Abstract
Activation of cytokine receptor-associated Janus kinases (JAKs) mediates most, if not all, of the cellular responses to peptide hormones and cytokines. Consequently, JAKs play a paramount role in homeostasis and immunity. Members of this family of tyrosine kinases control the cytokine/hormone-induced alterations in cell gene expression program. This function is largely mediated through an ability to signal towards activation of the signal transducer and activator of transcription proteins (STAT) as well as towards some other pathways. Importantly, JAKs are also instrumental in tightly controlling the expression of associated cytokine and hormone receptors, and, accordingly, in regulating the cell sensitivity to these cytokines and hormones. This review highlights the enzymatic and non-enzymatic mechanisms of this regulation and discusses the importance of the ambidextrous nature of JAK as a key signaling node that integrates the combining the functions of forward signaling and eliminative signaling. Attention to the latter aspect of JAK function may contribute to emancipating our approaches to the pharmacologic modulation of JAKs.
Keywords: JAK, kinase, cytokine, receptor, downregulation
JAKs as important receptor-associated physiologic regulators
Members of the Janus kinases (JAK) family play a key role in immunity and hormone signaling. These kinases’ function is instrumental in numerous important physiological processes. Tight regulation of JAK-dependent mechanisms is therefore essential for homeostatic integrity of many tissues as well as for their ability to cope with environmental challenges. Both loss and abnormal increase in JAK activity is often responsible for diverse diseases (reviewed in [Costa-Pereira et al., 2011; Kerr et al., 2003; Stark and Darnell, 2012]). Accordingly, tremendous medical significance of JAK is reflected by a growing number of research programs developed to identify and characterize pharmacologic agents that target these kinases [Wilks, 2008].
Tight association of JAKs with cognate cytokine receptors plays a key role in the mechanism of their activation and functions. This characteristic also positions JAKs as a unique group of protein kinases that, at least initially anchored to the cell membrane (unlike cytosolic and nuclear tyrosine kinases such as Src and Abl), yet do not have their own transmembrane domain for the membrane recruitment (unlike the receptor tyrosine kinases such as epidermal growth factor receptor). Four members of this family of tyrosine kinase (JAK1, JAK2, TYK2 and JAK3) have been discovered and extensively characterized [Wagner and Schmidt, 2011]. All these proteins harbor several common protein domains. The interaction of JAKs with the intracellular domain of diverse cytokine receptors is mediated by the four-point-one, ezrin, radixin and moesin (FERM) domain. Additional common motifs include the Src homology domain 2, the pseudokinase domain (initially considered to mediate the serine/threonine kinase activity) and the tyrosine kinase domain (reviewed in [Costa-Pereira et al., 2011; Kerr et al., 2003; Stark and Darnell, 2012; Strobl et al., 2011]). JAKs are often co-translated and subsequently largely remain tightly associated with intracellular domains of the cytokine receptors. This association per se might play an important role in receptor trafficking, presence on the cell surface and function. Specific examples of such regulation are outlined in the subsequent sections.
JAK at the cross-roads of cytokine signaling
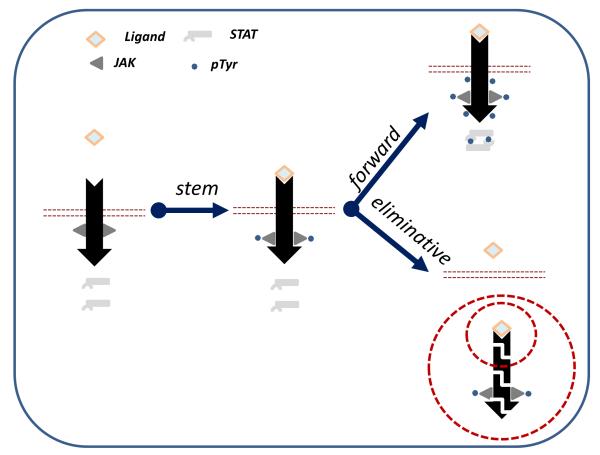
Activation of JAK represents the most proximal and often essential step in the signaling elicited by a given cytokine (Figure 1). JAK activity is required for majority of events that occur upon cytokine receptor activation. A few notable exceptions (often limited to a particular tissue or cell type) include JAK activity-independent activation of mitogen-activated protein kinase by erythropoietin in vascular smooth muscle cells [Ammarguellat et al., 2001], activation of this kinase as well as of Src by prolactin [Sakamoto et al., 2007], stimulation of the recruitment of protein kinase D2 (PKD2) to the interferon receptor [Zheng et al., 2011a], induction of CCL20 chemokine expression by interleukin (IL)-17 [Kao et al., 2005], and others.
Figure 1.
Interaction of a cytokine ligand with the extracellular domains of its cognate receptor initiates the cytokine signaling. Common elements of this signaling (“stem signaling”) is represented by the activation of JAKs resulting in their own phosphorylation. Activated JAKs then signal “forward” to phosphorylate the intracellular domains of the receptor and recruited STAT proteins that become activated and alter the transcriptional program of a cytokine-exposed cell. Concurrently, the “eliminative: signaling mediated by JAKs promotes downregulation of associated receptors often coupled with their proteolytic degradation via lysosomal or/and proteasomal pathway.
Most of the “forward” signaling, by which JAK enables the effects of the cytokines functions, is mediated via tyrosine phosphorylation and ensuing activation of the signal transducer and activator of transcription proteins (STAT). However, diverse STAT-independent effects of JAK activation include phosphorylation and cross-activation of unrelated signaling (e.g., insulin receptor substrate, [Wang et al., 1997]) and activation of additional signaling branches dependent on unrelated kinases (such as PI3K [Kaur et al., 2005], p38 kinase [Katsoulidis et al., 2005], and others) are documented in the literature. Given how many excellent reviews have been dedicated to the “forward” signaling via JAK, we decided not to focus on this topic in this review.
Instead, we wish to highlight and discuss the eliminative functions of JAKs, i.e. the role of these kinases in rapid and irreversible downregulation of cytokine receptors that limits the ability of a cell to respond to the subsequent encounters with a given cytokine (Figure 1, [Fuchs, 2012; Fuchs, 2013b; Huangfu and Fuchs, 2010]). Here we provide specific examples as per how the JAK-activating cytokines (e.g., type 1 interferons) or hormones (e.g., growth hormone) utilize the activation of JAK for decreasing receptor levels and terminating the signal. These examples suggest that JAK activation represents an important step in eliminating the cognate receptors and in ensuing restriction of the magnitude and duration of cytokine-elicited effects.
JAK: the Janus kinase, after all…
Historical excurse into JAKs naming and characterization is abundant on irony [Stark and Darnell, 2012]. Being convinced of importance of tyrosine kinases in diverse biological function, Andrew Wilks carried out a series of elegant, daring and largely successful studies to identify the novel members of this family. Two of cloned genes were named Just Another Kinase (JAK1 and JAK2). Being fond of the abbreviation JAK and having noticed a putative serine/threonine kinase motif in addition to a characteristic tyrosine kinase domain, Wilks proposed that JAK may have both tyrosine and serine/threonine kinase activities. Understandable analogy with a two-faced Roman god Janus shaped the acronym “JAK” to be representative of Janus kinase [Wilks, 2008; Wilks and Oates, 1996].
Years of subsequent biochemical characterization carried out by many investigators (regrettably, too many for each to be properly cited within the space constraints) convincingly demonstrated that all four members of JAK family do not possess an appreciable serine/threonine kinase activity. Yet, the name persisted, and, as we hope to demonstrate in the following section, for a very good reason. Indeed, Janus is the God of Gates and Doors; conversely, Janus kinases as signaling mediators are functioning right at the gateway of cytokine signaling (Figure 1). Janus, the God of beginnings and endings, has two faces to simultaneously look to the future and the past. Very appropriate of this allegory, Janus kinases mediate both positive (STAT activation) and negative regulatory (receptor downregulation) events elicited by cytokines and hormones. Therefore, JAKs should be considered the true Janus kinases in their ability to shape both the starting the cytokine signaling and terminating it by elimination of cytokine receptors and desensitization of the cell to additional ligand exposure (Figure 1).
