Abstract
We report here the use of novel self-assembling collagen-hyaluronic acid (HyA) membranes to deliver bone morphogenetic protein-2 (BMP-2) for orthopedic applications. Prior work has demonstrated that collagen-HyA membranes are formed initially through electrostatic interactions between the oppositely charged collagen and HyA molecules, and that membrane growth is driven by osmotic pressure imbalances between the collagen and HyA solutions. The purpose of this study was to investigate the potential of incorporating charged growth factors such as BMP-2 within the membrane for regenerative medicine applications. Membrane material properties, protein mass loss, and release kinetics of BMP-2, as well as biocompatibility and osteogenic potential in vitro and in vivo using a subcutaneous mouse model were assessed. Scanning electron microscopy and mechanical testing confirmed no loss of structural or mechanical integrity upon BMP-2 incorporation into the membranes. Slow and steady release of the growth factor was demonstrated with 17% of total loaded BMP-2 released over the course of 49 days. To test biocompatibility and osteogenic potential in vitro, human mesenchymal stem cells were cultured on collagen-HyA membranes and showed greater proliferation rates (for up to 28 days) on membranes without BMP-2, but a greater alkaline phosphatase activity and osteocalcin production on membranes releasing BMP-2. In vivo subcutaneous implantation of the membranes showed a minimal immune response with osteoblasts and mineral deposits present in the ectopic site for BMP-2-releasing membranes, further demonstrating the potential of the BMP-2-releasing membranes to induce osteogenic differentiation. This study presents a novel strategy to create self-assembled membranes using two biocompatible molecules that can deliver bioactive agents in a sustained manner to induce a local regenerative response.
Introduction
The use of collagen and hyaluronic acid (HyA) is prevalent in medicine because they are endogenous biopolymers, providing inherent biocompatibility and bioactivity to the material.1–6 For instance, collagen alone accounts for ∼30% of all vertebrate body proteins and more than 90% of the extracellular proteins in connective tissues.7 The use of collagen-based scaffolds has been shown to be advantageous because of their inherent cell-binding sites, availability, accessibility, and structural strength.8–10 HyA is a glycosaminoglycan copolymer of d-glucuronic acid and N-acetyl-d-glucosamine, and is a major intercellular component of connective tissues such as in the synovial fluid of joints, vitreous fluid of the eye, and scaffolding within cartilage and umbilical cord. HyA has been reported to play an important role in lubrication, wound healing, and cell proliferation, differentiation, and migration.11,12 Moreover, medical products containing HyA have been used for several decades confirming its biocompatibility, familiarity, and ease of adoption for clinical applications.13–15 The benefits of combining collagen and HyA into a biomaterial include enhanced effects on extracellular matrix protein production and material tunability.16,17
With these advantages in mind, we have recently reported on the self-assembly between collagen and HyA into membrane structures.18 Unlike previous studies that have taken advantage of these oppositely charged molecules to build up collagen-HyA membranes using a layer-by-layer multistep deposition process,5 the collagen-HyA membranes described here are fabricated using a one-step procedure.18 Collagen-HyA membranes are self-assembled by simply layering an equal volume of collagen solution on top of the denser HyA solution. While an initial, collagen-HyA diffusion barrier is instantaneously formed by electrostatic complexation at the interface of the two solutions, membrane growth is driven by the osmotic pressure imbalance between the collagen and HyA solutions.18
Notably, collagen-HyA membranes have the ability to bind and coat tissues, and are permeable to biomolecules.18 This supports their potential to deliver bioactive agents in a sustained manner for inducing a local regenerative response. In the present study, we incorporated bone morphogenetic protein-2 (BMP-2) within collagen-HyA membranes to assess its potential to act as a bioactive membrane. BMP-2 was specifically chosen because it is a well-known promoter of ectopic bone formation19–21 and can successfully induce mesenchymal stem cells (MSCs) to undergo osteoblast differentiation.22–24 A variety of tissue-engineering studies delivering BMP-2 from various scaffolds have been explored since delivery is maximized when combined with a carrier.25–27 Results from these studies, however, often had varied outcomes due to unfavorable release rates characterized by an initial burst release without sustained BMP-2 delivery over an extended period of time.28,29 A large initial burst release and retention of less than 5% after 14 days of BMP-2 was observed even for collagen sponges currently approved by the FDA for spinal fusion. This initial high load of BMP-2 has also been shown to lead to an adverse immunological response and increased postoperative tissue morbidity.29,30
In this study, we assess the mechanical properties and degradation rate of collagen-HyA membranes, as well as the kinetics of BMP-2 release from these novel materials. Since BMP-2 carries two negative charges,31 BMP-2 was added to the positively charged collagen molecules before combining with the HyA for membrane formation. The design of the bioactivity is based on the potential ability to bind and slowly release the electrostatically bound and physically entrapped BMP-2 from the membrane. The osteogenic potential of the BMP-2-releasing membranes is also evaluated by studying the differentiation of MSCs in vitro and the ability to induce bone formation within an ectopic, subcutaneous mouse model. Furthermore, the potential of our novel, bioactive membranes for controlled and localized release of growth factors for applications in regenerative medicine is discussed.
Materials and Methods
Collagen-HyA membrane formation
Type I collagen powder derived from the bovine Achilles tendon (Sigma Aldrich, St. Louis, MO) was homogenized into a 0.5% wt/vol solution in 0.05 M acetic acid. The HyA solution was prepared by dissolving 0.75 wt% sodium hyaluronate (2.6 MDa; Lifecore Biomedical, Chaska, MN) in MQ water. An equal volume of collagen solution was overlaid on top of the HyA solution and was allowed to incubate at room temperature for 24 h before washing with MQ water to remove excess HyA. Membranes used for BMP-2 release in vitro, and in vivo studies were fabricated by overlaying 350 μL of collagen solution over 350 μL of HyA solution in a 24-well plate. To incorporate human recombinant BMP-2 (Medtronic, Schiller Park, IL), 2 μg of human recombinant BMP-2 was added to the collagen solution before overlaying onto the HyA solution. All membrane thicknesses were ∼130 μm.
