Abstract
Background:
All relevant authorities recommend an interval of 10 years between normal screening colonoscopies. We assessed the timing of repeated colonoscopies after a negative screening colonoscopy finding in a population-based sample of Medicare patients.
Methods:
A 5% national sample of Medicare enrollees from 2000 through 2008 was used to identify average-risk patients undergoing screening colonoscopy between 2001 and 2003. Colonoscopy was classified as a negative screening examination finding if no indication other than screening were in the claims and if no biopsy, fulguration, or polypectomy was performed. Time to repeated colonoscopy was calculated.
Results:
Among 24 071 Medicare patients who had a negative screening colonoscopy finding in 2001 through 2003, 46.2% underwent a repeated examination in fewer than 7 years. In 42.5% of these patients (23.5% of the overall sample), there was no clear indication for the early repeated examination. In patients aged 75 to 79 years or 80 years or older at the time of the initial negative screening colonoscopy result, 45.6% and 32.9%, respectively, received a repeated examination within 7 years. In multivariable analyses, male sex, more comorbidities, and colonoscopy by a high-volume colonoscopist or in an office setting were associated with higher rates of early repeated colonoscopy without clear indication, while those 80 years or older had a reduced risk. There were also marked geographic variations, from less than 5% in some health referral regions to greater than 50% in others.
Conclusions:
A large proportion of Medicare patients who undergo screening colonoscopy do so more frequently than recommended. Current Medicare regulations intending to limit reimbursement for screening colonoscopy to every 10 years would not appear to be effective.
The adoption of screening for colorectal cancer (CRC) has been accompanied by declines in CRC mortality.1 Colonoscopy is the screening modality preferred by authorities such as the American Cancer Society because of its ability to identify and remove precancerous lesions, thus reducing cancer incidence.2-4 Considerable attention has been given to the underuse of CRC screening, particularly screening colonoscopy.4-8 Ethnic minorities and the uninsured have substantially lower rates of colonoscopy as well as other CRC screening modalities.5,8 Limitations in the current endoscopist workforce have been cited as a barrier to increasing screening rates.6,7
Less attention has been paid to possible overuse of screening colonoscopy.9-11 Overuse is important for several reasons. First, screening colonoscopy can have adverse effects, including hospitalization and death.12-14 Too frequent performance of the examination may shift the benefit to risk ratio by increasing complications without additional benefit. Second, colonoscopy screening is costly; it is important to restrain expenditures for unnecessary procedures. Third, colonoscopy is a limited resource, in terms of facilities and practitioners.6,7 Identifying and decreasing overuse of screening colonoscopy should free up resources to increase appropriate colonoscopy in inadequately screened populations.
Most patients at initial screening colonoscopy have no findings relevant to cancer, such as polyps.15-19 In such patients,
the time interval for a repeated examination recommended by all expert panels is 10 years.2-4 The objective of this study is to determine the frequency of early repeated colonoscopy after a negative screening colonoscopy finding among fee-for-service Medicare patients in the United States and to examine its association with demographic variables, health care provider specialty, and location of service.
METHODS
STUDY SUBJECTS
Our overall approach involved identifying subjects with a negative screening colonoscopy finding between 2001 and 2003 and determining what percentage of them had an additional colonoscopy between 2001 and 2008. The selection of the subjects is outlined in Figure 1. We used the claims and enrollment data for the 2000-2008 period from a 5% random national sample of Medicare beneficiaries. We first identified a cohort of patients 66 years or older with parts A and B Medicare and not enrolled in a health maintenance organization, who underwent a complete colonoscopy between 2001 and 2003 (n=236 145).
Figure 1.
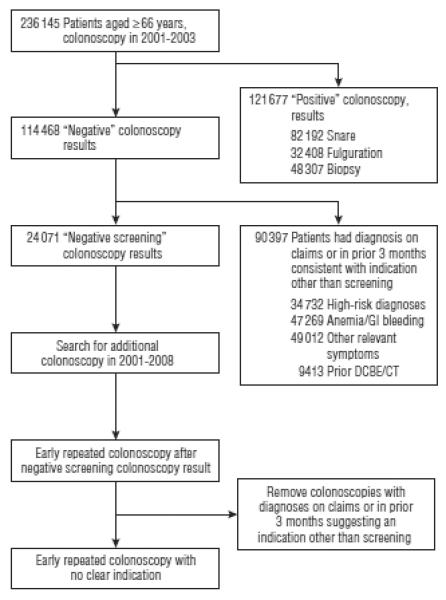
Schema for selection of the study cohorts. The numbers for the various indications in the right hand boxes add up to more than the total because some patients had multiple indications. GI indicates gastrointestinal tract; DCBE/CT, double-contrast barium enema/computed tomography.
The identification of screening colonoscopy is complicated by the fact that few colonoscopies are submitted using the screening code (eg, 4.6% in 2001 and 14.6% in 2007-2008), although approximately two-thirds of colonoscopies are estimated to be performed for CRC screening.8 In addition, Medicare will not reimburse for a screening colonoscopy within 10 years after a negative examination result, consistent with all guidelines.2-4
Our approach to identifying negative screening colonoscopy results was to define several successive cohorts from the 236 145 beneficiaries who underwent a complete colonoscopy between 2001 and 2003 (Figure 1). First, we eliminated all colonoscopies that were accompanied by a biopsy, fulguration, or any other procedure. This left 114 468 patients with what we considered a negative colonoscopy result. We should note that findings from colonoscopies with biopsies or other procedures might also be negative. For example, a biopsy result might be normal. However, we had no way to identify such cases, so all colonoscopies with a procedure were excluded. Also, the term negative is limited to cancer screening. These colonoscopies might have found other abnormalities, such as internal hemorrhoids or diverticula.
