Summary
This review focuses on research involving calorie restriction (CR) in humans and the resulting changes observed in endocrine and neuroendocrine systems. Special emphasis is given to the clinical science studies designed to investigate the effects of controlled, high-quality, energy-restricted diets on both biomarkers of longevity and on the development of chronic diseases of human aging.
Prolonged CR has been shown to extend both the median and maximal lifespan in a variety of lower species such as yeast, worms, fish, rats and mice. The biological mechanisms of this lifespan extension via CR are not fully elucidated, but possibly involve significant alterations in energy metabolism, oxidative damage, insulin sensitivity and functional changes in both neuroendocrine and sympathetic nervous systems. Most of the difficulty in characterizing the systemic endocrine and neuroendocrine changes with aging and CR is due to the limited capability to collect large and multiple blood samples from small animals, which are usually shorter lived, and hence the most studied. Ongoing studies of prolonged CR in humans are now making it possible to analyze changes in the “biomarkers of aging” to unravel some of the mechanisms of its anti-aging phenomenon.
With the incremental expansion of research endeavors in the area of energy restriction, data on the effects of CR in non-human primates and human subjects are becoming more accessible. Detailed analyses from controlled human trials involving long-term CR will allow investigators to link observed alterations from body composition and endocrine systems down to changes in molecular pathways and gene expression, with their possible effects on aging.
1. Introduction
Aging is associated with increased risk of metabolic disorders including overweight, obesity, insulin resistance, type 2 diabetes mellitus, atherosclerosis and cancer. Cross-sectional and longitudinal studies in humans and experimental animals suggest that over-consumption of energy-dense foods and lack of physical activity are the leading causes of weight gain, obesity and the related health issues (Calle et al., 2005). Research in the field of aging attempts to explore and unravel the mechanisms associated with four phenomena: 1) aging per se; 2) determinants of longevity; 3) age-associated disease; and 4) death (Hayflick, 2007). Aging is considered to be either ‘primary’-that is, the inevitable deterioration of cells, tissue structure and function that occurs independent of disease, lifestyle and environmental causes, or ‘secondary’- where the decline in tissue structure and function occurs as a result of these external influences (Holloszy and Fontana, 2007). Attenuation of primary aging therefore results in an increase in maximal lifespan, whereas delays in the progression of age-related disease or secondary aging increase mean lifespan. Calorie restriction (CR) is the only known non-pharmacological intervention that can slow primary aging and also has a protective effect against secondary aging. The impact of CR on aging and longevity in humans is not known.
2. Endocrine changes with human aging
The endocrine system is subject to numerous changes throughout a person’s lifespan (Figure 1). In general, most hormone deficiencies occur because of a reduced responsiveness of endocrine tissues to normal stimuli and a decreased hormone secretion from peripheral glands (Chahal and Drake, 2007). In addition, the hormone systems under neuroendocrine control are also dampened by the aging process. When considering age-associated changes in endocrine function, it is important to delineate which deficiencies occur as a consequence of primary aging compared to those which occur because of secondary aging or age-related disease. For primary aging, several hormone deficiencies are commonly observed in older individuals, including estrogen (menopause), testosterone (andropause), GH/IGF-1 (somatopause) and DHEA-S (adrenopause). The onset of age-related diseases is also associated with changes in endocrine function. Fasting insulin, for example, is typically elevated with aging, largely due to a resistance to insulin action in peripheral tissues caused by increased adiposity related to poor diet, physical inactivity and loss of fat-free mass (Moller et al., 2003). The thyroid gland is also a common casualty of aging. Thyroid dysfunction is common in the elderly, yet most of the thyroid deficiencies are associated with autoimmune complications rather than the aging process.
Figure 1. Impact of calorie restriction on factors related to aging.
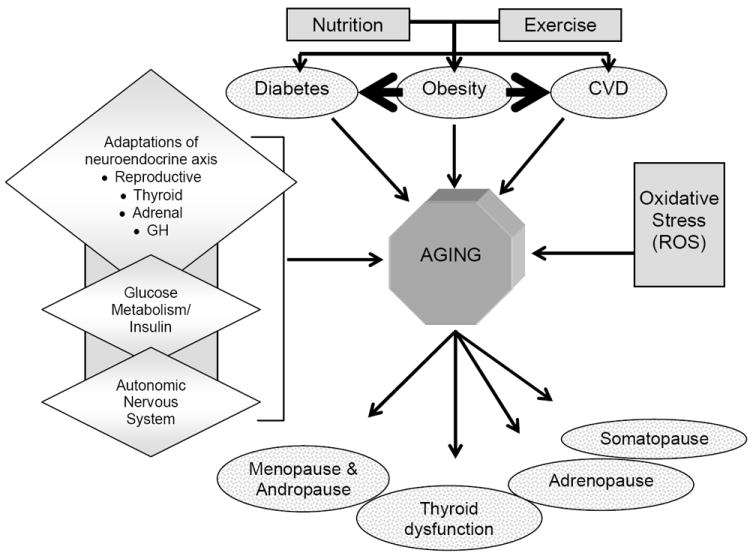
Non-mutually exclusive candidate mechanisms of CR include: Decreased oxidative damage due to reduced ROS generation and increased ROS removal; altered neuroendocrine function including growth hormone axis, thyroid axis, hypothalamic pituitary axis, autonomic nervous system, and carbohydrate metabolism; decreased incidence of chronic diseases such as obesity, diabetes and cardiovascular disease; and delayed onset of aging-related markers (i.e., glucose, insulin, DHEA-S and body temperature). Aging is then marked by changes in endocrine function which could occur as a result of the primary mechanisms of aging, or secondary due to lifestyle (nutrition and exercise) factors.
The clinical significance of endocrine changes with aging is highly variable. Aging is associated with body composition changes (accumulation of fat mass, loss of fat free mass), insulin resistance, altered plasma lipid profile, bone loss, decreased libido, reduced aerobic fitness (VO2max) and muscular strength, decreased protein synthesis and a decline in immune function (Hayflick, 2007). Hormone replacement paradigms with the intention to restore a youthful hormone profile and therefore reverse the clinical signs and symptoms of aging are the focus of many endocrine studies of aging. Hormone replacement strategies have been developed for some endocrine systems, e.g., estrogen replacement for the female reproductive system. However, the clinical application of hormone replacement therapy continues to be controversial among scientists and clinicians since preserving a young and middle-age hormone profile is not always safe and effective for improving clinical outcomes. Alternative non-pharmacological interventions proposed for lifespan extension - increased physical activity and/or calorie restriction, for instance - are increasing in popularity as a more attractive means of delaying age-associated endocrine changes and holding on to the fountain of youth.