Scanning electron microscopy
Scanning electron microscopy (SEM) samples were prepared by fixing membranes in 2% glutaraldehyde/3% sucrose in phosphate-buffered saline (PBS) (pH 7.4). Samples were dehydrated via a graded series of ethanol, critical point dried (Samdri-795; Tousimis, Rockville, MD), and sputter coated with 10 nm of gold before observing via SEM (Hitachi 3500°N; EPIC, Northwestern University, Evanston, IL). To assess the morphology of the membrane cross sections, dried samples were manually torn.
Mechanical properties
The elastic modulus under tension was measured using a mechanical tester from JLW Instruments (Chicago, IL). Membranes (0.8×3.5×0.13 cm) were kept hydrated before testing and pulled at a rate of 2 mm/min until failure, using a preload of 0.02N (n=3). A concentration of 2 μg BMP-2/350 μL collagen solution was used to fabricate BMP-2 membranes for mechanical testing. The membrane thickness was measured by freezing membranes, embedding in the Tissue Tek medium (Tissue Tek; Sakura Finetek, Torrance, CA), and manually measuring the cross section of 5–7-μm cryosections via light microscopy (Nikon Eclipse TE2000-U, Tokyo, Japan).
Degradation
Membranes were submerged in PBS (pH 7.4) at 37°C for degradation studies. At days 0, 7, 14, 21, 28, 35, and 49, membranes were digested by collagenase type I (100 μg enzyme/mL PBS (28 units) at 37°C for 48 h (Sigma Aldrich, n=10) to quantify the remaining protein content. The protein content was measured using the BCA assay according to the manufacturer's instructions (Thermo Scientific Pierce, Rockford, IL). Since collagen and HyA are both required to keep the membrane intact, the representative degradation rate of the collagen-HyA membranes was assessed by quantifying the remaining collagen content within the membrane for up to 49 days.
BMP-2 release kinetics
To investigate the release kinetics of BMP-2, membranes were submerged in 1 mL PBS (pH 7.4) at 37°C and samples (both PBS and membranes) were collected at 4 h and 1, 2, 3, 4, 7, 14, 21, 28, 35, and 49 days (n≥3). The amount of BMP-2 within the collected PBS solution samples and remaining within the membranes was quantified via ELISA according to the manufacturer's instructions (R&D Systems, Minneapolis, MN). The remaining BMP-2 within the membrane was obtained by enzymatically digesting the membranes as described earlier.
Zeta potential
Zeta potential measurements using 1 mL of 0.1 wt% HyA or collagen solutions were acquired using the Zetasizer Nano ZS (n=5; Malvern, Worcestershire, United Kingdom). The collagen with the BMP-2 solution (2 μg BMP-2/350 μL 0.5% collagen concentration) was also diluted accordingly before measurements were obtained.
Cell proliferation
Human MSCs (hMSCs) were purchased from Lonza (Walkersville, MD) and expanded in growth media containing the low-glucose DMEM (1 g/mL glucose) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37°C in humidified air (containing 5% CO2). Cells at passage 5 were used in this study and media were changed every 3 or 4 days.
Before cell seeding, membranes with and without BMP-2 incorporation were sterilized by immersion in 70% ethanol for 30 min and exposure to UV-C radiation (Hg lamp, 30 W) for 30 min. After 24 h of conditioning the samples in growth media at 37°C, 20,000 cells were suspended in 10 μL of media and seeded on the collagen side of the membrane (10,000 cells/cm2). Two hours postseeding, 2 mL of media was aliquoted into each well. To quantify hMSC attachment and proliferation, total DNA was quantified on days 4, 14, and 28 using Quant-iT Pico Green dsDNA Reagent (Invitrogen, Carlsbad, CA). Membranes were washed twice with PBS and cells were lysed by submerging the membrane in 0.1% Triton-X 100 and sonicating for 20 min. The lysate was used for the assay (n≥3). Cell morphology was determined via SEM as described earlier on day 1 and day 28.
Measurement of alkaline phosphatase activity and osteocalcin concentration
hMSCs were cultured and seeded on sterilized membranes as described above. At day 1, 14, and 28, the intracellular alkaline phosphatase (ALP) activity of hMSCs was assessed by measuring the release of p-nitrophenol from p-nitrophenyl. One aliquot of the cell lysate was added to an equal amount of a reaction buffer containing 10 mM p-nitrophenyl phosphate in a 50 mM glycine buffer, pH 10.5, supplemented with 0.5 mM MgCl2 (Sigma Aldrich). After 30 min at 37°C, the reaction was stopped by the addition of 0.05 M NaOH and measured by spectrophotometry at 410 nm. The standard curve was constructed from different dilutions of the p-nitrophenol stock solution. The osteocalcin concentration within the lysates was measured via ELISA following the manufacturer's instructions (Quidel, San Diego, CA). At least three samples were used and results were normalized to the total DNA amount.
Subcutaneous implantation in mouse model
Female BALB/c mice, aged 6–8 weeks (Charles River, Wilmington, MA) were anesthetized with isoflurane and one membrane (2 cm2×130 μm) was placed in a subcutaneous pocket on the dorsal side of each animal. A group of six mice were used per membrane type (no BMP-2 and BMP-2). Incisions (∼2 cm) were closed with 4-0 Ethilon sutures (Ethicon, Blue Ash, OH). Before implantation, membranes were sterilized by immersion in 70% ethanol for 30 min and exposed to UV-C radiation (Hg lamp, 30 W) for 30 min. Membranes were then washed with sterile PBS (pH 7.4) immediately before implantation. The surgical protocol (No. 2011-2498-3) followed NIH guidelines for the care and use of laboratory animals and was approved by Northwestern University's Animal Care and Use Committee (Chicago, IL).
Histology and immunohistochemistry
At 4 weeks postimplantation, explants were immediately frozen and stored in −80°C. Samples were embedded in Tissue Tek (Sakura Finetek) and sectioned (5–7-μm sections) for histology and immunohistochemistry (Microm HM 525; Fisher Scientific, Pittsburg, PA). Sections were stained with hematoxylin and eosin (H&E) and von Kossa and evaluated using light microscopy (Nikon Eclipse TE2000-U).
