Abstract
Protein tyrosine phosphatase 1B (PTP1B) is a non-transmembrane protein tyrosine phosphatase that has come into focus as a critical regulator of multiple signaling pathways. The role of PTP1B in breast cancer remains unclear with evidence suggesting that PTP1B can exert both tumor-suppressing and tumor-promoting effects. To better define the role of PTP1B in human breast cancer, and its relationship with HER2, we performed immunohistochemical studies on a large cohort of functionally annotated primary breast cancer specimens. 683 of 1,402 (49 %) evaluable primary breast cancers are positive for PTP1B. There is no statistically significant association between PTP1B expression and age, tumor size, T stage, histologic grade, lymph node status, or histological subtype. Of note, there is no significant association between PTP1B expression and HER2 expression (PTP1B expression 53.1 % in HER2+ cancers vs. 47.5 % in HER2– cancers, p = 0.0985). However, PTP1B expression is significantly associated with estrogen receptor expression (PTP1B expression 50.7 % in ER+ cancers vs. 43.1 % in ER– cancers, p = 0.0137) and intrinsic molecular subtype (PTP1B expression 53.9 % in the luminal B HER2+ subtype and 37.9 % in the basal-like subtype). Of note, multivariate analyses demonstrate that PTP1B is an independent predictor of improved survival in breast cancer (HR 0.779, p = 0.006). Taken together, we demonstrate in the largest study to date that (1) PTP1B is commonly expressed in breast cancer, (2) there is no association or functional impact of PTP1B expression in HER2+ breast cancer, and (3) PTP1B expression in breast cancer is associated with significantly improved clinical outcome. Until additional studies are performed, caution should be exercised in using PTP1B inhibitors in human breast cancer.
Keywords: PTP1B, Breast cancer, HER2
Introduction
Reversible tyrosine phosphorylation, which is controlled by the opposite actions of protein tyrosine kinases and protein tyrosine phosphatases, constitutes an important mechanism for regulating diverse physiological processes such as cell growth, intercellular communication, cell adhesion, and migration [1, 2]. The protein tyrosine phosphatase 1B (PTP1B) is a non-transmembrane protein tyrosine phosphatase that is located on the cytoplasmic face of the endoplasmic reticulum and has recently come into focus as an important regulator of signaling pathways involved in human diseases such as diabetes, obesity, and cancer [3]. The multiple substrates of PTP1B are associated with diverse cellular processes involving proliferation, apoptosis, intercellular adhesion, and differentiation and include receptor tyrosine kinases, transcription factors, and cytoskeletal proteins [3]. Of note, PTP1B dephosphorylates the insulin receptor and its primary substrates as well as the tyrosine kinase JAK2, a downstream element of the leptin signaling cascade and thus serves as an inhibitor of the insulin and leptin signaling pathways [4–6]. Other important substrates of PTP1B include the Src homology phosphotyrosyl phosphatase (SHP) 1 and 2, the epithelial growth factor receptor (EGFR), and the platelet-derived growth factor receptor (PDGFR) [1].
While the biology of PTP1B as a negative regulator of the insulin and leptin signaling pathways has been extensively studied, its role in cancer metabolism remains unclear. Experimental evidence suggests that PTP1B can exert both tumor-suppressing and tumor-promoting effects. As many oncogenes encode proteins with a tyrosine kinase activity that appears to be directly involved in the process of transformation, overexpression of protein tyrosine phosphatases, which remove phosphate from tyrosine residues, are intuitive tumor suppressors. Consistent with this hypothesis, studies have shown that PTP1B overexpression diminishes tumorigenecity in transformed mouse fibroblasts [7, 8], and several reports suggest that PTP1B can promote apoptosis through down-regulation of pro-survival signaling [9, 10], enhancement of stress signaling [11], or facilitation of caspase activities [3, 12]. Consistent with this hypothesis is the finding that p53 and PTP1B double knockout mice show an increase of B cell lymphomas and a decreased survival when compared to p53-only knockout mice [13].
In contrast to these findings, there is evidence that PTP1B might act as a tumor promoter. For example, upregulation of PTP1B was associated with increased tumor growth in colon cancer cell lines [14], and it has been shown that PTP1B is able to block anti-tumor signaling pathways in carcinoma and lymphoma cell lines [15]. Additional evidence for a role of PTP1B in promoting tumorigenesis has come from studies of human cancers. Immunohistochemical studies looking at the expression of PTP1B in tumor cells found that it was upregulated in 80 % of ovarian carcinomas [16] and also elevated in squamous cell carcinomas when compared to normal human skin [17]. In addition, a recent report by Lessard et al. [18] suggests a tumor-promoting role of PTP1B in human prostate cancer.