The next step was to exclude any colonoscopy with a diagnosis suggesting a nonscreening indication or if there were prior diagnoses or procedures suggesting that there might be an indication other than screening. We excluded colonoscopies if there was a claim for barium enema or abdominal computerized tomography in the prior 3 months, or if there was a diagnosis on the colonoscopy claim or on any inpatient or outpatient claim in the prior 3 months for anemia, gastrointestinal tract (GI) bleeding, change in bowel habits, abdominal pain, irritable bowel syndrome, ischemic bowel disease, history of colon cancer, or any other condition for which a colonoscopy might plausibly be indicated (see eAppendix; http://www.archinternmed.com). If the primary diagnosis on the colonoscopy claim was diverticular disease and there was evidence of diverticulitis on prior claims, those colonoscopies were excluded. We also excluded those with any diagnosis of CRC, inflammatory bowel disease, or familial polyposis syndromes on the colonoscopy claim or in the 12 months prior to colonoscopy or those who had procedures or diagnoses suggestive of colon cancer in the month after colonoscopy. All other colonoscopies were categorized as a negative screening colonoscopy result (N=24 071).
STUDY OUTCOME
The study outcome was the first repeated colonoscopy in the period starting at 3 months after the initial examination between 2001 and 2003 and ending at December 31, 2008. Beneficiaries were censored at death, loss of coverage, or end of follow-up. The repeated colonoscopies were classified as “probably indicated,” “possibly indicated,” or “no clear indication,” based on the following approach. First, we examined the diagnosis on the colonoscopy claim and also all diagnoses on inpatient and outpatient claims in the 3 months prior to the colonoscopy. If there was a diagnosis on the claim that might represent an indication for diagnostic colonoscopy, and if that or any other diagnosis consistent with an indication for diagnostic colonoscopy were present in the claims in the 3 months prior, the colonoscopy was considered as probably indicated. If a diagnosis consistent with an indication for diagnostic colonoscopy were present in only one of the sources (colonoscopy claim or claims in the prior 3 months), this was labeled as possibly indicated. Colonoscopies with no relevant diagnoses on the colonoscopy claim or in the prior 3 months were labeled as “without indication.” The indications for diagnostic colonoscopy are the same as those used to categorize the baseline colonoscopy and are listed in the eAppendix.
COVARIATES
Age, sex, race, and zip code and region of residence of each beneficiary were obtained from the Medicare enrollment file. Education for zip code areas were obtained from the 2000 Census data. Urban/rural was categorized by Rural-Urban Continuum Codes.20 Comorbidity measures were generated from all claims in the year prior to the baseline colonoscopy,21 with conditions possibly related to a clinical indication for colonoscopy (anemia and weight loss) excluded (see footnote to Table 1 for a list of included comorbidities). Physician specialty was obtained from Medicare Part B claims. Volume of colonoscopies by the endoscopist who performed the baseline colonoscopy was assessed between 2001 and 2003.
Table 1.
Characteristics of Cohort of Medicare Patients Who Underwent Normal Screening Colonoscopy Between 2001 and 2003 and the Percentage Who Had a Repeated Colonoscopy Within 7 Years
|
Early Repeated Colonoscopy, %a
|
|||
|---|---|---|---|
| Characteristic | No. of Patients | Any Early Repeated Colonoscopyb | Repeated With No Clear Indicationb |
| Total sample | 24 071 | 46.2 | 23.5 |
| Age at initial colonoscopy, y | |||
| 66-69 | 5591 | 49.3 | 28.0 |
| 70-74 | 9153 | 49.1 | 26.6 |
| 75-79 | 6014 | 45.6 | 20.7 |
| ≥80 | 3313 | 32.9 | 10.8 |
| Sex | |||
| Male | 10 633 | 48.5 | 26.0 |
| Female | 13438 | 44.5 | 21.6 |
| Race | |||
| White | 22 639 | 46.1 | 23.6 |
| Black | 1006 | 51.7 | 21.6 |
| Other | 426 | 40.3 | 22.0 |
| No. of comorbiditiesc | |||
| 0 | 7887 | 39.9 | 21.7 |
| 1 | 6723 | 47.8 | 26.2 |
| 2 | 4638 | 47.0 | 23.6 |
| ≥3 | 4823 | 52.6 | 22.0 |
| Education (% with >12-y education in zip code) | |||
| <6.9 | 5885 | 46.1 | 24.5 |
| 6.9 to <11.4 | 5848 | 45.9 | 24.5 |
| 11.4 to <17.5 | 5906 | 46.9 | 22.6 |
| ≥17.5 | 5898 | 46.0 | 22.2 |
| Region | |||
| New England | 1826 | 41.5 | 22.7 |
| Middle Atlantic | 3038 | 54.6 | 29.3 |
| East North Central | 4571 | 48.3 | 24.5 |
| West North Central | 2697 | 44.8 | 25.0 |
| South Atlantic | 5117 | 46.2 | 21.9 |
| East South Central | 1446 | 46.2 | 22.8 |
| West South Central | 2050 | 45.7 | 22.0 |
| Mountain | 1395 | 40.3 | 20.6 |
| Pacific | 1825 | 39.8 | 19.9 |
| Rural/urban | |||
| Metropolitan | 18494 | 46.9 | 24.0 |
| Nonmetropolitan urban | 4736 | 43.8 | 22.2 |
| Rural | 674 | 43.8 | 21.4 |
| Specialty of initial colonoscopist | |||
| Gastrointestinal | 13 693 | 46.6 | 23.6 |
| Generalist | 2643 | 43.7 | 20.6 |
| Surgeon | 6828 | 46.2 | 23.5 |
| Other/unknown | 907 | 47.6 | 27.3 |
| Place of serviced | |||
| Office | 1304 | 53.5 | 26.9 |
| Hospital | 14 996 | 46.1 | 23.3 |
| Ambulatory center | 6643 | 45.2 | 23.4 |
| Volume of initial colonoscopiste | |||
| ≤460 | 5796 | 42.8 | 20.8 |
| 461-780 | 5727 | 46.1 | 23.0 |
| 781-1200 | 5424 | 46.3 | 23.4 |
| >1200 | 5672 | 50.0 | 26.8 |
Percentage of patients in each category who undergo an early repeated colonoscopy The denominator for each category is given in the column labeled “No. of Patients.”
