Abstract
Recent evidence suggests that lifelong bilingualism may contribute to cognitive reserve (CR) in normal aging. However, there is currently no neuroimaging evidence to suggest that lifelong bilinguals can retain normal cognitive functioning in the face of age-related neurodegeneration. Here we explored this issue by comparing white matter (WM) integrity and gray matter (GM) volumetric patterns of older adult lifelong bilinguals (N = 20) and monolinguals (N = 20). The groups were matched on a range of relevant cognitive test scores and on the established CR variables of education, socioeconomic status and intelligence. Participants underwent high-resolution structural imaging for assessment of GM volume and diffusion tensor imaging (DTI) for assessment of WM integrity. Results indicated significantly lower microstructural integrity in the bilingual group in several WM tracts. In particular, compared to their monolingual peers, the bilingual group showed lower fractional anisotropy and/or higher radial diffusivity in the inferior longitudinal fasciculus/inferior fronto-occipital fasciculus bilaterally, the fornix, and multiple portions of the corpus callosum. There were no group differences in GM volume. Our results suggest that lifelong bilingualism contributes to CR against WM integrity declines in aging.
Keywords: cognitive reserve, brain reserve, bilingualism, DTI, aging
Introduction
Aging is associated with neurodegenerative changes that typically lead to cognitive decline. However, there is a great deal of heterogeneity in the relationship between cerebral declines and cognitive functioning in aging, with variability in task performance tending to increase with age (Christensen, et al., 1999). While neurodegenerative changes result in significant cognitive declines in some older adults, others seem to continue to function like young healthy adults (Cabeza, Anderson, Locantore, & McIntosh, 2002; Duarte, Ranganath, Trujillo, & Knight, 2006). Similar heterogeneity between brain burden and cognitive function exists with respect to age-related dementias such as Alzheimer's disease (AD). For example, while the majority of individuals with who meet criteria for pathological AD also meet clinical AD criteria, a significant number remain cognitively normal (Mortimer, 1997; Shaw, et al., 2009; Valenzuela & Sachdev, 2006).
The theory of cognitive reserve arose as an explanation for such mismatch between brain structure and cognitive functioning (Stern, 2002). Cognitive reserve (CR) theory holds that certain variables improve the brain's ability to cope with damage, effectively mitigating its effects on cognition (Stern, 2002, 2009). Three well established CR variables are education, intelligence, and socioeconomic status (SES) (Albert, et al., 1995; Christensen, 2001; Steffener & Stern, 2012). Uncovering other CR variables represents an important step toward maximizing the ability of older adults to live independently. In addition, this line of research has implications for early detection of dementia. Because individuals with high CR present with greater brain burden, typical cognitive screening tests may be insufficient for their early detection. A more complete understanding of the range of effective CR variables may aid early detection.
Recent evidence suggests that lifelong bilinguals may develop clinical AD symptoms at an older age than monolinguals (Bialystok, Craik, & Freedman, 2007; Craik, Bialystok, & Freedman, 2010). Bilingualism carries broad appeal as a potential reserve variable because it is primarily influenced by environmental factors such as country of birth, emigration, or attendance of a second language immersion school (Bialystok & Craik, 2010). In addition, whereas some CR variables like education are typically established as of one's 3rd decade of life, bilingualism can be practiced across one's lifespan. It is the continuous practice/experience of monitoring context for potential language switches and inhibiting the language not under active use that have been suggested as a basis for bilingual advantages in executive control (Abutalebi & Green, 2007; Bialystok & Craik, 2010; Green, 1998).
However, bilingual advantages in executive control are not uniformly observed (Hilchey & Klein, 2011; Paap & Greenberg, 2013). In addition, only one neuroimaging study has provided evidence suggesting that lifelong bilinguals can tolerate more neurodegenerative change than monolinguals at similar levels of cognitive functioning (Schweizer, Ware, Fischer, Craik, & Bialystok, 2012). In this previous computed tomography (CT) study, AD patients who were lifelong bilinguals had a larger width of the temporal horn ratio, suggesting medial temporal lobe (MTL) atrophy, than monolinguals at similar levels of cognitive impairment. While these results are promising, more research is clearly required to determine if lifelong bilingualism contributes to CR.
The case for bilingualism as a CR variable would be strengthened by evidence that bilingual older adults are capable of normal cognitive performance despite neuroimaging profiles typically associated with impaired cognition. Two neuroimaging profiles associated with age-related neurodegeneration involve reduced gray matter (GM) volume and white matter (WM) integrity (Good, et al., 2001; Madden, et al., 2012; Smith, 2012). Volumetric reduction of GM structures has been linked with poorer cognitive performance (Raz, Briggs, Marks, & Acker, 1999). More recently, age-related WM integrity reductions have been linked with poorer performance on a wide range of motor and cognitive tasks (reviewed in Madden, et al., 2012), in part because they reduce the fidelity of signal transmission between GM structures (Bartzokis, et al., 2010).
In the present study, we compared imaging measures of cerebral WM integrity and GM volume between groups of cognitively normal monolingual and bilingual older adults. Following the original approach to the study of CR (Stern, Alexander, Prohovnik, & Mayeux, 1992), we matched groups on relevant demographic and neuropsychological scores prior to imaging analyses. Groups were further matched on the previously established CR variables of education, SES and IQ to limit the influence of these variables on group differences. Analyses of the DTI data were conducted on each of the four main indices of the diffusion tensor (fractional anisotropy, mean diffusivity, radial diffusivity, and axial diffusivity) to provide a detailed comparison of potential WM integrity differences between matched bilingual and monolingual groups.