The role of PTP1B has been studied most extensively in breast cancer, with several studies focused on the potential association of PTP1B with the human epidermal growth factor receptor 2 (HER2), and the potential for PTP1B to modulate HER2 signaling. In an important study by Wiener et al. [19], 21 out of 29 human breast cancers stained strongly for PTP1B when compared to normal breast tissue, and overexpression of PTP1B was noted in 11 out of 12 HER2+ cases. This strong association between PTP1B and HER2 expression suggests that PTP1B and HER2 may cooperate in the pathogenesis of this subtype of breast cancers. Two recent studies independently support this hypothesis, showing that PTP1B deficiency delays tumor progression and protects against lung metastasis in an activated HER2-induced genetically engineered mouse model of human breast cancer [20, 21]. In this model, both genetic deletion of PTP1B activity in the NDL2 transgenic mice, as well as chemical inhibition of PTP1B, delayed the time of tumor onset [20]. Importantly, PTP1B-deficient mice were not universally protected against the development of mammary tumors, as mammary tumors actuated by the polyoma middle T antigen were not affected by loss of PTP1B [21]. PTP1B therefore seems to have a selective affirmatory role in HER2 signaling [22], and even seems to be required for HER2-dependent transformation of human breast epithelial cells [23]. In contrast, in an older study, it was shown that PTP1B was capable of suppressing oncogenic transformation by HER2 [8]. Supporting a possible oncogenic role of PTP1B in breast cancer is the finding that overexpression of PTP1B in the mouse mammary gland leads to spontaneous mammary tumor development, suggesting that this tyrosine phosphatase can act as an oncogene on its own [20]. In humans, overexpression of PTP1B in breast epithelial cells distorts the normal acinar morphology and causes uninhibited proliferation and loss of polarity [23].
The pharmaceutical industry has developed a considerable interest in PTP1B as a potential drug target, as its inhibition might help control a number of common diseases such as adult-onset diabetes and obesity. Based on the studies reviewed above highlighting a potential role for PTP1B in HER2-mediated breast cancer, investigators have also proposed targeting this molecule in human breast cancer. To further explore the role of PTP1B in human breast cancer, we conducted an immunohistochemical study on a breast cancer tissue microarray (TMA) encompassing a total of 1,402 formalin-fixed breast cancer cases with detailed clinical annotation and outcomes data. The aim of our study was to investigate the association between PTP1B and HER2 and other clinicopathological parameters in breast cancer, and to study the impact of PTP1B on prognosis. The data are reported according to the Reporting recommendations for tumor marker prognostic studies (REMARK) [24].
Materials and methods
Tissue microarray
We used a TMA encompassing 2,020 breast cancer tissue punches from 1,579 formalin-fixed and paraffin-embedded tumor samples collected from patients diagnosed with primary breast cancer between 1985 and 2007 at the Institute for Pathology, University of Basel and the Viollier Institute in Basel, Switzerland. Of these 2,020 tissue punches, a total of 1,402 were evaluable for our study. The tissue samples were brought into a TMA format as previously described [25]. In brief, 0.6-mm tissue cylinders were punched out of donor tumor tissue blocks and transferred into a recipient paraffin block using a semi-automated tissue arrayer. Each TMA contained a number of tumor punches ranging from 159 to 522. Histopathological data were obtained from the pathology reports, and raw patient survival data were obtained from the Cancer Registry of Basel or from the patient's attending physician. Retrieval of tissue and clinical data was performed according to the regulations of the local institutional review boards and data safety laws with specific regard to ethical standards and patient confidentiality. The mean follow-up time was 80.8 months (range 1–263 months), and the median age of the patients was 63 years (range 27–101 years). Demographic information of the patients can be found in Table 1.
Table 1.
Demographic data
| Clinicopathologic parameter | |
|---|---|
| Mean age at diagnosis (years) | 63.5 |
| Mean tumor size (mm) | 30.7 |
| n (%) | |
|---|---|
| Tumor grade | |
| 1 | 324 (23.1) |
| 2 | 576 (41.1) |
| 3 | 502 (35.8) |
| Tumor size | |
| pT1 | 385 (27.5) |
| pT2 | 738 (52.6) |
| pT3 | 107 (7.6) |
| pT4 | 172 (12.3) |
| Lymph node stage | |
| pN0 | 725 (51.8) |
| pN1 | 540 (38.6) |
| pN2 | 134 (9.6) |
| Histological subtype | |
| Invasive ductal | 993 (70.9) |
| Invasive lobular | 203 (14.5) |
| Mucinous | 38 (2.7) |
| Apocrine | 17 (1.2) |
| Cribriform | 41 (2.9) |
| Papillary | 19 (1.3) |
| Medullary | 45 (3.2) |
| Other | 44 (3.1) |
| Molecular subtype | |
| Luminal A (ER+ and/or PR+, HER2–, Ki-67 < 14 %) | 218 (15.6) |
| Luminal B (HER2-negative) ER+ and/or PR+, HER2–, Ki-67 ≥ 14 %) | 683 (48.9) |
| Luminal B (HER2-positive) (ER+ and/or PR+, HER2+) | 156 (11.2) |
| HER2 type (ER– or PR–, HER2+) | 111 (7.9) |
| Basal-like (ER–,PR–, HER2–) | 227 (16.2) |
Immunohistochemistry
For immunohistochemical staining, 4-μm sections of the TMA blocks were incubated overnight with the primary monoclonal anti-PTP1B antibody (Epitomics Inc., Catalog Nr. 2066-1, CA, USA) in a dilution of 1:200 after heat-induced antigen retrieval with Citrate buffer at pH 6. Standard DAB technique (Dako EnVision + System-labeled Polymer Anti-rabbit followed by Liquid DAB + Substrate Chromogen System) was employed for immunostaining. Counterstaining was performed with hematoxylin solution. The percentage of cells with a distinctive strong cytoplasmic staining was estimated. All cases with a cytoplasmic PTP1B expression of ≥5 % were considered positive for PTP1B expression. The staining intensity of ER, PR, and HER2 was scored as described previously [26]. In brief, tumors were considered positive for ER if they showed nuclear staining in more than 10 % of tumor cells with an intensity score between 1 and 3.