All comparisons on differences in percentage of patients undergoing any early repeated colonoscopy by patient or health care provider characteristic were significant by χ2 at P < .001 except education (not significant) and specialty of colonoscopist (not significant). All comparisons on differences in percentage of patients undergoing early repeated colonoscopy with no clear indication by patient or health care provider characteristic were significant by χ2 at P < .001 except race (not significant), education (significant at P= .03), rural/urban (significant at P= .003), place of service (significant at P= .02), and specialty of colonoscopist (not significant).
Comorbidities are the number of individual comorbidities from the index created by Elixhauser et al21 that appear in any claims in the 12 months prior to the repeated colonoscopy The diagnoses of anemia and weight loss were excluded because they might represent indications for the colonoscopy The diagnoses included in the index were congestive heart failure, cardiac valve disease, pulmonary circulation disease, peripheral vascular disease, hypertension, paralysis, neurological disorders, chronic pulmonary disease, diabetes mellitus without complication, diabetes mellitus with complication, hypothyroidism, renal failure, liver disease, peptic ulcer, acquired immune deficiency syndrome, lymphoma, metastatic cancer, solid tumor with or without metastasis, rheumatoid arthritis, coagulopathy obesity fluid and electrolyte disorders, alcohol abuse, drug abuse, psychoses, and depression.
Data on place of service was missing for 1128 patients (4.7%). Place of service is given for the initial colonoscopy.
Data on health care provider identification was missing for 1452 patients (6.0%). Volume was estimated as the number of fee-for-service Medicare patients, who were billed for any colonoscopy by the colonoscopist who performed the initial examination in the year of the initial examination.
STATISTICAL ANALYSIS
The rates of early repeated colonoscopy were estimated by the Kaplan-Meier method. Hospital referral regions described in the Dartmouth Atlas of Health Care22 were used to assess regional variation. Multivariable survival analyses were performed using the Cox proportional hazards model. All analyses were performed using SAS version 9.1 statistical software (SAS Institute, Cary, North Carolina).
RESULTS
Figure 2 shows the cumulative percentage of repeated colonoscopies over the 7 years following the initial colonoscopy in 2001 through 2003. Curves for 3 different cohorts are given: all patients who underwent complete colonoscopies in 2001 through 2003, all those with complete colonoscopies with negative results in 2001 through 2003, and those with complete negative colonoscopy results with no indication other than screening in 2001 through 2003. For the 236 145 patients who underwent complete colonoscopy in 2001 through 2003, the rates of repeated colonoscopy at 5 and 7 years were 43.3% and 59.5%, respectively. For those with negative examination results in 2001 through 2003, the repeated colonoscopy rates at 5 and 7 years were 31.9% and 50.1%, respectively, and for those with no indications other than screening and a negative examination result, the repeated colonoscopy rates at 5 and 7 years were 24.6% and 46.2%, respectively. With all 3 lines, one can note inflection points at approximately 3 and 5 years after the initial colonoscopy, suggesting that those colonoscopies were performed as part of a routine scheduled follow-up. All other analyses in this article relate to the cohort with a negative screening baseline colonoscopy finding in 2001 through 2003, the bottom line in Figure 2.
Figure 2.
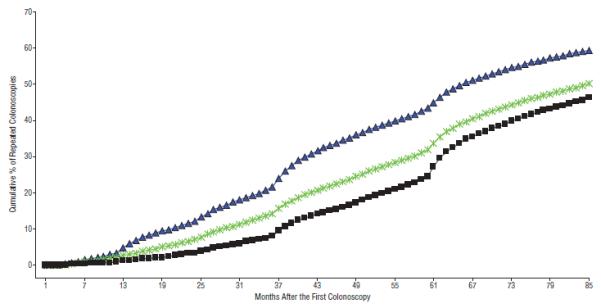
Cumulative percentage of repeated colonoscopies for patients 66 years or older who underwent a complete a colonoscopy between 2001 and 2003. The blue line is for all patients with a complete colonoscopy between 2001 and 2003 (N=236 145). The green line is for patients who had a negative colonoscopy result (n=114 468). The black line is for patients who had a negative colonoscopy finding with no indication other than screening (n=24 071).
