Abstract
Zebra finch song is a learned behavior dependent upon successful progress through a sensitive period of late-postnatal development. This learning is associated with maturation of distinct brain nuclei and the fiber tract interconnections between them. We have previously found remarkably distinct and dense CB1 cannabinoid receptor expression within many of these song control brain regions, implying a normal role for endocannabinoid signaling in vocal learning. Activation of CB1 receptors via daily treatments with exogenous agonist during sensorimotor stages of song learning (but not in adulthood) results in persistent alteration of song patterns. Now we are working to understand physiological changes responsible for this cannabinoid-altered vocal learning. We have found that song-altering developmental treatments are associated with changes in expression of endocannabinoid signaling elements, including CB1 receptors and the principal CNS endogenous agonist, 2-AG. Within CNS, 2-AG is produced largely through activity of the α isoform of the enzyme diacylglycerol lipase (DAGLα). To better appreciate the role of 2-AG production in normal vocal development we have determined the spatial distribution of DAGLα expression within zebra finch CNS during vocal development. Early during vocal development at 25 days, DAGLα staining is typically light and of fibroid processes. Staining peaks late in the sensorimotor stage of song learning at 75 days and is characterized by fiber, neuropil and some staining of both small and large cell somata. Results provide insight to the normal role for endocannabinoid signaling in the maturation of brain regions responsible for song learning and vocal-motor output, and suggest mechanisms by which exogenous cannabinoid exposure alters acquisition of this form of vocal communication.
Keywords: Vocal learning, Vocal development, Endocannabinoid, Drugs of abuse, Diacylglycerol lipase, 2-arachidonyl glycerol, CNS development
1. Introduction
Zebra finches learn their song during a sensitive period of late-postnatal development (reviewed by (Bottjer and Arnold, 1997). This has led to their importance as a model species for studying the neurobiology of vocal learning, a process that occurs during a period roughly encompassing adolescence. Song learning and production are controlled by discrete, interconnected brain regions collectively described as the song system. This system is comprised of a set of interconnected regions of cortex, basal ganglia and thalamus (see the introduction to (Bolhuis et al., 2012) for an excellent brief review).
The song system is comprised of two major pathways as illustrated in Figure 1. The first is a caudal vocal motor pathway involved in song production that includes cortical-like regions HVC and RA. HVC projects vocal output to RA that ultimately projects to drive vocal output to the syrinx. The second is a predominately rostral set of regions that have been described as the anterior forebrain pathway. This pathway includes cortical-like lMAN, basal ganglia Area X, and the thalamic region DLM. This anterior pathway is necessary for sensorimotor vocal learning and more subtle variability associated with adult song (Thompson et al., 2007). Notably, HVC also participates in the anterior pathway through projection to basal ganglia (Area X, (Nottebohm et al., 1982) and output of the anterior pathway ultimately connects to the vocal motor pathway through cortical lMAN projections to RA (Bottjer et al., 1989). Auditory cortex that includes L2 studied here, also participates in the song system through projections to the motor pathway (HVC, (Vates et al., 1996).
Figure 1.
Anatomical relationships of brain regions studied. (A) Low-power micrograph of anti-DAGLα and –CB1 receptor double-label immunohistochemistry illustrates locations of song regions. Higher-power double-labeling images are presented in Figures 12 and 13. (B) Diagram of relative locations and some known interconnections between the song regions studied. Cortical regions include lMAN, L2, HVC and RA. Area X is a region of basal ganglia. DLM and Ov are thalamic. Interconnections between regions forming the anterior forebrain pathway are illustrated with solid arrows, including lMAN output to vocal motor RA. Vocal motor pathways are illustrated with dashed arrows, including motor output to brainstem. Striatum that contains Area X is shaded. Thalamic regions not represented in the micrograph of Panel A (DLM and Ov) are in grey. Arrows indicate approximate dorsal and caudal directions. These arrows are 1 mm.
Accumulating evidence demonstrates that, early in vocal development, the anterior forebrain pathway predominates in control of vocal output. As sensorimotor development progresses, the anterior pathway becomes less important, and the motor pathway becomes the primary regulator of vocal output (Olveczky et al., 2011). Thus, the song system provides a compelling developmental model to investigate drug effects on learning-related processes of CNS maturation that occur during late-postnatal development.
As most humans troubled by drug abuse begin use during a similar adolescent period of post-natal development (Palmer et al., 2009), the zebra finch model has become useful for beginning to understand relationships between developmental exposure to CNS-active drugs, ethologically-relevant learning, and associated persistent neurophysiological effects that are mechanistically involved (Schneider, 2008). A persistent effect produced by cannabinoid agonists when administered during periods of sensorimotor vocal development, but not later in adulthood, is modulation of expression of endocannabinoid signaling elements, including the endogenous agonist, 2-arachidonylglyerol (2-AG), and CB1 cannabinoid receptor immunoreactivity (Soderstrom et al., 2011). 2-AG is the principal endogenous cannabinoid agonist within zebra finch brain (Soderstrom and Tian, 2004). Developmental cannabinoid treatments that alter zebra finch vocal learning (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004) persistently increase 2-AG levels selectively within rostral telencephalon (Soderstrom et al., 2011), notable for containing lMAN and Area X song regions that are essential for vocal learning (Bottjer et al., 1985; Sohrabji et al., 1990). Importantly, endocannabinoid release has been clearly demonstrated to reduce glutamatergic input to spiny neurons within learning-essential Area X (Thompson and Perkel, 2011), directly confirming functional involvement of endocannabinoid signaling in the song system.
2-AG signaling is initiated via hydrolytic metabolism of diacylglycerol by diacylglycerol lipases (reviewed by (Ligresti et al., 2005), of which the alpha isoform predominates in CNS (DAGLα, (Bisogno et al., 2003). Post-synaptic 2-AG production initiates retrograde, negative-feedback signaling that is triggered by neuronal activity. After release and diffusion, 2-AG binds to and activates presynaptic CB1 receptors, decreasing the probability of further transmitter release (reviewed by (Di Marzo, 2011). The CNS importance of DAGLα is clearly demonstrated by the complete loss of endocannabinoid-mediated suppression of hippocampal GABAergic signaling in knock-out mice (Gao et al., 2010).
Persistent elevation of 2-AG in rostral telencephalon of zebra finches exposed to cannabinoid agonists during sensorimotor development, but not adulthood, suggests a potential mechanism for cannabinoid-altered vocal learning. Elevated levels may be attributable to increased production, decreased metabolism, or a combination of both processes. The first step in studying the role of 2-AG production in cannabinoid-altered vocal learning is to appreciate the normal developmental pattern of synthetic enzyme expression. Therefore, through the experiments described below, we have established the developmental pattern of DAGLα immunoreactivity at distinct stages of zebra finch vocal development.
2. Materials and Methods
Except where noted, all materials and reagents were purchased from Sigma or Fisher Scientific. Immunochemicals were purchased from Vector laboratories (Burlingame, CA). We have employed the recently revised system of nomenclature in descriptions of zebra finch neuroanatomy (Reiner, 2004).
2.1 Animals
Male zebra finches bred in our aviary were used in these experiments. Prior to the start of experiments, birds were housed in flight aviaries with mixed seeds (SunSeed VitaFinch), grit, water, and cuttlebone freely available. Each flight aviary contained several perches. The light–dark cycle was controlled at LD 14:10 h and ambient temperature was maintained at 78° F.
Four groups of animals at the following ages (± 3) days were employed: 25, 50, 75 and adults > 100 days of age. To eliminate possible variance associated with staining conditions during immunohistochemistry, tissue from animals from each age group were always processed simultaneously. Four replicates of animals in each age group were employed (n = 4 per group, sixteen animals total). Animals within each replicate were euthanized and perfused with fixative in the morning as described in detail below. Animals were cared for and experiments conducted according to protocols approved by East Carolina University’s Animal Care and Use Committee.
2.2 Anti-DAGLα immunohistochemistry
For immunohistochemistry experiments, 30 μm sections of zebra finch brain were reacted with anti-DAGLα antibody (1:6000, Santa Cruz Biotechnology catalog # sc-133308). Other reagents were purchased from Vector Laboratories (Burlingame, CA). Birds were killed by Equithesin overdose and transcardially perfused with phosphate-buffered saline (PBS, pH = 7.4) followed by phosphate-buffered 4 % paraformaldehyde, pH = 7.0. After brains were removed and immersed overnight in buffered 4 % paraformaldehyde, brains were blocked down the midline and parasaggitally sectioned (lateral to medial) on a vibrating microtome. Immunocytochemistry was performed using a standard protocol reported in (Whitney et al., 2000) except that the anti-DAGLα primary antibody was employed. Briefly, tissue sections were rinsed in 0.1 % H2O2 for 30 min, blocked with 5 % goat serum for 30 min, and incubated overnight in blocking solution containing anti-DAGLα antibody (1:6000). After antibody exposure, sections were rinsed in PBS (pH = 7.4), incubated in blocking solution containing biotinylated anti-rabbit antiserum (1:500) for 1 hour, rinsed with PBS again, and then submerged in avidin-biotin-peroxidase complex solution for 1 hour. Antibody labeling was visualized with DAB solution. Control sections that were not incubated in primary antibody were not immunoreactive. For double-labeling with anti-DAGLα and CB1 receptor antibodies, higher concentrations (1:3000) of anti-DAGLα antibody were required to distinguish staining from that of CB1 receptor antibody staining done with nickel peroxidase as described in (Soderstrom and Tian, 2008).