Role of JAK in eliminative signaling by specific receptors
Type 1 interferon receptor
This receptor is composed by two diverse chains: IFNAR1 associated with TYK2 and IFNAR2 associated with JAK1 (reviewed in [Uze et al., 2007]). Maintenance of the basal levels of IFNAR1 on cell surface in human cells directly depends on its association with TYK2 [Gauzzi et al., 1997], which impedes its ligand-independent constitutive endocytosis [Payelle-Brogard and Pellegrini, 2010; Ragimbeau et al., 2003; Ragimbeau et al., 2001]. When bound to IFNAR1, this kinase masks the linear endocytic motif [Kumar et al., 2008], whose exposure to the cellular endocytic machinery could be further regulated by tyrosine phosphorylation and activity of protein tyrosine phosphatase PTP1B [Carbone et al., 2012]. Mouse IFNAR1 contains a different endocytic motif; as a result, the plasma membrane levels of mouse IFNAR1 do not depend on either TYK2 status [Karaghiosoff et al., 2000] or PTP1B activities [Carbone et al., 2012].
Downregulation of the entire receptor is driven by unmasking of IFNAR1 endocytic motifs mediated by the phosphorylation-dependent ubiquitination of IFNAR1 [Kumar et al., 2004; Kumar et al., 2003]. This ubiquitination facilitated by the β-Trcp E3 ubiquitin ligase accelerates receptor internalization and stimulates its post-internalization trafficking towards the lysosomal degradation [Kumar et al., 2007]. The recruitment of β-Trcp to IFNAR1 relies on IFNAR1 phosphorylation on serine residues within a specific phospho-degron [Kumar et al., 2004]. This phosphorylation (and ensuing IFNAR1 ubiquitination, endocytosis and degradation) could be mediate by cross-eliminative stimuli that do not require JAK activity [Liu et al., 2009a; Liu et al., 2008]. These stimuli include tobacco smoking products [HuangFu et al., 2008], non-ligand cytokines and growth factors [Huangfu et al., 2012; HuangFu et al., 2010; Zheng et al., 2011b], pathogens [Qian et al., 2011], activity of oncogenic proteins [Bhattacharya et al., 2011b], and stress conditions [Bhattacharya et al., 2012; Bhattacharya et al., 2010; Bhattacharya et al., 2011a; Liu et al., 2009b].
Nevertheless, the ligands (i.e. type 1 interferons) elicit a different specific pathway leading to the downregulation of IFNAR1. This pathway is largely dependent on activities of TYK2 and JAK1 [Liu et al., 2008; Marijanovic et al., 2006]. Activated JAKs signal towards IFNAR1 downregulation via stimulating the recruitment of β-Trcp as a result of increased serine phosphorylation within the IFNAR1 phospho-degron [Kumar et al., 2004; Marijanovic et al., 2006]. As neither TYK2 nor JAK1 possess the serine kinase activities, their effect is indirect. In the interferon-stimulated cells, another kinase - PKD2 - is recruited to IFNAR1. This kinase becomes activated as a result of JAK-mediated tyrosine phosphorylation within the plekstrin homology domain of PKD2 [Zheng et al., 2011a]. As a result, activated PKD2 phosphorylates the serines within IFNAR1 degron, stimulates the recruitment of β-Trcp and ensuing ubiquitination and degradation of IFNAR1 as well as attenuation of cellular responses to type 1 interferons [Zheng et al., 2011c].
Erythropoietin receptor
Erythropoietin activates its homodimeric JAK2-associated receptor (EpoR) to and promote erythropoiesis and elicit other important physiological effects such as regulating renal function and plasma volume, angiogenesis and cognitive effects, etc (reviewed in [Constantinescu et al., 1999]). Post-translational mechanisms dominate the regulation of the cell surface EpoR levels [Sinclair et al., 2008], the latter determine responsiveness of cells to Erythropoietin. Since the first demonstration of an essential role of associated JAK2 in Erythropoietin-induced signaling and transcriptional activation [Witthuhn et al., 1993], important studies gained the insight into the relationship between EpoR and JAK2 and their mutual regulation. Although early results were suggestive that ligand promotes the interaction between these two proteins [Miura et al., 1994], subsequent work strongly supports the notion that JAK2 is already associated with EpoR in naïve cells. Evidence for the latter paradigm include demonstrated ability of JAK2 to phosphorylate immature EpoR in the endoplasmic reticulum [Cohen et al., 1997] and important role of associated JAK2 in delivering the de novo synthesized EpoR to the cell surface [Huang et al., 2001].
EpoR homodimers are pre-formed prior to their interaction with the ligands [Constantinescu et al., 2001; Livnah et al., 1999]; the latter trigger activation via inducing a conformational change leading to JAK2 activation [Remy et al., 1999]. This dimerization is required even for enhanced signaling mediated by a constitutively active JAK2V617F oncogene found in patients with diverse myeloproliferative diseases and present in almost all patients with polycythemia vera [Lu et al., 2008]; accordingly, the integrity of the FERM domain in this mutant is essential for its high intrinsic activity [Wernig et al., 2008]. It would be expected that the latter activity should promote receptor elimination. Indeed, EpoR expression is reduced on early erythroblasts in mice expressing constitutively active JAK2V617F [Bumm et al., 2006].
Inactivation of EpoR-associated JAK2 and ensuing termination of erythropoietin-induced signaling is attributed to the effects of tyrosine protein phosphatases including SH-PTP1 [Klingmuller et al., 1995] and PTP1B [Cohen et al., 2004] as well of SOCS proteins that could target JAK2 and/or its association with the receptor [Sasaki et al., 2000]. Other regulators such as Spry1 [Sathyanarayana et al., 2012] or an adaptor protein Lnk also function as negative regulators of JAK2 signaling and downstream effects of erythropoietin [Tong et al., 2005]. It remains to be seen whether these mechanisms directly participate in regulating the EpoR turnover.
A C-terminal truncation in EpoR found in patients with primary familial and congenital polycythemia has been reported [Arcasoy et al., 1999; Forget et al., 2000; Furukawa et al., 1997; Watowich et al., 1999]. This alteration results in an impaired downregulation of EpoR [Sulahian et al., 2009] and sustained JAK2 activation and signaling [Arcasoy et al., 1999; Forget et al., 2000; Furukawa et al., 1997; Watowich et al., 1999]. Given that this mutant lacks the phospho-degron motif needed for the recruitment of β-Trcp E3 ubiquitin ligase, which has been shown to play a key role in EpoR downregulation and degradation [Meyer et al., 2007], it is plausible that sustained activation of JAK2 results from dissociation of its ability to signal forward from JAK2-mediated elimination of EpoR [Verdier et al., 2000; Walrafen et al., 2005]. A dominant nature of this mutation have been reported [Watowich et al., 1999] suggesting that the loss of degron (along with the loss of distal tyrosine residues [Arcasoy and Karayal, 2005] implicated in recruitment of PI3K elements, [Sulahian et al., 2009]) renders this receptor constitutively active.
In line with this possibility, JAK-stimulated ubiquitination of EpoR by β-Trcp may stimulate elimination of receptor via diverse mechanisms including involvement of lysosomal and proteasomal activities [Verdier et al., 2000; Walrafen et al., 2005]. Ubiquitination of EpoR plays a key role not only in receptor elimination but also in its signaling [Bulut et al., 2011]. Whereas a central role of β-Trcp E3 in the elimination of EpoR has been firmly established [Meyer et al., 2007], a potential role of other ubiquitin ligases such as p33RUL cannot be ruled out [Friedman et al., 2003]. The role of JAK2 in the function of these ligases remains to be elucidated.
Thrombopoietin receptor
Thrombopoietin receptor (TpoR) is capable of activating both JAK2 and TYK2. This activation in response to the ligand plays a key role in the differentiation of megakaryocytes, formation of platelets, and renewal of the hematopoietic stem cells (reviewed in [Kaushansky, 2009]). Association of TpoR with both JAK2 and TYK2 ensures a proper plasma membrane localization of the receptor in the absence of the ligand. Whereas, unlike for EpoR, a large fraction of the TpoR is processed to the mature Endo H-resistant form and reaches the cell surface even in the absence of JAK2, this association stabilizes the mature form of the receptors and protects it from internalization and other modes of degradation that may involve proteasomal activity [Royer et al., 2005; Tong et al., 2006].