Table 1 details the characteristics of the patients with negative screening colonoscopy findings in 2001 through 2003, and the percentage of patients who underwent a repeated colonoscopy examination within 7 years after the negative screening colonoscopy result, stratified by patient and health care provider characteristics. Because of the large sample, almost all variations are statistically significant. Therefore, it is important to focus on the magnitude of any differences, rather than statistical significance. Surprisingly, 45.6% of patients aged 75 to 79 years and one-third of patients 80 years or older at the time of the initial negative screening colonoscopy result underwent a repeated colonoscopy within 7 years. Male sex, black race, and more comorbidities were all associated with a higher rate of early repeated colonoscopy. The rate of early repeated colonoscopy by geographic region ranged from 39.8% to 54.6%. The rate of early repeated colonoscopy was greater in patients whose initial colonoscopy was performed in an office setting and whose initial colonoscopy was performed by a physician who performed a high volume of colonoscopies. Also given in Table 1 is the percentage of patients who underwent an early repeated colonoscopy with no clear indication other than screening.
Among the patients with a repeated colonoscopy within 7 years after a negative screening colonoscopy, 26.9% had a diagnosis on the colonoscopy claim consistent with a medical indication for the repeated examination. These included anemia, abdominal pain, constipation, change in bowel habits, hemorrhoids, and weight loss (a complete list is given in the eAppendix). The diagnoses listed on the claims for the repeated colonoscopies are given in Table 2, along with any diagnoses or procedures in the 3 months prior to the repeated examination that might represent an indication. For example, 1252 claims for repeated colonoscopies listed anemia or GI bleeding as the indication. In 887 of these, there were diagnoses or procedures in the prior 3 months that could be consistent with anemia or GI bleeding or that might represent another indication for colonoscopy, such as change in bowel habits, constipation, or weight loss. These repeated colonoscopies were classified as probably indicated. The remaining 365 of the 1252 repeated colonoscopies with an anemia/GI bleeding indication and no prior procedure or related diagnosis were classified as possibly indicated. In 1955 early repeated colonoscopies, diverticulosis (n=1588) or another unrelated diagnosis (n=367) were listed as the indication on the claim (Table 2). Diverticulosis is not an indication for colonoscopy, but it might be considered appropriate for evaluation of a prior episode of acute diverticulitis. Only 4.6% of the patients had such diagnosis in the prior claims, and 49.2% had 1 or more other potential indications for colonoscopy listed in the 3 months prior. Thus, 52.4% of the colonoscopies with a diverticulosis indication were listed as possibly indicated and the remaining 47.6% as without indication. Approximately half of the early repeated colonoscopies were accompanied by diagnoses that suggested that the colonoscopy was performed for screening. These diagnoses included benign neoplasm of the colon, history of polyps, screening for colon cancer, and family history of colon cancer. In 37.2% of those cases, there were procedures or diagnoses in the prior 3 months consistent with a nonscreening indication for colonoscopy. These cases were considered possibly indicated, and the remaining 62.8% were considered without indications.
Table 2.
Relevant Diagnoses or Procedures in the 3 Months Prior to Repeated Colonoscopy and Indications for the Repeated Colonoscopy Listed in the Medicare Claima
| Indication on Claim for Repeated Colonoscopy, No. (%) |
||||||
|---|---|---|---|---|---|---|
|
Relevant Diagnoses and Procedures
in the 3 mo Prior to Repeated Colonoscopy |
Anemia/GI
Bleeding (n=1252) |
Other
Relevant Diagnoses (n=906)b |
High Risk
Diagnoses (n=155)c |
Unrelated
Diagnoses (n=1955)d |
Diagnoses Consistent
With Screening (n=4340)e |
All
(n=8608) |
| Received DCBE/CT in 3 mo before the repeated colonoscopy |
128 (10.2) | 185 (20.4) | 29 (18.7) | 223 (11.4) | 282 (6.5) | 847 (9.8) |
| Diverticulitis in 3 mo before the repeated examination |
21 (1.7) | 46 (5.1) | 5 (3.2) | 90 (4.6) | 82 (1.9) | 244 (2.8) |
| Anemia/GI bleeding in 3 mo before the repeated examination |
766 (61.2) | 208 (23.0) | 50 (32.3) | 516 (26.4) | 765 (17.6) | 2305 (26.8) |
| Other relevant symptoms in 3 mo before the repeated examination |
369 (29.5) | 626 (69.1) | 53 (34.2) | 644 (32.9) | 1059 (24.4) | 2751 (32.0) |
| Probably indicated | 887 (70.8) | 710 (78.4) | 155 (100) | 0 | 0 | 1752 (20.3) |
| Possibly indicated | 365 (29.2) | 196 (21.6) | 0 | 1024 (52.4) | 1615 (37.2) | 3200 (37.2) |
| Without indications | 0 | 0 | 0 | 931 (47.6) | 2725 (62.8) | 3656 (42.5) |
Abbreviations: DCBE/CT, double-contrast barium enema/computed tomography; GI, gastrointestinal tract.
The distribution of relevant diagnoses and procedures in the 3 months before the repeated colonoscopy and the distribution of indications for the repeated colonoscopy listed on the Medicare claim (n = 8608).
Other relevant diagnoses include abdominal pain, diarrhea, constipation, ischemic bowel disease, irritable bowel syndrome, change in bowel habits, hemorrhoids, and weight loss. See the eAppendix (http://www.archinternmed.com) for specific codes.
High-risk diagnoses include inflammatory bowel disease (17.6%), history of colon cancer (64.0%), and others (18.4%).