Methods
Participants
A total of 83 right-handed community dwelling older adults were enrolled in the study. Of these participants, 20 were lifelong bilinguals and the remaining 63 were lifelong monolinguals. Informed consent was obtained from each participant under an approved University of Kentucky Institutional Review Board protocol. Exclusionary criteria for the study included the following: a major head injury and/or concussion, stroke, a neurological or psychiatric disorder, high blood pressure, hypercholesterolemia, diabetes, heart disease, the use of psychotropic medications, or the presence of metal fragments and/or metallic implants contraindicated for MRI.
Language status was determined via a detailed questionnaire about language history similar to that used in previous research in this area (Bialystok, Craik, & Ryan, 2006). The questionnaire included items about age and place of language acquisition, and a chart regarding proficiency of each language compared to a native speaker. Lifelong bilinguals had to have been speaking English and another language on a daily basis since the age of 10 years old or younger and had to rate themselves as completely proficient in their two languages (English proficiency was also assessed objectively as described below). Following previous work in this area (Bialystok & Craik, 2010), lifelong bilinguals spoke English and a variety of second languages, whereas lifelong monolinguals spoke only English and had no significant exposure to a second language. The specific non-English languages spoken by lifelong bilinguals in the present study were: African languages (Igbo, Swahili), Filipino, French, German, Indian languages (Gujarati, Hindi, Konkani), Spanish.
Following the original methodological approach to the study of CR (Stern, et al., 1992), a sub-group of 20 monolinguals were matched with the group of 20 bilinguals for sex ratio, education level and scores on a range of other cognitive measures described below (Table 1).
Table 1.
Group means, standard deviations (in brackets), and P-values for demographic and neuropsychological scores.
| Monolingual | Bilingual | P-Values | |
|---|---|---|---|
| Age | 64.4 (5.1) | 63.9 (4.0) | 0.76 |
| Sex (M/F) | 10/10 | 10/10 | 0.99 |
| Education | 17.5 (2.6) | 17.4 (2.2) | 0.97 |
| ISP | 21.6 (7.0) | 19.7 (8.7) | 0.44 |
| Cattell IQ score | 124.6 (20.2) | 127.6 (22.5) | 0.66 |
| MMSE | 28.2 (1.6)17 | 27.8 (1.2)16 | 0.39 |
| Vocabulary (PPVT) | 110.1 (13.9) | 106.3 (18.5) | 0.47 |
| Digits Span Forward | 10.6 (2.0) | 9.7 (2.1) | 0.23 |
| Digits Span Backward | 6.6 (1.8) | 6.6 (2.5) | 0.97 |
| Spatial Span Forward | 10.7 (1.6)17 | 10.2 (1.5)16 | 0.36 |
| Spatial Span Backward | 7.6 (1.4)17 | 7.2 (1.3)16 | 0.48 |
| Logical Memory I | 45.4 (4.7)17 | 44.3 (3.8)16 | 0.44 |
| Logical Memory II | 28.2 (5.8)17 | 26.8 (4.3)16 | 0.45 |
| Task Switching (RT) | 179 msec | 114 msec | 0.04 |
| Task Switching (% Errors) | 3.4 (3.5) | 2.6 (2.6) | 0.41 |
Notes. ISP, Index of Social Position; MMSE, Mini-Mental State Exam; PPVT, Peabody Picture Vocabulary Test. If score values were missing, the number of participants used in the comparison is shown as subscript.
Cognitive and Demographic Measures
The Mini-Mental State Exam (MMSE) is a 30-point test used to screen for cognitive impairment (Folstein, Folstein, & McHugh, 1975). It assesses orientation to time and place as well as basic memory functions.
The Peabody Picture Vocabulary Test (PPVT-III) is a culture-fair measure used to assess proficiency in English (Dunn, 1997). There are a total of 204 test items. Test items are presented on pages containing four black-and-white pictures. Participants were read a word and were asked to choose the picture on that page that best corresponds to the word.
The Hollingshead Two-Factor Index of Social Position (ISP) was used as a measure of socioeconomic states (SES) (Hollingshead, 1958). The ISP is based on an individual's occupation and highest level of formal education. It is calculated by assigning numeric values, from 1–7, to an individual's occupation and education. Scores are then weighted by multiplying by 7 (occupation) and 4 (education). Values are then summed to produce a social index. Lower values represent higher earning occupations and more years of education.
The Cattell Culture Fair (CCF) Intelligence Test (Cattell & Cattell, 1960). The CCF is a test of fluid intelligence that is not influenced by cultural background or verbal ability. The CCF (Scale 3) consists of 50 items and assesses inductive reasoning about relationships in shapes and figures.
The Digits Span Subtests of the Wechsler Memory Scale (WMS III) (Wechsler, 1997). The Digit Span tests assess verbal memory. Participants were read digit lists aloud and were instructed to repeat each set of digits verbally in the same order (digit forward; DF) and in reverse order (digit backward; DB). In both conditions participants received two trials. Standard termination procedures were followed and the totals for the DF and DB sets were based on the number of trials that were accurately reported in the correct order.