2.1. Menopause
The most dramatic, rapidly occurring endocrine change in women is menopause, which is marked by the cessation of menstrual bleeding. Estrogen concentrations are consistently low and as a result the gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH) are elevated. The hypoestrogenemia is accompanied by atrophic changes of the breasts, uterus and vagina, vasomotor instability (hot flashes) and, in many instances, bone loss (Lindsay et al., 1996). Menopause on average occurs at 51.4 years of age (Grady et al., 1992) and has not changed over time. Since life expectancy of women is increasing, it is expected that women will spend more than one third of their life in a hypoestrogenic state. Estrogen is probably the most studied hormone for supplementation and replacement. There are several advantages of continued estrogen replacement; the clinical symptoms of menopause (hot flashes etc.) are managed and the negative effects of low estrogen on bone and atherosclerosis are delayed (Grady et al., 1992). While estrogen therapy does not appear to influence lifespan expectancy in women (Grady et al., 1992), long-term use of hormone replacement therapies (estrogen only, and estrogen in combination with progesterone) is not associated with a reduced risk of myocardial infarction or coronary heart disease events (Hulley et al., 1998) and has been shown to increase the risk for breast cancer by 5-6% with each year of use (Li et al., 2003).
2.2. Andropause
Gonadal function in men is also affected by aging. Cross-sectional and longitudinal data in men provide consistent evidence that testosterone concentrations progressively decline. This has been termed andropause (Gray et al., 1991; Morley et al., 1997). Commencing around age 40, there is a decrease in free testosterone, resulting partially from a reduction in total testosterone but largely from a rise in sex hormone binding globulin (Harman et al., 2001). Clinically, lower levels of total and free testosterone in aging men is probably responsible for increased fat mass, loss of muscle and bone mass, erectile dysfunction, depression, insulin resistance and increased CVD risk (Hak et al., 2002). The use of testosterone treatment has been suggested as an anti-aging remedy in men (Tenover, 1997) however, clinical trials have not reached a consensus with regard to the beneficial effects versus safety of testosterone replacement therapy (Baum and Crespi, 2007).
2.3. Insulin
A common metabolic condition associated with aging is type 2 diabetes mellitus, the prevalence of which increases from 20-30 years of age onwards. The Framingham Heart study and the 3rd National Health and Nutrition Examination Survey (NHANES) survey demonstrated steep and steady increases in the incidence of type 2 diabetes mellitus with increasing age after the third decade (Harris et al., 1998; Wilson et al., 1986). The NHANES dataset indicates that the prevalence of impaired glucose tolerance is ~40% in Americans 60-74 years of age (Cowie et al., 2006; Harris et al., 1998). Impaired glucose tolerance and type 2 diabetes mellitus likely ensue from a combination of a resistance to insulin action in peripheral tissues followed by a reduced secretion of insulin from β-cells after increasing demand (Moller et al., 2003). Physiological changes associated with aging such as changes in body composition, decreased physical fitness, hormonal alterations (reduced GH/IGF-1), and the secondary effects of high levels of free fatty acids and glucose, may also contribute to the impairment of insulin secretion and action (Moller et al., 2003). Furthermore, obesity is associated with low grade inflammation that can lead to insulin resistance, impaired glucose tolerance and even diabetes (Bastard et al., 2006). It is difficult for researchers to demonstrate impairment in β-cell function with age because findings are influenced by confounding factors such as obesity and its duration, and physical inactivity. After controlling for insulin sensitivity, defects in insulin secretion are consistently demonstrated in aging (Chang and Halter, 2003).
2.4. Thyroid Function
Aging is associated with significant changes in the structure of the thyroid gland and the function of the hypothalamic-pituitary- thyroid hormone axis (Fisher, 1996). Thyroid hormones are secreted by the thyroid gland under the influence of thyroid stimulating hormone (TSH) from the pituitary and the hypothalamic-derived thyrotropin releasing hormone. The thyroid hormones, thyroxine (T4) and 3,5,3’-triiodothyronine (T3), primarily act to regulate the metabolic rate of cells and tissues. Thyroid hormones also stimulate thermogenesis and exert effects on temperature regulation which are related to their effects on energy metabolism. Studies characterizing the aging thyroid axis are discordant in their findings. Epidemiological studies identified that hypothyroidism increases in aged individuals (Canaris et al., 2000; Hollowell et al., 2002). In adults aged between 20-50 years, there is little or no change in serum T4, free T4 (fT4) or TSH concentrations. In individuals older than 50, a number of progressive changes occur, but the net effect on circulating T4 or TSH is minimal (Ferrari et al., 2008; Solomon, 1991), with thyroid hormone levels low but still within normal ranges (Mariotti et al., 1993; Weissel, 2006). However, a progressive reduction in T3 is observed in aged individuals, and is believed to be mostly due to reduced peripheral conversion of T4 to T3 (Fisher, 1996). Unfortunately, the clinical symptoms of thyroid dysfunction with age are not well described, because comparisons are most often made with younger individuals rather than those with clinical hypothyroidism (Ferrari et al., 2008).
2.5. Somatopause
The somatotropic axis consists of the pituitary-derived hormone, growth hormone (GH) and insulin-like growth factor 1 (IGF-1). The somatotropic axis functions to carry out anabolic and lipolytic functions. In concert with sex steroids (estrogens and testosterone), GH and IGF-1 interact to instigate growth during puberty, maintenance of body composition and peak physical conditioning (Veldhuis et al., 2005). Concentrations of GH decline progressively with increasing age in men and women (Finkelstein et al., 1972; Zadik et al., 1985). Studies of 24-hour GH kinetics demonstrate that basal secretion, the maximal pituitary secretory capacity, secretory events and plasma elimination kinetics are unchanged by the aging process (Iranmanesh et al., 1994; Veldhuis et al., 2005), but show a decline in the amount of GH secreted with each secretory event (Iranmanesh et al., 1994). Epidemiological studies correlate the GH decline with obesity, particularly an increase in abdominal adiposity, reduced insulin sensitivity, dyslipidemia and sarcopenia (Corpas et al., 1993).