To detect the presence of osteoblasts in vivo, the following primary antibodies were used: rabbit polyclonal anti-mouse osteopontin (1:200) and rabbit polyclonal anti-mouse osteocalcin (1:250) (Abcam, Cambridge, MA). Frozen sections were thawed, fixed in methanol, washed in PBS, and blocked in 0.1% Triton X-100 in PBS containing 5% FBS for 30 min. Primary antibodies were diluted in the blocking solution before slides were incubated for 2 h in a humidified chamber at room temperature. Secondary antibodies (goat polyclonal anti-rabbit-Cy5 or goat polyclonal anti-rabbit-FITC; Abcam) were diluted (1:1000) in the blocking solution, applied to the samples, and incubated for 90 min at room temperature. Slides were washed two times in PBS, mounted, counterstained with DAPI (Vectershield, Burlingame, CA), and evaluated using fluorescence microscopy (Nikon Eclipse TE2000-U).
Statistical analysis
A Student's t-test was used to compare means of pairs. Analysis of variance (ANOVA) with Newman–Keuls multiple comparison test post hoc analysis was used to determine significant differences among three or more means. A p-value of 0.05 or less was considered to be significant.
Results and Discussion
In these studies, collagen-HyA membranes are formed by simply layering a volume of positively charged collagen solution on top of a denser HyA solution of opposite charge (Fig. 1). Collagen-HyA membranes with osteogenic potential were fabricated by adding 2 μg of BMP-2 to the collagen solution before overlaying on top of the HyA solution. As demonstrated in prior work,18 the growth of the multilayered membrane to reach hundreds of microns in thickness is a result of the imbalance of osmotic pressure between the two solutions. Upon initial electrostatic complexation between the HyA and collagen, counterions are released from the HyA side, which increases the osmolarity of the HyA solution. This increase in osmolarity drives the movement of the solvent from the collagen solution side into the HyA side, and HyA molecules into the collagen side, causing more complexation until all of the collagen molecules are incorporated into the membrane. Therefore, to maximize growth factor incorporation into the membrane, BMP-2 was added to the collagen solution. Furthermore, since collagen is dissolved in dilute acetic acid, adding BMP-2 to the collagen solution is also advantageous because the low pH maintains BMP-2 bioactivity by reducing protein aggregation and conformation changes.32–34
FIG. 1.
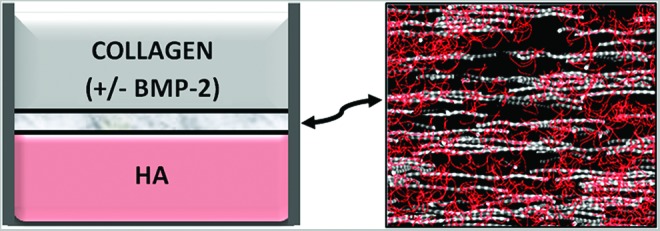
Schematic depicting the process of membrane formation. Self-assembling membranes are formed at the interface between collagen and HyA solutions creating a nanofibrous membrane structure that can be hundreds of microns thick. HyA, hyaluronic acid. Color images available online at www.liebertpub.com/tea
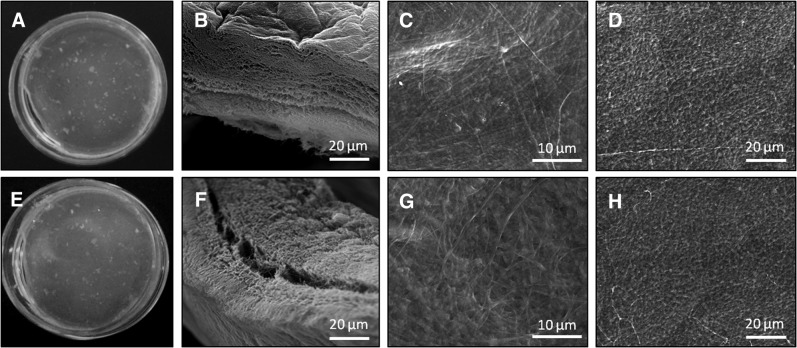
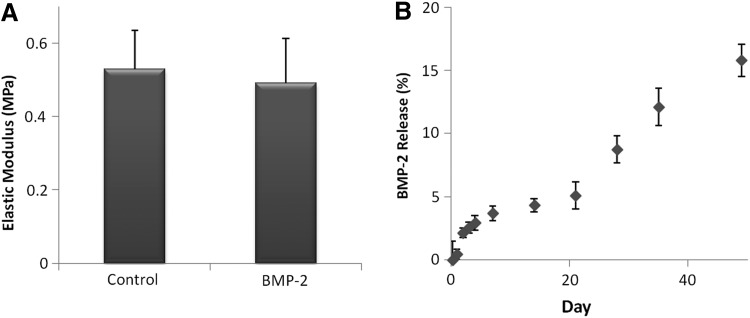
The incorporation of bioactive components into other self-assembling biomaterials has been shown to cause structural disruption, which can be directly reflected in the material mechanical properties.35 Since electrostatic interactions are a key component to collagen-HyA membrane self-assembly, the incorporation of BMP-2, which contains several positively and negatively charged amino acids,31,36 has the potential to disrupt membrane formation and the structural integrity of the membrane.35,37 However, SEM images of collagen-HyA membranes with or without BMP-2 incorporation demonstrated no change in the membrane microstructure upon BMP-2 incorporation (Fig. 2). The interface of both membranes showed a dense, multilayered fibrous structure, with compact surfaces on the collagen and HyA sides (Fig. 2B, F). The collagen side (Fig. 2C, G) has a more fibrous morphology and the HyA side shows a beaded surface architecture, which is consistent with our previous study (Fig. 2D, H).18 Furthermore, there was no significant difference in the elastic modulus of hydrated collagen-HyA membranes with (0.49±0.1 MPa) and without (0.52±0.1 MPa) BMP-2 when performed under tension (Fig. 3A). These mechanical property results are consistent with the minimal structural changes found under SEM.