The Spatial Span Subtests of the WMS III (Wechsler, 1997). The Spatial Span tests assess spatial memory. Participants viewed the examiner touch blocks in a specific order and were instructed to touch the same blocks in the same order (spatial forward; SF) and in reverse order (spatial backward; SB). In both conditions participants received two trials. Standard termination procedures were followed and the totals for the SF and SB sets were based on the number of trials that were accurately completed in the correct order.
The Logical Memory Subtests of the WMS III (Wechsler, 1997). The Logical Memory I test assesses the ability to remember information immediately after oral presentation and the Logical Memory II assesses the ability to remember information after a 25-35 minute delay. Standard scoring rules were applied.
Task Switching
The color-shape task switching paradigm assesses the executive functions of attention switching and inhibitory control. This paradigm was administered as part of a previous fMRI experiment described in detail elsewhere (Gold, Kim, Johnson, Kryscio, & Smith, 2013). The stimuli consisted of two possible shapes (circle or square), in one of two possible colors (red or blue), presented in the center of the computer screen. Tasks were indicated via cue words. In shape blocks, participants decided if a stimulus was a circle or square. In color blocks, participants decided if a stimulus was red or blue. In switch blocks, participants alternated between shape and color decisions. Participants indicated their responses via a left or right button press. Switch costs were computed by subtracting each subject's mean (RT or percentage of errors) in the non-switch condition from their mean (RT or percentage of errors) in the switch condition.
Imaging Data Acquisition
Imaging data were collected on a 3 Tesla Siemens TIM scanner at the Magnetic Resonance Imaging and Spectroscopy Center of University of Kentucky. High-resolution, 3D anatomic images were acquired using an MP-RAGE sequence [repetition time (TR) = 2100 ms, echo time (TE) = 2.93 ms, flip angle (FA) = 12°, 1 mm isotropic voxels]. Diffusion tensor imaging used a double spin echo EPI sequence (TR = 6900 ms, TE = 105 ms, FA = 90°, FOV = 224 mm2, in-plane resolution = 1.75 × 1.75 mm voxels, 40 contiguous 3-mm thick axial slices). The DTI images were acquired with 36 non-collinear encoding directions (b = 1000 s/mm2), and five images were acquired without diffusion weighting (b = 0 s/mm2, b0). A B0 field map sequence for subsequent geometric unwarping of DTI images. The field map images were collected using a double-echo EPI sequence (TE1 = 5.19 ms, TE2 = 7.65 ms).
Diffusion Tensor Imaging Analyses
Diffusion tensor imaging (DTI) data were preprocessed and analyzed using the Functional MRI of the Brain (FMRIB) software library (FSL v4.1.5). Each diffusion-weighted volume was corrected for motion and residual eddy current distortion using a 12-parameter affine alignment to the corresponding b0 image, via FMRIB's Linear Image Registration Tool (FLIRT: http://www.fmrib.ox.ac.uk/fsl). Images were then corrected for static field inhomogeneity distortions using B0 field maps. Brain masks were then generated from the b0 images using FMRIB's brain extraction tool (BET v2.1) to exclude non-brain voxels from all subsequent processing (S. M. Smith, et al., 2006). Next, FMRIB's Diffusion Toolbox (FDT v2.0) was used to fit the diffusion tensor and calculate eigenvalues, fractional anisotropy (FA), axial diffusivity (DA), radial diffusivity (DR), and mean diffusivity (MD).
Registration of FA images into MNI152 space and subsequent voxelwise analyses followed a series of procedures known as Tract-Based Spatial Statistics [TBSS v1.2; (Smith et al., 2006)], as described in detail in our previous work (Johnson, Kim, Clasey, Bailey, & Gold, 2012). Briefly, the initial step in this process was to remove likely outliers from the fitted tensor by eroding brain edge artifacts and zeroing the end slices. Next, all participants' FA images were aligned to the FMRIB58_FA_1mm template using a nonlinear registration approach based on free-form deformations and B-Splines (Rueckert, et al., 1999). The FA images were then affine registered and resampled to 1×1×1 mm MNI152 space. Transformations derived from the FA maps were then applied to the other diffusivity maps (DR, DA and MD) for matched processing of all image volumes.
All MNI-transformed FA images were then averaged to create a mean FA image used to generate a common WM tract skeleton. An FA value of 0.2 was used to threshold the skeleton in order to minimize partial voluming effects after warping across subjects. Next, each participant's spatially normalized FA image was projected onto the FA skeleton in order to account for residual misalignments between participants after the initial nonlinear registration. Finally, each subject's DR, DA and MD maps in MNI space were projected onto the common tract skeleton, using the pipeline for non-FA data provided by TBSS, which employs the projection vectors from each individual's FA-to-skeleton transformation (Smith et al., 2006).
Voxelwise statistical analyses were performed via a permutation-based inference for nonparametric statistical thresholding using FSL's “randomise”. Between group comparisons of FA/MD/DR/DA values within the tract skeleton were tested using a two-sample t-test. A permutation nonparametric test (using the maximum number of 5000 permutations) was employed using a threshold-free cluster enhancement (TFCE). Results were then thresholded at P < 0.05 (corrected for multiple comparisons). Anatomical locations of significant clusters were detected using the Johns Hopkins University WM tractography atlas and the International Consortium of Brain Mapping-DTI white matter labels atlas.
