)00560-3. [DOI] [PubMed] [Google Scholar]
- DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73:759–768. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC. Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 2006;147:23–30. doi: 10.1210/en.2005-0973. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Proulx K, Woods SC. Central administration of melanin-concentrating hormone increases alcohol and sucrose/quinine intake in rats. Alcohol Clin Exp Res. 29:958–964. doi: 10.1097/01.alc.0000167741.42353.10. [DOI] [PubMed] [Google Scholar]
- Duncan EA, Tamashiro KL, Nguyen MM, Gardner SR, Woods SC, Sakai RR. The impact of moderate daily alcohol consumption on aggression and the formation of dominance hierarchies in rats. Psychopharm. 2006;189:83–94. doi: 10.1007/s00213-006-0536-7. [DOI] [PubMed] [Google Scholar]
- Francis K, Baker BI. Developmental changes in melanin-concentrating hormone in Rana temporaria. Gen Comp Endocrinol. 2005;98:157–165. doi: 10.1006/gcen.1995.1056. 1995. [DOI] [PubMed] [Google Scholar]
- Francis K, Suzuki M, Baker BI. Responses of melanin-concentrating hormone mRNA to salt water challenge in the rainbow trout. Neuroendocrinology. 1997;66:195–202. doi: 10.1159/000127238. [DOI] [PubMed] [Google Scholar]
- Georgescu D, Sears RM, Hommel JD, Barrot M, Bolanos CA, Marsh DJ, Bednarek MA, Bibb JA, Maratos-Flier E, Nestler EJ, DiLeone RJ. The hypothalamic neuropeptide melanin-concentrating hormone acts in the nucleus accumbens to modulate feeding behavior and forced-swim performance. J Neurosci. 2005;25:2933–2940. doi: 10.1523/JNEUROSCI.1714-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Samson HH. Induction and maintenance of ethanol self-administration without food deprivation in the rat. Psychopharmacology. 1985;86:475–479. doi: 10.1007/BF00427912. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Kawauchi H, Kawazoe I, Tsubokawa M, Kishida M, Baker BI. Characterization of melanin-concentrating hormone in chum salmon pituitaries. Nature. 1983;305:321–323. doi: 10.1038/305321a0. [DOI] [PubMed] [Google Scholar]
- Kela J, Salmi P, Rimondini-Giorgini R, Heilig M, Wahlestedt C. Behavioural analysis of melanin-concentrating hormone in rats: evidence for orexigenic and anxiolytic properties. Regul Pept. 2003;114:109–114. doi: 10.1016/s0167-0115(03)00114-9. [DOI] [PubMed] [Google Scholar]
- Kratz CM, Levitsky DA, Lustick SL. Long term effects of quinine on food intake and body weight in the rat. Physiol Behav. 1978;21:321–324. doi: 10.1016/0031-9384(78)90088-4. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22:2855–2862. doi: 10.1111/j.1460-9568.2005.04461.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. Entrainment by a palatable meal induces food-anticipatory activity and c-Fos expression in reward-related areas of the brain. Neuroscience. 2005;133:293–303. doi: 10.1016/j.neuroscience.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Mistlberger R, Rusak B. Palatable daily meals entrain anticipatory activity rhythms in free-feeding rats: dependence on meal size and nutrient content. Physiol Behav. 1987;41:219–226. doi: 10.1016/0031-9384(87)90356-8. [DOI] [PubMed] [Google Scholar]
- Monzon ME, De Barioglio SR. Response to novelty after i.c.v. injection of melanin-concentrating hormone (MCH) in rats. Physiol Behav. 1999;67:813–817. doi: 10.1016/s0031-9384(99)00117-1. [DOI] [PubMed] [Google Scholar]
- Monzon ME, Varas MM, De Barioglio SR. Anxiogenesis induced by nitric oxide synthase inhibition and anxiolytic effect of melanin-concentrating hormone (MCH) in rat brain. Peptides. 2001;22:1043–1047. doi: 10.1016/s0196-9781(01)00439-9. [DOI] [PubMed] [Google Scholar]
- Moore TO, June HL, Lewis MJ. Ethanol-induced stimulation and depression on measures of locomotor activity: effects of basal activity levels in rats. Alcohol. 1993;10:537–540. doi: 10.1016/0741-8329(93)90078-3. [DOI] [PubMed] [Google Scholar]