Unrelated diagnoses included diverticulosis (81.2%), diagnoses or symptoms related to the upper GI (5.8%), unspecified functional GI disorder (5.2%), and other (7.8%). Diverticulosis is not an indication for colonoscopy, except as a follow-up examination after acute diverticulitis, or with bleeding. Of the patients in this group, 90 had a prior diagnosis of diverticulitis.
The major diagnoses under codes consistent with screening are benign neoplasm of colon/rectum (54.2%), history of colonic polyps (24.5%), screening for colon cancer (10.7%), and family history of GI cancer (7.4%).
Using that strategy, we classified 57.5% of the early repeated colonoscopies as possibly or probably indicated, while 42.5% were without indications (Table 2). Of the 3656 claims for colonoscopies without clear indications, only 86 (2%) were denied reimbursement by Medicare. As noted previously, the curves of cumulative percentage of early repeated colonoscopies in Figure 1 show inflection points with noticeable increases in the rate of repeated colonoscopies at 3 and 5 years, suggesting that those procedures might have been routinely scheduled rather than in response to symptoms. To investigate this, we selected colonoscopies that occurred 59 to 65 months after the baseline colonoscopy and calculated the percentage that were without indication. That percentage was 61.5%, compared with 42.5% for the entire sample.
We repeated the analyses in Table 1 and Table 2, excluding those with a family history of GI cancer both from the initial negative screening colonoscopy cohort and from those identified as undergoing early repeated colonoscopy with no clear indication. Using that method, we found that the percentage of patients undergoing early repeated colonoscopy and early repeated colonoscopy without clear indication were similar to those in Table 1 (45.5% vs 46.2% and 21.8% vs 23.5%, respectively).
Table 3 presents the results of a multivariable analysis examining the independent associations of patient and health care provider characteristics with the hazard of early repeated colonoscopy. We present a model for the hazard of any repeated colonoscopy within 7 years and also a model assessing hazard for patients who had no clear indication for the repeated examination.
Table 3.
Multivariable Analysis of Patient and Health Care Provider Characteristics Associated With Early Repeated Colonoscopy in All Patients With an Initial Negative Screening Colonoscopy Finding in 2001 Through 2003
| HR (95% CI) |
||
|---|---|---|
| Characteristic | Any Early Repeated Colonoscopy |
Early Repeat Colonoscopy Without Clear Indication |
| Age at initial colonoscopy, y | ||
| 66-69 | 1 [Reference] | 1 [Reference] |
| 70-74 | 1.00 (0.94-1.05) | 0.92 (0.85-1.00) |
| 75-79 | 0.93 (0.88-0.99) | 0.76 (0.69-0.83) |
| ≥80 | 0.66 (0.61-0.71) | 0.40 (0.35-0.46) |
| Sex | ||
| Male | 1 [Reference] | 1 [Reference] |
| Female | 0.87 (0.84-0.91) | 0.78 (0.73-0.83) |
| Race | ||
| White | 1 [Reference] | 1 [Reference] |
| Black | 1.19 (1.08-1.33) | 0.95 (0.79-1.15) |
| Other | 0.89 (0.75-1.07) | 0.96 (0.74-1.26) |
| Total No. of comorbidities | ||
| 0 | 1 [Reference] | 1 [Reference] |
| 1 | 1.30 (1.23-1.38) | 1.29 (1.19-1.40) |
| 2 | 1.34 (1.26-1.42) | 1.24 (1.13-1.36) |
| ≥3 | 1.68 (1.58-1.78) | 1.18 (1.07-1.31) |
| Zip code education (% with <12-y education in zip codes) |
||
| <7.5 | 1 [Reference] | 1 [Reference] |
| 7.5 to <12.5 | 0.96 (0.90-1.02) | 0.95 (0.87-1.05) |
| 12.5 to <18.5 | 0.98 (0.92-1.05) | 0.92 (0.86-1.02) |
| ≥18.5 | 0.95 (0.89-1.01) | 0.89 (0.80-0.98) |
| Region | ||
| New England | 1 [Reference] | 1 [Reference] |
| Middle Atlantic | 1.52 (1.37-1.68) | 1.47 (1.26-1.70) |
| East North Central | 1.25 (1.13-1.37) | 1.14 (0.99-1.32) |
| West North Central | 1.20 (1.08-1.33) | 1.15 (0.99-1.35) |
| South Atlantic | 1.13 (1.03-1.25) | 0.95 (0.82-1.09) |
| East South Central | 1.31 (1.16-1.47) | 1.14 (0.95-1.37) |
| West South Central | 1.26 (1.13-1.41) | 1.13 (0.96-1.34) |
| Mountain | 1.06 (0.93-1.20) | 0.95 (0.79-1.15) |
| Pacific | 1.01 (0.90-1.14) | 0.87 (0.72-1.04) |
| Rural/urban | ||
| Metropolitan | 1 [Reference] | 1 [Reference] |
| Nonmetropolitan urban | 0.92 (0.87-0.98) | 0.89 (0.81-0.98) |
| Rural | 0.92 (0.80-1.06) | 0.87 (0.70-1.08) |
| Specialtya | ||
| GI | 1 [Reference] | 1 [Reference] |
| Generalist | 0.98 (0.91-1.06) | 0.96 (0.86-1.08) |
| Surgeon | 1.06 (1.01-1.12) | 1.09 (1.00-1.18) |
| Other/unknown | 1.04 (0.93-1.16) | 1.15 (0.98-1.36) |
| Place of servicea | ||
| Hospital | 1 [Reference] | 1 [Reference] |
| Office | 1.19 (1.08-1.30) | 1.16 (1.01-1.34) |
| Ambulatory Center | 0.94 (0.89-0.99) | 0.95 (0.88-1.04) |
| Volume of initial colonoscopista | ||
| ≤440 | 1 [Reference] | 1 [Reference] |
| 441-740 | 1.07 (1.00-1.14) | 1.12 (1.01-1.24) |
| 741-1180 | 1.07 (1.00-1.15) | 1.14 (1.03-1.27) |
| >1180 | 1.26 (1.18-1.35) | 1.44 (1.31-1.59) |
Abbreviations: CI, confidence interval; GI, gastrointestinal tract; HR, hazard ratio.