Structural Volumetric Analyses
Voxel-based morphometry (VBM; http://www.fil.ion.ucl.ac.uk/spm) was performed using SPM8, as described in detail in our recent work (Gold, Jiang, Jicha, & Smith, 2010). Briefly, preprocessing of images included segmentation, bias correction, and spatial normalization, incorporated into a single generative model using SPM prior probability templates (Ashburner & Friston, 2005). Gray matter (GM) images were normalized to their own custom templates in MNI space using a set of non-linear basis functions. A modulation step was also incorporated into the preprocessing model in order to explore regional differences in absolute volume. Intracranial volume (ICV) was estimated as the sum of GM, WM, and cerebrospinal fluid (CSF) volume for each participant (for use as a nuisance covariate in statistical analysis). Normalized, modulated GM images were smoothed using an 8 mm FWHM isotropic Gaussian kernel.
The preprocessed GM data were analyzed within the framework of the GLM. Statistical parametric maps of between-group differences in GM volumes were determined using a full-factorial model (a two-sample t-test) with unequal variance and ICV as a nuisance covariate. Second-level, group linear contrasts were then conducted on parameter estimates from the model. The voxel values for the contrasts constituted a statistical parametric map (SPM) of the t statistic. Differences between groups were assessed using a statistical threshold of P < 0.05 (FWE corrected for multiple comparisons).
Results
DTI Data
Figure 1 presents the results from the group comparisons of FA. The anatomic underlay is the MNI152 standard brain. The bilingual group had lower FA (shown in red) compared to the monolingual group in a number of regions, including the inferior longitudinal fasciculus/inferior fronto-occipital fasciculus (ILF/IFOF), the fornix and multiple portions of the corpus callosum. There were no regions in which the monolingual group showed lower FA than the bilingual group.
Figure 1.
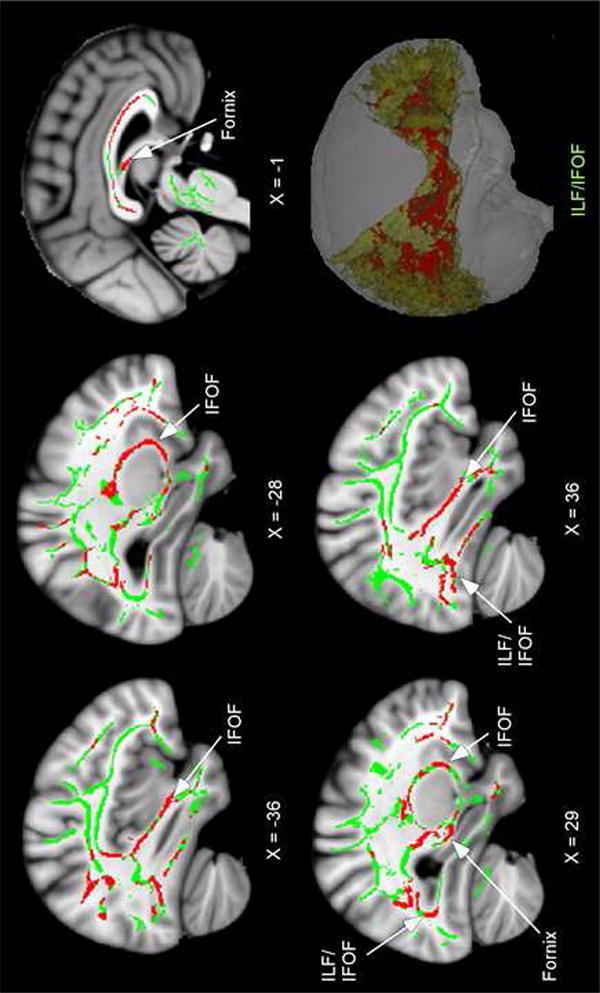
Lower FA in lifelong bilinguals compared to monolinguals. The anatomic underlay is the MNI152 T1-weighted 1mm brain. The registered average FA skeleton is represented in green. Regions of lower FA in the lifelong bilinguals are displayed in red. The numbers below sagittal sections represent x coordinates in MNI space. The bottom right image shows a 3-D rendering of the FSL mask of the ILF/IFOF tract (greenish-yellow) in order to visualize the widespread effects of lower FA (in red) in this tract. Note: ILF; inferior longitudinal fasciculus, IFOF; inferior fronto-occipital fasciculus.
Figure 2 presents the group comparisons of radial diffusivity (DR). The bilingual group showed several regions of increased DR relative to the monolingual group. Figure 2 shows regions of higher DR in the bilingual group in addition to regions of lower FA (from Figure 1), to visualize FA/DR overlap (orange), regions of selectively higher DR in the bilingual group that do not overlap with lower FA (blue), and regions of lower FA in the bilingual group in which selective DR alterations were not observed (red). The majority of regions of higher DR in the bilingual group overlapped with regions in which they showed lower FA. Regions of higher DR and lower FA in the bilingual group included the IFOF and corpus callosum, as well as smaller parietal and occipital tracts.
Figure 2.