2.6. Adrenopause
Although aging is not associated with consistent changes in adrenocorticotrophic hormone (ACTH) or cortisol levels, concentrations of dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEA-S) decrease. This age-associated decline is termed “adrenopause” (Nawata et al., 2004). When growth and development are completed and adulthood is reached, DHEA and DHEA-S levels start to decline. By 70-80 years of age, peak DHEA-S concentrations are only 10-20% of those in young adulthood. Studies of calorie restriction in non-human primates at the National Institute of Aging identified DHEA-S concentration as a good marker of aging in these animals (Lane et al., 1997) and in men from the Baltimore Longitudinal Study of Aging (Roth et al., 2002a). DHEA-S therefore appears to be important for longevity. Some of the hypothesized anti-aging effects of DHEA-S include stimulation of the immune system (Okabe et al., 1995) and anti-aging effects on insulin sensitivity (Nakashima et al., 1995), body composition changes (Taniguchi et al., 1995), atherosclerosis (Taniguchi et al., 1996), bone (Nawata et al., 1995) and dementia (Yanase et al., 1996). For these reasons, DHEA has been studied as a potential hormone replacement strategy in aging men and women (Arlt et al., 1999; Morales et al., 1994). The few completed randomized controlled trials found an increased well-being (Arlt et al., 1999; Morales et al., 1994), improvements in depression and anxiety, increases in sex steroid levels (Arlt et al., 1999) and improvements in bone turnover (Baulieu et al., 2000).
3. Calorie restriction and aging
A non-pharmacological approach to counteracting the effects of aging on the health and longevity of organisms has been achieved through prolonged calorie restriction (CR). Since the 1930’s, CR has been shown to retard the aging process (McCay et al., 1935), extending the median and maximal lifespan in various animal species (Walford et al., 1987). While the exact mechanisms through which CR enhances lifespan have yet to be fully elucidated, several hypotheses have been proposed. CR reduces metabolic rate (although this is not seen in some studies) and oxidative damage, delays the incidence of age-related diseases including diabetes, cancers and cardiovascular disease, and has been shown to alter neuroendocrine activity in animals (Heilbronn and Ravussin, 2003). Increased lifespan through prolonged CR has been demonstrated in yeast and worms (Furuyama et al., 2002; Motta et al., 2004), flies (Kapahi et al., 2004), fish, mice and rats (Pugh et al., 1999; Shimokawa et al., 2003; Yamaza et al., 2004). Results from studies on rhesus monkeys suggest that prolonged CR opposes many age-associated pathophysiological changes. These studies demonstrate alterations in a variety of body systems and include learning and behavior changes (Pugh et al., 1999), lower body temperatures (Lane et al., 1996), lower plasma insulin concentrations (Roth et al., 2002a), and reductions in resting energy expenditure (Blanc et al., 2003). Since many changes associated with prolonged CR are important for the health and survival of humans, and excessive caloric intake is associated with morbidity and development of chronic diseases, it has become an important research objective to assess the feasibility, the safety and the effects of prolonged CR in well-controlled randomized human trials.
3.1. Calorie restriction may alter “rate of living”
The aging process may be influenced by CR through a reduction in the metabolic “rate of living” (Sacher and Duffy, 1979), leading ultimately to less oxidative damage. The rate of living theory posits that metabolic rate changes following dietary manipulation are due to several factors, including decreased tissue mass, reduced energy intake with decreased thermic effect of foods, and diminished size of the metabolizing mass (Heilbronn and Ravussin, 2003). An ongoing controversy among investigators in animal studies appears to be whether chronic CR leads to “metabolic adaptation”, that is, a reduction in the metabolic rate which is out of proportion to the loss of tissue mass of the organism. In 1985, a FAO/WHO/UNU report defined metabolic adaptation as “a process by which a new or different steady state is reached in response to a change or difference in the intake of food or nutrients.” Given this contextual definition, metabolic (Weyer et al., 2000), social or behavioral changes may be viewed as adaptations. While the rate of living theory of aging and lifespan extension remains one of the most popular hypothesis for the mechanism of CR and lifespan extension, it has been recently challenged by a number of esteemed CR investigators (Masoro, 2005; Speakman, 2005). Underlying problems in its interpretation include the inherent difficulty to compare metabolic rates across species and the widespread evaluation of resting metabolic rate as a measure of energy metabolism (Speakman, 2005).
3.2. Caloric restriction and oxidative stress
The “free radical theory of aging” or “oxidative stress” hypotheses are well-supported theories of aging. It is widely accepted that the metabolic rate of an organism plays a major role in the rate of aging, and is inversely related to its lifespan (Sohal and Allen, 1985). Since 1-3% of consumed oxygen is associated with the production of reactive oxygen species (ROS) (Alexeyev et al., 2004), the production of these highly reactive molecules from normal aerobic metabolism is also in direct proportion to an organism’s metabolic rate.
The influence of CR on the aging process has been evaluated, in part, on the basis of longevity, disease patterns, and age-associated biological changes (Barger et al., 2003). Additionally, many investigators have shown that modulation of the oxidative stress of an organism through prolonged CR is able to retard the aging process in various species, including mammals (Dandona et al., 2001; Jung et al., 2004; Lee and Yu, 1990; Sohal and Weindruch, 1996; Weindruch et al., 1986). For example, Dandona et al. (Dandona et al., 2001) found that protein carbonylation, a common measure of oxidative damage associated with age (Navarro and Boveris, 2004), was reduced after 4 weeks of CR in obese humans. Bevilacqua et al. demonstrated that even in response to short- and medium-term CR (40%) mitochondrial proton leaks are reduced with consequential reductions in VO2, ROS production, and cellular damage (Bevilacqua et al., 2004).