Specialty, place of service, and volume were all characteristics of the baseline colonoscopy, not the early repeated examination, because there would be no comparison data if the characteristics of the early repeated examination were used. Colonoscopist volume was the number of colonoscopies performed between 2001 and 2003 on Medicare fee-for-service patients by the colonoscopist performing the baseline colonoscopy.
In both models, the hazard was lower in patients 75 and older at the time of their initial examination. It was also lower in women, in those with fewer comorbidities, in those residing in zip codes with lower educational levels, and in those residing outside of large metropolitan areas. Patients receiving their initial examination in an office setting were more likely to undergo an early repeated examination, as were those whose initial examinations were by endoscopists in with high volumes of colonoscopies. The variation by census region was similar to the unadjusted analyses, with patients in the New England, Mountain, and Pacific regions at the lowest risk for early repeated colonoscopy.
We further explored the geographic variation in early repeated colonoscopy after a negative screening colonoscopy finding by examining those rates at the level of the 306 US health referral regions (HRRs). Those analyses were restricted to patients with no clear indication for the repeated examination. Figure 3 shows marked variation, with rates higher than 50% in Pueblo, Colorado, and Bryan, Texas, and lower than 5% in many HRRs. Of interest is the juxtaposition of HRRs with very high and very low rates in the same state, such as in Colorado, Texas, Wisconsin, and California.
Figure 3.
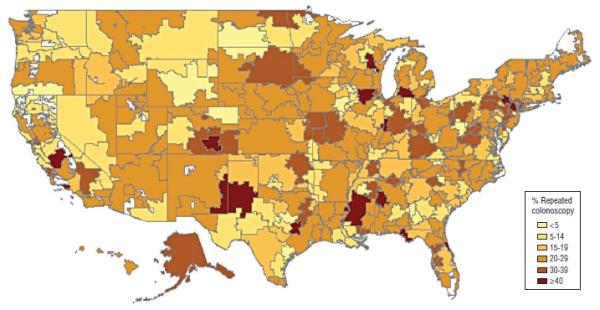
Percentage of Medicare fee-for-service enrollees who underwent early repeated colonoscopy with no clear indication, by health referral region. All patients underwent a negative baseline screening colonoscopy between 2001 and 2003.
COMMENT
The analyses in this study were based on a number of assumptions. One is that colonoscopy results can be classified as negative if not accompanied by a biopsy, polypectomy, or other procedure. We reasoned that the class of abnormalities associated with colon cancer would almost always trigger a procedure. This strategy would tend to underestimate the true prevalence of negative examination results; eg, biopsy finding can be negative. Another assumption is that the colonoscopies were performed for screening purposes rather than for evaluating a sign or symptom. We reasoned that a diagnostic colonoscopy for conditions such as anemia, GI bleeding, change in bowel habit, or abdominal pain would produce relevant diagnoses in the colonoscopy claim or in physician claims in the prior 3 months.
An alternative approach would be to limit the baseline cohort to those with a charge for a screening rather than a diagnostic colonoscopy. This is problematic because many endoscopists continued to use diagnostic codes for those cases even after Medicare initiated reimbursement for screening colonoscopy in 2001 for men and women 50 years or older at average risk. For example, the percentage of all Medicare colonoscopies with a screening code was only 4.6% in 2001 and 14.6% in 2007 through 2008, while an estimated two-thirds of all colonoscopies are performed for CRC screening.8 In one validation study, approximately half of the procedures that had a nonscreening indication in the Medicare claims were listed as screening in the medical record.23
The final cohort of negative screening colonoscopy results represented only 10.2% of all complete colonoscopies in 2001 through 2003. This is clearly a gross underestimate of the true percentage of screening colonoscopies.8 However, the method we used to create the cohort should ensure a very high specificity for negative screening colonoscopy results. This method also tends to underestimate the rate of early repeated colonoscopies. We examined a large number of ways to stratify the cohort of 236 145 individuals with complete colonoscopies in 2001 through 2003. The rate of repeated colonoscopies within 7 years was never lower than 45%. When we examined all patients who received colonoscopies between 2001 and 2003, the repeat rate at 7 years was 59.5%. Thus, the actual practice of colonoscopy in the community seems quite different from the assumptions made in assessing the cost-effectiveness of CRC screening by colonoscopy.24
Overall, 57.5% of the early repeated colonoscopies were accompanied by a diagnosis that might represent a legitimate indication for an examination. This proportion dropped to 38.5% when we examined the colonoscopies done around the inflection point at 60 months shown in Figure 1. The rapid increase in colonoscopies in the period around 60 months suggests that those might have been routinely scheduled, a concept supported by the lower percentage of legitimate indications included in the claims for these examinations.