TpoR is known to be downregulated in cells exposed to the ligand [Sato et al., 1998]. TpoR-dependent activation of JAK2 and TYK2 within the context of thrombopoietin signaling is negatively regulated by the presence of a unique amphipathic motif at the junction between the transmembrane and cytoplasmic domains [Staerk et al., 2006]. Either the role of this domain in receptor stability or the function of JAK2 and TYK2 in the ligand remains to be determined. However, consistent with a hypothesis that JAKs contribute to TpoR elimination, this receptor is robustly downregulated in cells that harbor constitutively active JAK2V617F [Pecquet et al., 2012]. Constitutively active mutant forms of TpoR have been reported in some myeloproliferative neoplasms [Kota et al., 2008; Marty et al., 2009], the exact effect of these mutations on the rate of receptor downregulation remains to be elucidated.
Growth hormone receptor
Growth hormone receptor (GHR) is tightly associated with JAK2 and relies on JAK2 for mediating most, if not all, of its signaling pathways (reviewed in [Frank, 2002; Frank and Fuchs, 2008]). Association of the GHR with JAK2 ensure proper GHR maturation [He et al., 2005; He et al., 2003] and plays a paramount role in enabling the trafficking of de novo synthesized receptor to the cell surface [He et al., 2005; Loesch et al., 2007]. The fate of already matured GHR is also influenced by JAK2 interaction given that the proteolytic turnover rate for this receptor is grossly accelerated in cells lacking JAK2 even in the absence of the ligand [He et al., 2005]. Studies that examined the role of catalytically active JAK2 in the ligand-inducible ubiquitination, endocytosis and degradation of GHR so far paint a rather complicated scenario. GHR is ubiquitinated (and this ubiquitination is stimulated by GHR), however, the role of ubiquitination per se in GHR endocytosis has been disputed based on receptor truncation analysis. Yet a role of several E3 ubiquitin ligases including SOCS, Triad1, CHIP and β-Trcp in GHR downregulation has been suggested [Hassink et al., 2012; Landsman and Waxman, 2005; Slotman et al., 2012; Strous and Gent, 2002; van Kerkhof et al., 2007; van Kerkhof et al., 2011]. JAK2 was proposed to negatively regulate ubiquitin-mediated endocytosis [Putters et al., 2011], however, numerous other studies using pharmacological [Moulin et al., 2003; Saito et al., 1994] or molecular approaches [Deng et al., 2007] strongly suggest that JAK2 activation within the context of growth hormone signaling promotes downregulation of this receptor. Future studies aimed to tease apart constitutive versus ligand-inducible and ubiquitination-driven versus ubiquitination-independent mechanisms should shed the light on the role of JAK2 in elimination of GHR.
Prolactin receptor
Several forms of prolactin receptor (long, intermediate, ΔS1, and two short forms) have been described in mammalian cells (reviewed in [Clevenger and Kline, 2001; Swaminathan et al., 2008a]). The pre-formed homodimer of the long form of this receptor is known to mediate the entire plethora of signaling events triggered by prolactin including the activation of associated JAK2 shown by several groups to represent a key mediator of effects elicited by this hormone [Dusanter-Fourt et al., 1994; Rui et al., 1994]. This long form (known to be stabilized in breast cancer cells [Li et al., 2006]) undergoes constitutive phosphorylation-dependent ubiquitination, endocytosis and lysosomal degradation that is dependent on the glycogen synthase kinase 3β and β-Trcp E3 ubiquitin ligase [Li et al., 2004; Plotnikov et al., 2008; Plotnikov et al., 2009].
Importantly, within the context of prolactin signaling, activation of JAK2 (as well as Src) appears to further stimulate the degradation of prolactin receptor. Among the mechanisms underlying this phenomenon are ubiquitination-stimulated receptor internalization [Swaminathan et al., 2008b], trafficking of already internalized receptor into the lysosomes [Varghese et al., 2008] and proteolytic processing of the receptor at the membrane followed by proteasomal degradation of the fragments [Lu et al., 2005; Piazza et al., 2009]. Intriguingly, a recent possibility of JAK2-mediated cross-talk between prolactin and growth hormone in the regulation of respective receptors has been recently suggested [Xu et al., 2012].
Prolactin represents a major activator of JAK2 in mammary glands. Unlike myeloproliferative diseases featuring constitutively active JAK2 mutants, these types of aberrations are not found in breast cancers. However, constitutively active variants of prolactin receptor that mediate an augmented JAK2 activation in mammary fibroadenomas have been recently identified [Bogorad et al., 2008; Courtillot et al., 2010]. Relative susceptibility of these mutant receptors to basal and ligand-inducible downregulation remains to be elucidated.
Other receptors
Stabilizing effects of JAK1 on cell surface levels of the oncostatin M receptor [Radtke et al., 2002; Radtke et al., 2006] and IL-9Rα, and IL-2Rβ [Malka et al., 2008] have been reported. Similar regulation by JAK3 was proposed for the common γ-chain of IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 receptor complexes [Hofmann et al., 2004]. The ligand-stimulated internalization and degradation of interleukin-7 receptor-α has been demonstrated to depend on the activity of associated JAK3 in T cells [Henriques et al., 2010]. Whereas ubiquitination of the interleukin-10 receptor appears to be constitutive [Jiang et al., 2011], an acceleration of its degradation was shown to be stimulated by the ligand [Wei et al., 2006] suggesting at least some sort of involvement for the JAK-dependent stem signaling.
Gp130 is a common chain for many cytokine receptors such as IL-6 (in combination with IL6R chain) or leukemia inhibitory factor (reviewed in [Silver and Hunter, 2010]. Ligand treatment robustly promotes downregulation of IL6R [Zohlnhofer et al., 1992]. IL6-induced activation of associated JAK2 precedes post-translational modification of gp130 followed by its internalization [Wang and Fuller, 1994] that is dependent on a di-leucine endocytic motif [Dittrich et al., 1996; Dittrich et al., 1994; Doumanov et al., 2006]. A phosphorylation of a serine adjacent to this motif that stimulates its unmasking and subsequent receptor downregulation and degradation can be induced by diverse ligands that utilize gp130 as a component of their receptor [Blanchard et al., 2001; Gibson et al., 2000]. This phosphorylation is mediated by the MK2 kinase that could be activated not only by a ligand (in a manner presumably dependent on JAK activity) but also by numerous stress stimuli that could stimulate MK2 in a JAK-independent manner [Hideshima et al., 2003; Radtke et al., 2010]. Accordingly, JAK activation was shown to be not essential for overall gp130 internalization [Thiel et al., 1998a; Thiel et al., 1998b]. Given that other cytokines (for example, interferon-α [Anthes et al., 1995]) are capable of joining the plethora of stimuli that could cross-eliminate gp130, the experimental assessment of the role of JAK solely within the context of the ligand-dependent signaling appears problematic.
The common beta chain shared by the cognate receptors for interleukin-3, interleukin-5 and the granulocyte-macrophage colony-stimulating factor was shown to be ubiquitinated, internalized and degraded in a manner dependent on activity of associated JAK2 [Martinez-Moczygemba and Huston, 2001] and, perhaps, of JAK1 that is associated with the interleukin-5-specific alpha chain [Martinez-Moczygemba et al., 2007]. Similarly to the JAK-inducible IFNAR1 ubiquitination [Kumar et al., 2007], specific ubiquitin-acceptor sites appear to be responsible for the downregulation of common chain induced by interleukin-5 [Lei et al., 2011].
Medical aspects of JAK-mediated eliminative signaling
Given obvious medical importance of balancing activity of JAK in cells, an intensive search for pharmacologic modulators of these kinases is warranted [Wilks, 2008]. Both approaches to activate and to inhibit JAKs are being considered. Use of hormones/cytokines to stimulate JAK activities and ensuing “forward” signaling has been obviously used within the context of the hormone/cytokine action. Such therapeutic approaches include the use of growth hormone in replacement therapy in deficient patients [Giannoulis et al., 2012], use of erythropoietin to combat anemia [San Miguel and Garcia-Sanz, 1998] as well to protect kidney, liver and neural tissues from injury [Moore et al., 2011; Sargin et al., 2010], use of interferons to treat cancers, chronic viral infections and multiple sclerosis [Borden et al., 2007; Fuchs, 2013a], and many others. Conversely, the inhibitors of JAKs are proposed to be used as immunosuppressants, anti-inflammatory and anti-cancer agents [Borie et al., 2004; O'Neill, 2006; Zhao et al., 2005]. Ongoing clinical trials are examining the efficacy of diverse JAK-targeting agents against psoriasis [Kwatra et al., 2012], rheumatoid arthritis [Cohen and Fleischmann, 2010; Vaddi and Luchi, 2012], and myeloproliferative neoplasias [Stein et al., 2011; Tefferi and Pardanani, 2011].