- Morens C, Norregaard P, Receveur JM, van Dijk G, Scheurink AJ. Effects of MCH and a MCH1-receptor antagonist on (palatable) food and water intake. Brain Res. 2005;1062:32–38. doi: 10.1016/j.brainres.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Paivarinta P, Korpi ER. Voluntary ethanol drinking increases locomotor activity in alcohol-preferring AA rats. Pharmacol Biochem Behav. 1993;44:127–132. doi: 10.1016/0091-3057(93)90289-6. [DOI] [PubMed] [Google Scholar]
- Paxinos GC. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- Pissios P, Maratos-Flier E. Melanin-concentrating hormone: from fish skin to skinny mammals. Trends Endocrinol Metab. 2003;14:243–248. doi: 10.1016/s1043-2760(03)00079-1. [DOI] [PubMed] [Google Scholar]
- Presse F, Nahon JL. Differential regulation of melanin-concentrating hormone gene expression in distinct hypothalamic areas under osmotic stimulation in rat. Neuroscience. 1993;55:709–720. doi: 10.1016/0306-4522(93)90436-j. [DOI] [PubMed] [Google Scholar]
- Qu D, Ludwig DS, Gammeltoft S, Piper M, Pelleymounter MA, Cullen MJ, Mathes WF, Przypek R, Kanarek R, Maratos-Flier E. A role for melanin-concentrating hormone in the central regulation of feeding behaviour. Nature. 1996;380:243–247. doi: 10.1038/380243a0. [DOI] [PubMed] [Google Scholar]
- Read GW, Cutting W, Furst A. Comparison of excited phases after sedatives and tranquilizers. Psychopharmacologia. 1960;1:346–350. doi: 10.1007/BF00404231. [DOI] [PubMed] [Google Scholar]
- Roy M, David NK, Danao JV, Baribault H, Tian H, Giorgetti M. Genetic Inactivation of Melanin-Concentrating Hormone Receptor Subtype 1 (MCHR1) in Mice Exerts Anxiolytic-Like Behavioral Effects. Neuropsychopharmacology. 2006;31:112–120. doi: 10.1038/sj.npp.1300805. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Ackroff K. Glucose- and fructose-conditioned flavor preferences in rats: taste versus postingestive conditioning. Physiol Behav. 1994;56:399–405. doi: 10.1016/0031-9384(94)90213-5. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Camacho RE, Sloan Stribling D, Zhou D, Bednarek MA, Hreniuk DL, Feighner SD, Tan CP, Howard AD, Van der Ploeg LH, MacIntyre DE, Hickey GJ, Strack AM. Chronic MCH-1 receptor modulation alters appetite, body weight and adiposity in rats. Eur J Pharmacol. 2003;475:37–47. doi: 10.1016/s0014-2999(03)02146-0. [DOI] [PubMed] [Google Scholar]
- Smith DG, Davis RJ, Rorick-Kehn L, Morin M, Witkin JM, McKinzie DL, Nomikos GG, Gehlert DR. Melanin-Concentrating Hormone-1 Receptor Modulates Neuroendocrine, Behavioral, and Corticolimbic Neurochemical Stress Responses in Mice. Neuropsychopharmacology. 2005;31:1135–45. doi: 10.1038/sj.npp.1300913. [DOI] [PubMed] [Google Scholar]
- Smith DG, Tzavara ET, Shaw J, Luecke S, Wade M, Davis R, Salhoff C, Nomikos GG, Gehlert DR. Mesolimbic dopamine super-sensitivity in melanin-concentrating hormone-1 receptor-deficient mice. J Neurosci. 2005;25:914–922. doi: 10.1523/JNEUROSCI.4079-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan CP, Sano H, Iwaasa H, Pan J, Sailer AW, Hreniuk DL, Feighner SD, Palyha OC, Pong SS, Figueroa DJ, Austin CP, Jiang MM, Yu H, Ito J, Ito M, Ito M, Guan XM, MacNeil DJ, Kanatani A, Van der Ploeg LH, Howard AD. Melanin-concentrating hormone receptor subtypes 1 and 2: species-specific gene expression. Genomics. 2002;79:785–792. doi: 10.1006/geno.2002.6771. [DOI] [PubMed] [Google Scholar]
- Tolliver GA, Sadeghi KG, Samson HH. Ethanol preference following the sucrose-fading initiation procedure. Alcohol. 1988;5:9–13. doi: 10.1016/0741-8329(88)90036-5. [DOI] [PubMed] [Google Scholar]
- Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- Woods SC, Hutton RA, Makous W. Conditioned insulin secretion in the albino rat. Proc Soc Exp Biol Med. 1970;133:964–968. doi: 10.3181/00379727-133-34605. [DOI] [PubMed] [Google Scholar]
- Woods SC, Kulkosky PJ. Classically conditioned changes of blood glucose level. Psychosom Med. 1976;38:201–219. doi: 10.1097/00006842-197605000-00006. [DOI] [PubMed] [Google Scholar]
- Woods SC, Ramsay DS. Pavlovian influences over food and drug intake. Behav Brain Res. 2000;110:175–182. doi: 10.1016/s0166-4328(99)00194-1. [DOI] [PubMed] [Google Scholar]
- Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol. 1977;91:128–133. doi: 10.1037/h0077307. [DOI] [PubMed] [Google Scholar]