The estimates of repeated colonoscopy within 7 years produces a highly conservative estimate of potential overuse of screening colonoscopy, given that no guidelines recommend a screening interval of fewer than 10 years after a negative examination result.2-4 We lack full 10-year follow-up data, since Medicare initiated reimbursement for screening colonoscopy for normal-risk individuals in 2001.
Several other findings of this study bear consideration. First, one-third of patients 80 years or older at their initial negative screening examination result underwent a repeated screening examination within 7 years. This is of special concern, given the increased potential for complications and decreased benefit of this examination in the very old.9,16,25 The US Preventive Services Task Force recommends against routine screening in those aged between 75 and 84 years and against any screening of those 85 years or older.25 A second finding of concern is that older patients with 3 or more comorbidities were much more likely to undergo early repeated screening colonoscopy. Because increasing comorbidity is closely linked to decreasing life expectancy, any potential benefit from identifying and removing precancerous lesions would be considerably lower in these patients.16,26,27 It is unclear why such patients are actually more likely to be tested. We had removed from the comorbidity measure any diagnoses that might be indications for colonoscopy. It may be that patients with multiple comorbidities see more medical providers, increasing their risk of being recommended for another screening colonoscopy.
A limitation of this study is the lack of information on the quality of the initial colonoscopy. Early repeated colonoscopies could result from incomplete or poor-quality initial colonoscopies. However, we eliminated patients with incomplete initial colonoscopies from the analyses. Also, one would expect repeated colonoscopies to follow up on suspicious findings to occur relatively soon after the initial colonoscopy, which is not the temporal pattern we found (Figure 2).
Another limitation is that we studied patients 66 years or older with fee-for-service Medicare coverage. The results may not be applicable to younger patients, and we doubt that they are applicable to patients in health maintenance organizations.
The evidence base for the determination of optimal spacing for screening colonoscopy is limited. Indeed, there are no randomized prospective trials of screening colonoscopy, though both observational data and our understanding of tumor biology are strongly supportive.1,25,28 An understanding of tumor biology also underlies the recommendations on frequency of screening colonoscopy.25,28,29 Investigators at the Mayo Clinic identified 226 patients with colonic polyps found by barium enema examination in the precolonoscopy era.28 Three-quarters of the polyps were 10 to 15 mm in diameter, and the rest were larger. These patients were then followed up with periodic barium enema examinations. The rate of conversion to cancer of these large polyps was 2.5% at 5 years and 8% at 10 years, with greater than 70% without nodal involvement or distant spread at surgery.28 This relatively slow rate of progression to cancer of large polyps underlies the recommendation for a 10-year interval following a negative colonoscopy result or a colonoscopy finding of a few small polyps.23
Few quality indicators deal with the overuse of diagnostic tests or treatments for a condition.30 Medicare regulations preclude reimbursement for screening colonoscopy within 10 years of a negative examination result. However, only 2% of the claims for early repeated colonoscopies without indication were denied. Identification of claims for early repeated colonoscopy without an indication other than screening is complicated by the seeming underuse of the screening code in colonoscopies billed to Medicare.
Early repeated colonoscopies without clear indication compose a substantial proportion of the present endoscopist workload and also represents substantial Medicare expenditures. There also seems to be considerable divergence between the current recommendations and the opinions of colonoscopists on appropriate screening intervals,31,32 even though those recommendations have been stable over the past 3 decades.2,3 A recent Institute of Medicine report recommended the development of monitoring systems to identify patterns of overuse of CRC screening.9 Our analyses, when applied to 100% Medicare data (in contrast to the 5% sample used), should be able to identify individual endoscopists with patterns of potential overuse, such as early repeated colonoscopy without indications or screening colonoscopy in very old patients. Such findings could then trigger audits involving medical chart reviews. Pressure from patients may also lead to inappropriate screening.33 Given the increasing public interest in and ownership of cancer screening,33,34 public information campaigns that emphasize both the necessity for CRC screening as well as the dangers of overuse may prove beneficial in reducing overuse.
Acknowledgments
Funding/Support: This study was supported by grants K05CA134923 and R01CA134275 from the National Institutes of Health and grant RP101207 from the Cancer Prevention and Research Institute of Texas.
Footnotes
Financial Disclosure: None reported.
Online-Only Material: The eAppendix is available at http://www.archinternmed.com.