Staining was examined in various brain regions at 100 and 1000 X using an Olympus BX51 microscope with Nomarski DIC optics. Images were captured using a Spot Insight QE digital camera and Image-Pro Plus software (MediaCybernetics, Silver Spring, MD).
The selectivity of the anti-DAGLα antibody used was assessed via Western blotting. 25 μg of zebra finch brain protein was separated via SDS-PAGE using a 10 % gel. These proteins were transferred to a PVDF membrane, blocked with a 1% casein solution (Vector catalog #sp-5020), and probed with a 1:200 dilution of antibody in 1 % casein solution, followed by a 1:3,000 dilution of HRP-conjugated secondary antibody. The goat anti-rabbit secondary antibody was detected by enhanced chemiluminescence (GE healthcare catalog #RPN2106). Staining in the presence and absence of 20 μM of the immunizing peptide was determined.
2.3 Measurement of optical density of anti-DAGLα staining
For each age and brain region, four 100 X grey scale images were captured and borders of brain regions of interest were traced using ImagePro-Plus analysis software. The analysis software computed mean optical densities (OD) within regions of interest. Images for each of the four experiments were captured in single sessions under identical, calibrated exposure conditions using the light preset control of the Olympus BX-51 microscope. Illumination was not standardized across the four replicates. However, identical illumination conditions were maintained within each replicate by capturing all images by using the microscope light preset switch and not altering the microscope optical system or Image Pro software acquisition settings. Thus, variance in illumination across replicates was spread evenly over age groups.
2.4 Data analysis and statistics
Data analyses and statistical procedures were performed using Prism GraphPad, Sigma Stat and Microsoft Excel PC software. Data from the four experiments were pooled and mean OD compared across age group and brain region using two-way ANOVA and SNK post-tests where appropriate.
2.5 Digital photography
Images shown in Figures 3 – 9, and those used for the density measures in Figures 10 and 11, were taken at 100, and 1000 X using an Olympus BX51 microscope equipped with Nomarski optics and a Spot Insight QE digital camera. The digital camera was controlled by Image-Pro Plus software. The tissue shown in these figures were antibody-reacted together. The color images obtained were background-corrected using an image of a blank coverslipped slide, converted to 8-bit grey scale and stored in TIFF format using Image-Pro Plus software. These TIFF images were then arranged into figures using Adobe Illustrator software. Figures created with Adobe Illustrator were saved as image files in TIFF format. No brightness, contrast or other image modifications were made. Images shown in Figures 1, 12 and 13 were taken at 12.5, 200 and 1000X using an Olympus BX51 microscope under brightfield illumination.
Figure 3.
Developmental DAGLα antibody staining of the lateral magnocellular nucleus of the anterior nidopallium (lMAN). Staining within lMAN progresses from fiber staining at 25 days (A and B) to somatic staining later in development. Somata staining is only most distinct at 75 Days (E and F). 100 X bar = 200 μm, 1000 X bar = 20 μm.
Figure 9.
Developmental anti-DAGLα antibody staining of cerebellum. At 25 days distinct staining of the Purkinje cell layer is apparent (A). This staining consists of what appear to be puncta the migrating cell bodies surrounding larger Purkinje cells. The Purkinje cells themselves appear stained pericellularly, with axonal regions proximal to and emerging from cell bodies also stained. Light neuropil staining is present within the molecular layer (B). By 50 days the staining of Purkinje cells is more intense (C). Somatic staining of Purkinje cells has increased in density, and surrounding neuropil is also stained with occasional distinct puncta. Fiber staining within the molecular layer has become distinctly and densely stained. Some pericellular neuropil staining is present within the granule cell layer (D). At 75 days, staining intensity within all layers has peaked (E). Purkinje cell bodies are more distinctly stained than at prior stages of development. The fiber staining within the molecular layer has become more distinct, with smaller fibers stained. Pericellular staining of neuropil surrounding granule cells is also apparent (F). By 100 days, staining intensity has decreased in all layers, and appears very similar to that observed at 25 days (G). Staining of Purkinje cell bodies is markedly reduced, and somata of smaller cells within the Purkinje layer, possibly basket or stellate cells, are distinctly but lightly stained (B). 100 X bar = 200 μm, 1000 X bar = 20 μm.
Figure 10.
Comparison of optical density (OD) of anti-DAGLα labeling in lMAN, Area X, HVC, RA and molecular layer of cerebellum across different age groups. For each age and brain region, four 100 X grey scale images were captured and borders of brain regions of interest were traced using ImagePro-Plus analysis software. The analysis software computed optical densities within regions of interest. Images for each of the four experiments represented were captured in single sessions under identical, calibrated exposure conditions. Within Area X, HVC and RA, a general pattern of increased labeling density at 75 days, followed by decreased staining at adulthood is observed. This pattern of increased DAGLα expression associated with the sensory-motor stage of vocal learning contrasts with CB1 cannabinoid receptor expression patterns that peak at 50 days and are maintained through 75 days (Soderstrom and Tian, 2006). RA = robust nucleus of the arcopallium, HVC is used as a proper name, Area X = Area X of the medial striatum, lMAN = lateral magnocellular nucleus of the anterior nidopallium.
Figure 11.
Comparison of optical density (OD) of anti-DAGLα labeling in L2, DLM and OV across different age groups. For each age and brain region, four 100 X grey scale images were captured and borders of brain regions of interest were traced using ImagePro-Plus analysis software. The analysis software computed mean optical densities within regions of interest. Images for each of the four experiments represented were captured in single sessions under identical, calibrated exposure conditions. Within all brain regions examined, a general pattern of increased labeling density at 75 days, followed by decreased staining at adulthood is observed. This pattern of increased DAGLα expression associated with the sensory-motor stage of vocal learning contrasts with CB1 cannabinoid receptor expression patterns that peak at 50 days and are maintained through 75 days (Soderstrom and Tian, 2006). Nif = forebrain nucleus interface of the nidopallium, DLM = nucleus dorsolateralis anterior thalami, pars medialis, OV = nucleus ovoidalis.
Figure 12.
Double-label immunohistochemistry with anti-DAGLα (rust colored stain) and –CB1 receptor (blue-grey colored stain) antibodies. Staining of cortical (HVC, RA, lMAN) and basal ganglia (Area X) regions from tissue of a 75 day-old male are shown at 200 and 1000X. Staining features shared by both antibodies include somata and neuropil. In the case of DAGLα, somatic staining is of both small- (< 5 μm) and mid-sized (~ 10 μm) features, where CB1 appears restricted to the small class. Smaller CB1 features may be small cell bodies, or perhaps more likely, terminal fields. Staining of HVC is notable for negative staining of large somata (~ 20 μm) and dense CB1 neuropil that occludes DAGLα staining. Within RA, the neuropil staining is less robust, and distinct somatic DAGLα and CB1 staining are evident. Occasionally these somata appear associated (arrows). Staining in lMAN is notable for larger diameter DAGLα stained somata (~ 15 μm) and dense CB1 neuropil. The striatal region Area X shows moderate neuropil staining and distinct small somata that occasionally appear associated (arrows).
Figure 13.
Double-label immunohistochemistry with anti-DAGLα (rust colored stain) and –CB1 receptor (blue-grey colored stain) antibodies. Staining of cortical (L2), thalamic (DLM and OV) and cerebellar regions from tissue of a 75 day-old male are shown at 200 and 1000X. General features include staining of neuropil and small somata by both antibodies. Staining within auditory field L2 is notable for DAGLα positive bipolar cells characteristic of this region (arrow). Staining patterns are similar within thalamic regions DLM and OV. DAGLα staining of mid-sized somata (10 μm) is evident. Occasionally, CB1-stained smaller somata appear associated with larger DAGLα stained cell bodies (arrows). Cerebellum is notable for frequent intensely CB1-positive puncta surrounding DAGLα-expressing Purkinje cells.