Understanding the eliminative aspect of JAK’s function adds another dimension to the development and practical use of both activators and inhibitors of these kinases within the context of diverse pathologic conditions. Potential development of potent therapeutic modalities aimed at the eliminative function of JAK might be beneficial for various patient groups. Agents that could promote dissociation of JAK from its cognate receptor may act to desensitize the cell to a given cytokine/hormone and prevent its potentially harmful effect. As both type 1 interferons and TYK2 play a key role in augmenting the lethality during the septic shock [Karaghiosoff et al., 2003], patients suffering from this condition could benefit from agents that promote dissociation of TYK2 from IFNAR1 are expected to inactivate TYK2 and promote the loss of IFNAR1 from the cell surface.
Additional efforts could be expanded towards the agents or their combination that may selectively target either “forward” or “eliminative” signaling outcomes. For example, if inhibitors of the constitutively JAK2V617F are combined with another agent that maintains a high level of degradation of JAK2-associated receptors, the cumulative efficacy of treatment in patients with myeloproliferative diseases could be increased. Future progress in this area of research may lead to development of novel and exciting therapeutic approaches.
Acknowledgments
The authors apologize to those colleagues whose work was not cited due to a lack of space and declare the lack of conflict of interest. Work in the lab of S.Y.F. is supported by NIH/NCI grants CA092900 and CA142425.
REFERENCES
- Ammarguellat F, Llovera M, Kelly PA, Goffin V. Low doses of EPO activate MAP kinases but not JAK2-STAT5 in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2001;284:1031–8. doi: 10.1006/bbrc.2001.5085. [DOI] [PubMed] [Google Scholar]
- Anthes JC, Zhan Z, Gilchrest H, Egan RW, Siegel MI, Billah MM. Interferon-alpha down-regulates the interleukin-6 receptor in a human multiple myeloma cell line, U266. Biochem J. 1995;309:175–80. doi: 10.1042/bj3090175. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcasoy MO, Harris KW, Forget BG. A human erythropoietin receptor gene mutant causing familial erythrocytosis is associated with deregulation of the rates of Jak2 and Stat5 inactivation. Exp Hematol. 1999;27:63–74. doi: 10.1016/s0301-472x(98)00003-4. [DOI] [PubMed] [Google Scholar]
- Arcasoy MO, Karayal AF. Erythropoietin hypersensitivity in primary familial and congenital polycythemia: role of tyrosines Y285 and Y344 in erythropoietin receptor cytoplasmic domain. Biochim Biophys Acta. 2005;1740:17–28. doi: 10.1016/j.bbadis.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S, Huangfu WC, Dong G, Qian J, Baker DP, Karar J, Koumenis C, Diehl JA, Fuchs SY. Anti-tumorigenic effects of Type 1 interferon are subdued by integrated stress responses. Oncogene. 2012 doi: 10.1038/onc.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, HuangFu WC, Liu J, Veeranki S, Baker DP, Koumenis C, Diehl JA, Fuchs SY. Inducible priming phosphorylation promotes ligand-independent degradation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2010;285:2318–25. doi: 10.1074/jbc.M109.071498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Qian J, Tzimas C, Baker DP, Koumenis C, Diehl JA, Fuchs SY. Role of p38 protein kinase in the ligand-independent ubiquitination and down-regulation of the IFNAR1 chain of type I interferon receptor. J Biol Chem. 2011a;286:22069–76. doi: 10.1074/jbc.M111.238766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya S, Zheng H, Tzimas C, Carroll M, Baker DP, Fuchs SY. Bcr-abl signals to desensitize chronic myeloid leukemia cells to IFNalpha via accelerating the degradation of its receptor. Blood. 2011b;118:4179–87. doi: 10.1182/blood-2010-12-325373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard F, Wang Y, Kinzie E, Duplomb L, Godard A, Baumann H. Oncostatin M regulates the synthesis and turnover of gp130, leukemia inhibitory factor receptor alpha, and oncostatin M receptor beta by distinct mechanisms. J Biol Chem. 2001;276:47038–45. doi: 10.1074/jbc.M107971200. [DOI] [PubMed] [Google Scholar]
- Bogorad RL, Courtillot C, Mestayer C, Bernichtein S, Harutyunyan L, Jomain JB, Bachelot A, Kuttenn F, Kelly PA, Goffin V, Touraine P. Identification of a gain-of-function mutation of the prolactin receptor in women with benign breast tumors. Proc Natl Acad Sci U S A. 2008;105:14533–8. doi: 10.1073/pnas.0800685105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borden EC, Sen GC, Uze G, Silverman RH, Ransohoff RM, Foster GR, Stark GR. Interferons at age 50: past, current and future impact on biomedicine. Nat Rev Drug Discov. 2007;6:975–90. doi: 10.1038/nrd2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borie DC, O'Shea JJ, Changelian PS. JAK3 inhibition, a viable new modality of immunosuppression for solid organ transplants. Trends Mol Med. 2004;10:532–41. doi: 10.1016/j.molmed.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Bulut GB, Sulahian R, Ma Y, Chi NW, Huang LJ. Ubiquitination regulates the internalization, endolysosomal sorting, and signaling of the erythropoietin receptor. J Biol Chem. 2011;286:6449–57. doi: 10.1074/jbc.M110.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumm TG, Elsea C, Corbin AS, Loriaux M, Sherbenou D, Wood L, Deininger J, Silver RT, Druker BJ, Deininger MW. Characterization of murine JAK2V617F-positive myeloproliferative disease. Cancer Res. 2006;66:11156–65. doi: 10.1158/0008-5472.CAN-06-2210. [DOI] [PubMed] [Google Scholar]
- Carbone CJ, Zheng H, Bhattacharya S, Lewis JR, Reiter AM, Henthorn P, Zhang ZY, Baker DP, Ukkiramapandian R, Bence KK, Fuchs SY. Protein tyrosine phosphatase 1B is a key regulator of IFNAR1 endocytosis and a target for antiviral therapies. Proc Natl Acad Sci U S A. 2012;109:19226–31. doi: 10.1073/pnas.1211491109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevenger CV, Kline JB. Prolactin receptor signal transduction. Lupus. 2001;10:706–18. doi: 10.1191/096120301717164949. [DOI] [PubMed] [Google Scholar]
- Cohen J, Altaratz H, Zick Y, Klingmuller U, Neumann D. Phosphorylation of erythropoietin receptors in the endoplasmic reticulum by pervanadate-mediated inhibition of tyrosine phosphatases. Biochem J. 1997;327:391–7. doi: 10.1042/bj3270391. Pt 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Oren-Young L, Klingmuller U, Neumann D. Protein tyrosine phosphatase 1B participates in the down-regulation of erythropoietin receptor signalling. Biochem J. 2004;377:517–24. doi: 10.1042/BJ20031420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Fleischmann R. Kinase inhibitors: a new approach to rheumatoid arthritis treatment. Curr Opin Rheumatol. 2010;22:330–5. doi: 10.1097/BOR.0b013e3283378e6f. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Ghaffari S, Lodish HF. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Keren T, Socolovsky M, Nam H, Henis YI, Lodish HF. Ligand-independent oligomerization of cell-surface erythropoietin receptor is mediated by the transmembrane domain. Proc Natl Acad Sci U S A. 2001;98:4379–84. doi: 10.1073/pnas.081069198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Pereira AP, Bonito NA, Seckl MJ. Dysregulation of janus kinases and signal transducers and activators of transcription in cancer. Am J Cancer Res. 2011;1:806–16. [PMC free article] [PubMed] [Google Scholar]