REFERENCES
- 1.Edwards BK, Ward E, Kohler BA, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116(3):544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levin B, Lieberman DA, McFarland B, et al. American Cancer Society Colorectal Cancer Advisory Group. US Multi-Society Task Force. American College of Radiology Colon Cancer Committee Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134(5):1570–1595. doi: 10.1053/j.gastro.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, American College of Gastroenterology American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected] Am J Gastroenterol. 2009;104(3):739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 4.Davila RE, Rajan E, Baron TH, et al. Standards of Practice Committee. American Society for Gastrointestinal Endoscopy ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63(4):546–557. doi: 10.1016/j.gie.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Vital signs: colorectal cancer screening among adults aged 50-75 years—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59(26):808–812. [PubMed] [Google Scholar]
- 6.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? Gastroenterology. 2004;127(6):1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 7.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: data from the National Cancer Institute Survey of Colorectal Cancer Screening Practices. Am J Med. 2003;115(2):129–133. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 8.Chao A, Connell CJ, Cokkinides V, Jacobs EJ, Calle EE, Thun MJ. Underuse of screening sigmoidoscopy and colonoscopy in a large cohort of US adults. Am J Public Health. 2004;94(10):1775–1781. doi: 10.2105/ajph.94.10.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden DJ, Harris R, Porterfield DS, et al. Enhancing the Use and Quality of Colorectal Cancer Screening: Evidence Report/Technology Assessment No. 190 (Prepared by the RTI International-University of North Carolina Evidence-based Practice Center under Contract No. 290-2007-10056-I) Agency for Healthcare Research and Quality; Rockville, MD: Feb, 2010. AHRQ publication 10-E-002. [Google Scholar]
- 10.Song Y, Skinner J, Bynum J, Sutherland J, Wennberg JE, Fisher ES. Regional variations in diagnostic practices. N Engl J Med. 2010;363(1):45–53. doi: 10.1056/NEJMsa0910881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Ann Intern Med. 2010;152(10):668–676. doi: 10.7326/0003-4819-152-10-201005180-00239. [DOI] [PubMed] [Google Scholar]
- 12.Levin TR, Zhao W, Conell C, et al. Complications of colonoscopy in an integrated health care delivery system. Ann Intern Med. 2006;145(12):880–886. doi: 10.7326/0003-4819-145-12-200612190-00004. [DOI] [PubMed] [Google Scholar]
- 13.Arora G, Mannalithara A, Singh G, Gerson LB, Triadafilopoulos G. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–664. doi: 10.1016/j.gie.2008.09.008. 3, pt 2. [DOI] [PubMed] [Google Scholar]
- 14.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150(12):849–857. doi: 10.7326/0003-4819-150-12-200906160-00008. W152. [DOI] [PubMed] [Google Scholar]
- 15.Prajapati DN, Saeian K, Binion DG, et al. Volume and yield of screening colonoscopy at a tertiary medical center after change in Medicare reimbursement. Am J Gastroenterol. 2003;98(1):194–199. doi: 10.1111/j.1572-0241.2003.07172.x. [DOI] [PubMed] [Google Scholar]
- 16.Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. JAMA. 2006;295(20):2357–2365. doi: 10.1001/jama.295.20.2357. [DOI] [PubMed] [Google Scholar]
- 17.Schoenfeld P, Cash B, Flood A, et al. CONCeRN Study Investigators. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352(20):2061–2068. doi: 10.1056/NEJMoa042990. [DOI] [PubMed] [Google Scholar]
- 18.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343(3):162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 19.Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343(3):169–174. doi: 10.1056/NEJM200007203430302. [DOI] [PubMed] [Google Scholar]
- 20.US Department of Agriculture Measuring rurality: rural-urban continuum codes. http://www.ers.usda.gov/Data/RuralUrbanContinuumCodes/. Accessed June 4, 2010.
- 21.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 22.The Dartmouth Atlas of Health Care Zip code crosswalk files. http://www.dartmouthatlas.org/tools/downloads.aspx. Accessed June 4, 2010.
- 23.Schenck AP, Klabunde CN, Warren JL, et al. Data sources for measuring colorectal endoscopy use among Medicare enrollees. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2118–2127. doi: 10.1158/1055-9965.EPI-07-0123. [DOI] [PubMed] [Google Scholar]
- 24.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284(15):1954–1961. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 25.US Preventive Services Task Force Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149(9):627–637. doi: 10.7326/0003-4819-149-9-200811040-00243. [DOI] [PubMed] [Google Scholar]
- 26.Braithwaite RS, Fiellin D, Justice AC. The payoff time: a flexible framework to help clinicians decide when patients with comorbid disease are not likely to benefit from practice guidelines. Med Care. 2009;47(6):610–617. doi: 10.1097/MLR.0b013e31819748d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gross CP, McAvay GJ, Krumholz HM, Paltiel AD, Bhasin D, Tinetti ME. The effect of age and chronic illness on life expectancy after a diagnosis of colorectal cancer: implications for screening. Ann Intern Med. 2006;145(9):646–653. doi: 10.7326/0003-4819-145-9-200611070-00006. [DOI] [PubMed] [Google Scholar]
- 28.Stryker SJ, Wolff BG, Culp CE, Libbe SD, Ilstrup DM, MacCarty RL. Natural history of untreated colonic polyps. Gastroenterology. 1987;93(5):1009–1013. doi: 10.1016/0016-5085(87)90563-4. [DOI] [PubMed] [Google Scholar]
- 29.Peipins LA, Sandler RS. Epidemiology of colorectal adenomas. Epidemiol Rev. 1994;16(2):273–297. doi: 10.1093/oxfordjournals.epirev.a036154. [DOI] [PubMed] [Google Scholar]
- 30.McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003;348(26):2635–2645. doi: 10.1056/NEJMsa022615. [DOI] [PubMed] [Google Scholar]
- 31.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? a national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141(4):264–271. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 32.Krist AH, Jones RM, Woolf SH, et al. Timing of repeat colonoscopy: disparity between guidelines and endoscopists’ recommendation. Am J Prev Med. 2007;33(6):471–478. doi: 10.1016/j.amepre.2007.07.039. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz LM, Woloshin S, Fowler FJ, Jr, Welch HG. Enthusiasm for cancer screening in the United States. JAMA. 2004;291(1):71–78. doi: 10.1001/jama.291.1.71. [DOI] [PubMed] [Google Scholar]
- 34.Cram P, Fendrick AM, Inadomi J, Cowen ME, Carpenter D, Vijan S. The impact of a celebrity promotional campaign on the use of colon cancer screening: the Katie Couric effect. Arch Intern Med. 2003;163(13):1601–1605. doi: 10.1001/archinte.163.13.1601. [DOI] [PubMed] [Google Scholar]