3. Results
3.1 Anti-DAGLα staining of zebra finch telencephalic song regions
3.1.1 Lateral magnocellular nucleus of the anterior nidopallium (lMAN)
lMAN is one of three regions known to be essential for zebra finch vocal development (Bottjer et al., 1984). At 25 days anti-DAGLα was light but discernible against surrounding regions (Fig 3A). Higher magnification revealed distinct somatic staining of mostly irregular-shaped cells, with some neuropil staining and staining of fibers that appeared to surround regions of unstained cells (Fig 3B). By 50 days, staining was still light but discernible (Fig 3C), and characterized by somatic staining of more regularly-shaped cells, and less evident neuropil and fiber staining (Fig 3D). The most distinct lMAN staining was observed at 75 days, but was still light relative to that found in other telencephalic song regions at the same developmental stage (Fig 3E). Cell body staining density had increased. Larger cells were now labeled with increased staining of surrounding neuropil (Fig 3F). By 100 days, staining intensities had waned to levels similar to those observed at 25 days (Fig 3G). Somatic staining was still evident, but surrounding neuropil appeared to no longer be stained (Fig 3H). Staining intensity of lMAN was remarkable in that it is the only region measured that was not significantly increased at 75 days (Fig 10A), although staining of adult lMAN was of significantly lower intensity that that observed at 75 days. Distinctly low-intensity staining of adult lMAN was similar to the pattern observed previously for anti-zebra finch CB1 receptor staining (Soderstrom and Tian, 2006). Despite significant differences in staining intensity, the pattern of staining at each developmental stage showed distinct cellular, and subcellular features (summarized in Table 1).
Table 1.
Summary of Anti-DAGLa Staining Features by Age and Brain Region
| Region/Age (days) | Large Cells (15–20 μ) | Small Cells (5–10 μ) | Fibers | Diffuse Neuropil | Small Puncta | Figure |
|---|---|---|---|---|---|---|
| lMAN/25 | 0 | + | ++ | + | + | 2A,B |
| lMAN/50 | + | + | 0 | 0 | 0 | 2C,D |
| lMAN/75 | ++ | + | 0 | ++ | 0 | 2E,F |
| lMAN/100 | + | ++ | 0 | 0 | 0 | 2G,H |
|
| ||||||
| Area X/25 | 0 | + | +++ | + | + | 3A,B |
| Area X/50 | + | ++ | 0 | 0 | 0 | 3C,D |
| Area X/75 | ++ | ++ | + | +++ | + | 3E,F |
| Area X/100 | 0 | ++ | 0 | + | 0 | 3G,H |
|
| ||||||
| HVC/25 | 0 | + | ++ | + | 0 | 4A,B |
| HVC/50 | + | ++ | + | + | + | 4C,D |
| HVC/75 | ++ | + | + | ++ | + | 4E,F |
| HVC/100 | + | ++ | 0 | + | 0 | 4G,H |
|
| ||||||
| RA/25 | 0 | + | ++ | + | + | 5A,B |
| RA/50 | + | + | + | + | + | 5C,D |
| RA/75 | + | +++ | + | +++ | +++ | 5E,F |
| RA/100 | 0 | + | + | + | + | 5G,H |
|
| ||||||
| L2/25 | 0 | + | + | + | + | 6A,B |
| L2/50 | 0 | + | 0 | + | 0 | 6C,D |
| L2/75 | + | ++ | + | ++ | ++ | 6E,F |
| L2/100 | + | + | 0 | + | 0 | 6G,H |
|
| ||||||
| DLM/25 | + | + | + | + | + | 7A,B |
| DLM/50 | 0 | + | + | + | + | 7D,E |
| DLM/75 | + | ++ | ++ | +++ | ++ | 7G,H |
| DLM/100 | + | + | ++ | ++ | ++ | 7J,K |
|
| ||||||
| Ov/25 | + | + | 0 | + | + | 7A,C |
| Ov/50 | + | ++ | 0 | + | + | 7D,F |
| Ov/75 | + | ++ | ++ | +++ | ++ | 7G,I |
| Ov/100 | + | + | + | ++ | + | 7J,L |
|
| ||||||
| Cer/25 | + | + | + | + | 0 | 8A,B |
| Cer/50 | +++ | 0 | +++ | ++ | + | 8C,D |
| Cer/75 | +++ | 0 | +++ | ++ | + | 8E,F |
| Cer/100 | + | + | + | + | 0 | 8G,H |
0 = not apparent/very infrequent, + = apparent but infrequent, ++ = readily apparent, +++ = remarkably apparent
3.1.2 Area X within songbird medial striatum (Area X)
As illustrated in Figure 1, the striatal region Area X projects to DLM of thalamus which, in turn, projects to lMAN (Sohrabji et al., 1990). Developmental lesions of any one of these three brain regions will prevent song learning (Johnson and Bottjer, 1993). Area X is particularly interesting in-terms of effects of abused drugs because it receives a prominent dopaminergic input from the zebra finch ventral tegmental area (Bottjer, 1993) in a manner similar to mammalian ventral striatum that is implicated in drug reward (Lewis et al., 1981). Similar to the pattern observed in lMAN described above, distinct anti-DAGLα staining of Area X was present at 25 days (Fig 4A). Higher magnification revealed some somatic staining of small cells, and diffuse staining of neuropil, some of whi ch was intense and fibrous and appeared to surround unstained cells (Fig 4B). Overall intensity at 50 days appeared similar to that at 25 (Fig 4C), and was characterized by some somatic staining of larger cells than at 25 days (Fig 4D). Staining of fibrous neuropil had resolved. Peak intensity of staining within Area X was observed at 75 days (Fig 4E). This was characterized by dramatically increased staining intensity of neuropil, some of which appeared punctuate, and fibrous (Fig 4F). By 100 days, staining intensity had waned (Fig 4G), and was associated with very little neuropil staining, and light staining of some small somata (Fig 4H). The overall staining intensities measured at 25, 50 and 100 days did not significantly differ; only that measured at 75 days was significantly different, and of higher intensity, than those measured at other stages of development (Fig 10C).
Figure 4.
Developmental anti-DAGLα antibody staining of Area X of the medial striatum. Staining in this region is low at 25 Days (A), and is characterized by light staining of a few somata and more dense, but infrequent fiber staining (B). Staining intensity is still low at 50 days (C), although fibers have disappeared and lightly-stained cell bodies have become more frequent (D). Staining intensity is greatly increased by 75 days (E), with many small puncta intensely stained, generally dense neuropil staining, and moderate staining of a fraction of cell bodies (F). Staining is reduced by 100 days, and appears similar to that at 50 days (G and H). 100 X bar = 200 μm, 1000 X bar = 20 μm.
3.1.3 HVC
HVC is a caudal-dorsal vocal pre-motor nucleus of telencephalon essential for song output that projects to RA (Nottebohm et al., 1976). Light, but distinct anti-DAGLα staining was discernible within HVC of 25-day old group animals (e.g. Fig 5A). Higher magnification revealed that this staining is somatic, within cells of generally small size, and light, diffuse, neuropil and fibers (Fig 5B). By 50 days, neuropil labeling became more pronounced (Fig 5C), with sparse densely-stained fibers and puncta (Fig 5D). Staining intensity was significantly increased at 75 days (Fig 5E), although the pattern of cell, neuropil and fiber staining appeared similar to that at 50 days (Fig 5F). Staining intensities waned by adulthood (Fig 5G) and consisted of somatic staining of regularly-shaped small cells with light neuropil staining (Fig 5H). Optical density of anti-DAGLα staining within HVC showed relatively low levels in 25-day old animals with a small but significant increase at 50 days (Fig 10B). Levels peaked at 75 days and then diminished to 25 day levels at adulthood.
Figure 5.
Developmental anti-DAGLα antibody staining within HVC. Staining at 25 days is barely discernable above background (A), and is associated with light staining of cell bodies and more distinct, but infrequent fiber staining (B). The intensity of cell body staining increases by 50 days (C) with reduced fiber staining (D). Staining intensity peaks at 75 days (E). Labeled fibers are still present with a fraction of cell bodies stained distinctly densely (F). By 100 days, the intensity of staining has diminished (G), fiber-staining is absent and only small round cell bodies appear labeled (H). 100 X bar = 200 μm, 1000 X bar = 20 μm.
3.1.4 Robust nucleus of the arcopallium (RA)
RA is a caudal telencephalic nucleus essential for vocal motor output. It receives input from HVC and, in turn innervates the tracheosyringeal portion of the hypoglossal nucleus (nXIIts) that ultimately projects to the muscles of the syrinx vocal organ (reviewed by Bottjer and Johnson, 1997). The pattern of anti-DAGLα staining within RA is summarized in Fig 5. Staining in 25 day olds was already distinct, despite the small, undeveloped size of RA at this age (Fig 6A). This staining is characterized by small stained soma and neuropil with occasional densely-stained puncta that surround large unstained cells (Fig 6B). This pattern and intensity of staining is largely maintained at 50 days (Fig 6C), with the exception of less frequent punctuate staining (Fig 6D). Staining intensity is significantly increased at 75 days (Fig 6E) and is characterized by more intense neuropil staining, and more frequent intensely stained puncta, some of which appear to follow fibers (Fig 6F). This intense staining wanes by adulthood (Fig 6G) although somatic staining is still apparent with diffuse neuropil and occasional puncta (Fig 6H). Optical density measures do not differ across measures taken from 25 day-old, 50 day-old and adult tissues. RA staining at 75 days was significantly more intense than at any of the other ages (Fig 10D).