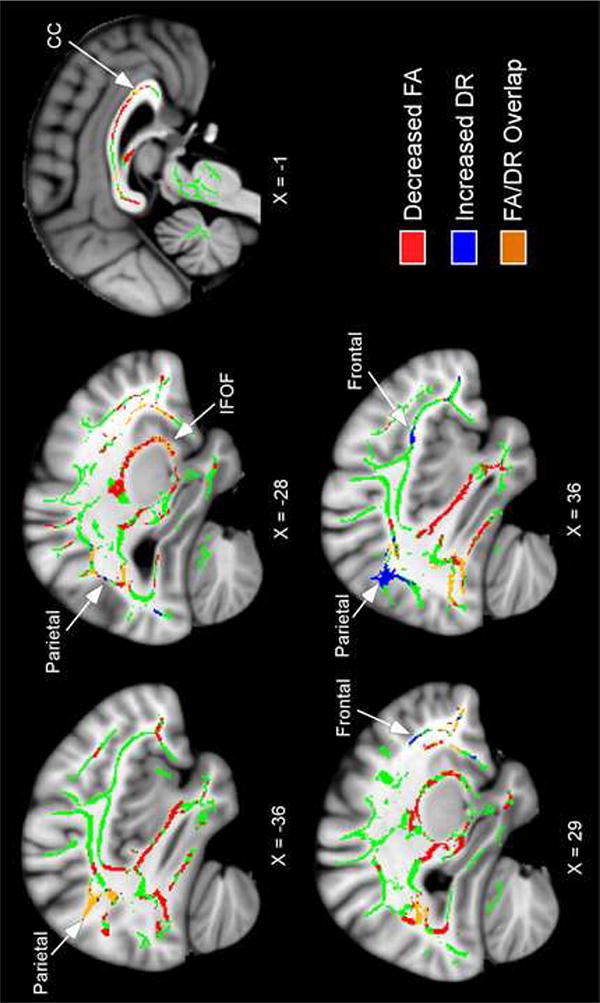
Higher DR in lifelong bilinguals compared to monolinguals. The anatomic underlay, registered average FA skeleton and coordinate numbers are described in the Figure 1 legend. Both regions of higher DR and lower FA in the bilingual group are shown to visualize their overlap (orange). Regions of selectively higher DR in the bilingual group that do not overlap with lower FA are illustrated in blue, and regions of lower FA in the bilingual group in which no DR alterations were observed are illustrated in red. Note: IFOF; inferior fronto-occipital fasciculus, CC; corpus callosum.
The bilingual group only showed a few regions of higher DR that did not overlap with lower FA, which were observed in WM of the parietal and frontal lobes. In contrast, there were no regions in which the monolingual group showed higher DR compared to the bilingual group. In addition, there were no group differences in either direction in axial diffusivity (DA) or mean diffusivity (MD).
VBM Data
There were no voxelwise differences in GM volume between the bilingual and monolingual groups in either direction. Even when a liberal uncorrected significance level (P < 0.001, cluster 50) was employed, no group differences in GM volume between language groups were observed.
Discussion
Our results add to a growing literature suggesting that lifelong bilingualism may contribute to cognitive reserve (CR) in aging. At similar levels of cognitive functioning, seniors who were lifelong bilinguals showed significantly lower cerebral white matter (WM) integrity compared to their monolingual peers. Strengths of our study include rigorous between-group matching on a range of cognitive test scores, control for the well-established CR variables of education, SES, and IQ, and exploration of each of the four main components of the diffusion tensor.
The most prominent DTI-based group difference observed was lower fractional anisotropy (FA) in the bilingual group in the inferior longitudinal fasciculus/inferior fronto-occipital fasciulus (ILF/IFOF). In addition, the bilingual group also showed lower FA than the monolingual group in the fornix, and in multiple portions of the corpus callosum. Several of these tracts contain fibers with connections to MTL structures such as the hippocampus. For example, the fornix serves as a major source of cholinergic projections between basal frontal structures and the hippocampus. In addition, reductions observed in posterior portions of the ILF/IFOF showed strong linear spatial characteristics consistent with the known trajectory of a group of fibers connecting the amygdala/hippocampal head region and the ventral visual association cortex (Smith, et al., 2009).
Interestingly, the regions showing lower FA in the bilingual group form part of the brain's memory circuitry, and represent a subset of tracts that are affected in Alzheimer's disease (Stebbins & Murphy, 2009). In particular, the observed pattern of lower FA in the ILF/IFOF, fornix, and splenium of the corpus callosum in the bilingual group has also been observed in studies of Alzheimer's disease and MCI (Stebbins & Murphy, 2009). The preservation of cognitive functioning in bilinguals, despite lower WM integrity in a subset of tracts prominently affected in AD, is consistent with data suggesting that lifelong bilinguals may develop clinical AD symptoms at an older age than monolinguals (Bialystok, et al., 2007; Craik, et al., 2010).
The lifelong bilingual group also showed higher radial diffusivity (DR) than the monolingual group in several WM tracts, most of which overlapped regions of lower FA in the lifelong bilingual group. The pattern of lower FA and higher DR is thought to reflect reduced myelin, small vessel alterations, and reductions in axonal structure/coherence (Bronge, Bogdanovic, & Wahlund, 2002; Moseley, 2002). In contrast, no between group differences were observed in axial diffusivity (DA) or mean diffusivity (MD) indices in which alterations are thought to reflect loss of neurons and glia (Bronge, et al., 2002; Moseley, 2002). Similarly, there were no group differences in MRI-based GM volume, a measure on which declines reflect advanced stages of neurodegeneration including the loss of neurons, synapses and glia (Bobinski, et al., 2000). The overall pattern of findings is suggestive of moderate neurodegeneration in the bilingual group.