FIG. 2.
Structure of self-assembling collagen-HyA membrane. Digital images (A, E) and SEM images of the interface (B, F), collagen side (C, G), and HyA side (D, H) of the self-assembled collagen-HyA membrane without (top) and with (bottom) BMP-2 incorporation. BMP-2, bone morphogenetic protein-2; SEM, scanning electron microscopy.
FIG. 3.
Tensile modulus of hydrated collagen-HyA membranes (A, n=3) and BMP-2 release profile of BMP-2-incorporated membranes for up to 49 days (B, n≥3).
Collagen-HyA membranes were observed to stay intact for up to 49 days at 37°C in vitro, losing ∼15.3% of total protein (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tea), confirming their stability and potential for sustained growth factor delivery in vivo. The release kinetics of BMP-2 from collagen-HyA membranes was also measured for up to 49 days (Fig. 3B). A slow and sustained release of BMP-2 was observed, without an initial burst release that is characteristic to other BMP-2-loaded scaffolds. Although the washing steps to eliminate excess HyA solution immediately upon membrane formation may contribute to such behavior, both the physical entrapment of BMP-2 within the collagen-HyA membrane and the ionic complexation between the negatively charged carboxyl groups of HyA and the net positively charged BMP-2 protein may also contribute to enhanced noncovalent immobilization, prolonged release of the protein, and lack of a burst release.38
Approximately 17% of the total BMP-2 incorporated was released by day 49. The release profile showed a distinct transition in the release rate between days 14–21 and 21–49. The faster release rate after day 21 may be due to degradation and changes in the membrane structure over time. Based on the average linear slope of the release profile, total growth factor release is projected to be completed by week 48. This slow release profile may be advantageous since the amount of BMP-2 retained in the membrane (i.e., in direct contact with adhered cells) may significantly contribute to an enhanced biological response. Prolonged and local BMP-2 release is crucial for bone applications since MSC differentiation in vitro occurs between 14–21 days,39,40 and bone formation in vivo has been reported to take place between 4–8 weeks.41,42 Since the degradation rate of membranes is slower than the release profile of BMP-2, the release of BMP-2 is likely attributed to both degradation as well as diffusion of the growth factor out of the membrane as the charged groups of BMP-2 and HyA become screened by ionic salts in the media, weakening their interactions. Collagen molecules, which are positively charged when dissolved in 0.05 M acetic acid or pH 3.4 as prepared in this study, have a zeta potential of 51.2±2.9 mV and decreases upon the addition of BMP-2 (46.9±1.2 mV), which suggests that collagen molecules have a higher affinity to the highly negative anionic HyA (−90.9±15.6 mV) over BMP-2 proteins (Table 1).20,43 As a result, diffusion of BMP-2 out of the membrane is likely to contribute to its total release.44
Table 1.
Zeta Potential of Collagen and Hyaluronic Acid Solutions
| ζP (mV) | |
|---|---|
| Hyaluronic acid | −90.9±15.6 |
| Collagen | 51.2±2.9 |
| Collagen+BMP-2 | 46.9±1.2 |
BMP-2, bone morphogenetic protein-2.
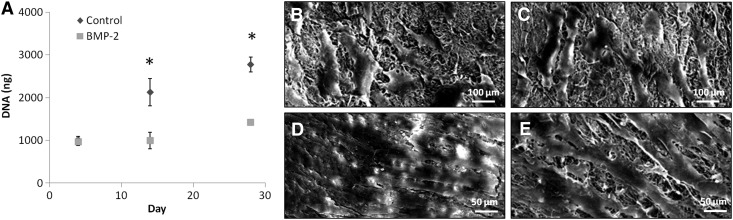
When human MSCs were seeded on collagen-HyA membranes, cells adhered and spread on both BMP-2 and control membranes at day 4 (Fig. 4). Although cell attachment was similar at day 4 between the membrane types (control 1002.6±85.4 vs. BMP-2 964.7±87.4 ng DNA), cell proliferation at day 14 and 28 was found to be significantly greater on membranes without BMP-2 (p≤0.05, control d14: 2126.1±316.7 vs. BMP-2 d14: 998.5±194.0, control d28: 2769.8±174.2 vs. BMP-2 d28: 1415.7±3.7 ng DNA, Fig. 3A). SEM images on day 28 (Fig. 4D) showed a cell sheet covering the entire surface of control membranes. Although cell proliferation was evident on membranes containing BMP-2 at day 28 in comparison to day 4, cells were not confluent as observed on the control membranes (Fig. 4E). This slower rate of cell proliferation on BMP-2-releasing membranes is consistent with cells that are undergoing differentiation.45 Although hMSCs are known to spontaneously differentiate upon becoming confluent, cells on the collagen-HA membranes without BMP-2 did not demonstrate a greater osteogenic differentiation compared to those on the BMP-2-releasing membranes. Thus, the sustained delivery of BMP-2 from these novel collagen-HA membranes is a significant component of the observed osteogenic differentiation of the seeded hMSCs.43
FIG. 4.
MSC proliferation on control and BMP-2 membranes. Quantification of cell proliferation for up to 28 days (A, n≥3, *p≤0.05) and SEM images of MSC attachment at day 4 on control (B) and BMP-2 membranes (C), and MSC growth at day 28 on control (D) and BMP-2 membranes (E). MSC, mesenchymal stem cell.