Figure 6.
Anti-DAGLα antibody staining of the robust nucleus of the arcopallium (RA) at different stages of vocal development. Tissue from all ages shown were reacted simultaneously as described in the Methods section. Staining at 25 days is low compared to surrounding regions (A), and is comprised primarily of neuropil surrounding unstained cell bodies (B). Staining within RA becomes distinct by 50 days (C) and is associated with staining of both neuropil and cell bodies (D). The density of staining greatly increases by 75 days (E), and in addition to cell body and neuropil staining, a new pattern densely-stained puncta is found (F). By 100 days, the staining density has decreased (G), and is more characteristic of the pattern observed at 50 days, with a few remaining lightly-stained puncta (H). 100 X bar = 200 μm, 1000 X bar = 20 μm.
3.2 Anti-DAGLα Staining of Auditory and Thalamic Nuclei
3.2.1 Auditory Field L2
Field L2 is a thalamorecipient auditory region of the song bird telencephalon. Early in development, at 25 days, DAGLα staining was barely discernible above background (Fig 7A). This staining was comprised of light staining of diffuse neuropil and occasional small cell bodies (Fig 7B). This pattern was maintained through 50 days (Fig 7C and D). Distinct L2 staining was observed at 75 days (Fig 7E). Higher magnification revealed that this labeling consisted of stained cell bodies, diffuse neuropil, and some distinct intense puncta and fiber staining (Fig 7F). By 100 days, staining intensity had diminished, and was associated with light staining of diffuse neuropil and occasional cell somata (Fig 7H). Optical density measures did not differ across measures taken from 25 day-old, 50 day-old and adult tissues. L2 staining at 75 days was significantly more intense than at any of the other ages (Fig 11A).
Figure 7.
Developmental anti-DAGLα antibody staining within Field L2. At 25 days staining is barely discernable (A), and is limited to a few cell bodies (B). At 50 days staining is increased slightly (C) with a larger fraction of stained cell bodies and diffuse staining of surrounding neuropil (D). Staining intensity peaks at 75 days (E) and, in addition to cell body and neuropil staining, now also includes some fiber staining and darkly-stained small puncta (F). Staining intensities are reduced at 100 days (G), and exhibit a pattern similar to that at 50 days (H). 100 X bar = 200 μm, 1000 X bar = 20 μm.
3.2.2 Dorsal lateral nucleus of the medial thalamus (DLM) and nucleus ovoidalis (OV)
DLM is one of three nuclei known to be essential for vocal learning (Johnson and Bottjer, 1993). OV is involved in relaying auditory information to Field L2 and shares homology with mammalian medial geniculate (Mello and Clayton, 1994; Vates et al., 1996). Despite differing system roles, anti-DAGLα staining patterns within these two thalamic regions are remarkably similar. At 25 days, staining in both regions is distinct (Fig 8A). The staining within DLM was associated with light diffuse neuropil, with several moderately-stained small cell bodies (8B). Intensely stained circular and rod-shaped puncta were particularly notable. Staining within OV was similar to that of DLM, with the exception of a larger number of intensely-stained circular puncta, and a lower number of rod-shaped (Fig 8C). At 50 days the general intensity of staining was similar to that at 25 days (Fig 8D), but within DLM the intense puncta staining was no longer present (Fig 8E). There were still intensely-stained circular puncta within OV, but the rod-shaped were no longer present (Fig 8F). At 75 days staining in both regions had dramatically increased (Fig 8G). Within DLM, neuropil staining had become prominent with distinct patches of high density labeling (Fig 8H). A few cell bodies were densely stained, and several intensely-stained rod-shaped puncta were present. Within OV, staining was mostly of neuropil, with a few intense puncta (Fig 8I). Some distinct cell body staining was also apparent. At 100 days staining intensity had decreased in both regions, although to a greater extent in OV than DLM (Fig 8J). The pattern of staining in DLM was very similar to that observed at 75 days, with cell bodies, neuropil and puncta staining evident (Fig 8K). Staining within OV had decreased in intensity. OV staining was characterized by a few moderately-stained large cell bodies, and sparse puncta, both rod- and circular shaped (Fig 8L). Within both DLM and OV, optical density measures did not differ across measures taken from 25 day-old, 50 day-old and adult tissues (Fig 11B and C). For both regions, staining at 75 days was significantly more intense than at any of the other ages.
Figure 8.
Developmental anti-zebra finch CB1 antibody staining of thalamic regions DLM (nucleus dorsolateralis anterior thalami, pars medialis) and OV (nucleus ovoidalis). In 100 X images OV is rostral-ventral (lower-left) and DLM is caudal-dorsal (upper-right). Center column contains 1000X images of DLM (panels B, E, H, K). The right column contains 1000X images of OV (panels C, F, I, L). At 25 days, staining in both regions is distinct (A). The staining within DLM is associated with light diffuse neuropil, with several moderately-stained small cell bodies (B). Intensely stained circular and rod-shaped puncta are particularly notable. Staining within OV is similar to that of DLM, with the exception of a larger number of intensely-stained circular puncta, and a lower number of rod-shaped (C). At 50 days the general intensity of staining is similar to that at 25 days (D), but within DLM the intense puncta staining is no longer present (E). There are still intensely-stained circular puncta within OV, but the rod-shaped are no longer present (F). At 75 days staining in both regions has dramatically increased (G). Within DLM, neuropil staining has become prominent with distinct patches of high density labeling (H). A few cell bodies are densely stained, and several intensely-stained rod-shaped puncta are present. Within OV, staining is mostly of neuropil, with a few intense puncta (I). Some, distinct cell body staining is also apparent. At 100 days staining intensity has decreased in both regions, although to a greater extent in OV than DLM (J). The pattern of staining in DLM is very similar to that observed at 75 days, with cell bodies, neuropil and puncta staining evident (K). Staining within OV has decreased in intensity (L). OV staining is characterized by a few moderately-stained large cell bodies, and sparse puncta, both rod- and circular shaped. 100 X bar = 200 μm, 1000 X bar = 20 μm.
3.2.3 Anti-DAGLα immunoreactivity in cerebellum
Cerebellum is notable for distinctly-dense expression of CB1 cannabinoid receptors across vertebrata (Elphick and Egertova, 2001), and some limited evidence suggests that this structure may contribute to vocal learning (cf. (Person et al., 2008). Cerebellum has been the subject of a comprehensive analysis of the expression pattern of proteins known to be involved in the endocannabinoid signaling system of rats, including DAGLα (Suarez et al., 2008). Because of these factors, we have included this structure in the present study for comparative purposes. As described in the mouse studies, we saw distinct staining of Purkinje cell bodies, and labeling of molecular layer neuropil with varying intensity across development. Given complexities of analyzing staining densities in granule cell, Purkinje cell and molecular layers of cerebellum, we did not measure OD values of this structure as a function of development. What follows is a description of staining patterns observed at each time point.
At 25 days, Purkinje cell staining was already present, while labeling within the molecular and granule cell layers appeared no greater than background (Fig 9A). Interestingly, moderately-dense somatic staining of what appear to be granule cells surrounding larger Purkinje cells was observed (Fig 9B). By 50 days, staining intensity had significantly increased (Fig 9C). Purkinje cell bodies were more densely stained, and fibers and neuropil within the molecular layer were also labeled (Fig 9D). Peak DAGLα staining intensity was observed at 75 days, with a pattern consistent with that reported for Wistar rats, i.e. staining of Purkinje cell bodies, and dense staining of neuropil within the molecular layer (Suarez et al., 2008) (Fig 9E). In contrast to the pattern observed in rat, some pericellular staining around granule cells was also observed (Fig 9F). By adulthood, staining within the molecular and granule cell layers was markedly reduced (Fig 9G). Purkinje cells were still distinctly, although lightly-labeled. The overall pattern of staining at adulthood is very similar to that observed in the 25 day old group (Fig 9H).
3.2.3 Double labeling with anti-DAGLα and –CB1 receptor antibodies
To begin to determine the relative distribution of the enzyme responsible for endocannabinoid production and the receptor ultimately activated, a series of double label immunhistochemistry reactions were done using tissue taken from a 75 day-old male. Results of these procedures are summarized in Figures 12 and 13.