3.3. Caloric restriction and endocrine alterations
It is likely that the neuroendocrine system mediates many of the metabolic consequences of CR. Caloric excess is associated with a variety of endocrine perturbations (Smith, 1996). Similarly, the endocrine changes associated with caloric deprivation (CR or starvation) are well described in rodents as reviewed by Shimokawa (Shimokawa and Higami, 2001). Many of these alterations have been described in humans and include a fall in T3, an increase in cortisol secretion (Fichter et al., 1986) and a decrease in gonadal function. One area of discord between the human and rodent studies of CR is the somatotropic axis. GH secretion is decreased in rodents in response to CR, whereas humans that lose weight have an increase in GH secretion (Smith, 1996). Obesity per se is associated with increases in levels of free IGF -1 (Frystyk et al., 1995; Nam et al., 1997) and free fatty acids (FFA), both of which are known to decrease GH secretion through a negative feedback mechanism (Lee et al., 1995). Weight loss decreases the levels of both FFA and free IGF-1 and therefore should increase GH secretion. It has long been hypothesized that the neuroendocrine system coordinates and integrates some of the anti-aging actions of CR (Everitt et al., 1980; Meites, 1989; Nelson, 1994), but little is known about the neuroendocrine pathways that are altered by chronic CR (Nelson, 1994).
4. Endocrine alterations with CR in humans
The National Institute on Aging is sponsoring a randomized clinical trial, CALERIE (Comprehensive Assessment of the Long-term Effect of Reducing Intake of Energy) to test the effects of 25% CR for 2 years in non-obese (22≤BMI<28) healthy men and women aged 25-45 years. Three clinical sites are involved in the trial: Washington University in St. Louis, MO; Tufts University in Boston, MA; and the Pennington Biomedical Research Center in Baton Rouge, LA. The protocol and endpoints for this multi-center trial were developed from three independent Phase 1 trials conducted at each clinical site (Das et al., 2007; Heilbronn et al., 2006; Racette et al., 2006).
The Phase 1 study conducted at the Pennington Center involved 48 men and women, randomized to one of four treatment groups for 6 months (Civitarese et al., 2007; Larson-Meyer et al., 2006; Martin et al., 2007a; Martin et al., 2007b; Martin et al., 2007c; Redman et al., 2007; Williamson et al., 2007). For the CR group, individuals were restricted to 75% of their weight maintenance energy requirements (a 25% CR). The other groups were: 1) CR plus exercise group for which the calorie deficit was also 25% from weight maintenance but half (12.5%) was achieved by CR and half (12.5%) by increasing energy expenditure with structured aerobic exercise; 2) a low calorie diet group where participants consumed 890 kcal/d to achieve a 15% weight loss and thereafter followed a weight maintenance diet; and 3) a healthy diet control group that followed a weight-maintaining diet based on the American Heart Association Diet, Step 1.
Six months of CR produced favorable alterations in physiological, biochemical and hormonal outcomes. The 12 participants assigned to this treatment group completed the study and reported no development of eating disorder symptoms (Williamson et al., 2007) or reductions in quality of life indices (Williamson et al., 2008). There was a progressive reduction in body mass throughout the study, and after 6 months the group lost 10.4±0.9% of their body mass (Redman et al., 2007). Body composition analysis by DXA and multi-slice CT showed that the loss of tissue mass was attributable to significant reductions in both fat mass (CR:-24±3%) and fat-free mass (CR:-4±1%) and included a 27% decrease in both visceral and subcutaneous fat depots. Interestingly, the fat distribution within the abdomen was not altered by CR (Redman et al., 2007). We also observed a reduction in subcutaneous abdominal mean fat cell size by ~20%, and a lowering of hepatic lipid by ~37% but no change in skeletal muscle lipid content (Larson-Meyer et al., 2006).
With regard to longevity, two out of the three biomarkers of longevity (Roth et al., 2002a, b) were improved with 6 months of CR (Heilbronn et al., 2006). Significant reductions were observed in both fasting insulin concentrations and core body temperature. Interestingly, in parallel with the decrease in core temperature measured by telemetry, we observed a lowering of sedentary energy expenditure (24-hour energy expenditure measured in a metabolic chamber) but, more importantly, the lowering of the metabolic rate was larger than expected on the basis of weight and tissue loss (Heilbronn et al., 2006). In addition, these physiological responses were associated with a reduction in DNA damage probably due to lower production of ROS.
4.1. CALERIE and insulin
Epidemiological studies and studies of calorie-restricted rodents and monkeys hypothesize that insulin is a biomarker of aging and longevity. Data from the Baltimore Longitudinal Study of Aging support the association between longevity and insulin in humans (Lane et al., 1995; Roth et al., 2002a). In that study, men with plasma insulin concentration below median (after adjustment for BMI and age) had increased longevity. However, these subjects were not known to be in CR. A cross-sectional study by Fontana et al. (2004) compared individuals on self-imposed nutritionally adequate CR for an average of 6 years (CRONIES) to normal weight controls and found that CR subjects had lower serum insulin and glucose (Fontana et al., 2004). In a 12-month study of 20% CR in 50-60 year old men and women, fasting insulin concentrations were decreased and insulin sensitivity (measured during an OGTT) was increased (Weiss et al., 2006). In our study, 6 months of CR reduced fasting insulin concentrations by 29±6% (from 9.4±1.5 to 6.3±0.9 IU/mL) (Heilbronn et al., 2006). We also observed a 40% improvement in insulin sensitivity in the CR group, although this did not reach statistical significance (p=0.08) (Larson-Meyer et al., 2006). However, the acute insulin response to glucose (AIRg), was significantly decreased from baseline (CR: 29±7%, p<0.01), indicating an improvement in β-cell responsiveness to glucose.
4.2. CALERIE and thyroid function
Short-term studies of CR in humans have reported alterations in thyroid function. Four weeks of complete fasting resulted in a decrease in T3 and increase in reverse T3 (rT3) which was believed to be caused by the reduction in metabolic rate (Vagenakis et al., 1975). The CRONIES have significantly lower T3 but not T4 or TSH concentrations compared to age, sex and weight- matched controls (Fontana et al., 2006). The eight individuals confined in Biosphere 2 lost a considerable amount of weight during the first 6 months of their 2-year experiment due to a shortage of food and also had a decrease in thyroid hormones (Walford et al., 2002). Long-term CR in non-human primates (10 years of ~40%CR) resulted in a significant decrease in T4 levels in the CR animals, but no difference in T3 levels (DeLany et al., 1999). In the CALERIE study, plasma T3 concentrations were reduced from baseline in the CR group after 3 (p<0.01) and 6 months (p<0.02) of the intervention (Heilbronn et al., 2006). Similar results were found for change in plasma T4 in response to the treatment. When the data of the subjects in the three calorie-restricted groups were combined into one intervention sample, we observed significant linear relationships between the change in plasma thyroid hormones and the degree of metabolic adaptation in sedentary energy expenditure measured over 24h in a respiratory chamber at month 3 of intervention (T3; r=0.40, p=0.006 and T4; r=0.29, p=0.05) (Heilbronn et al., 2006).