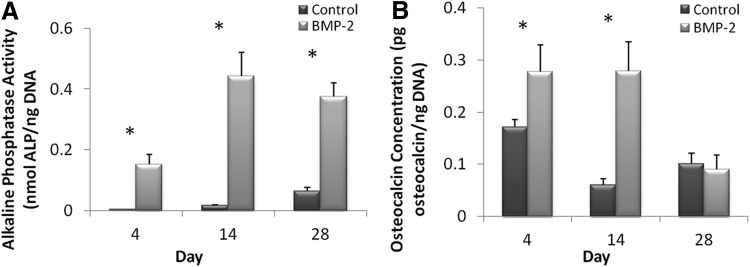
Osteogenic differentiation of mesenchymal cells by BMP-2 signaling can be monitored by the induction of the ALP activity and osteocalcin expression.46 To assess the osteogenic potential of collagen-HyA membranes with and without BMP-2, intracellular ALP and osteocalcin production were quantified at days 4, 14, and 28 postseeding. The ALP activity of cells cultured on BMP-2-releasing membranes was significantly greater at all time points in comparison to the ALP activity of cells on control membranes with the highest activity on day 14 (p≤0.05, control d4: 0.004±0.0001 vs. BMP-2 d4: 0.152±0.033, control d14: 0.017±0.002 vs. BMP-2 d14: 0.443±0.783, and control d28: 0.064±0.012 vs. BMP-2 d28: 0.376±0.045 nmol ALP/ng DNA, Fig. 5A). The intracellular osteocalcin concentration was also found to be significantly higher at day 4 and day 14 for the BMP-2-releasing membrane group compared to the control membrane group, with the greatest production on day 14 (p≤0.05, control d4: 0.172±0.014 vs. BMP-2 d4: 0.278±0.052, control d14: 0.060±0.014 vs. BMP-2 d14: 0.279±0.056 pg osteocalcin/ng DNA, Fig. 5B). However, unlike the ALP activity, the osteocalcin concentration for the BMP-2 membrane group decreased on day 28 and was similar to control membranes (control: 0.101±0.020 vs. BMP-2: 0.091±0.027 pg osteocalcin/ng DNA).
FIG. 5.
Osteogenic potential of BMP2-incorporated membranes in vitro. The intracellular ALP activity of MSCs on control and BMP-2 membranes for up to 28 days (A, n≥3, *p≤0.05) and osteocalcin expression of MSCs on control and BMP-2 membranes (B, n≥3, *p≤0.05). ALP, alkaline phosphatase.
When osteoprogenitor cells differentiate into osteoblasts in vivo, early stage osteoblasts express collagen type I and secrete ALP into the surrounding extracellular matrix, preparing the matrix for mineralization and indicating bone maturation. The osteoblasts then deposit hydroxyapatite, and proteins, such as osteocalcins, which bind to hydroxyapatite are expressed.47 Osteocalcin, a noncollagenous matrix protein, is synthesized by mineralizing osteoblasts and is an important marker for bone regeneration.44,48 By day 3, both the early osteoblast marker ALP and the late marker osteocalcin were expressed, and peak activity and concentration of ALP and osteocalcin was found at day 14. Therefore, our assays confirm the potency of BMP-2-releasing membranes to drive MSCs into the osteoblast lineage in vitro. Although it was expected that osteocalcin levels would be greater than the ALP activity at day 28, since it is known to be a late osteoblast marker, we observed a decrease in osteocalcin expression and a continuation of ALP expression. The decrease of osteocalcin on day 28 may be caused by the changing amount of BMP-2 remaining locally within the collagen-HA membranes as more BMP-2 is being released over time. Prior work has shown similar variations of osteocalcin expression over time with different loaded amounts of BMP-2 being released from HyA scaffolds.38
To confirm the efficacy of osteoblast differentiation in vivo, membranes were implanted subcutaneously in a mouse model for 4 weeks. This model is ideal as a proof-of-concept to demonstrate biocompatibility and functionality of novel delivery systems. Many studies have shown that a carrier incorporating BMP-2 can induce bone formation at the ectopically implanted site by activating a set of cellular events, including chemotaxis of uncommitted mesenchymal cells and differentiation of these cells into osteoblasts.49,50 Without a carrier, a large concentration of BMP-2 must be administered to observe bone formation, whereas delivery using a carrier usually requires less growth factors since it can be retained locally for more prolonged periods of time for continual BMP-2 release and exposure to the surrounding cells.49,51 Observations from previous studies suggest that a certain level of BMP-2 must be released to enhance bone formation. A biomaterial like the collagen-HyA membranes that can achieve slow and localized release of the growth factor over time at the site of implantation may therefore be an ideal carrier for inducing bone formation in vivo.
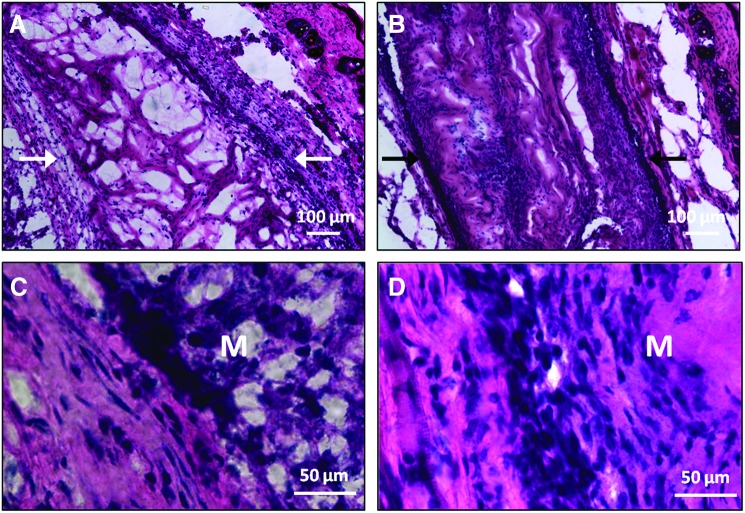
H&E staining of explanted membranes showed the presence of the collagen-HyA membrane after 4 weeks in vivo (Fig. 6). Cells infiltrated both collagen-HyA membranes with and without the incorporation of BMP-2. A thin and continuous fibrous capsule also surrounded both membranes, which is consistent with other studies evaluating collagen or HyA biomaterials implanted at this location.52–56 Using the fibrous capsule as a reference point for the membrane edges, the approximate thickness of membranes with and without BMP-2 after implantation was found to be similar. However, membranes without BMP-2 contained multiple voids within its structure, whereas membranes with BMP-2 showed a dense structure in which the membrane seemed to be completely filled with cells and the extracellular matrix (Fig. 6B, D). This increase in biosynthesis may be explained by the ability of BMP-2 to elevate the production of extracellular matrix components such as collagen and aggrecan.57–59
FIG. 6.