Statistical analysis
The distributions of patient and clinical characteristics between PTP1B-positive and PTP1B-negative tumors were compared using Chi-square test, Wilcoxon rank sum test, or two-sample t test, as appropriate. The strength of association between PTP1B expression and other continuous or ordinal factors (such as tumor size and ER expression) was also described using Spearman's correlation coefficients (ρ). Overall survival (OS) was defined as the time from the first operation to death due to any cause. Survivors were censored at the date of last contact. Survival curves by PTP1B status were estimated using the Kaplan–Meier product-limit method and compared by log-rank test. Univariate Cox proportional hazard models were fit to identify factors significantly related to OS. To assess whether PTP1B was an independent predictor of survival, a multivariate Cox model was constructed to adjust other patient/clinical characteristics that were significant in the univariate analyses. Two-way interaction terms between PTP1B and other factors in the multivariate Cox model were also assessed. All analyses were two-sided and significance was set at a p value of 0.05. Statistical analyses were performed using SAS (SAS Institutes, Cary, NC).
Results
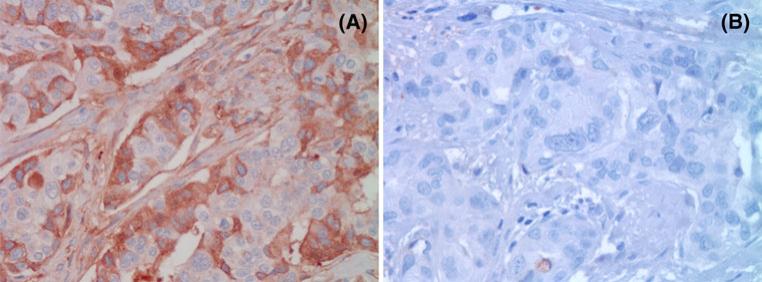
Consistent with the known intracellular localization of PTP1B, breast cancers expressing PTP1B showed strong cytoplasmic staining of PTP1B (Fig. 1). Using a threshold of ≥5 % of cancer cells staining positive for PTP1B, a total of 683 (49 %) of the 1,402 evaluable primary breast cancers were positive for PTP1B. PTP1B expression was not significantly associated with tumor size, age at diagnosis, tumor stage, tumor grade, lymph node status (Table 2), or histological subtype (data not shown). Of note, there was no significant association between PTP1B expression and HER2 expression (PTP1B expression 53.1 % in HER2+ cancers vs. 47.5 % in HER2– cancers, p = 0.0985, and Spearman's ρ = –0.04, Table 2). However, we did find a small, but statistically significant association between PTP1B expression and ER status (PTP1B expression 50.7 % in ER+ cancers vs. 43.1 % in ER– cancers, p = 0.0137, and Spearman's ρ = –0.07, Table 2). In addition, PTP1B expression is associated with the intrinsic subtypes of breast cancer, as defined by the St. Gallen consensus conference. The breast cancer intrinsic subtypes were originally defined by gene expression profiling [27, 28] but can be approximated using immunohistochemistry for estrogen receptor (ER), progesterone receptor (PR), Ki-67, and HER2 [29, 30]. These subtypes are known to have differing epidemiological risk factors, prognosis, and response to therapy [29]. PTP1B expression was highest in the luminal B HER2+ subtype (53.9 %) and lowest in the basal-like subtype (37.9 %, p = 0.008) (Table 3).
Fig. 1.
a PTP1B staining of a primary invasive ductal breast cancer with cytoplasmic staining of 50 % of cells (PTP1B, ×400), b invasive ductal breast cancer negative for PTP1B staining (PTP1B, ×400)
Table 2.
Association between PTP1B expression and clinicopatho-logical parameters
| Clinicopathologic parameter | PTP1B- positive | PTP1B- negative | Spearman's ρ | p value |
|---|---|---|---|---|
| Mean tumor size (mm) | 30.6 | 30.7 | 0.019 | 0.4797 |
| Mean age at diagnosis (years) | 63.1 | 63.8 | –0.029 | 0.3159 |
| n (%) | n (%) | Spearman's ρ | p value | |
|---|---|---|---|---|
| Tumor stage | 0.017 | 0.6283 | ||
| pT1 | 178 (46.2) | 207 (53.7) | ||
| pT2 | 371 (50.3) | 367 (49.7) | ||
| pT3 | 52 (48.6) | 55 (51.4) | ||
| pT4 | 82 (47.7) | 90 (52.3) | ||
| Tumor grade | –0.018 | 0.4753 | ||
| 1 | 157 (48.5) | 167 (51.5) | ||
| 2 | 291 (50.5) | 285 (49.5) | ||
| 3 | 235 (46.8) | 267 (53.2) | ||
| Lymph node stage | –0.026 | 0.6210 | ||
| pN0 | 363 (50.1) | 362 (49.9) | ||
| pN1 | 257 (47.6) | 283 (52.4) | ||
| pN2 | 63 (47.0) | 71 (52.9) | ||
| Estrogen receptor | –0.066 | 0.0137 | ||
| ER+ | 527 (50.7) | 513 (49.3) | ||
| ER– | 153 (43.1) | 202 (56.9) | ||
| HER2 | –0.044 | 0.0985 | ||
| HER2+ | 142 (53.1) | 125 (46.8) | ||
| HER2– | 537 (47.5) | 592 (52.4) |
Table 3.