4.3. CALERIE and the somatotropic axis
Aging is marked by a reduction in both GH and IGF-1 concentrations in healthy adults resulting from a reduced amount of GH secreted at each burst without alterations of burst frequency or GH half life (Veldhuis et al., 2005). Unlike rodents, weight loss via energy restriction in humans increases GH (Smith, 1996). After 6 months of CR, 11-hour mean GH concentrations were not changed nor was the secretory dynamics in terms of the number of secretion events, secretion amplitude and secretion mass (unpublished data). Ghrelin, a GH secretagogue, was significantly increased from baseline but IGF-1 was unaffected. Despite a significant reduction in weight and visceral fat and an improvement in insulin sensitivity, mean GH concentrations were not altered by the 6-month intervention. This agrees with the observation that both GH and IGF-1 were not affected by the chronic food shortage experienced by the individuals in Biosphere 2 (Walford et al., 2002).
4.4. Calorie restriction and DHEA-S
Given the evidence from cross-sectional (Orentreich et al., 1984) and longitudinal studies (Orentreich et al., 1992) that DHEA-S declines with age, DHEA-S is considered to be a reliable endocrine marker of human aging and longevity (Roth et al., 2002a). It was hypothesized that CR will delay or attenuate the age-associated decline in DHEA-S. In our 6-month study in young individuals (37±2 years), we observed no alteration in DHEA-S (Heilbronn et al., 2006). Similarly, DHEA-S was not changed with 2 years of energy restriction in the individuals within Biosphere 2 (Walford et al., 2002). To our knowledge, there has been no report of DHEA-S levels in those individuals from the Calorie Restriction Society (CRONIES) who are self-imposing CR. The lack of agreement between the human and non-human primate data is believed due to first, the chronological age of the subjects at the onset of CR, and second, to the duration of CR. Young adult monkeys undergoing CR for 3-6 years had an age-related decline in DHEA-S of 3% compared to 30% in monkeys fed ad libitum (Lane et al., 1997). In contrast, CR initiated in older animals (~22 years) did not attenuate the age-associated decline in DHEA-S (Urbanski et al., 2004). These explanations remain to be tested in longer term studies of CR in humans.
5. Conclusion
The term “biomarker of aging” implies a parameter that reflects physiologic or functional age; it must show significant age-related changes, be slowed or reversed by treatments that increase longevity (e.g., calorie restriction), and must be measured reliably. Numerous biomarkers have been identified in rodents and primates, including temperature and hormones DHEA-S and insulin (Figure 2). Since calorie restriction is the most robust means of delaying aging and reducing age-related diseases in several animal species, there is scientific interest to conduct randomized controlled trials of CR in humans. Human aging is marked by the dysregulation of many body systems including the endocrine system. Changes in endocrine systems include those in the reproductive axis, thyroid axis, somatotropic axis, the pancreas and adrenal glands. Studies of hormone replacement, while controversial and for the most part unconvincing, do provide intriguing evidence to suggest the effects of age maybe by restoring an attenuated youthful or middle-age hormonal profile. Calorie restriction in animals instigates numerous hormonal changes, including decreased insulin, DHEA-S, T3 and GH. While the exact mechanisms by which CR and longevity are linked are not known, it is likely that changes in the endocrine system play an important role in longevity. Longer-term studies are needed to adequately characterize any endocrine changes with CR in humans, and to determine whether the age-associated endocrine alterations are attenuated or delayed with CR. Additional data will become available in the near future, as the second phase of the CALERIE study is currently underway, testing the effects of 25% CR for 24 months in approximately 160 participants compared to 80 controls.
Figure 2. Can calorie restriction improve biological age and extend chronological age?
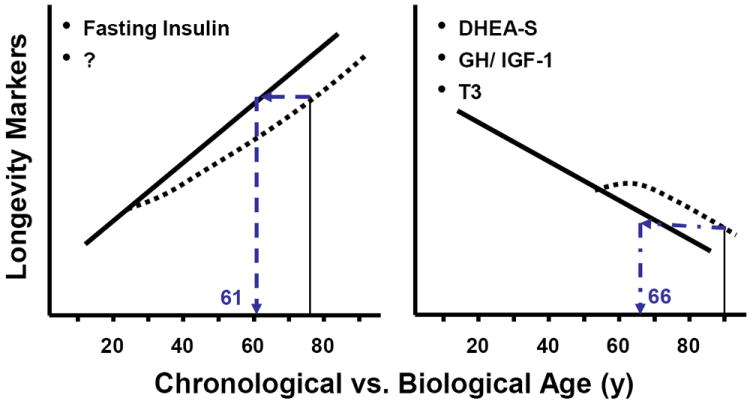
This figure illustrates three validated biomarkers of longevity. It is hypothesized that calorie restriction will change the trajectory of these biomarkers and therefore improve biological age and extend chronological age. For example, the left panel shows an individual aged 75 years who engages in prolonged calorie restriction from age 25 would have a reduced plasma insulin concentration which corresponds to a biological age 14 years younger. Similarly, the individual on the right at 90 years with prolonged calorie restriction will be biologically similar to an individual aged 66 years.
Table 1.
Summary of endocrine changes with calorie restriction in humans; a comparison across previously published studies.
| Variable | CALERIE | Biosphere 2 | CRONIES |
|---|---|---|---|
| DHEA-S | ↔ | ↔ | |
| GH | ↔ | ↔ | |
| IGF-1 | ↔ | ↔ | |
| Insulin | ↓ | ↓ | ↓ |
| T3 | ↓ | ↓ | ↓ |
| T4 | ↓ | ↔ | ↔ |
DHEA-S = dehydroepiandrosterone sulfate, GH = growth hormone, IGF-1 = insulin-like growth factor 1, T3 = triiodothyronine, T4 = thyroxine, ↓ = decreased by calorie restriction, ↑ = increased by calorie restriction, ↔ = not changed by calorie restriction.