Our results are consistent with those of two other recent neuroimaging studies suggesting that lifelong bilingualism contributes to CR (Luk, Bialystok, Craik, & Grady, 2011; Schweizer, et al., 2012). However, each of these studies yielded different results in terms of specific brain structure differences between bilingual and monolingual groups. The study by Luk et al. (2011) reported higher WM integrity in cognitively normal bilinguals than cognitive normal monolinguals. The study by Schweizer et al. (2012) found that AD patients who were lifelong bilinguals had more MTL atrophy than monolinguals at similar levels of cognitive impairment. Finally, we found lower WM integrity in cognitively normal bilinguals than cognitive normal monolinguals in the absence of volumetric atrophy differences.
The differing brain structure results of these studies may not be as contradictory as they first appear when considering that cross-sectional designs provide a snapshot of participants' neurocognitive profile at one point in time and do not indicate how these profiles have changed over time. It is likely that early acquisition and usage of a second language has positive effects on brain structure and function (Abutalebi, et al., 2012; Della Rosa, et al., 2013; Mechelli, et al., 2004). However, whether developmental structural and/or functional gains associated with bilingualism are evident at a given time point in older adulthood is likely to depend upon multiple factors, importantly including the extent of intervening neuropathological accumulation. Cognitively normal older adults vary considerably in neuropathological burden, with a significant number meeting criteria for pathological AD (Shaw, et al., 2009; Valenzuela & Sachdev, 2006).
It is thus possible that the different direction of effects between our results and those of Luk et al. (2011) could in part reflect a higher incidence of preclinical AD in our bilingual participants. This possibility would appear consistent with the specific WM tracts showing reduced integrity in our bilingual participants. As noted above, our bilinguals showed reduced integrity of a subset of tracts that are affected in early stage AD. Of particular relevance is the specific pattern of decreased FA and increased DR in the ILF/IFOF of our bilingual group. This specific pattern in the IFOF has been reported in multiple studies of cognitively normal seniors at high risk for future AD based on genetics and/or family history (reviewed in Gold, Johnson, Powell, & Smith, 2012). In addition, the pattern of decreased FA and increased DR is a prominent finding in aMCI (Bosch, et al., 2012).
Additional support for the possibility of interactions between preclinical dementia and CR comes from a series of studies showing that the direction of correlations between education and brain structure (i.e. positive or negative) depended upon the presence/absence of a biomarker for AD. In an initial study, older adults with higher education were found to have lower WM integrity than a matched group with lower education (Arenaza-Urquijo, et al., 2011). However, subsequent studies indicated that in individuals without an AD biomarker (assessed via levels of amyloid β in cerebrospinal fluid), higher education was associated with higher WM integrity (Arenaza-Urquijo, Landeau, et al., 2013). In contrast, in individuals with an AD biomarker, higher education was associated with lower WM integrity (Arenaza-Urquijo, Molinuevo, et al., 2013). Future research on bilingualism as a potential CR variable should explore this issue directly by collecting CSF or amyloid imaging data.
The more advanced neurodegenerative changes in bilingual participants of the Schweizer et al., (2012) study compared to those in our study (i.e. MTL atrophy) may also be due in part to differences in neuropathological burden (i.e. greater neuropathology in their AD participants than our cognitively normal participants). Results from the Schweizer et al., (2012) study suggest that lifelong bilinguals appear capable of better cognitive performance than expected by their degree of MTL atrophy in clinical AD. Our findings suggest that lifelong bilinguals appear capable of normal cognitive performance despite moderate neurodegeneration (i.e., microscopic WM changes in the absence of gross tissue loss). Our findings thus appear broadly consistent with those of Schweizer et al., (2012), with the more moderate neurodegeneration in our bilingual participants being consistent with their continued cognitive performance with the normal range.
The present results, when considered in the context of our recent fMRI study with the same participants, suggest that our bilingual participants may be circumventing WM integrity reductions in some tracts through flexible, efficient use of intact executive networks. In our recent fMRI study, we found that older bilinguals switched between tasks faster than monolinguals while showing lower BOLD response in several frontal ‘executive regions’ (Gold, et al., 2013). The lateral frontal regions showing ‘more efficient’ response in bilinguals are in part connected with parietal and subcortical portions of the ‘executive control network’ via WM tracts such as the superior longitudinal fasciculus and the anterior limb of the internal capsule. These WM tracts were largely preserved in our bilingual group. It is possible that our bilingual participants could be using intact executive networks to compensate for damage to other networks, such as MTL-related memory systems. This would be consistent with a view that flexible, efficient use of executive systems may be a key mechanism through which CR could operate (Stern, 2009).
As noted above, the present study has several caveats that motivate future work in this field. First, the cross-sectional design used in this study is unable to provide definitive information on cause-and-effect relationships between lifelong bilingualism and WM integrity. A related caveat common to cross-sectional studies is that results could have been influenced by factors other than the cognitive variable of interest alone (in this case language experience). Although groups were carefully matched on demographic characteristics, neuropsychological scores, and scores on several other potential CR variables, it is possible that they differed in some other unknown way for which we could not adjust or control. The present study also included only extreme groups (i.e. lifelong bilinguals and monolinguals), which precluded correlation analyses. An important topic for future research will be to determine if degree of bilingual experience correlates with cognition and brain structure in older adults. Finally, both our monolingual and bilingual groups had relatively high mean IQs. Future work will be required to determine if our findings generalize to language groups with average IQs.