Association between PTP1B expression and breast cancer intrinsic subtypes
| Molecular subtype | PTP1B-positive (n) | % | PTP1B-negative | % | p value |
|---|---|---|---|---|---|
| Luminal A (ER+ and/or PR+, HER2–, Ki-67 <14 %) | 107 | 49.1 | 111 | 50.9 | <0.008 |
| Luminal B (HER2-negative) ER+ and/or PR+, HER2–, Ki-67 ≥ 14 %) | 344 | 50.4 | 339 | 49.6 | |
| Luminal B (HER2-positive) (ER+ and/or PR+, HER2+) | 84 | 53.9 | 72 | 46.1 | |
| HER2 type (ER– or PR–, HER2+) | 58 | 52.2 | 53 | 47.8 | |
| Basal-like (ER–,PR–, HER2–) | 86 | 37.9 | 141 | 62.1 |
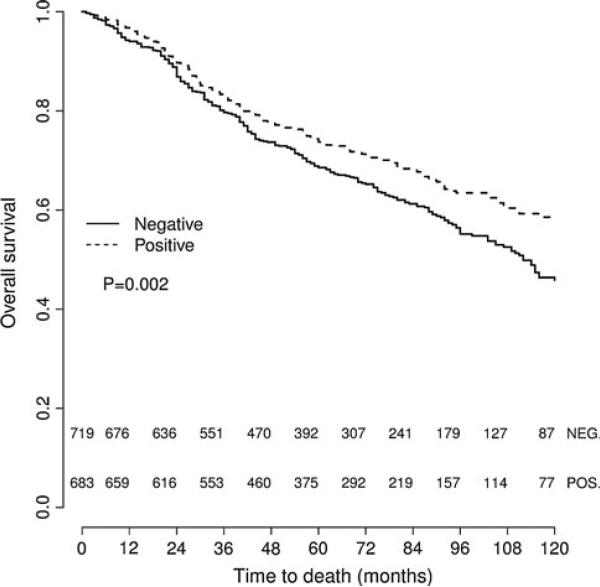
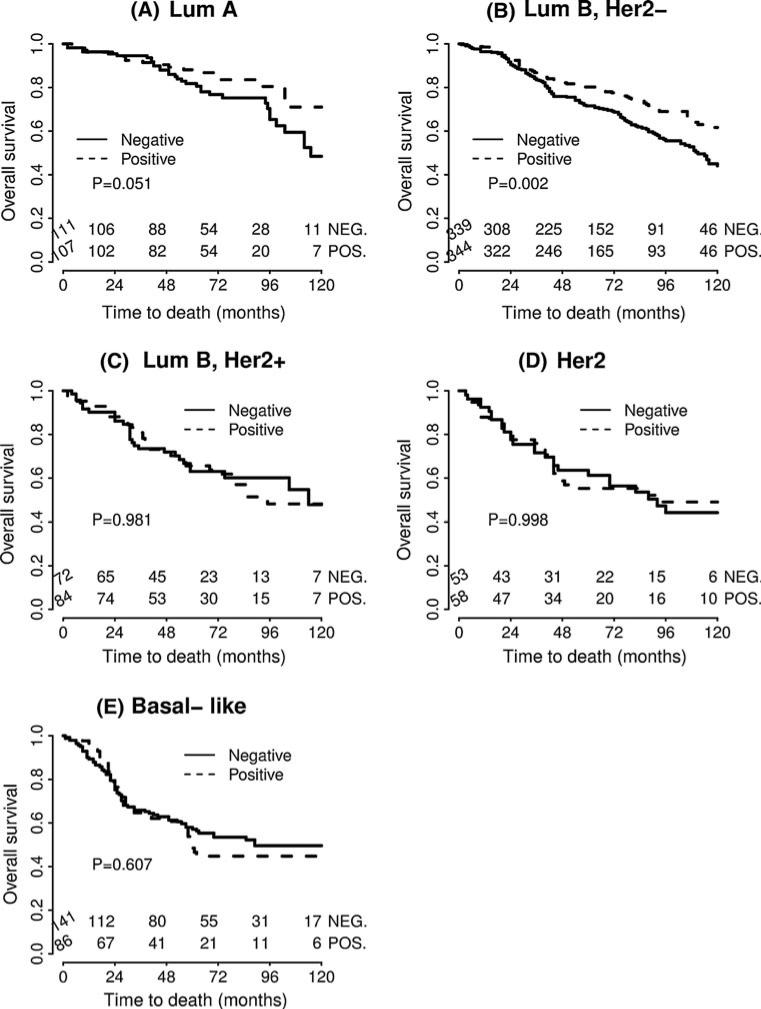
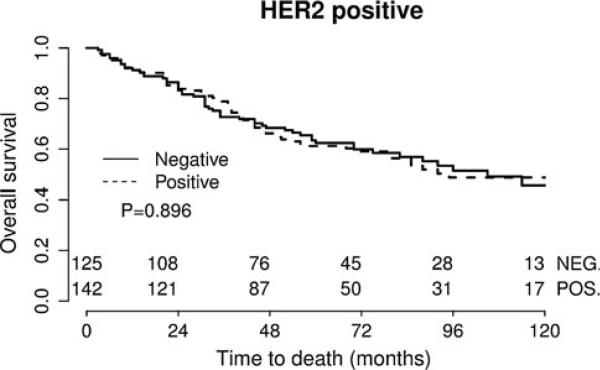
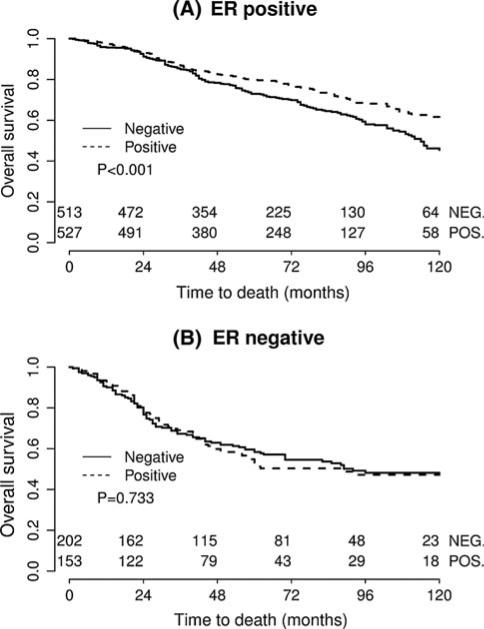
Studying the impact of PTP1B expression on survival, we found that in univariate survival analyses, breast cancer cases with positive PTP1B expression had a significantly better OS (hazard ratio (HR) = 0.763, p = 0.002) (Table 4; Fig. 2). In multivariate analyses, after adjusting for age, grade, tumor size, lymph node status, and intrinsic subtype, PTP1B remained an independent positive prognostic factor for OS (HR = 0.779, p = 0.006) (Table 5). In subset analyses of the specific intrinsic subtypes, PTP1B expression proved to be an independent prognostic factor for OS in the luminal B HER2– type, where positive PTP1B expression was associated with significantly better OS (HR = 0.661, p = 0.002) (Table 4; Fig. 3). The luminal A subtype also showed a strong trend toward improved OS with PTP1B expression (HR = 0.568, p = 0.051) (Table 4; Fig. 3), but this was not statistically significant. Of note, PTP1B expression did not have a significant impact on OS in HER2+ cases (Table 4; Fig. 5). However, PTP1B expression did have a positive effect on OS in ER+ cases (HR = 0.693, p < 0.001), but not in ER– cases (HR = 1.082, p = 0.733) (Table 4; Fig. 4).
Table 4.