References
- Calle EE, Teras LR, Thun MJ. Adiposity and physical activity as predictors of mortality. N Engl J Med. 2005;352:1381–4. doi: 10.1056/NEJM200503313521322. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Biological aging is no longer an unsolved problem. Ann N Y Acad Sci. 2007;1100:1–13. doi: 10.1196/annals.1395.001. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Fontana L. Caloric restriction in humans. Exp Gerontol. 2007;42:709–12. doi: 10.1016/j.exger.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal HS, Drake WM. The endocrine system and ageing. J Pathol. 2007;211:173–80. doi: 10.1002/path.2110. [DOI] [PubMed] [Google Scholar]
- Moller N, Gormsen L, Fuglsang J, Gjedsted J. Effects of ageing on insulin secretion and action. Horm Res. 2003;60:102–4. doi: 10.1159/000071233. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Bush TL, Grady D, Speroff L, Lobo RA. Therapeutic controversy: Estrogen replacement in menopause. J Clin Endocrinol Metab. 1996;81:3829–38. doi: 10.1210/jcem.81.11.8923821. [DOI] [PubMed] [Google Scholar]
- Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, Ernster VL, Cummings SR. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–37. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA. 1998;280:605–13. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- Li CI, Malone KE, Porter PL, Weiss NS, Tang MT, Cushing-Haugen KL, Daling JR. Relationship between long durations and different regimens of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–63. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–25. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism. 1997;46:410–3. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–31. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87:3632–9. doi: 10.1210/jcem.87.8.8762. [DOI] [PubMed] [Google Scholar]
- Tenover JL. Testosterone and the aging male. J Androl. 1997;18:103–6. [PubMed] [Google Scholar]
- Baum NH, Crespi CA. Testosterone replacement in elderly men. Geriatrics. 2007;62:15–8. [PubMed] [Google Scholar]
- Harris MI, Flegal KM, Cowie CC, Eberhardt MS, Goldstein DE, Little RR, Wiedmeyer HM, Byrd-Holt DD. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults. The Third National Health and Nutrition Examination Survey, 1988-1994. Diabetes Care. 1998;21:518–24. doi: 10.2337/diacare.21.4.518. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Anderson KM, Kannel WB. Epidemiology of diabetes mellitus in the elderly. The Framingham Study. Am J Med. 1986;80:3–9. doi: 10.1016/0002-9343(86)90532-2. [DOI] [PubMed] [Google Scholar]
- Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, Saydah SH, Williams DE, Geiss LS, Gregg EW. Prevalence of diabetes and impaired fasting glucose in adults in the U.S. population: National Health And Nutrition Examination Survey 1999-2002. Diabetes Care. 2006;29:1263–8. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, Feve B. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. doi: 10.1152/ajpendo.00366.2002. [DOI] [PubMed] [Google Scholar]
- Fisher DA. Physiological variations in thyroid hormones: physiological and pathophysiological considerations. Clin Chem. 1996;42:135–9. [PubMed] [Google Scholar]
- Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. The Colorado thyroid disease prevalence study. Arch Intern Med. 2000;160:526–34. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, Braverman LE. Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) J Clin Endocrinol Metab. 2002;87:489–99. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Ferrari E, Cravello L, Falvo F, Barili L, Solerte SB, Fioravanti M, Magri F. Neuroendocrine features in extreme longevity. Exp Gerontol. 2008;43:88–94. doi: 10.1016/j.exger.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Solomon DH. Effect on aging on thyroid hormone metabolism. Blackwell Scientific; Oxford: 1991. [Google Scholar]
- Mariotti S, Barbesino G, Caturegli P, Bartalena L, Sansoni P, Fagnoni F, Monti D, Fagiolo U, Franceschi C, Pinchera A. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab. 1993;77:1130–4. doi: 10.1210/jcem.77.5.8077303. [DOI] [PubMed] [Google Scholar]
- Weissel M. Disturbances of thyroid function in the elderly. Wien Klin Wochenschr. 2006;118:16–20. doi: 10.1007/s00508-005-0504-y. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Erickson D, Iranmanesh A, Miles JM, Bowers CY. Sex-steroid control of the aging somatotropic axis. Endocrinol Metab Clin North Am. 2005;34:877–93. viii. doi: 10.1016/j.ecl.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Finkelstein JW, Roffwarg HP, Boyar RM, Kream J, Hellman L. Age-related change in the twenty-four-hour spontaneous secretion of growth hormone. J Clin Endocrinol Metab. 1972;35:665–70. doi: 10.1210/jcem-35-5-665. [DOI] [PubMed] [Google Scholar]
- Zadik Z, Chalew SA, McCarter RJ, Jr, Meistas M, Kowarski AA. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J Clin Endocrinol Metab. 1985;60:513–6. doi: 10.1210/jcem-60-3-513. [DOI] [PubMed] [Google Scholar]
- Iranmanesh A, Grisso B, Veldhuis JD. Low basal and persistent pulsatile growth hormone secretion are revealed in normal and hyposomatotropic men studied with a new ultrasensitive chemiluminescence assay. J Clin Endocrinol Metab. 1994;78:526–35. doi: 10.1210/jcem.78.3.8126122. [DOI] [PubMed] [Google Scholar]
- Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- Nawata H, Yanase T, Goto K, Okabe T, Nomura M, Ashida K, Watanabe T. Adrenopause. Horm Res. 2004;62(Suppl 3):110–4. doi: 10.1159/000080509. [DOI] [PubMed] [Google Scholar]
- Lane MA, Ingram DK, Ball SS, Roth GS. Dehydroepiandrosterone sulfate: a biomarker of primate aging slowed by calorie restriction. J Clin Endocrinol Metab. 1997;82:2093–6. doi: 10.1210/jcem.82.7.4038. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002a:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Okabe T, Haji M, Takayanagi R, Adachi M, Imasaki K, Kurimoto F, Watanabe T, Nawata H. Up-regulation of high-affinity dehydroepiandrosterone binding activity by dehydroepiandrosterone in activated human T lymphocytes. J Clin Endocrinol Metab. 1995;80:2993–6. doi: 10.1210/jcem.80.10.7559886. [DOI] [PubMed] [Google Scholar]