Hematoxylin and eosin staining of explanted membranes at 4 weeks. Control (membrane without BMP-2) (A) and BMP-2 membrane (B) in lower magnification and control (C) and BMP-2 (D) membranes in higher magnification at the implant–tissue interface. Arrows point to the edges of the membrane, where a fibrous capsule is present. M, membrane. Color images available online at www.liebertpub.com/tea
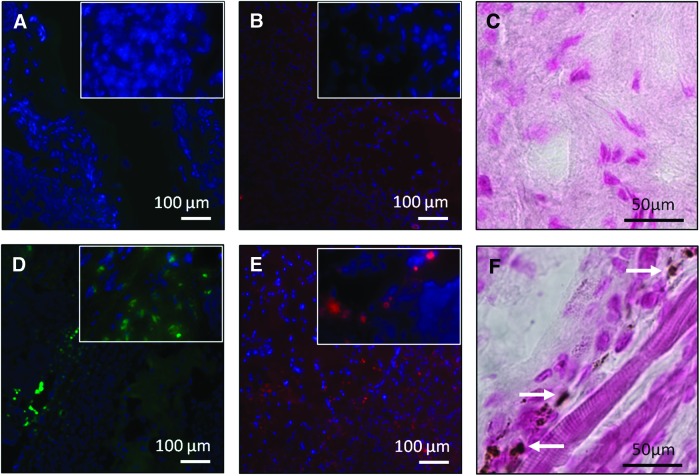
To assess osteogenesis in vivo, osteoblasts were identified through osteocalcin and osteopontin staining (Fig. 7). Osteopontin is another osteogenic marker that is highly expressed in osteoblasts during matrix mineralization and is known to play a role in bone remodeling.60 Cells stained for both osteocalcin and osteopontin were observed in BMP-2-releasing membranes at 4 weeks, compared to control membranes, which did not show cells stained with either marker. Mineral deposition, assessed by von Kossa staining, was also found only in BMP-2-releasing membranes (Fig. 7F). Furthermore, since osteoblasts are known to secrete the extracellular matrix, the dense structure observed in Figure 6 may also be due to the increased presence of active osteoblasts.61 Collectively, our in vitro and in vivo data confirm the ability of BMP-2-releasing collagen-HyA membranes to promote osteogenesis. Notably, growth factor delivery from these collagen-HyA membranes is slow and steady, in contrast to a sudden bolus growth factor release characteristic of other carriers.29,30 Although our release data confirm BMP-2 release for longer than 4 weeks, future studies assessing osteogenesis at longer time points and in bone defect models will confirm its full regenerative potential in vivo.21 Furthermore, the enhancement of mechanical properties through the incorporation of hydroxyapatite or crosslinking strategies may further tailor these membranes for orthopedic applications.18,40
FIG. 7.
Osteogenic potential of collagen-HyA membranes at 4 weeks in a subcutaneous mouse model. Immunofluorescent staining showed no cells with positive osteopontin (A) and osteocalcin (B) staining in control membranes, whereas positive staining for osteopontin (D) and osteocalcin (E) were found present within BMP-2 membranes, confirming the presence of osteoblasts. Positive immunofluorescent staining for osteopontin and osteocalin is shown in green and red, respectively, and nuclei DAPI counterstaining is shown in blue. Inset shows cells with positive staining in higher magnification. Unlike control membranes (C), von Kossa staining showed areas of mineral deposition in BMP-2 membranes (F) stained in brown. Arrows denote areas of mineral deposition. Color images available online at www.liebertpub.com/tea
Self-assembling membranes composed of collagen and HyA offer novel ways to deliver sustained and local growth factor release to promote bone formation. Through their ability to spontaneously organize into a well-defined structure without external instruction, these membranes have the potential to complement minimally invasive surgical procedures, which differ from other prefabricated collagen-HyA membranes.5 Furthermore, these membranes have the potential to promote highly localized regeneration and support healing at interfaces between various types of tissues, such as in the bone–tendon junction. Moreover, collagen-HyA membranes could also be used to deliver other growth factors for different regenerative medicine targets such as their use as bioactive dressings for wound-healing applications or as adjuncts to enhance the bioactivity of current graft implants.
Conclusions
Herein we demonstrate the ability of self-assembling collagen-HyA membranes to incorporate and release bioactive components. These membranes maintain structural and mechanical integrity upon BMP-2 incorporation and demonstrate a steady and sustained delivery of the growth factor in vitro for up to 49 days. BMP-2-releasing membranes promote osteogenesis as demonstrated through osteoblast differentiation in vitro using hMSCs. Moreover, the osteogenic potential of BMP-2-releasing membranes is further demonstrated by the presence of osteoblasts and mineral deposition when implanted subcutaneously in a mouse model. Self-assembling collagen-HyA membranes can be potentially used in many regenerative medicine applications, including their application as bioactive adjuncts in minimally invasive surgical procedures.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the financial support from the IBNAM-Baxter Early Career Award granted to E. Chung that supported this research. Experiments made use of the following facilities at the Northwestern University: Electron Probe Instrumentation Center (EPIC) of the NUANCE Center, Biological Imaging Facility (BIF), and the Institute for BioNanotechnology in Medicine (IBNAM). The NUANCE Center is supported by the NSF-NSEC, NSF-MRSEC, Keck Foundation, the State of Illinois, and the Northwestern University.
Disclosure Statement
No competing financial interests exist.