Univariate analyses for the effect of PTP1B on OS, as well as the interaction effect between PTP1B and other factors (ER and HER2 status, intrinsic subtype)
| Hazard ratio (95 % CI) | p value | |
|---|---|---|
| All cases | ||
| PTP1B ≥5 % | 0.763 (0.641–0.908) | 0.002 |
| Interaction with ER status | 0.019* | |
| ER+ | 0.693 (0.561–0.858) | 0.001 |
| ER– | 1.082 (0.797–1.468) | 0.733 |
| Interaction with HER2 status | 0.129* | |
| HER2+ | 0.972 (0.677–1.396) | 0.896 |
| HER2– | 0.706 (0.578–0.862) | <0.001 |
| Interaction with intrinsic subtype | 0.049* | |
| Luminal A | 0.568 (0.321–1.003) | 0.051 |
| Luminal B (HER2–) | 0.661 (0.510–0.856) | 0.002 |
| Luminal B (HER2+) | 0.994 (0.607–1.629) | 0.981 |
| HER2 type | 0.999 (0.586–1.705) | 0.998 |
| Basal-like | 1.107 (0.751–1.632) | 0.607 |
p values for interaction terms
Fig. 2.
Kaplan–Meier survival curve for OS depending on PTP1B expression (univariate analysis). PTP1B positive cases show significantly better OS
Table 5.
Multivariate analysis for the effect of PTP1B on OS
| Clinicopathologic parameter | Hazard ratio (95 % CI) | p value |
|---|---|---|
| PTPB1 = 0 % (reference) | 1 | |
| PTP1B ≥5 % | 0.779 (0.651–0.930) | 0.006 |
| Age (per 1-year) | 1.037 (1.030–1.045) | <0.001 |
| Tumor grade | ||
| BRE grade 1 (reference) | 1 | |
| 2 | 1.622 (1.227–2.145) | <0.001 |
| 3 | 2.614 (1.955–3.495) | <0.001 |
| Tumor stage | ||
| pT1 (reference) | 1 | |
| pT2 | 1.6523 (1.272–2.147) | <0.001 |
| pT3 | 2.139 (1.483–3.086) | <0.001 |
| pT4 | 2.375 (1.713–3.291) | <0.001 |
| Lymph node stage | ||
| pN1 (reference) | 1 | |
| pN1 | 1.378 (1.130–1.681) | <0.001 |
| pN2 | 2.634 (1.997–3.475) | 0.002 |
| Molecular subtype | ||
| Luminal A (reference) | 1 | |
| Luminal B (HER2–) | 1.086 (0.801–1.474) | 0.594 |
| Luminal B (HER2+) | 1.3 (0.893–1.894) | 0.171 |
| HER2 type | 1.207 (0.800–1.821) | 0.369 |
| Basal-like | 1.871 (1.319–2.654) | <0.001 |
Fig. 3.