- Nakashima N, Haji M, Sakai Y, Ono Y, Umeda F, Nawata H. Effect of dehydroepiandrosterone on glucose uptake in cultured human fibroblasts. Metabolism. 1995;44:543–8. doi: 10.1016/0026-0495(95)90065-9. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Yanase T, Haji M, Ishibashi K, Takayanagi R, Nawata H. The antiobesity effect of dehydroepiandrosterone in castrated or noncastrated obese Zucker male rats. Obes Res. 1995;3(Suppl 5):639S–643S. doi: 10.1002/j.1550-8528.1995.tb00480.x. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Yanase T, Kobayashi K, Takayanagi R, Nawata H. Dehydroepiandrosterone markedly inhibits the accumulation of cholesteryl ester in mouse macrophage J774-1 cells. Atherosclerosis. 1996;126:143–54. doi: 10.1016/0021-9150(96)05902-3. [DOI] [PubMed] [Google Scholar]
- Nawata H, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M. Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995;53:165–74. doi: 10.1016/0960-0760(95)00031-t. [DOI] [PubMed] [Google Scholar]
- Yanase T, Fukahori M, Taniguchi S, Nishi Y, Sakai Y, Takayanagi R, Haji M, Nawata H. Serum dehydroepiandrosterone (DHEA) and DHEA-sulfate (DHEA-S) in Alzheimer’s disease and in cerebrovascular dementia. Endocr J. 1996;43:119–23. doi: 10.1507/endocrj.43.119. [DOI] [PubMed] [Google Scholar]
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341:1013–20. doi: 10.1056/NEJM199909303411401. [DOI] [PubMed] [Google Scholar]
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78:1360–7. doi: 10.1210/jcem.78.6.7515387. [DOI] [PubMed] [Google Scholar]
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, de Lacharriere O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proc Natl Acad Sci U S A. 2000;97:4279–84. doi: 10.1073/pnas.97.8.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon th eultimate body size. J Nutr. 1935:63–79. [PubMed] [Google Scholar]
- Walford RL, Harris SB, Weindruch R. Dietary restriction and aging: historical phases, mechanisms and current directions. J Nutr. 1987;117:1650–4. doi: 10.1093/jn/117.10.1650. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–4. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L. Mammalian SIRT1 represses forkhead transcription factors. Cell. 2004;116:551–63. doi: 10.1016/s0092-8674(04)00126-6. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–90. doi: 10.1016/j.cub.2004.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–65. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y, Tsuchiya T, Otani H, Komatsu T, Chiba T, Yamaza H. Life span extension by reduction of the growth hormone-insulin-like growth factor-1 axis: relation to caloric restriction. Faseb J. 2003;17:1108–9. doi: 10.1096/fj.02-0819fje. [DOI] [PubMed] [Google Scholar]
- Yamaza H, Komatsu T, Chiba T, Toyama H, To K, Higami Y, Shimokawa I. A transgenic dwarf rat model as a tool for the study of calorie restriction and aging. Exp Gerontol. 2004;39:269–72. doi: 10.1016/j.exger.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–64. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc S, Schoeller D, Kemnitz J, Weindruch R, Colman R, Newton W, Wink K, Baum S, Ramsey J. Energy expenditure of rhesus monkeys subjected to 11 years of dietary restriction. J Clin Endocrinol Metab. 2003;88:16–23. doi: 10.1210/jc.2002-020405. [DOI] [PubMed] [Google Scholar]
- Sacher GA, Duffy PH. Genetic relation of life span to metabolic rate for inbred mouse strains and their hybrids. Fed Proc. 1979;38:184–8. [PubMed] [Google Scholar]
- Weyer C, Pratley RE, Salbe AD, Bogardus C, Ravussin E, Tataranni PA. Energy expenditure, fat oxidation, and body weight regulation: a study of metabolic adaptation to long-term weight change. J Clin Endocrinol Metab. 2000:1087–94. doi: 10.1210/jcem.85.3.6447. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mech Ageing Dev. 2005;126:913–22. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208:1717–30. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Allen RG. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- Alexeyev MF, Ledoux SP, Wilson GL. Mitochondrial DNA and aging. Clin Sci (Lond) 2004;107:355–64. doi: 10.1042/CS20040148. [DOI] [PubMed] [Google Scholar]
- Barger JL, Walford RL, Weindruch R. The retardation of aging by caloric restriction: its significance in the transgenic era. Exp Gerontol. 2003;38:1343–51. doi: 10.1016/j.exger.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, Prabhala A, Afzal A, Garg R. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- Jung KJ, Ishigami A, Maruyama N, Takahashi R, Goto S, Yu BP, Chung HY. Modulation of gene expression of SMP-30 by LPS and calorie restriction during aging process. Exp Gerontol. 2004;39:1169–77. doi: 10.1016/j.exger.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Lee DW, Yu BP. Modulation of free radicals and superoxide dismutases by age and dietary restriction. Aging (Milano) 1990;2:357–62. doi: 10.1007/BF03323951. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction, and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. J Nutr. 1986;116:641–54. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Navarro A, Boveris A. Rat brain and liver mitochondria develop oxidative stress and lose enzymatic activities on aging. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1244–9. doi: 10.1152/ajpregu.00226.2004. [DOI] [PubMed] [Google Scholar]
- Bevilacqua L, Ramsey JJ, Hagopian K, Weindruch R, Harper ME. Effects of short- and medium-term calorie restriction on muscle mitochondrial proton leak and reactive oxygen species production. Am J Physiol Endocrinol Metab. 2004;286:E852–61. doi: 10.1152/ajpendo.00367.2003. [DOI] [PubMed] [Google Scholar]
- Smith SR. The Endocrinology of Obesity. In: Bray G, editor. Endocrinology and Metabolism Clinics of North America. W.B Saunders; 1996. pp. 921–942. [DOI] [PubMed] [Google Scholar]
- Shimokawa I, Higami Y. Leptin and anti-aging action of caloric restriction. J Nutr Health Aging. 2001:43–8. [PubMed] [Google Scholar]