In conclusion, our results suggest that lifelong bilingualism contributes to CR against neurodegenerative declines in normal aging. Older adults who are lifelong bilinguals appear to be capable of similar levels of cognitive functioning as their monolingual peers despite significantly lower WM integrity. Our findings should help stimulate further research on the neural bases of understudied CR variables such as bilingualism, toward an ultimate goal of fostering lifestyle variables that maximize the ability of older adults to live independently.
Highlights.
We explored whether lifelong bilingualism contributes to cognitive reserve.
Bilingual and monolingual older adults participated.
Groups were matched on demographic variables and cognitive test scores.
Bilinguals had lower white matter integrity than monolinguals.
Lifelong bilingualism appears to contribute to cognitive reserve.
Acknowledgments
This study was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG033036 and the National Science Foundation under award number BCS 0814302. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting agencies. We thank Sara Cilles and Quenio Ferreira for their assistance in recruiting participants. In addition, we wish to thank our study volunteers for their participation in this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abutalebi J, Della Rosa PA, Green DW, Hernandez M, Scifo P, Keim R, Cappa SF, Costa A. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cereb Cortex. 2012;22:2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Abutalebi J, Green DW. Bilingual language production: the neurocognition of language representation and control. Journal of Neurolinguistics. 2007;20:242–275. [Google Scholar]
- Albert MS, Jones K, Savage CR, Berkman L, Seeman T, Blazer D, Rowe JW. Predictors of cognitive change in older persons: MacArthur studies of successfulaging. Psychol Aging. 1995;10:578–589. doi: 10.1037//0882-7974.10.4.578. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Bosch B, Sala-Llonch R, Sole-Padulles C, Junque C, Fernandez-Espejo D, Bargallo N, Rami L, Molinuevo JL, Bartres-Faz D. Specific anatomic associations between white matter integrity and cognitive reserve in normal and cognitively impaired elders. Am J Geriatr Psychiatry. 2011;19:33–42. doi: 10.1097/JGP.0b013e3181e448e1. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Landeau B, La Joie R, Mevel K, Mezenge F, Perrotin A, Desgranges B, Bartres-Faz D, Eustache F, Chetelat G. Relationships between years of education and gray matter volume, metabolism and functional connectivity in healthy elders. Neuroimage. 2013;83C:450–457. doi: 10.1016/j.neuroimage.2013.06.053. [DOI] [PubMed] [Google Scholar]
- Arenaza-Urquijo EM, Molinuevo JL, Sala-Llonch R, Sole-Padulles C, Balasa M, Bosch B, Olives J, Antonell A, Llado A, Sanchez-Valle R, Rami L, Bartres-Faz D. Cognitive reserve proxies relate to gray matter loss in cognitively healthy elderly with abnormal cerebrospinal fluid amyloid-beta levels. J Alzheimers Dis. 2013;35:715–726. doi: 10.3233/JAD-121906. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Lu PH, Tingus K, Mendez MF, Richard A, Peters DG, Oluwadara B, Barrall KA, Finn JP, Villablanca P, Thompson PM, Mintz J. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialystok E, Craik FI, Freedman M. Bilingualism as a protection against the onset of symptoms of dementia. Neuropsychologia. 2007;45:459–464. doi: 10.1016/j.neuropsychologia.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FI, Ryan J. Executive control in a modified antisaccade task: Effects of aging and bilingualism. J Exp Psychol Learn Mem Cogn. 2006;32:1341–1354. doi: 10.1037/0278-7393.32.6.1341. [DOI] [PubMed] [Google Scholar]
- Bialystok E, Craik FIM. Cognitive and linguisitc processing in the bilingual mind. Current Directions in Psychological Science. 2010;19:19–23. [Google Scholar]
- Bobinski M, de Leon MJ, Wegiel J, Desanti S, Convit A, Saint Louis LA, Rusinek H, Wisniewski HM. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]
- Bosch B, Arenaza-Urquijo EM, Rami L, Sala-Llonch R, Junque C, Sole-Padulles C, Pena-Gomez C, Bargallo N, Molinuevo JL, Bartres-Faz D. Multiple DTI index analysis in normal aging, amnestic MCI and AD. Relationship with neuropsychological performance. Neurobiol Aging. 2012;33:61–74. doi: 10.1016/j.neurobiolaging.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Bronge L, Bogdanovic N, Wahlund LO. Postmortem MRI and histopathology of white matter changes in Alzheimer brains. A quantitative, comparative study. Dement Geriatr Cogn Disord. 2002;13:205–212. doi: 10.1159/000057698. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cattell RB, Cattell AKS. Handbook for the individual or group culture fair intelligence test. USA: IPAT; 1960. Chapter Chapter. [Google Scholar]
- Christensen H. What cognitive changes can be expected with normal ageing? Aust N Z J Psychiatry. 2001;35:768–775. doi: 10.1046/j.1440-1614.2001.00966.x. [DOI] [PubMed] [Google Scholar]
- Christensen H, Mackinnon AJ, Korten AE, Jorm AF, Henderson AS, Jacomb P, Rodgers B. An analysis of diversity in the cognitive performance of elderly community dwellers: individual differences in change scores as a function of age. Psychol Aging. 1999;14:365–379. doi: 10.1037//0882-7974.14.3.365. [DOI] [PubMed] [Google Scholar]