- Courtillot C, Chakhtoura Z, Bogorad R, Genestie C, Bernichtein S, Badachi Y, Janaud G, Akakpo JP, Bachelot A, Kuttenn F, Goffin V, Touraine P. Characterization of two constitutively active prolactin receptor variants in a cohort of 95 women with multiple breast fibroadenomas. J Clin Endocrinol Metab. 2010;95:271–9. doi: 10.1210/jc.2009-1494. [DOI] [PubMed] [Google Scholar]
- Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ. Determinants of growth hormone receptor down-regulation. Mol Endocrinol. 2007;21:1537–51. doi: 10.1210/me.2007-0138. [DOI] [PubMed] [Google Scholar]
- Dittrich E, Haft CR, Muys L, Heinrich PC, Graeve L. A di-leucine motif and an upstream serine in the interleukin-6 (IL-6) signal transducer gp130 mediate ligand-induced endocytosis and down-regulation of the IL-6 receptor. J Biol Chem. 1996;271:5487–94. doi: 10.1074/jbc.271.10.5487. [DOI] [PubMed] [Google Scholar]
- Dittrich E, Rose-John S, Gerhartz C, Mullberg J, Stoyan T, Yasukawa K, Heinrich PC, Graeve L. Identification of a region within the cytoplasmic domain of the interleukin-6 (IL-6) signal transducer gp130 important for ligand-induced endocytosis of the IL-6 receptor. J Biol Chem. 1994;269:19014–20. [PubMed] [Google Scholar]
- Doumanov JA, Daubrawa M, Unden H, Graeve L. Identification of a basolateral sorting signal within the cytoplasmic domain of the interleukin-6 signal transducer gp130. Cell Signal. 2006;18:1140–6. doi: 10.1016/j.cellsig.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Dusanter-Fourt I, Muller O, Ziemiecki A, Mayeux P, Drucker B, Djiane J, Wilks A, Harpur AG, Fischer S, Gisselbrecht S. Identification of JAK protein tyrosine kinases as signaling molecules for prolactin. Functional analysis of prolactin receptor and prolactin-erythropoietin receptor chimera expressed in lymphoid cells. EMBO J. 1994;13:2583–91. doi: 10.1002/j.1460-2075.1994.tb06548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget BG, Degan BA, Arcasoy MO. Familial polycythemia due to truncations of the erythropoietin receptor. Trans Am Clin Climatol Assoc. 2000;111:38–44. discussion 44-5. [PMC free article] [PubMed] [Google Scholar]
- Frank SJ. Receptor dimerization in GH and erythropoietin action--it takes two to tango, but how? Endocrinology. 2002;143:2–10. doi: 10.1210/endo.143.1.8607. [DOI] [PubMed] [Google Scholar]
- Frank SJ, Fuchs SY. Modulation of growth hormone receptor abundance and function: roles for the ubiquitin-proteasome system. Biochim Biophys Acta. 2008;1782:785–94. doi: 10.1016/j.bbadis.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AD, Nimbalkar D, Quelle FW. Erythropoietin receptors associate with a ubiquitin ligase, p33RUL, and require its activity for erythropoietin-induced proliferation. J Biol Chem. 2003;278:26851–61. doi: 10.1074/jbc.M210039200. [DOI] [PubMed] [Google Scholar]
- Fuchs SY. Ubiquitination-mediated regulation of interferon responses. Growth Factors. 2012;30:141–8. doi: 10.3109/08977194.2012.669382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY. Hope and Fear for Interferon: The Receptor-centric Outlook on the Future of Interferon Therapy. J Interferon Cytokine Res. 2013a doi: 10.1089/jir.2012.0117. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs SY. Hope and fear for interferon: the receptor-centric outlook on the future of interferon therapy. J Interferon Cytokine Res. 2013b;33:211–25. doi: 10.1089/jir.2012.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa T, Narita M, Sakaue M, Otsuka T, Kuroha T, Masuko M, Azegami T, Kishi K, Takahashi M, Utsumi J, Koike T, Aizawa Y. Primary familial polycythaemia associated with a novel point mutation in the erythropoietin receptor. Br J Haematol. 1997;99:222–7. doi: 10.1046/j.1365-2141.1997.3583172.x. [DOI] [PubMed] [Google Scholar]
- Gauzzi MC, Barbieri G, Richter MF, Uze G, Ling L, Fellous M, Pellegrini S. The amino-terminal region of Tyk2 sustains the level of interferon alpha receptor 1, a component of the interferon alpha/beta receptor. Proc Natl Acad Sci U S A. 1997;94:11839–44. doi: 10.1073/pnas.94.22.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannoulis MG, Martin FC, Nair KS, Umpleby AM, Sonksen P. Hormone replacement therapy and physical function in healthy older men. Time to talk hormones? Endocr Rev. 2012;33:314–77. doi: 10.1210/er.2012-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson RM, Schiemann WP, Prichard LB, Reno JM, Ericsson LH, Nathanson NM. Phosphorylation of human gp130 at Ser-782 adjacent to the Di-leucine internalization motif. Effects on expression and signaling. J Biol Chem. 2000;275:22574–82. doi: 10.1074/jbc.M907658199. [DOI] [PubMed] [Google Scholar]
- Hassink G, Slotman J, Oorschot V, Van Der Reijden BA, Monteferrario D, Noordermeer SM, Van Kerkhof P, Klumperman J, Strous GJ. Identification of the ubiquitin ligase Triad1 as a regulator of endosomal transport. Biol Open. 2012;1:607–14. doi: 10.1242/bio.2012778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ. JAK2 enhances the stability of the mature GH receptor. Endocrinology. 2005;145:4755–4765. doi: 10.1210/en.2005-0514. [DOI] [PubMed] [Google Scholar]
- He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol. 2003;17:2211–27. doi: 10.1210/me.2003-0256. [DOI] [PubMed] [Google Scholar]
- Henriques CM, Rino J, Nibbs RJ, Graham GJ, Barata JT. IL-7 induces rapid clathrin-mediated internalization and JAK3-dependent degradation of IL-7Ralpha in T cells. Blood. 2010;115:3269–77. doi: 10.1182/blood-2009-10-246876. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Hayashi T, Akiyama M, Mitsiades N, Mitsiades C, Podar K, Munshi NC, Richardson PG, Anderson KC. Proteasome inhibitor PS-341 abrogates IL-6 triggered signaling cascades via caspase-dependent downregulation of gp130 in multiple myeloma. Oncogene. 2003;22:8386–93. doi: 10.1038/sj.onc.1207170. [DOI] [PubMed] [Google Scholar]
- Hofmann SR, Lam AQ, Frank S, Zhou YJ, Ramos HL, Kanno Y, Agnello D, Youle RJ, O'Shea JJ. Jak3-independent trafficking of the common gamma chain receptor subunit: chaperone function of Jaks revisited. Mol Cell Biol. 2004;24:5039–49. doi: 10.1128/MCB.24.11.5039-5049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell. 2001;8:1327–38. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- Huangfu WC, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1:725–34. doi: 10.1177/1947601910382901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuangFu WC, Liu J, Harty RN, Fuchs SY. Cigarette smoking products suppress anti-viral effects of Type I interferon via phosphorylation-dependent downregulation of its receptor. FEBS Lett. 2008;582:3206–10. doi: 10.1016/j.febslet.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu WC, Qian J, Liu C, Liu J, Lokshin AE, Baker DP, Rui H, Fuchs SY. Inflammatory signaling compromises cell responses to interferon alpha. Oncogene. 2012;31:161–72. doi: 10.1038/onc.2011.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HuangFu WC, Qian J, Liu C, Rui H, Fuchs SY. Melanoma cell-secreted soluble factor that stimulates ubiquitination and degradation of the interferon alpha receptor and attenuates its signaling. Pigment Cell Melanoma Res. 2010;23:838–40. doi: 10.1111/j.1755-148x.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Lu Y, Yuan L, Liu J. Regulation of interleukin-10 receptor ubiquitination and stability by beta-TrCP-containing ubiquitin E3 ligase. PLoS One. 2011;6:e27464. doi: 10.1371/journal.pone.0027464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao CY, Huang F, Chen Y, Thai P, Wachi S, Kim C, Tam L, Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J Immunol. 2005;175:6676–85. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M, Neubauer H, Lassnig C, Kovarik P, Schindler H, Pircher H, McCoy B, Bogdan C, Decker T, Brem G, Pfeffer K, Muller M. Partial impairment of cytokine responses in Tyk2-deficient mice. Immunity. 2000;13:549–60. doi: 10.1016/s1074-7613(00)00054-6. [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, Levy D, Decker T, Muller M. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003;4:471–7. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- Katsoulidis E, Li Y, Mears H, Platanias LC. The p38 mitogen-activated protein kinase pathway in interferon signal transduction. J Interferon Cytokine Res. 2005;25:749–56. doi: 10.1089/jir.2005.25.749. [DOI] [PubMed] [Google Scholar]