Because both DAGLα and CB1 antibodies were raised in the same animal, double immunofluoresence was impossible, and a less elegant sequential peroxidase procedure was employed. This technique does not eliminate tissue depth, and so true relative location of staining cannot be firmly established. Also, higher DAGLα antibody concentrations were required to distinguish it from that of CB1. However, general patterns can be appreciated. Staining features shared by both antibodies include somata and neuropil. In the case of DAGLα, somatic staining is of both small- (< 5 μm) and mid-sized (~ 10 μm) features, where CB1 appears restricted to the small class. Smaller CB1–stained features may be small cell bodies, or perhaps more likely, terminal fields.
Double-labeling of cortical regions lMAN, HVC, RA and the basal ganglia region Area X is summarized in Figure 12. Staining of HVC is notable for negative staining of large somata (~ 20 μm) and dense CB1 neuropil that occludes DAGLα staining. Within RA, the neuropil staining is less robust, and distinct somatic DAGLα and CB1 staining are evident. Occasionally these somata appear associated (arrows). Staining in lMAN is notable for larger diameter DAGLα stained somata (~ 15 μm) and dense CB1 neuropil. The striatal region Area X shows moderate neuropil staining and distinct small somata that occasionally appear associated (arrows).
Staining of the cortical auditory Field L2, thalamic regions Ov and DLM, and cerebellum are summarized in Figure 13. Staining within auditory field L2 is notable for DAGLa positive bipolar cells characteristic of this region (arrow, (Fortune and Margoliash, 1992). Staining patterns are similar within thalamic regions DLM and Ov where DAGLα staining of mid-sized somata (10 μm) is evident. Occasionally, CB1-stained smaller somata appear associated with larger DAGLα stained cell bodies (arrows). Cerebellum is notable for frequent intensely CB1-positive puncta surrounding DAGLa-expressing Purkinje cells.
4. Discussion
We have previously found that 2-AG is the principal endogenous cannabinoid agonist produced in zebra finch brain (Soderstrom and Tian, 2004). Zebra finch brain 2-AG levels are acutely increased by restricted access to food, that also reduces vocal output (Soderstrom and Tian, 2004). Following repeated daily cannabinoid treatments that alter sensorimotor vocal learning, 2-AG levels are persistently elevated in rostral telencephalon (notable for containing vocal learning-essential regions Area X and lMAN), and persistently decreased in cerebellum (Soderstrom et al., 2011). In addition to demonstrating presence of distinct mechanisms of control of 2-AG levels in cerebellum and telencephalon, these results also suggest mechanisms of 2-AG synthesis may play a role in altered vocal learning (Soderstrom and Johnson, 2003). The present experiments represent the initial stages of determining the relevance of the principal enzyme responsible for CNS 2-AG synthesis, DAGLα, to normal vocal learning, and are the first to document this expression in the context of postnatal CNS development.
4.1 General findings
The significance of biosynthetic enzyme activity in the control of endocannabinoid signaling has only recently begun to receive experimental attention (reviewed by (Di Marzo, 2011). Thus, the present study is the first to document in a detailed manner the spatial distribution of DAGLα expression during late-postnatal CNS maturation. In contrast to expression of CB1 cannabinoid receptors, detailed knowledge of the spatial distribution of 2-AG synthetic enzyme expression is currently limited to mouse cerebellum (Bisogno et al., 2003; Suarez et al., 2008) and therefore we have included this non-song region that is well-conserved across vertebrates in the current study.
More general DAGLα expression has been described within regions of mouse brain including; striatum, thalamus, hippocampus and cortex (Yoshida et al., 2006). General DAGLα expression in mammalian cortex is consistent with avian expression within lMAN, L2, HVC and RA, considered homologous to regions of mammalian frontal (lMAN, (Brainard and Doupe, 2000) auditory (L2, (Wild and Krutzfeldt, 2010) and motor (HVC and RA, (Daou et al., 2013; Yip et al., 2012) cortex. Moderate expression levels were reported within mouse caudate putamen, a region of striatum that shares similarity with striatal Area X studied here. Anti-DAGLα staining of mouse thalamus was distinctly-dense, consistent with what we found in avian thalamic regions DLM and Ov, avian homologs of the mammalian basal ganglia-thalamocortical pathway (Luo et al., 2001; Reiner et al., 1998) and thalamic auditory nuclei (Wild et al., 1993), respectively. Messenger RNA expression levels were also measured within these regions. Interestingly, thalamic and cortical DAGLα mRNA expression was not reflected in similarly distinct protein expression (compare Fig 1B and C in (Yoshida et al., 2006). Distinctly high densities of DAGLα protein expression was also described in mouse hippocampus. Although avian species have a region described as hippocampus that plays a role in spatial memory and therefore has some functional homology, the distinct organization and circuitry of mammalian hippocampus not been established within the avian region (Mayer et al., 2013).
In most brain regions studied, with the notable exception of lMAN, there was a clear pattern of peak DAGLα staining intensity at 75 days, late in the sensorimotor stage of vocal learning. It is important to note that our density measures are not quantitative, and so we are unable to address the question of protein amounts expressed. However, relative expression levels at different periods of development may be appreciated, as has been done earlier with CB1 receptor expression (Soderstrom and Tian, 2006). Optical density measures summarized in Figures 10 and 11 represent the means of four independent experiments. Background staining varied across these experiments, but because all four developmental groups were equally represented within each replicate, variance attributable to different staining conditions across experiments was spread equally across groups.
Unlike CB1 receptor expression, the pattern of DAGLα expression was notably dense and distinct in some areas surrounding song regions. For example, DAGLα expression is clearly distinct, and varies as a function of development in arcopallial regions surrounding RA (See Fig 6A, C, E and G). Early in vocal development at 25 days, expression levels are generally low, and levels outside of song regions are similar to that within song regions. This may indicate a more general, CNS-wide role for 2-AG production early in the vocal development period. This possibility is further supported by the general pattern of fiber staining at 25 days that is consistent with endocannabinoid expression within maturing neuronal processes during post-natal development in other species (Soderstrom and Gilbert, 2013).
Distinctly high-level DAGLα expression at 75 days was associated with elevated neuropil staining that was much lighter or absent at other ages. Staining of cell bodies was also more intense at 75 days, but was typically present at other ages also, suggesting a basal capacity for 2-AG production throughout late-postnatal CNS development. It is notable that somatic staining, with some exceptions discussed below, was generally associated with smaller cells of five to ten microns in diameter. This suggests the types of most cells that are DAGLα-expressing are likely smaller glia or interneurons, such as the spiny neurons within Area X established to be subject to endocannabinoid modulation (Thompson and Perkel, 2011). Less frequent and the clearly negative staining of larger diameter cells within HVC (see Fig 12) suggest that projection neurons, comprising critical learning- and vocal motor-related interconnections between song regions, typically do not somatically release 2-AG. An exception to this rule is expression within large cells late in sensorimotor learning at 75 days of age. At this developmental stage, staining of large diameter cells, consistent in size with projection neurons, are apparent in varying frequency within all regions evaluated, with particularly distinct expression in lMAN, Area X, HVC and cerebellum. Thus, it appears that activity-dependent release of 2-AG may occur from some projection neurons late in sensorimotor learning at 75 days. This suggests an important endocannabinoid role in development of interconnections between song regions during this developmental period. Interestingly, cannabinoid agonist exposure at 75 – 100 days has distinct effects to reduce note numbers produced later in adulthood, suggesting this time period is important for memory maintenance and/or integration of the song template (Soderstrom and Tian, 2004).
A convincing weight of evidence indicates that endocannabinoid signaling is retrograde, and functions to reduce presynaptic transmitter release (Elphick and Egertova, 2001). Assuming that this general rule applies to zebra finch song regions, distinct DAGLα staining of neuropil at 75 days is likely due to post-synaptic dendritic expression. This suggests that endocannabinoid modulation of neural transmission received dendritically is of particular importance to the sensorimotor development of most song regions. This is consistent with altered densities of dendritic spines found within Area X and HVC following vocal development-altering cannabinoid treatments (Gilbert and Soderstrom, 2011). Moreover, since dendritic spines are largely restricted to excitatory signaling (reviewed by (Hoogenraad and Akhmanova, 2010), this also suggests that modulation of NMDA and AMPA receptor signaling is of particular importance during the “plastic song” phase of sensorimotor learning at 75 days.