Kaplan–Meier survival curve for OS depending on PTP1B expression for the intrinsic breast cancer subtypes. PTP1B expression is an independent prognostic factor for OS in the luminal B HER2– type. The luminal A subtype also showed a strong trend toward improved OS with PTP1B expression
Fig. 5.
Kaplan–Meier survival curve for OS depending on PTP1B expression in the HER2+ subgroup. PTP1B expression has significant impact on OS in HER2+ cases
Fig. 4.
Kaplan–Meier survival curve for OS depending on PTP1B expression in the ER+ and ER– subgroups. PTP1B expression has a positive effect on OS in ER+ cases, but not in ER– cases
Discussion
In our study, we evaluated the expression of PTP1B in a large cohort of functionally annotated primary breast cancer specimens. We observed that PTP1B is expressed in 49 % of primary breast cancers. This is in contrast to the report by Wiener et al. [19], who observed PTP1B expression in 73 % of primary breast cancers. Their collective, however, was fairly small (n = 29), and the authors used a different evaluation system where they assigned expression intensities of 0–3+ to the tissues and considered 2+ and 3+ as positive. The authors also used a non-commercially available antibody which consists of a different clone, which could have led to a different staining pattern. As all our positive cases showed a strong staining intensity of 3+, we decided to estimate the percentage of positive cells and to take all cases that showed cytoplasmic staining ≥5 % of cancer cells as positive.
Our results show that expression of PTP1B in primary breast cancers is associated with significantly better OS. Therefore, in contrast to the experience with murine models of breast cancer, PTP1B appears to play a tumor-suppressive role in human breast cancer. This may be through dephosphorylation of dysregulated protein tyrosine kinases and concomitant inhibition of oncogenic signaling pathways. One well-described substrate for PTP1B is EGFR, a protein that is associated with worse OS in breast cancer and has recently been implicated in the promotion of breast cancer brain metastases [31, 32]. It could be that PTP1B plays a role in the dephosphorylation of EGFR in breast cancer, limiting invasion and migration of breast cancer cells. In fact, several studies have shown that PTP1B is able to strengthen and stabilize cell–cell adhesion [33, 34]. Alternatively, the effect of PTP1B on breast cancer could be mediated through the modulation of other signaling pathways. For example, several reports suggest that PTP1B promotes apoptosis through down-regulation of pro-survival signaling [9, 10, 12].
The study by Wiener et al. [19] showed a significant correlation between the expression of PTP1B and HER2, an observation that was not replicated in our larger cohort of 1,402 evaluable cases. As such, our data does not support the hypothesis put forward by several studies suggesting that there is a biologic association, and possible functional interaction, between these two proteins in breast cancer [19–21, 23]. We did observe a small positive correlation between the expression of PTP1B and ER in our series, and PTP1B expression had a significant positive effect on OS in ER+, but not ER– patients. This association has not been described in the literature to date, and the mechanisms linking ER to PTP1B have yet to be investigated.
The pharmaceutical industry has long focused on PTP1B as a potential therapeutic target for diabetes and obesity. Several companies have created potent and selective PTP1B inhibitors, and the question has been raised whether these inhibitors could also be used in breast cancer therapy [22, 35], particularly for HER2+ breast cancers [35]. Our results, however, suggest that (1) expression of PTP1B in human breast cancer might represent a favorable condition and (2) PTP1B may not have any functional implication in human HER2+ breast cancers. Further studies investigating the functional mechanisms of PTP1B in breast cancer are needed. Until we know more about the role of this enzyme in human breast cancer, caution should be exercised in using PTP1B inhibitors in a clinical setting with breast cancer patients.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standard Retrieval of tissue and clinical data was performed according to the regulations of the local institutional review boards (Ethikkommission Beider Basel, EKBB) and data safety laws with specific regard to ethical standards and patient confidentiality.
Contributor Information
S. Soysal, Department of Surgery, University Hospital Basel, Basel, Switzerland Department of Surgery, Washington University School of Medicine, St. Louis, USA.
E. C. Obermann, Institute of Pathology, University Hospital Basel, Schönbeinstrasse 40, 4031 Basel, Switzerland
F. Gao, Division of Biostatistics, Washington University School of Medicine, St. Louis, USA
D. Oertli, Department of Surgery, University Hospital Basel, Basel, Switzerland
W. E. Gillanders, Department of Surgery, Washington University School of Medicine, St. Louis, USA
C. T. Viehl, Department of Surgery, University Hospital Basel, Basel, Switzerland
S. Muenst, Department of Surgery, Washington University School of Medicine, St. Louis, USA Institute of Pathology, University Hospital Basel, Schönbeinstrasse 40, 4031 Basel, Switzerland.