- Kaur S, Uddin S, Platanias LC. The PI3' kinase pathway in interferon signaling. J Interferon Cytokine Res. 2005;25:780–7. doi: 10.1089/jir.2005.25.780. [DOI] [PubMed] [Google Scholar]
- Kaushansky K. Molecular mechanisms of thrombopoietin signaling. J Thromb Haemost. 2009;7(Suppl 1):235–8. doi: 10.1111/j.1538-7836.2009.03419.x. [DOI] [PubMed] [Google Scholar]
- Kerr IM, Costa-Pereira AP, Lillemeier BF, Strobl B. Of JAKs, STATs, blind watchmakers, jeeps and trains. FEBS Lett. 2003;546:1–5. doi: 10.1016/s0014-5793(03)00411-3. [DOI] [PubMed] [Google Scholar]
- Klingmuller U, Lorenz U, Cantley LC, Neel BG, Lodish HF. Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell. 1995;80:729–38. doi: 10.1016/0092-8674(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Kota J, Caceres N, Constantinescu SN. Aberrant signal transduction pathways in myeloproliferative neoplasms. Leukemia. 2008;22:1828–40. doi: 10.1038/leu.2008.236. [DOI] [PubMed] [Google Scholar]
- Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, Li Y, Baker DP, Peng J, Lukacs GL, Fuchs SY. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179:935–50. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279:46614–20. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003;22:5480–90. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar KG, Varghese B, Banerjee A, Baker DP, Constantinescu SN, Pellegrini S, Fuchs SY. Basal ubiquitin-independent internalization of interferon alpha receptor is prevented by Tyk2-mediated masking of a linear endocytic motif. J Biol Chem. 2008;283:18566–72. doi: 10.1074/jbc.M800991200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwatra SG, Dabade TS, Gustafson CJ, Feldman SR. JAK inhibitors in psoriasis: a promising new treatment modality. J Drugs Dermatol. 2012;11:913–8. [PubMed] [Google Scholar]
- Landsman T, Waxman DJ. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J Biol Chem. 2005;280:37471–80. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- Lei JT, Mazumdar T, Martinez-Moczygemba M. Three lysine residues in the common beta chain of the interleukin-5 receptor are required for Janus kinase (JAK)-dependent receptor ubiquitination, endocytosis, and signaling. J Biol Chem. 2011;286:40091–103. doi: 10.1074/jbc.M111.273482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Clevenger CV, Minkovsky N, Kumar KG, Raghunath PN, Tomaszewski JE, Spiegelman VS, Fuchs SY. Stabilization of prolactin receptor in breast cancer cells. Oncogene. 2006;25:1896–902. doi: 10.1038/sj.onc.1209214. [DOI] [PubMed] [Google Scholar]
- Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY. Negative regulation of prolactin receptor stability and signaling mediated by SCF(beta-TrCP) E3 ubiquitin ligase. Mol Cell Biol. 2004;24:4038–48. doi: 10.1128/MCB.24.9.4038-4048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carvalho LP, Bhattacharya S, Carbone CJ, Kumar KG, Leu NA, Yau PM, Donald RG, Weiss MJ, Baker DP, McLaughlin KJ, Scott P, Fuchs SY. Mammalian casein kinase 1alpha and its leishmanial ortholog regulate stability of IFNAR1 and type I interferon signaling. Mol Cell Biol. 2009a;29:6401–12. doi: 10.1128/MCB.00478-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, HuangFu WC, Kumar KG, Qian J, Casey JP, Hamanaka RB, Grigoriadou C, Aldabe R, Diehl JA, Fuchs SY. Virus-induced unfolded protein response attenuates antiviral defenses via phosphorylation-dependent degradation of the type I interferon receptor. Cell Host Microbe. 2009b;5:72–83. doi: 10.1016/j.chom.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Plotnikov A, Banerjee A, Suresh Kumar KG, Ragimbeau J, Marijanovic Z, Baker DP, Pellegrini S, Fuchs SY. Ligand-independent pathway that controls stability of interferon alpha receptor. Biochem Biophys Res Commun. 2008;367:388–93. doi: 10.1016/j.bbrc.2007.12.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livnah O, Stura EA, Middleton SA, Johnson DL, Jolliffe LK, Wilson IA. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science. 1999;283:987–90. doi: 10.1126/science.283.5404.987. [DOI] [PubMed] [Google Scholar]
- Loesch K, Deng L, Wang X, He K, Jiang J, Frank SJ. Endoplasmic reticulum-associated degradation of growth hormone receptor in Janus kinase 2-deficient cells. Endocrinology. 2007;148:5955–65. doi: 10.1210/en.2007-0455. [DOI] [PubMed] [Google Scholar]
- Lu JC, Piazza TM, Schuler LA. Proteasomes mediate prolactin-induced receptor down-regulation and fragment generation in breast cancer cells. J Biol Chem. 2005;280:33909–16. doi: 10.1074/jbc.M508118200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Huang LJ, Lodish HF. Dimerization by a cytokine receptor is necessary for constitutive activation of JAK2V617F. J Biol Chem. 2008;283:5258–66. doi: 10.1074/jbc.M707125200. [DOI] [PubMed] [Google Scholar]
- Malka Y, Hornakova T, Royer Y, Knoops L, Renauld JC, Constantinescu SN, Henis YI. Ligand-independent homomeric and heteromeric complexes between interleukin-2 or -9 receptor subunits and the gamma chain. J Biol Chem. 2008;283:33569–77. doi: 10.1074/jbc.M803125200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J. 2006;397:31–8. doi: 10.1042/BJ20060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston DP. Proteasomal regulation of betac signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization. J Clin Invest. 2001;108:1797–806. doi: 10.1172/JCI13877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Moczygemba M, Huston DP, Lei JT. JAK kinases control IL-5 receptor ubiquitination, degradation, and internalization. J Leukoc Biol. 2007;81:1137–48. doi: 10.1189/jlb.0706465. [DOI] [PubMed] [Google Scholar]
- Marty C, Chaligne R, Lacout C, Constantinescu SN, Vainchenker W, Villeval JL. Ligand-independent thrombopoietin mutant receptor requires cell surface localization for endogenous activity. J Biol Chem. 2009;284:11781–91. doi: 10.1074/jbc.M808703200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer L, Deau B, Forejtnikova H, Dumenil D, Margottin-Goguet F, Lacombe C, Mayeux P, Verdier F. beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007;109:5215–22. doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- Miura O, Nakamura N, Quelle FW, Witthuhn BA, Ihle JN, Aoki N. Erythropoietin induces association of the JAK2 protein tyrosine kinase with the erythropoietin receptor in vivo. Blood. 1994;84:1501–7. [PubMed] [Google Scholar]
- Moore EM, Bellomo R, Nichol AD. Erythropoietin as a novel brain and kidney protective agent. Anaesth Intensive Care. 2011;39:356–72. doi: 10.1177/0310057X1103900306. [DOI] [PubMed] [Google Scholar]
- Moulin S, Bouzinba-Segard H, Kelly PA, Finidori J. Jak2 and proteasome activities control the availability of cell surface growth hormone receptors during ligand exposure. Cell Signal. 2003;15:47–55. doi: 10.1016/s0898-6568(02)00054-2. [DOI] [PubMed] [Google Scholar]