4.2 Findings within brain regions
4.2.1 Cerebellum
Cerebellum has received early attention in regard to DAGLα expression as it expresses an abundant population of CB1 cannabinoid receptors (including within zebra finch (Soderstrom and Tian, 2006) and mouse cerebellum (Cristino et al., 2006), and is comprised of a clearly-defined population of neuronal cell types with established circuitry. This allows patterns of receptor expression and endocannabinoid synthetic enzymes to be studied in-terms of known functional relationships (reviewed by (Bower, 2002). This approach has proven profitable in the case of mouse, and now also zebra finch DAGLα that are both distinctly and densely expressed within cell bodies of Purkinje cells, and their dendritic fields extending into the molecular layer. Purkinje dendrites receive excitatory input from granule cells via ascending axonal segments and parallel fibers, and also from the inferior olive via climbing fibers (see Fig 9 and (Bisogno et al., 2003; Suarez et al., 2008). In contrast to DAGLα, CB1 cannabinoid receptors are not expressed in Purkinje cell bodies, but are present at high density in surrounding inhibitory basket cell terminals, and within inhibitory terminals of stellate cell interneurons impinging on Purkinje dendrites within the molecular layer (Ashton et al., 2004).
This presynaptic receptor localization on inhibitory terminals, and postsynaptic presence of DAGLα responsible for endocannabinoid production and release, explains established neurophysiological effects of endocannabinoid signaling to produce Purkinje cell depolarization-induced suppression of inhibition (DSI, reviewed by (Diana and Marty, 2004). Purkinje cell depolarization results in calcium-dependent activation of DAGLα and release of 2-AG (Bisogno et al., 2003). Released 2-AG freely diffuses to presynaptic CB1-expressing inhibitory terminals and activates cannabinoid receptors. Cannabinoid receptor-induced signaling reduces presynaptic calcium concentrations, decreasing the probability of inhibitory transmitter release, which in this circuit, further potentiates Purkinje cell activation. Thus, DAGLα activation within cerebellar Purkinje cell neurons effects a feed-forward amplification of activation through retrograde reduction of inhibitory input (Tanimura et al., 2009).
This feed-forward endocannabinoid-mediated system capable of producing DSI appears to be present in avian cerebellum (Fig 9), although some notable differences are also apparent. First, DAGLα expression densities appear lower in the avian molecular layer than those reported for mouse cerebellum (Bisogno et al., 2003; Suarez et al., 2008; Yoshida et al., 2006). Although this apparent difference may be attributable to methodological immunostaining differences (as our procedure was carefully optimized not to saturate signal, in order that potential intensity differences across age groups could be detected) the possibility for reduced DAGLα production in Purkinje dendrites of birds, relative to rodents, remains. This may mean that zebra finches are less sensitive to endocannabinoid-induced DSI, a question that will require electrophysiology to answer.
A second notable difference in zebra finch cerebellar DAGLα expression is distinct staining of neuropil surrounding granule cells (see Fig 9F). Cerebellar granule cells receive inhibitory input through Golgi cell terminals, and excitatory input from mossy fibers that originate from various regions notably including telencephalon and the vestibular system (Pakan et al., 2008). Avian presence of DAGLα in terminals of extra cerebellar origin may indicate an additional endocannabinoid-mediated modulatory system not present in terrestrial species, possibly relevant to specialized flight and song learning-related vocal-motor behaviors. Vocal learning relevance is suggested by peak peri-granule cell DAGLα expression late in the sensorimotor stage of song learning at 75 days, with much lower levels observed at other ages (compare Fig 9F to 9B, D and H).
As mentioned above, earlier results indicate that developmental exposure to cannabinoid agonist during sensorimotor vocal learning in these birds results in persistent reduction in song stereotypy and the number of notes produced in adulthood (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004). More recently, we have discovered that this same song-altering developmental cannabinoid treatment also persistently reduces 2-AG levels in cerebellum (Soderstrom et al., 2011). Because endocannabinoid signaling in cerebellum promotes activation of neuronal signaling, reduced 2-AG levels are likely to lead to a general decrease in cerebellar activity. This raises the possibility that persistently reduced song stereotypy may involve vocal-motor disruption related to reduced cerebellar responsiveness.
4.2.2 Thalamic regions DLM and OV
We have included the thalamic regions DLM and OV due to their involvement in relaying auditory and vocal-motor information to and from telencephalic song regions (Person and Perkel, 2007; Vates et al., 1996). CB1 cannabinoid receptors in these regions of thalamus are associated with diffuse neuropil and puncta consistent in size and shape with terminal fields surrounding unstained cell bodies (see Fig 8, (Soderstrom and Tian, 2006). Distinct DAGLα staining of cell bodies (Fig 8K and L) makes sense in-terms of the retrograde, presynaptic mechanism described for endocannabinoid signaling in several other systems (reviewed by (Elphick and Egertova, 2001).
DAGLα expression in these thalamic regions does not significantly increase between 25 and 50 days, but dramatically peaks by 75 days. This temporal pattern of expression is interesting when compared to that of CB1 receptor expression that peaks at 50 days, and has already decreased by 75 days (Soderstrom and Tian, 2006). Thus, CB1 receptor expression appears to precede that of the enzyme responsible for production of the principal endogenous agonist. This pattern of DAGLα expression following that of the receptor expression is also evident within a subset of telencephalic song regions, including Area X, RA and HVC. The significance of these timing differences remains puzzling. It may be the case that another endogenous agonist (e.g. anandamide) is important early in sensorimotor vocal development. Perhaps more likely is that CB1 receptors accompany axon terminals that establish connections relatively early, and DAGLα expression is induced as a function of fiber tract maturation (and related increased efficiency of neuronal signaling) that is known to be completed toward the end of the sensorimotor stage of song learning (Herrmann and Bischof, 1986).
4.2.3 Auditory Field L2
As auditory feedback is essential for song learning, the auditory thalamorecipient Field L2 is likely important to normal vocal development (Nordeen and Nordeen, 1992). CB1 receptor expression within this region is notably distinct (see Fig 1, 2 and 5 (Soderstrom and Tian, 2006). Anti-CB1 receptor staining within L2 is so distinct that the antibody has been used as a marker for this region (Nagel and Doupe, 2008). It is notable that DAGLα staining within L2 does not appear as dense and distinct as that within other densely CB1 receptor-expressing regions, such as cerebellum. This difference likely indicates distinct mechanisms of endocannabinoid regulation across the regions, the nature of which remains unknown. It may be the case that a high density of CB1 receptors increases sensitivity to modest levels of 2-AG production, consistent with a spare receptor-type mechanism for endocannabinoid modulation of auditory input. It is also possible that other endocannabinoids like anandamide are more critical to cannabinoid signaling within L2. At high power, DAGLα staining appears to be of diffuse neuropil and lightly-stained, elongated cell bodies, likely the bipolar, type 3 oriented cells described by (Fortune and Margoliash, 1992), and see Fig 7H and 13).
Figure 2.
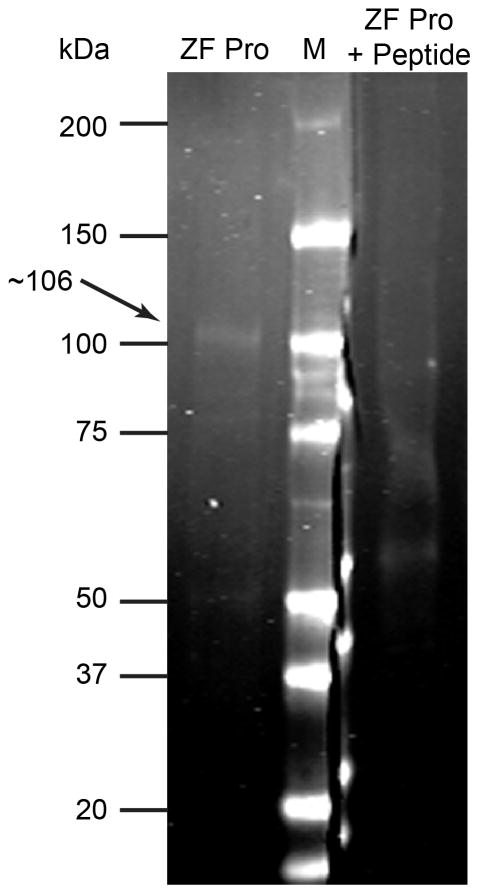
Selectivity of the anti-DAGLα antibody is demonstrated by its labeling of a single prominent band of about 106 kDa (estimated by non-linear regression using protein marker migration to generate a standard curve). 25 μg of zebra finch protein (ZF Pro) was separated by SDS-PAGE and transferred to a nylon membrane and probed with the primary antibody. Bound antibody was visualized with an IR dye-conjugated secondary antibody. Note lack of staining when the primary antibody was pre-absorbed with 20 μM of the immunizing peptide (ZF Pro + Peptide).