References
- 1.Ferrari E, Tinti M, Costa S, Corallino S, Nardozza AP, Chatraryamontri A, Ceol A, Cesareni G, Castagnoli L. Identification of new substrates of the protein-tyrosine phosphatase PTP1B by Bayesian integration of proteome evidence. J Biol Chem. 2011;286(6):4173–4185. doi: 10.1074/jbc.M110.157420. doi:10.1074/jbc.M110.157420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6(4):307–320. doi: 10.1038/nrc1837. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 3.Lessard L, Stuible M, Tremblay ML. The two faces of PTP1B in cancer. Biochim Biophys Acta. 2010;1804(3):613–619. doi: 10.1016/j.bbapap.2009.09.018. doi: 10.1016/j.bbapap.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Mok A, Cao H, Zinman B, Hanley AJ, Harris SB, Kennedy BP, Hegele RA. A single nucleotide polymorphism in protein tyrosine phosphatase PTP-1B is associated with protection from diabetes or impaired glucose tolerance in Oji-Cree. J Clin Endocrinol Metab. 2002;87(2):724–727. doi: 10.1210/jcem.87.2.8253. [DOI] [PubMed] [Google Scholar]
- 5.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283(5407):1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 6.Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2(4):497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- 7.Woodford-Thomas TA, Rhodes JD, Dixon JE. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown-Shimer S, Johnson KA, Hill DE, Bruskin AM. Effect of protein tyrosine phosphatase 1B expression on transformation by the human neu oncogene. Cancer Res. 1992;52(2):478–482. [PubMed] [Google Scholar]
- 9.Sangwan V, Paliouras GN, Cheng A, Dube N, Tremblay ML, Park M. Protein-tyrosine phosphatase 1B deficiency protects against Fas-induced hepatic failure. J Biol Chem. 2006;281(1):221–228. doi: 10.1074/jbc.M507858200. doi:10.1074/jbc.M507858200. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Rodriguez A, Escribano O, Alba J, Rondinone CM, Benito M, Valverde AM. Levels of protein tyrosine phosphatase 1B determine susceptibility to apoptosis in serum-deprived hepatocytes. J Cell Physiol. 2007;212(1):76–88. doi: 10.1002/jcp.21004. doi:10.1002/jcp.21004. [DOI] [PubMed] [Google Scholar]
- 11.Gu F, Nguyen DT, Stuible M, Dube N, Tremblay ML, Chevet E. Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem. 2004;279(48):49689–49693. doi: 10.1074/jbc.C400261200. doi:10.1074/jbc.C400261200. [DOI] [PubMed] [Google Scholar]
- 12.Akasaki Y, Liu G, Matundan HH, Ng H, Yuan X, Zeng Z, Black KL, Yu JS. A peroxisome proliferator-activated receptor-gamma agonist, troglitazone, facilitates caspase-8 and -9 activities by increasing the enzymatic activity of protein-tyrosine phosphatase-1B on human glioma cells. J Biol Chem. 2006;281(10):6165–6174. doi: 10.1074/jbc.M505266200. doi:10.1074/jbc.M505266200. [DOI] [PubMed] [Google Scholar]
- 13.Dube N, Bourdeau A, Heinonen KM, Cheng A, Loy AL, Tremblay ML. Genetic ablation of protein tyrosine phosphatase 1B accelerates lymphomagenesis of p53-null mice through the regulation of B-cell development. Cancer Res. 2005;65(21):10088–10095. doi: 10.1158/0008-5472.CAN-05-1353. doi:10.1158/0008-5472.CAN-05-1353. [DOI] [PubMed] [Google Scholar]
- 14.Zhu S, Bjorge JD, Fujita DJ. PTP1B contributes to the oncogenic properties of colon cancer cells through Src activation. Cancer Res. 2007;67(21):10129–10137. doi: 10.1158/0008-5472.CAN-06-4338. doi:10.1158/0008-5472.CAN-06-4338. [DOI] [PubMed] [Google Scholar]
- 15.Lu X, Malumbres R, Shields B, Jiang X, Sarosiek KA, Natkunam Y, Tiganis T, Lossos IS. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112(10):4098–4108. doi: 10.1182/blood-2008-03-148726. doi:10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiener JR, Hurteau JA, Kerns BJ, Whitaker RS, Conaway MR, Berchuck A, Bast RC., Jr Overexpression of the tyrosine phosphatase PTP1B is associated with human ovarian carcinomas. Am J Obstet Gynecol. 1994;170(4):1177–1183. doi: 10.1016/s0002-9378(94)70118-0. [DOI] [PubMed] [Google Scholar]
- 17.Nanney LB, Davidson MK, Gates RE, Kano M, King LE., Jr Altered distribution and expression of protein tyrosine phosphatases in normal human skin as compared to squamous cell carcinomas. J Cutan Pathol. 1997;24(9):521–532. doi: 10.1111/j.1600-0560.1997.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 18.Lessard L, Labbe DP, Deblois G, Begin LR, Hardy S, Mes-Masson AM, Saad F, Trotman LC, Giguere V, Tremblay ML. PTP1B is an androgen receptor-regulated phosphatase that promotes the progression of prostate cancer. Cancer Res. 2012;72(6):1529–1537. doi: 10.1158/0008-5472.CAN-11-2602. doi:10.1158/0008-5472.CAN-11-2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiener JR, Kerns BJ, Harvey EL, Conaway MR, Iglehart JD, Berchuck A, Bast RC., Jr Overexpression of the protein tyrosine phosphatase PTP1B in human breast cancer: association with p185c-erbB-2 protein expression. J Natl Cancer Inst. 1994;86(5):372–378. doi: 10.1093/jnci/86.5.372. [DOI] [PubMed] [Google Scholar]