- O'Neill LA. Targeting signal transduction as a strategy to treat inflammatory diseases. Nat Rev Drug Discov. 2006;5:549–63. doi: 10.1038/nrd2070. [DOI] [PubMed] [Google Scholar]
- Payelle-Brogard B, Pellegrini S. Biochemical monitoring of the early endocytic traffic of the type I interferon receptor. J Interferon Cytokine Res. 2010;30:89–98. doi: 10.1089/jir.2009.0044. [DOI] [PubMed] [Google Scholar]
- Pecquet C, Diaconu CC, Staerk J, Girardot M, Marty C, Royer Y, Defour JP, Dusa A, Besancenot R, Giraudier S, Villeval JL, Knoops L, Courtoy PJ, Vainchenker W, Constantinescu SN. Thrombopoietin receptor down-modulation by JAK2 V617F: restoration of receptor levels by inhibitors of pathologic JAK2 signaling and of proteasomes. Blood. 2012;119:4625–35. doi: 10.1182/blood-2011-08-372524. [DOI] [PubMed] [Google Scholar]
- Piazza TM, Lu JC, Carver KC, Schuler LA. SRC family kinases accelerate prolactin receptor internalization, modulating trafficking and signaling in breast cancer cells. Mol Endocrinol. 2009;23:202–12. doi: 10.1210/me.2008-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikov A, Li Y, Tran TH, Tang W, Palazzo JP, Rui H, Fuchs SY. Oncogene-mediated inhibition of glycogen synthase kinase 3 beta impairs degradation of prolactin receptor. Cancer Res. 2008;68:1354–61. doi: 10.1158/0008-5472.CAN-07-6094. [DOI] [PubMed] [Google Scholar]
- Plotnikov A, Varghese B, Tran TH, Liu C, Rui H, Fuchs SY. Impaired turnover of prolactin receptor contributes to transformation of human breast cells. Cancer Res. 2009;69:3165–72. doi: 10.1158/0008-5472.CAN-08-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putters J, da Silva Almeida AC, van Kerkhof P, van Rossum AG, Gracanin A, Strous GJ. Jak2 is a negative regulator of ubiquitin-dependent endocytosis of the growth hormone receptor. PLoS One. 2011;6:e14676. doi: 10.1371/journal.pone.0014676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian J, Zheng H, Huangfu WC, Liu J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Pathogen recognition receptor signaling accelerates phosphorylation-dependent degradation of IFNAR1. PLoS Pathog. 2011;7:e1002065. doi: 10.1371/journal.ppat.1002065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radtke S, Hermanns HM, Haan C, Schmitz-Van De Leur H, Gascan H, Heinrich PC, Behrmann I. Novel role of Janus kinase 1 in the regulation of oncostatin M receptor surface expression. J Biol Chem. 2002;277:11297–305. doi: 10.1074/jbc.M100822200. [DOI] [PubMed] [Google Scholar]
- Radtke S, Jorissen A, de Leur HS, Heinrich PC, Behrmann I. Three dileucine-like motifs within the interbox1/2 region of the human oncostatin M receptor prevent efficient surface expression in the absence of an associated Janus kinase. J Biol Chem. 2006;281:4024–34. doi: 10.1074/jbc.M511779200. [DOI] [PubMed] [Google Scholar]
- Radtke S, Wuller S, Yang XP, Lippok BE, Mutze B, Mais C, de Leur HS, Bode JG, Gaestel M, Heinrich PC, Behrmann I, Schaper F, Hermanns HM. Cross-regulation of cytokine signalling: pro-inflammatory cytokines restrict IL-6 signalling through receptor internalisation and degradation. J Cell Sci. 2010;123:947–59. doi: 10.1242/jcs.065326. [DOI] [PubMed] [Google Scholar]
- Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. EMBO J. 2003;22:537–47. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragimbeau J, Dondi E, Vasserot A, Romero P, Uze G, Pellegrini S. The receptor interaction region of Tyk2 contains a motif required for its nuclear localization. J Biol Chem. 2001;276:30812–8. doi: 10.1074/jbc.M103559200. [DOI] [PubMed] [Google Scholar]
- Remy I, Wilson IA, Michnick SW. Erythropoietin receptor activation by a ligand-induced conformation change. Science. 1999;283:990–3. doi: 10.1126/science.283.5404.990. [DOI] [PubMed] [Google Scholar]
- Royer Y, Staerk J, Costuleanu M, Courtoy PJ, Constantinescu SN. Janus kinases affect thrombopoietin receptor cell surface localization and stability. J Biol Chem. 2005;280:27251–61. doi: 10.1074/jbc.M501376200. [DOI] [PubMed] [Google Scholar]
- Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269:5364–8. [PubMed] [Google Scholar]
- Saito Y, Teshima R, Yamazaki T, Ikebuchi H, Sawada J. Ligand-induced internalization and phosphorylation-dependent degradation of growth hormone receptor in human IM-9 cells. Mol Cell Endocrinol. 1994;106:67–74. doi: 10.1016/0303-7207(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Creamer BA, Triplett AA, Wagner KU. The Janus kinase 2 is required for expression and nuclear accumulation of cyclin D1 in proliferating mammary epithelial cells. Mol Endocrinol. 2007;21:1877–92. doi: 10.1210/me.2006-0316. [DOI] [PubMed] [Google Scholar]
- San Miguel JF, Garcia-Sanz R. Recombinant human erythropoietin in the anaemia of multiple myeloma and non-Hodgkin's lymphoma. Med Oncol. 1998;15(Suppl 1):S29–34. [PubMed] [Google Scholar]
- Sargin D, Friedrichs H, El-Kordi A, Ehrenreich H. Erythropoietin as neuroprotective and neuroregenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract Res Clin Anaesthesiol. 2010;24:573–94. doi: 10.1016/j.bpa.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Yasukawa H, Shouda T, Kitamura T, Dikic I, Yoshimura A. CIS3/SOCS-3 suppresses erythropoietin (EPO) signaling by binding the EPO receptor and JAK2. J Biol Chem. 2000;275:29338–47. doi: 10.1074/jbc.M003456200. [DOI] [PubMed] [Google Scholar]
- Sathyanarayana P, Dev A, Pradeep A, Ufkin M, Licht JD, Wojchowski DM. Spry1 as a novel regulator of erythropoiesis, EPO/EPOR target, and suppressor of JAK2. Blood. 2012;119:5522–31. doi: 10.1182/blood-2011-11-392571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Fuse A, Niimi H, Fielder PJ, Avraham H. Binding and regulation of thrombopoietin to human megakaryocytes. Br J Haematol. 1998;100:704–11. doi: 10.1046/j.1365-2141.1998.00622.x. [DOI] [PubMed] [Google Scholar]
- Silver JS, Hunter CA. gp130 at the nexus of inflammation, autoimmunity, and cancer. J Leukoc Biol. 2010;88:1145–56. doi: 10.1189/jlb.0410217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair AM, Rogers N, Busse L, Archibeque I, Brown W, Kassner PD, Watson JE, Arnold GE, Nguyen KC, Powers S, Elliott S. Erythropoietin receptor transcription is neither elevated nor predictive of surface expression in human tumour cells. Br J Cancer. 2008;98:1059–67. doi: 10.1038/sj.bjc.6604220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotman JA, da Silva Almeida AC, Hassink GC, van de Ven RH, van Kerkhof P, Kuiken HJ, Strous GJ. Ubc13 and COOH terminus of Hsp70-interacting protein (CHIP) are required for growth hormone receptor endocytosis. J Biol Chem. 2012;287:15533–43. doi: 10.1074/jbc.M111.302521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staerk J, Lacout C, Sato T, Smith SO, Vainchenker W, Constantinescu SN. An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor. Blood. 2006;107:1864–71. doi: 10.1182/blood-2005-06-2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark GR, Darnell JE., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–14. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BL, Crispino JD, Moliterno AR. Janus kinase inhibitors: an update on the progress and promise of targeted therapy in the myeloproliferative neoplasms. Curr Opin Oncol. 2011;23:609–16. doi: 10.1097/CCO.0b013e32834d1b22. [DOI] [PubMed] [Google Scholar]
- Strobl B, Stoiber D, Sexl V, Mueller M. Tyrosine kinase 2 (TYK2) in cytokine signalling and host immunity. Front Biosci. 2011;16:3214–32. doi: 10.2741/3908. [DOI] [PubMed] [Google Scholar]
- Strous GJ, Gent J. Dimerization, ubiquitylation and endocytosis go together in growth hormone receptor function. FEBS Lett. 2002;529:102–9. doi: 10.1016/s0014-5793(02)03187-3. [DOI] [PubMed] [Google Scholar]