References
- 1.Friess W. Collagen — biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45:113. doi: 10.1016/s0939-6411(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 2.Chan B.P., et al. Self-assembled collagen–human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28:4652. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 3.Powell H.M. Supp D.M. Boyce S.T. Influence of electrospun collagen on wound contraction of engineered skin substitutes. Biomaterials. 2008;29:834. doi: 10.1016/j.biomaterials.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 4.Leach J.B. Schmidt C.E. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., et al. Natural polyelectrolyte films based on layer-by layer deposition of collagen and hyaluronic acid. Biomaterials. 2005;26:3353. doi: 10.1016/j.biomaterials.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Segura T. Chung P.H. Shea L.D. DNA delivery from hyaluronic acid-collagen hydrogels via a substrate-mediated approach. Biomaterials. 2005;26:1575. doi: 10.1016/j.biomaterials.2004.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee C.H. Singla A. Lee Y. Biomedical applications of collagen. Int J Pharm. 2001;221:1. doi: 10.1016/s0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- 8.Sinani V.A., et al. Collagen coating promotes biocompatibility of semiconductor nanoparticles in stratified LBL films. Nano Lett. 2003;3:1177. [Google Scholar]
- 9.Nimni M.E. Cheung D. Strates B. Kodama M. Sheikh K. Chemically modified collagen: a natural biomaterial for tissue replacement. J Biomed Mater Res. 1987;21:741. doi: 10.1002/jbm.820210606. [DOI] [PubMed] [Google Scholar]
- 10.Rosso F., et al. Smart materials as scaffolds for tissue engineering. J Cell Physiol. 2005;203:465. doi: 10.1002/jcp.20270. [DOI] [PubMed] [Google Scholar]
- 11.Segura T., et al. Crosslinked hyaluronic acid hydrogels: a strategy to functionalize and pattern. Biomaterials. 2005;26:359. doi: 10.1016/j.biomaterials.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 12.Kogan G. Šoltés L. Stern R. Gemeiner P. Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett. 2007;29:17. doi: 10.1007/s10529-006-9219-z. [DOI] [PubMed] [Google Scholar]
- 13.Kuo J. Practical Aspects of Hyaluronan Based Medical Products. Boca Raton: CRC/Taylor & Francis; 2006. [Google Scholar]
- 14.Kawasaki K. Ochi M. Uchio Y. Adachi N. Matsusaki M. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142. doi: 10.1002/(SICI)1097-4652(199905)179:2<142::AID-JCP4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 15.Longaker M.T., et al. Studies in fetal wound healing V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 1991;213:292. doi: 10.1097/00000658-199104000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehlers E.M. Behrens P. Wünsch L. Kühnel W. Russlies M. Effects of hyaluronic acid on the morphology and proliferation of human chondrocytes in primary cell culture. Ann Anat. 2001;183:13. doi: 10.1016/S0940-9602(01)80007-8. [DOI] [PubMed] [Google Scholar]
- 17.Tang S. Vickers S.M. Hsu H.-P. Spector M. Fabrication and characterization of porous hyaluronic acid–collagen composite scaffolds. J Biomed Mater Res A. 2007;82A:323. doi: 10.1002/jbm.a.30974. [DOI] [PubMed] [Google Scholar]
- 18.Chung E.J. Jakus A.E. Shah R.N. In situ forming collagen–hyaluronic acid membrane structures: mechanism of self-assembly and applications in regenerative medicine. Acta Biomater. 2013;9:5153. doi: 10.1016/j.actbio.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi A., et al. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro. J Cell Biol. 1991;113:681. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto N., et al. Smad1 and Smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- 21.Kim C.-S., et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005;26:2501. doi: 10.1016/j.biomaterials.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Karageorgiou V., et al. Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells. J Biomed Mater Res A. 2004;71A:528. doi: 10.1002/jbm.a.30186. [DOI] [PubMed] [Google Scholar]
- 23.Cowan C.M., et al. Bone morphogenetic protein 2 and retinoic acid accelerate in vivo bone formation, osteoclast recruitment, and bone turnover. Tissue Eng. 2005;11:645. doi: 10.1089/ten.2005.11.645. [DOI] [PubMed] [Google Scholar]
- 24.Tsuchida H. Hashimoto J. Crawford E. Manske P. Lou J. Engineered allogeneic mesenchymal stem cells repair femoral segmental defect in rats. J Orthop Res. 2003;21:44. doi: 10.1016/S0736-0266(02)00108-0. [DOI] [PubMed] [Google Scholar]
- 25.Geiger M. Li R.H. Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003;55:1613. doi: 10.1016/j.addr.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Kempen D.H.R., et al. Retention of in vitro and in vivo BMP-2 bioactivities in sustained delivery vehicles for bone tissue engineering. Biomaterials. 2008;29:3245. doi: 10.1016/j.biomaterials.2008.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li C. Vepari C. Jin H.-J. Kim H.J. Kaplan D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 28.Zara J.N., et al. High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A. 2011;17:1389. doi: 10.1089/ten.tea.2010.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shields L.B.E., et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine (Phila Pa 1976) 2006;31:542. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 30.Uludag H. D'Augusta D. Palmer R. Timony G. Wozney J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J Biomed Mater Res. 1999;46:193. doi: 10.1002/(sici)1097-4636(199908)46:2<193::aid-jbm8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Dong X. Wang Q. Wu T. Pan H. Understanding adsorption-desorption dynamics of BMP-2 on hydroxyapatite (001) surface. Biophys J. 2007;93:750. doi: 10.1529/biophysj.106.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luca L., et al. Physical instability, aggregation and conformational changes of recombinant human bone morphogenetic protein-2 (rhBMP-2) Int J Pharm. 2010;391:48. doi: 10.1016/j.ijpharm.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Luca L. Rougemont A.-L. Walpoth B.H. Gurny R. Jordan O. The effects of carrier nature and pH on rhBMP-2-induced ectopic bone formation. J Control Release. 2010;147:38. doi: 10.1016/j.jconrel.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Nishiyama N. Suzuki K. Nagatsuka A. Yokota I. Nemoto K. Dissociation states of collagen functional groups and their effects on the priming efficacy of HEMA bonded to collagen. J Dent Res. 2003;82:257. doi: 10.1177/154405910308200403. [DOI] [PubMed] [Google Scholar]