- 20.Julien SG, Dube N, Read M, Penney J, Paquet M, Han Y, Kennedy BP, Muller WJ, Tremblay ML. Protein tyrosine phosphatase 1B deficiency or inhibition delays ErbB2-induced mammary tumorigenesis and protects from lung metastasis. Nat Genet. 2007;39(3):338–346. doi: 10.1038/ng1963. doi:10.1038/ng1963. [DOI] [PubMed] [Google Scholar]
- 21.Bentires-Alj M, Neel BG. Protein-tyrosine phosphatase 1B is required for HER2/Neu-induced breast cancer. Cancer Res. 2007;67(6):2420–2424. doi: 10.1158/0008-5472.CAN-06-4610. doi:10.1158/0008-5472.CAN-06-4610. [DOI] [PubMed] [Google Scholar]
- 22.Yip SC, Saha S, Chernoff J. PTP1B: a double agent in metabolism and oncogenesis. Trends Biochem Sci. 2010;35(8):442–449. doi: 10.1016/j.tibs.2010.03.004. doi:10.1016/j.tibs.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arias-Romero LE, Saha S, Villamar-Cruz O, Yip SC, Ethier SP, Zhang ZY, Chernoff J. Activation of Src by protein tyrosine phosphatase 1B Is required for ErbB2 transformation of human breast epithelial cells. Cancer Res. 2009;69(11):4582–4588. doi: 10.1158/0008-5472.CAN-08-4001. doi: 10.1158/0008-5472.CAN-08-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM. REporting recommendations for tumor MAR-Ker prognostic studies (REMARK). Breast Cancer Res Treat. 2006;100(2):229–235. doi: 10.1007/s10549-006-9242-8. doi:10.1007/s10549-006-9242-8. [DOI] [PubMed] [Google Scholar]
- 25.Bubendorf L, Nocito A, Moch H, Sauter G. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol. 2001;195(1):72–79. doi: 10.1002/path.893. doi: 10.1002/path.893. [DOI] [PubMed] [Google Scholar]
- 26.Tapia C, Schraml P, Simon R, Al-Kuraya KS, Maurer R, Mirlacher M, Novotny H, Spichtin H, Mihatsch MJ, Sauter G. HER2 analysis in breast cancer: reduced immunoreactivity in FISH non-informative cancer biopsies. Int J Oncol. 2004;25(6):1551–1557. [PubMed] [Google Scholar]
- 27.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. doi:10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 28.Prat A, Perou CM. Deconstructing the molecular portraits of breast cancer. Mol Oncol. 2011;5(1):5–23. doi: 10.1016/j.molonc.2010.11.003. doi:10.1016/j.molonc.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. doi:10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, Heikkila P, Heikkinen T, Nevanlinna H, Akslen LA, Begin LR, Foulkes WD, Couch FJ, Wang X, Cafourek V, Olson JE, Baglietto L, Giles GG, Severi G, McLean CA, Southey MC, Rakha E, Green AR, Ellis IO, Sherman ME, Lissowska J, Anderson WF, Cox A, Cross SS, Reed MW, Provenzano E, Dawson SJ, Dunning AM, Humphreys M, Easton DF, Garcia-Closas M, Caldas C, Pharoah PD, Huntsman D. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. doi:10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nie F, Yang J, Wen S, An YL, Ding J, Ju SH, Zhao Z, Chen HJ, Peng XG, Wong ST, Zhao H, Teng GJ. Involvement of epidermal growth factor receptor overexpression in the promotion of breast cancer brain metastasis. Cancer. 2012 doi: 10.1002/cncr.27553. doi:10.1002/cncr.27553. [DOI] [PubMed] [Google Scholar]
- 32.Kallel I, Rebai M, Khabir A, Farid NR, Rebai A. Genetic polymorphisms in the EGFR (R521 K) and estrogen receptor (T594T) genes, EGFR and ErbB-2 protein expression, and breast cancer risk in Tunisia. J Biomed Biotechnol. 2009;2009:753683. doi: 10.1155/2009/753683. doi: 10.1155/2009/753683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Balsamo J, Arregui C, Leung T, Lilien J. The nonreceptor protein tyrosine phosphatase PTP1B binds to the cytoplasmic domain of N-cadherin and regulates the cadherin-actin linkage. J Cell Biol. 1998;143(2):523–532. doi: 10.1083/jcb.143.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura Y, Patrushev N, Inomata H, Mehta D, Urao N, Kim HW, Razvi M, Kini V, Mahadev K, Goldstein BJ, McKinney R, Fukai T, Ushio-Fukai M. Role of protein tyrosine phosphatase 1B in vascular endothelial growth factor signaling and cell–cell adhesions in endothelial cells. Circ Res. 2008;102(10):1182–1191. doi: 10.1161/CIRCRESAHA.107.167080. doi: 10.1161/CIRCRESAHA.107.167080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonks NK, Muthuswamy SK. A brake becomes an accelerator: PTP1B—a new therapeutic target for breast cancer. Cancer Cell. 2007;11(3):214–216. doi: 10.1016/j.ccr.2007.02.022. doi:10.1016/j.ccr.2007.02.022. [DOI] [PubMed] [Google Scholar]