4.2.4 HVC
HVC is a pre-motor region that receives prominent auditory input from Nif, L2 and thalamic Uva, and projects to vocal motor output region RA, as well as learning-essential striatal region Area X (reviewed by (Bottjer and Johnson, 1997). HVC has an important role in sequencing of notes within song (Kozhevnikov and Fee, 2007). Peak DAGLα expression at 75 days within HVC consists of both cell body and diffuse neuropil staining. Note that some large cell bodies, with diameters of 15 – 20 microns are distinctly stained at 75 days (Fig 5F). Staining of large cells is also apparent at 50 days, although intensities are lower (Fig 5D). The size of these cell bodies may represent projection neurons, given their location within HVC (Kozhevnikov and Fee, 2007), likely those that project to RA. Interestingly, a population of large cells consistent with somata of projection neurons that do not express DAGLα were revealed by negative staining for CB1 receptor (see Fig 12). At 50 and 100 days, staining of smaller cells with diameters of about five microns, predominates. The sizes of these smaller cells are consistent with interneurons or glia. Thus it appears that endocannabinoid signaling may be particularly important to modulating RA projections late in sensorimotor learning at 75 days, and more relevant to internal modulation of interneuron and glial activity during other periods.
Lower level staining at 25, 50 and 100 days is attributable to reduced neuropil staining relative to that observed at 75 days. Assuming that the post-synaptic, retrograde signaling role for 2-AG operating within mammalian hippocampus and vertebrate cerebellum is maintained within HVC, this pattern of reduced neuropil expression suggests that 2-AG is released from dendrites at 75 days, late in the sensorimotor stage of vocal development, but to a lesser degree at other ages, including adulthood. This implies another distinctly-important role for endogenous cannabinoid signaling in the normal late-postnatal development of HVC. It is notable that densities of dendritic spines within this region are elevated following cannabinoid agonist exposure during sensorimotor development (Gilbert and Soderstrom, 2011). Thus, inappropriately elevated densities of dendritic spines may involve a disregulation of normal endocannabinoid signaling important for maturation of neuronal circuitry within HVC, specifically experience-associated “pruning” of dendritic spines (Bock and Braun, 1998).
4.2.5 RA
The vocal motor output region, RA receives stimulation from both HVC and lMAN. RA activity is largely dependent upon that within HVC that “drives” the downstream region (Mooney and Konishi, 1991). Similar to the patterns observed in other regions, a coordinated pattern of somatic DAGLα staining of small cells surrounded by neuropil staining is also observed within RA. In addition to this consistent staining pattern, there was also interesting intense staining of granular puncta (see Fig 6F). In some cases this punctate staining appeared organized along fiber processes. Other puncta appeared as small spheres within cytoplasmic regions of large cells, possibly indicating intracellular DAGLα stores within a subpopulation of cells within RA. This spheroid staining within RA appears at all ages, but is most abundant at 75 days.
4.2.6 Area X
Turning to the learning-essential song regions of rostral telencephalon, Area X of striatum is clearly necessary for successful vocal learning, but doesn’t play a clear role in production of already learned song (Sohrabji et al., 1990). This region is of particular interest in the context of abused drugs as it receives a prominent dopaminergic input from the ventral tegmentum, and thus shares similarities with mammalian ventral striatum responsible for drug reward (Bottjer, 1993; Casto and Ball, 1994).
DAGLα expression within Area X follows a pattern of cell body labeling at all ages, with dramatically increased neuropil staining at 75 days. Cell body labeling is consistent with the mechanism established within cerebellum and cannabinoid-mediated DSI of Purkinje cell activation. As discussed above, peak levels late in sensorimotor learning implies an important role in the process of normal vocal development, which is further supported by the fact that cannabinoid-altered vocal development is associated with increased densities of dendritic spines within Area X (Gilbert and Soderstrom, 2011). In addition, we have found that developmental, but not adult cannabinoid treatments persistently increase 2-AG levels in rostral telencephalon, a significant portion of which is comprised of Area X (Soderstrom et al., 2011). This suggests that vocal development-altering cannabinoid treatments may upregulate DAGLα expression within Area X and possibly lMAN also. This possibility is currently being tested.
Taken together, these findings suggest that exogenous agonist exposure during sensorimotor learning disrupts normal endocannabinoid signaling essential for establishing proper circuitry within learning-essential Area X. Altered developmental course results in persistent changes in neurophysiology, and in the expression of endocannabinoid signaling elements.
4.2.7 lMAN
DAGLα staining within lMAN is notable for having the least variance in density across stages of development, and lack of distinctly high-level expression at 75 days. This region is also critical for vocal learning (Bottjer et al., 1984), and shows early expansion during development at about 25 days, followed by a regression to adulthood (Bottjer et al., 1985). Although lMAN is not essential for production of learned song, it influences activity within the vocal motor region HVC, and therefore is implicated in modulating vocal output (Thompson et al., 2007). Interestingly, lesions of lMAN cause a rapid correction of song patterns disrupted by HVC microlesions, supporting a role for lMAN in producing song variability that may be essential to vocal-motor feedback, and the refinement of song during sensorimotor learning (Thompson and Johnson, 2007).
Although comparison of optical density measurements across developmental groups did not reveal significant intensity differences, there are clear differences in the patterns of fiber expression at 25 days, and staining of small, five to ten micron somata at later ages (compare Fig 3B and Fig 3D, F and H). The lack of variable DAGLα density measures may indicate that endocannabinoid signaling plays a more modest role in lMAN maturation than within other song regions. This possibility is interesting in the context of lMAN as an “error generator”, implying that exogenous cannabinoid exposure will have a lesser effect on reducing song variability, but greater effects within auditory and vocal motor regions that may disrupt appropriate song error responses. Accumulating evidence demonstrates that a shift from lMAN to HVC control over vocal output during this period is responsible for the transition from early variable output that is refined with experience to become a mature, stereotyped song (Olveczky et al., 2011). Thus, less dynamic developmental DAGLα expression within lMAN documented here may reflect the decreased role this region plays in vocal learning over the developmental period studied. Developmental expression differences associated with the transition of lMAN to HVC vocal control may help explain the distinct effects of exogenous cannabinoid agonists to reduce note numbers and song stereotypy (Soderstrom and Johnson, 2003; Soderstrom and Tian, 2004).
5. Conclusions
We have found a clear developmental regulation of expression the enzyme DAGLα within zebra finch brain regions critical for vocal learning and production. As DAGLα is responsible for release of the principal endocannabinoid in zebra finch brain, 2-AG, these results add to accumulating evidence of the importance of endocannabinoid signaling during late-postnatal CNS maturation. These results also suggest mechanisms by which exogenous cannabinoid exposure may disregulate normal development, and contribute to established persistently-altered learned behaviors that follow developmental cannabinoid exposure.
Highlights.
DAGLα is the enzyme responsible for production of the principal endogenous cannabinoid in zebra finch CNS, 2-arachidonyl glycerol (2-AG).
DAGLα is distinctly expressed in a subset of brain regions that control vocal learning and production.
We examine the CNS DAGLα expression at 25, 50, 75 and 100 days, spanning sensorimotor vocal learning.
DAGLα staining generally peaks at 75 days of age, late in the sensorimotor song learning period.
Developmental regulation of DAGLα expression contributes to evidence for an endocannabinoid role in sensorimotor learning.
Acknowledgments
This work was supported by NIH NIDA grant R01DA020109.
Abbreviations
- lMAN
Lateral magnocellular nucleus of anterior nidopallium
- RA
Robust nucleus of arcopallium
- L2
Auditory Field L2
- DLM
Medial portion of the dorsolateral nucleus of the thalamus
- Ov
Nucleus Ovoidalis
Footnotes
Ethical Statement
The studies described in this manuscript involve the use of vertebrate animals.