- 35.Chow L.W., et al. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials. 2011;32:1574. doi: 10.1016/j.biomaterials.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Utesch T. Daminelli G. Mroginski M.A. Molecular dynamics simulations of the adsorption of bone morphogenetic protein-2 on surfaces with medical relevance. Langmuir. 2011;27:13144. doi: 10.1021/la202489w. [DOI] [PubMed] [Google Scholar]
- 37.Mücksch C. Urbassek H.M. Adsorption of BMP-2 on a hydrophobic graphite surface: a molecular dynamics study. Chem Phys Lett. 2011;510:252. doi: 10.1021/la201972f. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.D. Valentini R.F. Retention and activity of BMP-2 in hyaluronic acid-based scaffolds in vitro. J Biomed Mater Res. 2002;59:573. doi: 10.1002/jbm.10011. [DOI] [PubMed] [Google Scholar]
- 39.Jaiswal N. Haynesworth S.E. Caplan A.I. Bruder S.P. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295. [PubMed] [Google Scholar]
- 40.Chung E.J. Sugimoto M.J. Ameer G.A. The role of hydroxyapatite in citric acid-based nanocomposites: surface characteristics, degradation, and osteogenicity in vitro. Acta Biomater. 2011;7:4057. doi: 10.1016/j.actbio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Bruder S.P., et al. Bone regeneration by implantation of purified, culture-expanded human mesenchymal stem cells. J Orthop Res. 1998;16:155. doi: 10.1002/jor.1100160202. [DOI] [PubMed] [Google Scholar]
- 42.Marcus R. Normal and abnormal bone remodeling in man. Annu Rev Med. 1987;38:129. doi: 10.1146/annurev.me.38.020187.001021. [DOI] [PubMed] [Google Scholar]
- 43.DiGirolamo C.M., et al. Propagation, senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate, differentiate. Br J Haematol. 1999;107:275. doi: 10.1046/j.1365-2141.1999.01715.x. [DOI] [PubMed] [Google Scholar]
- 44.Picart C., et al. Buildup mechanism for poly(l-lysine)/hyaluronic acid films onto a solid surface. Langmuir. 2001;17:7414. [Google Scholar]
- 45.Chung Y.-I., et al. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle-hydrogel complex. J Control Release. 2007;121:91. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 46.Ryoo H.M. Lee M.H. Kim Y.J. Critical molecular switches involved in BMP-2-induced osteogenic differentiation of mesenchymal cells. Gene. 2006;366:51. doi: 10.1016/j.gene.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Denhardt D.T. Guo X. Osteopontin: a protein with diverse functions. FASEB J. 1993;7:1475. [PubMed] [Google Scholar]
- 48.Owen T.A., et al. Progressive development of the rat osteoblast phenotype in vitro: reciprocal relationships in expression of genes associated with osteoblast proliferation and differentiation during formation of the bone extracellular matrix. J Cell Physiol. 1990;143:420. doi: 10.1002/jcp.1041430304. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto M. Takahashi Y. Tabata Y. Controlled release by biodegradable hydrogels enhances the ectopic bone formation of bone morphogenetic protein. Biomaterials. 2003;24:4375. doi: 10.1016/s0142-9612(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 50.Wozney J.M. Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Relat Res. 1998;346:26. [PubMed] [Google Scholar]
- 51.Thies R.S., et al. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130:1318. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 52.Yang J. Webb A.R. Pickerill S.J. Hageman G. Ameer G.A. Synthesis and evaluation of poly(diol citrate) biodegradable elastomers. Biomaterials. 2006;27:1889. doi: 10.1016/j.biomaterials.2005.05.106. [DOI] [PubMed] [Google Scholar]
- 53.Fujisato T. Sajiki T. Liu Q. Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold. Biomaterials. 1996;17:155. doi: 10.1016/0142-9612(96)85760-7. [DOI] [PubMed] [Google Scholar]
- 54.Lemperle G. Morhenn V. Charrier U. Human Histology and Persistence of Various Injectable Filler Substances for Soft Tissue Augmentation. Aesthetic Plast Surg. 2003;27:354. doi: 10.1007/s00266-003-3022-1. [DOI] [PubMed] [Google Scholar]
- 55.Jeyanthi R. Panduranga Rao K. In vivo biocompatibility of collagenpoly(hydroxyethyl methacrylate) hydrogels. Biomaterials. 1990;11:238. doi: 10.1016/0142-9612(90)90004-a. [DOI] [PubMed] [Google Scholar]
- 56.Goreish H.H. Lewis A.L. Rose S. Lloyd A.W. The effect of phosphorylcholine-coated materials on the inflammatory response and fibrous capsule formation: in vitro and in vivo observations. J Biomed Mater Res A. 2004;68A:1. doi: 10.1002/jbm.a.10141. [DOI] [PubMed] [Google Scholar]
- 57.Tsumaki N., et al. Bone morphogenetic protein signals are required for cartilage formation and differently regulate joint development during skeletogenesis. J Bone Miner Res. 2002;17:898. doi: 10.1359/jbmr.2002.17.5.898. [DOI] [PubMed] [Google Scholar]
- 58.Davidson E.N.B., et al. Elevated extracellular matrix production, degradation upon bone morphogenetic protein-2 (BMP-2) stimulation point toward a role for BMP-2 in cartilage repair, remodeling. Arthritis Res Ther. 2007;9 doi: 10.1186/ar2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cowan C.M., et al. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007;13:501. doi: 10.1089/ten.2006.0141. [DOI] [PubMed] [Google Scholar]
- 60.Rickard D.J. Sullivan T.A. Shenker B.J. Leboy P.S. Kazhdan I. Induction of rapid osteoblast differentiation in rat bone marrow stromal cell cultures by dexamethasone and BMP-2. Dev Biol. 1994;161:218. doi: 10.1006/dbio.1994.1022. [DOI] [PubMed] [Google Scholar]
- 61.Xiao G., et al. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 Cells. J Bone Miner Res. 2002;17:101. doi: 10.1359/jbmr.2002.17.1.101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.