- Fichter MM, Pirke KM, Holsboer F. Weight loss causes neuroendocrine disturbances: experimental study in healthy starving subjects. Psychiatry Res. 1986:61–72. doi: 10.1016/0165-1781(86)90042-9. [DOI] [PubMed] [Google Scholar]
- Frystyk J, Vestbo E, Skjaerbaek C, Mogensen CE, Orskov H. Free insulin-like growth factors in human obesity. Metabolism. 1995:37–44. doi: 10.1016/0026-0495(95)90219-8. [DOI] [PubMed] [Google Scholar]
- Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997:355–9. doi: 10.1038/sj.ijo.0800412. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim KR, Lee HC, Cho JH, Nam MS, Nam SY, Song YD, Lim SK, Huh KB. Acipimox potentiates growth hormone response to growth hormone- releasing hormone by decreasing serum free fatty acid levels in hyperthyroidism. Metabolism. 1995:1509–12. doi: 10.1016/0026-0495(95)90154-x. [DOI] [PubMed] [Google Scholar]
- Everitt AV, Seedsman NJ, Jones F. The effects of hypophysectomy and continuous food restriction, begun at ages 70 and 400 days, on collagen aging, proteinuria, incidence of pathology and longevity in the male rat. Mech Ageing Dev. 1980:161–72. doi: 10.1016/0047-6374(80)90092-5. [DOI] [PubMed] [Google Scholar]
- Meites J. Evidence that underfeeding acts via the neuroendocrine system to influence aging processes. Prog Clin Biol Res. 1989:169–80. [PubMed] [Google Scholar]
- Nelson JF. Neuroendocrine involvement in the retardation of aging by food restriction: A hypothesis. In: Yu BP, editor. Modulation of Aging Processes by Dietary Restriction. CRC Press; Boca Raton, FL: 1994. pp. 37–55. [Google Scholar]
- Das SK, Gilhooly CH, Golden JK, Pittas AG, Fuss PJ, Cheatham RA, Tyler S, Tsay M, McCrory MA, Lichtenstein AH, Dallal GE, Dutta C, Bhapkar MV, Delany JP, Saltzman E, Roberts SB. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;85:1023–30. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette SB, Weiss EP, Villareal DT, Arif H, Steger-May K, Schechtman KB, Fontana L, Klein S, Holloszy JO. One year of caloric restriction in humans: feasibility and effects on body composition and abdominal adipose tissue. J Gerontol A Biol Sci Med Sci. 2006;61:943–50. doi: 10.1093/gerona/61.9.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civitarese AE, Carling S, Heilbronn LK, Hulver MH, Ukropcova B, Deutsch WA, Smith SR, Ravussin E. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson-Meyer DE, Heilbronn LK, Redman LM, Newcomer BR, Frisard MI, Anton S, Smith SR, Alfonso A, Ravussin E. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–44. doi: 10.2337/dc05-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Anton SD, Han H, York-Crowe E, Redman LM, Ravussin E, Williamson DA. Examination of cognitive function during six months of calorie restriction: results of a randomized controlled trial. Rejuvenation Res. 2007a;10:179–90. doi: 10.1089/rej.2006.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CK, Anton SD, York-Crowe E, Heilbronn LK, VanSkiver C, Redman LM, Greenway FL, Ravussin E, Williamson DA. Empirical evaluation of the ability to learn a calorie counting system and estimate portion size and food intake. Br J Nutr. 2007b;98:439–44. doi: 10.1017/S0007114507708802. [DOI] [PubMed] [Google Scholar]
- Martin CK, Heilbronn LK, de Jonge L, Delany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity (Silver Spring) 2007c;15:2964–73. doi: 10.1038/oby.2007.354. [DOI] [PubMed] [Google Scholar]
- Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E. Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrinol Metab. 2007;92:865–72. doi: 10.1210/jc.2006-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H, Ravussin E. Measurement of dietary restraint: validity tests of four questionnaires. Appetite. 2007;48:183–92. doi: 10.1016/j.appet.2006.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DA, Martin CK, Anton SD, York-Crowe E, Han H, Redman LM, Ravussin E. Is caloric restriction associated with development of eating disorder syndromes? Results from the CALERIE trial. Health Psychol. 2008;27:S32–S42. doi: 10.1037/0278-6133.27.1.S32. [DOI] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science. 2002b;297:811. doi: 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Lane MA, Baer DJ, Tilmont EM, Rumpler WV, Ingram DK, Roth GS, Cutler RG. Energy balance in rhesus monkeys (Macaca mulatta) subjected to long- term dietary restriction. J Gerontol A Biol Sci Med Sci. 1995:B295–302. doi: 10.1093/gerona/50a.5.b295. [DOI] [PubMed] [Google Scholar]
- Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagenakis AG, Burger A, Portnary GI, Rudolph M, O’Brian JR, Azizi F, Arky RA, Nicod P, Ingbar SH, Braverman LE. Diversion of peripheral thyroxine metabolism from activating to inactivating pathways during complete fasting. J Clin Endocrinol Metab. 1975;41:191–4. doi: 10.1210/jcem-41-1-191. [DOI] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO, Premachandra BN. Effect of long-term calorie restriction with adequate protein and micronutrients on thyroid hormones. J Clin Endocrinol Metab. 2006;91:3232–5. doi: 10.1210/jc.2006-0328. [DOI] [PubMed] [Google Scholar]
- Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–24. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- DeLany JP, Hansen BC, Bodkin NL, Hannah J, Bray GA. Long-term calorie restriction reduces energy expenditure in aging monkeys. J Gerontol A Biol Sci Med Sci. 1999;54:B5–11. doi: 10.1093/gerona/54.1.b5. discussion B12-3. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551–5. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Brind JL, Vogelman JH, Andres R, Baldwin H. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J Clin Endocrinol Metab. 1992;75:1002–4. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- Urbanski HF, Downs JL, Garyfallou VT, Mattison JA, Lane MA, Roth GS, Ingram DK. Effect of caloric restriction on the 24-hour plasma DHEAS and cortisol profiles of young and old male rhesus macaques. Ann N Y Acad Sci. 2004;1019:443–7. doi: 10.1196/annals.1297.081. [DOI] [PubMed] [Google Scholar]