Use of vertebrate animals was done according to protocols compliant with all ethical standards and approved by the Animal Care and Use Committee of East Carolina University.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Ashton JC, Appleton I, Darlington CL, Smith PF. Immunohistochemical localization of cannabinoid CB1 receptor in inhibitory interneurons in the cerebellum. Cerebellum. 2004;3:222–226. doi: 10.1080/14734220410019011. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J, Braun K. Differential emotional experience leads to pruning of dendritic spines in the forebrain of domestic chicks. Neural Plast. 1998;6:17–27. doi: 10.1155/NP.1998.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhuis JJ, Gobes SM, Terpstra NJ, den Boer-Visser AM, Zandbergen MA. Learning-related neuronal activation in the zebra finch song system nucleus HVC in response to the bird’s own song. PLoS One. 2012;7:e41556. doi: 10.1371/journal.pone.0041556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW. The distribution of tyrosine hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Arnold AP. Developmental plasticity in neural circuits for a learned behavior. Annu Rev Neurosci. 1997;20:459–481. doi: 10.1146/annurev.neuro.20.1.459. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Glaessner SL, Arnold AP. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J Neurosci. 1985;5:1556–1562. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Bower JM. The organization of cerebellar cortical circuitry revisited: implications for function. Ann N Y Acad Sci. 2002;978:135–155. doi: 10.1111/j.1749-6632.2002.tb07562.x. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ball GF. Characterization and localization of D1 dopamine receptors in the sexually dimorphic vocal control nucleus, area X, and the basal ganglia of European starlings. J Neurobiol. 1994;25:767–780. doi: 10.1002/neu.480250703. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocellis L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Daou A, Ross MT, Johnson F, Hyson RL, Bertram R. Electrophysiological characterization and computational models of HVC neurons in the zebra finch. J Neurophysiol. 2013;110:1227–1245. doi: 10.1152/jn.00162.2013. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Endocannabinoid signaling in the brain: biosynthetic mechanisms in the limelight. Nat Neurosci. 2011;14:9–15. doi: 10.1038/nn.2720. [DOI] [PubMed] [Google Scholar]
- Diana MA, Marty A. Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE) Br J Pharmacol. 2004;142:9–19. doi: 10.1038/sj.bjp.0705726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elphick MR, Egertova M. The neurobiology and evolution of cannabinoid signalling. Philos Trans R Soc Lond B Biol Sci. 2001;356:381–408. doi: 10.1098/rstb.2000.0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Cytoarchitectonic organization and morphology of cells of the field L complex in male zebra finches (Taenopygia guttata) J Comp Neurol. 1992;325:388–404. doi: 10.1002/cne.903250306. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, Reisenberg M, Shen R, Zhang MY, Strassle BW, Lu P, Mark L, Piesla MJ, Deng K, Kouranova EV, Ring RH, Whiteside GT, Bates B, Walsh FS, Williams G, Pangalos MN, Samad TA, Doherty P. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–2024. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert MT, Soderstrom K. Late-postnatal cannabinoid exposure persistently elevates dendritic spine densities in area X and HVC song regions of zebra finch telencephalon. Brain Res. 2011 doi: 10.1016/j.brainres.2011.06.019. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann K, Bischof HJ. Delayed development of song control nuclei in the zebra finch is related to behavioral development. J Comp Neurol. 1986;245:167–175. doi: 10.1002/cne.902450204. [DOI] [PubMed] [Google Scholar]
- Hoogenraad CC, Akhmanova A. Dendritic spine plasticity: new regulatory roles of dynamic microtubules. Neuroscientist. 2010;16:650–661. doi: 10.1177/1073858410386357. [DOI] [PubMed] [Google Scholar]
- Johnson F, Bottjer SW. Induced cell death in a thalamic nucleus during a restricted period of zebra finch vocal development. J Neurosci. 1993;13:2452–2462. doi: 10.1523/JNEUROSCI.13-06-02452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J Comp Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Cascio MG, Di Marzo V. Endocannabinoid metabolic pathways and enzymes. Curr Drug Targets CNS Neurol Disord. 2005;4:615–623. doi: 10.2174/156800705774933104. [DOI] [PubMed] [Google Scholar]
- Luo M, Ding L, Perkel DJ. An avian basal ganglia pathway essential for vocal learning forms a closed topographic loop. J Neurosci. 2001;21:6836–6845. doi: 10.1523/JNEUROSCI.21-17-06836.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer U, Watanabe S, Bischof HJ. Spatial memory and the avian hippocampus: Research in zebra finches. Journal of Physiology-Paris. 2013;107:2–12. doi: 10.1016/j.jphysparis.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R, Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci U S A. 1991;88:4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KI, Doupe AJ. Organizing principles of spectro-temporal encoding in the avian primary auditory area field L. Neuron. 2008;58:938–955. doi: 10.1016/j.neuron.2008.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Auditory feedback is necessary for the maintenance of stereotyped song in adult zebra finches. Behav Neural Biol. 1992;57:58–66. doi: 10.1016/0163-1047(92)90757-u. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Olveczky BP, Otchy TM, Goldberg JH, Aronov D, Fee MS. Changes in the neural control of a complex motor sequence during learning. J Neurophysiol. 2011;106:386–397. doi: 10.1152/jn.00018.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakan JM, Graham DJ, Iwaniuk AN, Wylie DR. Differential projections from the vestibular nuclei to the flocculus and uvula-nodulus in pigeons (Columba livia) J Comp Neurol. 2008;508:402–417. doi: 10.1002/cne.21623. [DOI] [PubMed] [Google Scholar]
- Palmer RH, Young SE, Hopfer CJ, Corley RP, Stallings MC, Crowley TJ, Hewitt JK. Developmental epidemiology of drug use and abuse in adolescence and young adulthood: Evidence of generalized risk. Drug Alcohol Depend. 2009;102:78–87. doi: 10.1016/j.drugalcdep.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. J Comp Neurol. 2008;508:840–866. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- Person AL, Perkel DJ. Pallidal neuron activity increases during sensory relay through thalamus in a songbird circuit essential for learning. J Neurosci. 2007;27:8687–8698. doi: 10.1523/JNEUROSCI.2045-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL. Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Brain Res Rev. 1998;28:235–285. doi: 10.1016/s0165-0173(98)00016-2. [DOI] [PubMed] [Google Scholar]
- Reiner AP, DJ, Bruce L, Butler AB, Csillag A, Kunzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised Nomenclature for Avian Telencephalon and Some Related Brainstem Nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol. 2008;13:253–263. doi: 10.1111/j.1369-1600.2008.00110.x. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Gilbert MT. Cannabinoid mitigation of neuronal morphological change important to development and learning: insight from a zebra finch model of psychopharmacology. Life Sci. 2013;92:467–475. doi: 10.1016/j.lfs.2012.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Johnson F. Cannabinoid exposure alters learning of zebra finch vocal patterns. Brain Res Dev Brain Res. 2003;142:215–217. doi: 10.1016/s0165-3806(03)00061-0. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Poklis JL, Lichtman AH. Cannabinoid exposure during zebra finch sensorimotor vocal learning persistently alters expression of endocannabinoid signaling elements and acute agonist responsiveness. BMC Neurosci. 2011;12:3. doi: 10.1186/1471-2202-12-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Distinct periods of cannabinoid sensitivity during zebra finch vocal development. Brain Res Dev Brain Res. 2004;153:225–232. doi: 10.1016/j.devbrainres.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. Developmental pattern of CB1 cannabinoid receptor immunoreactivity in brain regions important to zebra finch (Taeniopygia guttata) song learning and control. J Comp Neurol. 2006;496:739–758. doi: 10.1002/cne.20963. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Tian Q. CB(1) cannabinoid receptor activation dose dependently modulates neuronal activity within caudal but not rostral song control regions of adult zebra finch telencephalon. Psychopharmacology (Berl) 2008;199:265–273. doi: 10.1007/s00213-008-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Suarez J, Bermudez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509:400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Tanimura A, Kawata S, Hashimoto K, Kano M. Not glutamate but endocannabinoids mediate retrograde suppression of cerebellar parallel fiber to Purkinje cell synaptic transmission in young adult rodents. Neuropharmacology. 2009;57:157–163. doi: 10.1016/j.neuropharm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Johnson F. HVC microlesions do not destabilize the vocal patterns of adult male zebra finches with prior ablation of LMAN. Dev Neurobiol. 2007;67:205–218. doi: 10.1002/dneu.20287. [DOI] [PubMed] [Google Scholar]
- Thompson JA, Perkel DJ. Endocannabinoids mediate synaptic plasticity at glutamatergic synapses on spiny neurons within a basal ganglia nucleus necessary for song learning. J Neurophysiol. 2011;105:1159–1169. doi: 10.1152/jn.00676.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JA, Wu W, Bertram R, Johnson F. Auditory-dependent vocal recovery in adult male zebra finches is facilitated by lesion of a forebrain pathway that includes the basal ganglia. J Neurosci. 2007;27:12308–12320. doi: 10.1523/JNEUROSCI.2853-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Whitney O, Soderstrom K, Johnson F. Post-transcriptional regulation of zenk expression associated with zebra finch vocal development. Brain Res Mol Brain Res. 2000;80:279–290. doi: 10.1016/s0169-328x(00)00178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild JM, Karten HJ, Frost BJ. Connections of the auditory forebrain in the pigeon (Columba livia) J Comp Neurol. 1993;337:32–62. doi: 10.1002/cne.903370103. [DOI] [PubMed] [Google Scholar]
- Wild JM, Krutzfeldt NO. Neocortical-like organization of avian auditory ‘cortex’. Commentary on Wang Y, Brzozowska-Prechtl A, Karten HJ (2010): Laminar and columnar auditory cortex in avian brain. Proc Natl Acad Sci USA. 2010;107:12676–12681. doi: 10.1159/000320215. Brain Behav Evol 76, 89–92. [DOI] [PubMed] [Google Scholar]
- Yip ZC, Miller-Sims VC, Bottjer SW. Morphology of axonal projections from the high vocal center to vocal motor cortex in songbirds. J Comp Neurol. 2012;520:2742–2756. doi: 10.1002/cne.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]