- Sulahian R, Cleaver O, Huang LJ. Ligand-induced EpoR internalization is mediated by JAK2 and p85 and is impaired by mutations responsible for primary familial and congenital polycythemia. Blood. 2009;113:5287–97. doi: 10.1182/blood-2008-09-179572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Varghese B, Fuchs SY. Regulation of prolactin receptor levels and activity in breast cancer. J Mammary Gland Biol Neoplasia. 2008a;13:81–91. doi: 10.1007/s10911-008-9068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan G, Varghese B, Thangavel C, Carbone CJ, Plotnikov A, Kumar KG, Jablonski EM, Clevenger CV, Goffin V, Deng L, Frank SJ, Fuchs SY. Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol. 2008b;196:R1–7. doi: 10.1677/JOE-07-0554. [DOI] [PubMed] [Google Scholar]
- Tefferi A, Pardanani A. JAK inhibitors in myeloproliferative neoplasms: rationale, current data and perspective. Blood Rev. 2011;25:229–37. doi: 10.1016/j.blre.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Thiel S, Behrmann I, Dittrich E, Muys L, Tavernier J, Wijdenes J, Heinrich PC, Graeve L. Internalization of the interleukin 6 signal transducer gp130 does not require activation of the Jak/STAT pathway. Biochem J. 1998a;330:47–54. doi: 10.1042/bj3300047. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel S, Dahmen H, Martens A, Muller-Newen G, Schaper F, Heinrich PC, Graeve L. Constitutive internalization and association with adaptor protein-2 of the interleukin-6 signal transducer gp130. FEBS Lett. 1998b;441:231–4. doi: 10.1016/s0014-5793(98)01559-2. [DOI] [PubMed] [Google Scholar]
- Tong W, Sulahian R, Gross AW, Hendon N, Lodish HF, Huang LJ. The membrane-proximal region of the thrombopoietin receptor confers its high surface expression by JAK2-dependent and -independent mechanisms. J Biol Chem. 2006;281:38930–40. doi: 10.1074/jbc.M607524200. [DOI] [PubMed] [Google Scholar]
- Tong W, Zhang J, Lodish HF. Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways. Blood. 2005;105:4604–12. doi: 10.1182/blood-2004-10-4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uze G, Schreiber G, Piehler J, Pellegrini S. The receptor of the type I interferon family. Curr Top Microbiol Immunol. 2007;316:71–95. doi: 10.1007/978-3-540-71329-6_5. [DOI] [PubMed] [Google Scholar]
- Vaddi K, Luchi M. JAK inhibition for the treatment of rheumatoid arthritis: a new era in oral DMARD therapy. Expert Opin Investig Drugs. 2012;21:961–73. doi: 10.1517/13543784.2012.690029. [DOI] [PubMed] [Google Scholar]
- van Kerkhof P, Putters J, Strous GJ. The ubiquitin ligase SCF(betaTrCP) regulates the degradation of the growth hormone receptor. J Biol Chem. 2007;282:20475–83. doi: 10.1074/jbc.M702610200. [DOI] [PubMed] [Google Scholar]
- van Kerkhof P, Westgeest M, Hassink G, Strous GJ. SCF(TrCP) acts in endosomal sorting of the GH receptor. Exp Cell Res. 2011;317:1071–82. doi: 10.1016/j.yexcr.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Varghese B, Barriere H, Carbone CJ, Banerjee A, Swaminathan G, Plotnikov A, Xu P, Peng J, Goffin V, Lukacs GL, Fuchs SY. Polyubiquitination of prolactin receptor stimulates its internalization, postinternalization sorting, and degradation via the lysosomal pathway. Mol Cell Biol. 2008;28:5275–87. doi: 10.1128/MCB.00350-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdier F, Walrafen P, Hubert N, Chretien S, Gisselbrecht S, Lacombe C, Mayeux P. Proteasomes regulate the duration of erythropoietin receptor activation by controlling down-regulation of cell surface receptors. J Biol Chem. 2000;275:18375–81. doi: 10.1074/jbc.275.24.18375. [DOI] [PubMed] [Google Scholar]
- Wagner KU, Schmidt JW. The two faces of Janus kinases and their respective STATs in mammary gland development and cancer. J Carcinog. 2011;10:32. doi: 10.4103/1477-3163.90677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–8. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- Wang HY, Zamorano J, Yoerkie JL, Paul WE, Keegan AD. The IL-4-induced tyrosine phosphorylation of the insulin receptor substrate is dependent on JAK1 expression in human fibrosarcoma cells. J Immunol. 1997;158:1037–40. [PubMed] [Google Scholar]
- Wang Y, Fuller GM. Phosphorylation and internalization of gp130 occur after IL-6 activation of Jak2 kinase in hepatocytes. Mol Biol Cell. 1994;5:819–28. doi: 10.1091/mbc.5.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watowich SS, Xie X, Klingmuller U, Kere J, Lindlof M, Berglund S, de la Chapelle A. Erythropoietin receptor mutations associated with familial erythrocytosis cause hypersensitivity to erythropoietin in the heterozygous state. Blood. 1999;94:2530–2. [PubMed] [Google Scholar]
- Wei SH, Ming-Lum A, Liu Y, Wallach D, Ong CJ, Chung SW, Moore KW, Mui AL. Proteasome-mediated proteolysis of the interleukin-10 receptor is important for signal downregulation. J Interferon Cytokine Res. 2006;26:281–90. doi: 10.1089/jir.2006.26.281. [DOI] [PubMed] [Google Scholar]
- Wernig G, Gonneville JR, Crowley BJ, Rodrigues MS, Reddy MM, Hudon HE, Walz C, Reiter A, Podar K, Royer Y, Constantinescu SN, Tomasson MH, Griffin JD, Gilliland DG, Sattler M. The Jak2V617F oncogene associated with myeloproliferative diseases requires a functional FERM domain for transformation and for expression of the Myc and Pim proto-oncogenes. Blood. 2008;111:3751–9. doi: 10.1182/blood-2007-07-102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilks AF. The JAK kinases: not just another kinase drug discovery target. Semin Cell Dev Biol. 2008;19:319–28. doi: 10.1016/j.semcdb.2008.07.020. [DOI] [PubMed] [Google Scholar]
- Wilks AF, Oates AC. The JAK/STAT pathway. Cancer Surv. 1996;27:139–63. [PubMed] [Google Scholar]
- Witthuhn BA, Quelle FW, Silvennoinen O, Yi T, Tang B, Miura O, Ihle JN. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell. 1993;74:227–36. doi: 10.1016/0092-8674(93)90414-l. [DOI] [PubMed] [Google Scholar]
- Xu J, Sun D, Jiang J, Deng L, Zhang Y, Yu H, Bahl D, Langenheim JF, Chen WY, Fuchs SY, Frank SJ. The Role of Prolactin Receptor in GH Signaling in Breast Cancer Cells. Mol Endocrinol. 2012 doi: 10.1210/me.2012-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZJ, Vainchenker W, Krantz SB, Casadevall N, Constantinescu SN. Role of tyrosine kinases and phosphatases in polycythemia vera. Semin Hematol. 2005;42:221–9. doi: 10.1053/j.seminhematol.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Zheng H, Qian J, Baker DP, Fuchs SY. Tyrosine phosphorylation of protein kinase D2 mediates ligand-inducible elimination of the Type 1 interferon receptor. J Biol Chem. 2011a;286:35733–41. doi: 10.1074/jbc.M111.263608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Qian J, Carbone CJ, Leu NA, Baker DP, Fuchs SY. Vascular endothelial growth factor-induced elimination of the type 1 interferon receptor is required for efficient angiogenesis. Blood. 2011b;118:4003–6. doi: 10.1182/blood-2011-06-359745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Qian J, Varghese B, Baker DP, Fuchs S. Ligand-stimulated downregulation of the alpha interferon receptor: role of protein kinase D2. Mol Cell Biol. 2011c;31:710–20. doi: 10.1128/MCB.01154-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zohlnhofer D, Graeve L, Rose-John S, Schooltink H, Dittrich E, Heinrich PC. The hepatic interleukin-6 receptor. Down-regulation of the interleukin-6 binding subunit (gp80) by its ligand. FEBS Lett. 1992;306:219–22. doi: 10.1016/0014-5793(92)81004-6. [DOI] [PubMed] [Google Scholar]