- Craik FI, Bialystok E, Freedman M. Delaying the onset of Alzheimer disease: bilingualism as a form of cognitive reserve. Neurology. 2010;75:1726–1729. doi: 10.1212/WNL.0b013e3181fc2a1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Rosa PA, Videsott G, Borsa VM, Canini M, Weekes BS, Franceschini R, Abutalebi J. A neural interactive location for multilingual talent. Cortex. 2013;49:605–608. doi: 10.1016/j.cortex.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Duarte A, Ranganath C, Trujillo C, Knight RT. Intact recollection memory in high-performing older adults: ERP and behavioral evidence. J Cogn Neurosci. 2006;18:33–47. doi: 10.1162/089892906775249988. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn ES. The Peabody Picture Vocabulary Test-III. Circle Pines, MN: American Guidance Service; 1997. Chapter Chapter. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gold BT, Jiang Y, Jicha GA, Smith CD. Functional Response in Ventral Temporal Cortex Differentiates Mild Cognitive Impairment From Normal Aging. Human Brain Mapping. 2010;31:1249–1259. doi: 10.1002/hbm.20932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Johnson NF, Powell DK, Smith CD. White matter integrity and vulnerability to Alzheimer's disease: preliminary findings and future directions. Biochim Biophys Acta. 2012;1822:416–422. doi: 10.1016/j.bbadis.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Kim C, Johnson NF, Kryscio RJ, Smith CD. Lifelong bilingualism maintains neural efficiency for cognitive control in aging. J Neurosci. 2013;33:387–396. doi: 10.1523/JNEUROSCI.3837-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Green DW. Mental control of the bilingual lexico-semantic system. Bilingualism: Language and Cognition. 1998;1:67–81. [Google Scholar]
- Hilchey MD, Klein RM. Are there bilingual advantages on nonlinguistic interference tasks? Implications for the plasticity of executive control processes. Psychon Bull Rev. 2011;18:625–658. doi: 10.3758/s13423-011-0116-7. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Social Class and Mental Illness. New York: John Wiley & Sons; 1958. Chapter Chapter. [Google Scholar]
- Johnson NF, Kim C, Clasey JL, Bailey A, Gold BT. Cardiorespiratory fitness is positively correlated with cerebral white matter integrity in healthy seniors. Neuroimage. 2012;59:1514–1523. doi: 10.1016/j.neuroimage.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk G, Bialystok E, Craik FI, Grady CL. Lifelong bilingualism maintains white matter integrity in older adults. J Neurosci. 2011;31:16808–16813. doi: 10.1523/JNEUROSCI.4563-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Burzynska A, Potter GG, Chen NK, Song AW. Diffusion tensor imaging of cerebral white matter integrity in cognitive aging. Biochim Biophys Acta. 2012;1822:386–400. doi: 10.1016/j.bbadis.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Crinion JT, Noppeney U, O'Doherty J, Ashburner J, Frackowiak RS, Price CJ. Structural plasticity in the bilingual brain - Proficiency in a second language and age at acquisition affect grey-matter density. Nature. 2004;431:757–757. doi: 10.1038/431757a. [DOI] [PubMed] [Google Scholar]
- Mortimer JA. Brain reserve and the clinical expression of Alzheimer's disease. Geriatrics. 1997;52(Suppl 2):S50–53. [PubMed] [Google Scholar]
- Moseley M. Diffusion tensor imaging and aging - a review. NMR Biomed. 2002;15:553–560. doi: 10.1002/nbm.785. [DOI] [PubMed] [Google Scholar]
- Paap KR, Greenberg ZI. There is no coherent evidence for a bilingual advantage in executive processing. Cogn Psychol. 2013;66:232–258. doi: 10.1016/j.cogpsych.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Raz N, Briggs SD, Marks W, Acker JD. Age-related deficits in generation and manipulation of mental images: II. The role of dorsolateral prefrontal cortex. Psychol Aging. 1999;14:436–444. doi: 10.1037/0882-7974.14.3.436. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE transactions on medical imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Schweizer TA, Ware J, Fischer CE, Craik FI, Bialystok E. Bilingualism as a contributor to cognitive reserve: evidence from brain atrophy in Alzheimer's disease. Cortex. 2012;48:991–996. doi: 10.1016/j.cortex.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, Petersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD. Structural imaging in early pre-states of dementia. Biochim Biophys Acta. 2012;1822:317–324. doi: 10.1016/j.bbadis.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CD, Lori NF, Akbudak E, Sorar E, Gultepe E, Shimony JS, McKinstry RC, Conturo TE. MRI diffusion tensor tracking of a new amygdalo-fusiform and hippocampo-fusiform pathway system in humans. J Magn Reson Imaging. 2009;29:1248–1261. doi: 10.1002/jmri.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffener J, Stern Y. Exploring the neural basis of cognitive reserve in aging. Biochim Biophys Acta. 2012;1822:467–473. doi: 10.1016/j.bbadis.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Mayeux R. Inverse relationship between education and parietotemporal perfusion deficit in Alzheimer's disease. Ann Neurol. 1992;32:371–375. doi: 10.1002/ana.410320311. [DOI] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Brain reserve and dementia: a systematic review. Psychol Med. 2006;36:441–454. doi: 10.1017/S0033291705006264. [DOI] [PubMed] [Google Scholar]
- Wechsler DS. Wechsler Adult Intelligence Scale-3rd Edition (WAIS-3®) San Antonio, TX: Harcourt Assessment; 1997. Chapter Chapter. [Google Scholar]


