Abstract
New techniques for tissue engineering (TE) are rapidly emerging. The basic concept of autologous TE is to isolate cells from small biopsy specimens, and to expand these cells in culture for subsequent seeding onto biodegradable scaffolds. Nanocrystalline diamond films have attracted the attention of researchers from a variety of different areas in recent years, due to their unique and exceptional properties. In this approach, human dental stem cells (hDSCs) were characterized by flow cytometry and grown on diamond films with hydrogen (H)-terminated and oxygen (O)-terminated surfaces for 28 days, and then removed by lysis and washing with distilled water. Energy dispersive spectroscopy analysis was performed, showing that the regions with O-terminated surfaces contained much higher levels of deposited calcium, oxygen, and phosphorus. These results suggest that the extracellular matrix was considerably more developed in the O-terminated regions, as compared with the H-terminated regions. In addition, optical microscopy of hDSCs cultured on the diamond substrate with H- and O-terminated surfaces, before washing with distilled water, showed preferential directions of the cells arrangement, where orthogonal lines suggest that the cells appeared to be following the O-terminated regions or hydrophilic surface. These findings suggest that O-terminated diamond surfaces prepared on biodegradable scaffolds can be useful for mineralized dental tissue formation.
Introduction
Recent advances in the identification and characterization of dental stem cells (DSC) for tooth tissue engineering (TTE) applications indicate that within the next decade, bioengineering approaches may successfully be used to regenerate dental tissues1 and whole teeth.2–9 Strategies for TTE, as first described by Langer and Vacanti10 and Kaigler and Mooney,1 can be classified into three types—conductive, inductive, and cellular transplantation—the latter of which has guided the approach taken by this group for TTE.
New techniques for TE are rapidly emerging, and many innovative clinical technologies are likely to improve patient care and treatment during this century. The ability to use autologous cell-seeded scaffolds for TE applications fulfills two important roles—cell delivery and provision of a three-dimensional space and support for cell growth.11 Existing challenges for successful dental TE applications include identifying optimal combinations of cell types, scaffold biodegradable materials, and scaffold design to generate complex, bioengineered dental tissues exhibiting similar physical, mechanical, and functional characteristics to naturally formed teeth. Suitable scaffold materials must exhibit proper porosity, chemical composition, and mechanical properties to support the growth and differentiation of dental tissues, as well as serve as gene and/or growth factor delivery vehicles.
Two major cell types are involved in dental hard tissue formation—mesenchymal neural crest cell-derived odontoblasts, and the dental epithelial cell-derived ameloblasts. The commitment and differentiation of odontoblasts are regulated by the sequential and reciprocal interactions between the oral epithelium and the underlying neural crest-derived mesenchyme.12,13 Odontoblastic processes facilitate the secretion of dentin matrix, largely composed of the noncollagenous proteins dentin sialophosphoprotein (DSPP) and dentin matrix protein 1 (DMP1). The deposition of apatite minerals onto the dentin matrix results in mature calcified dentin formation. Ameloblasts, which align and polarize along the dentin–enamel junction, secrete the enamel matrix composed of the hydrophobic proteins amelogenin, ameloblastin, enamelin, amelotin, and tuftelin.14 In this way, dentin and enamel are formed, the two mineralized tissues that comprise the tooth crowns.
Nanocrystalline diamond (NCD) films have attracted the attention of researchers from a variety of different areas in recent years, due to their unique and exceptional properties.15 Combining diamond with smoother surfaces creates higher toughness, and a wide band gap and high electron emission efficiency. Dental TE applications, in particular, could be facilitated by the use of NCD films, whose three-dimensional coating could be used to promote dental cell adhesion and differentiation.16 A biocompatible material, diamond surfaces can also be modified by introducing hydrogen (H)-terminated or oxygen (O)-terminated surfaces. More complex surfaces can also be created using microlithography, to create selected surface areas with different terminations. The two different diamond surface (H and O terminated) modifications do not only result in altered electrical properties, but also affect hydrophobicity and hydrophilicity.17
Focused on the need to identify alternative scaffold materials that can guide the size and shape of bioengineered human dental tissues, and to better understand the manner through which scaffold materials instruct dental progenitor cell differentiation, the objective of the present study was to characterize hDSC synthesized extracellular matrix (ECM) formed on hydrogenated and oxidized diamond films surfaces. The H-terminated and O-terminated diamond surfaces were characterized and compared using atomic force microscopy, Kelvin force microscopy (KFM), and scanning electron microscopy (SEM). The contact angles of the diamond surfaces were used to evaluate relative wettability. SEM and energy dispersive spectroscopy (EDS) were used to characterize hDSC behavior on these diamond surfaces.
This research was performed at the Federal University of São Paulo (UNIFESP) and Thin Films Laboratory of Institute of Physics of the University of São Paulo, using protocols approved by the ethics committee of UNIFESP–Federal University of São Paulo—Paulista School of Medicine. Discarded human tooth tissues were used only with the free and enlightened consent of the participants. This research was developed at CTCMol, Center of Cellular and Molecular Therapy—UNIFESP-EPM (Federal University of São Paulo, Paulista School of Medicine) in São Paulo, Brazil and Tufts University, Boston, MA, under the supervision of Dr. Pamela C. Yelick.
Materials and Methods
hDSC isolation and culture
Human tooth tissues were collected from individual patients scheduled for diagnostic third molar extraction, as determined by professional dentists from public and/or private clinics, according to ethics committee recommendations (CEP–0595/01 and CONEP −13083). Isolated human enamel and pulp organ tissues were placed in Petri dishes containing prewarmed (37°C) Hank's balanced salt solution (HBSS; Gibco BRL). Human tooth tissues were digested for 30 to 40 min with 0.4 mg/mL type II collagenase (Sigma-Aldrich), and 0.2 mg/mL Dispase I (Boehringer Mannheim). Following enzyme digestion, the tissues were dissociated by trituration, and washed five times in 50% of Dulbecco's modified Eagle's medium (Gibco BRL) containing 10% fetal bovine serum (FBS), 5 mL glutamax, 50 units/mL penicillin, 50 mg/mL streptomycin, 2.5 mg/mL ascorbic acid, and 50% F12 medium (Sigma-Aldrich Corp.). Single-cell tooth bud suspensions were generated by filtration through a Falcon 40-micron cell strainer.3,8
hDSC characterization by flow cytometry
hDSCs were centrifuged at 1500 rpm for 5 min and transferred into 96 V-bottom well plates (Nunc) in 100 μL of staining buffer (phosphate-buffered saline [PBS] supplemented with 0.1% sodium azide [Sigma] and 1% FBS, pH 7.4–7.6) with the surface monoclonal antibodies panel. Cells were incubated at 4°C in darkness for 30 min, washed twice, and resuspended in 100 μL of fixation buffer (1% paraformaldehyde [Polysciences] in PBS, pH 7.4–7.6). Samples were acquired on a FACSCanto, using FACSDiva software (BD Biosciences), and then analyzed with FlowJo software (Tree Star). Fluorescence voltages were determined using matched unstained cells. Compensation was carried out with CompBeads (BD Biosciences) single stained with CD3-PerCP, CD4-FITC, CD8-APC-Cy7, CD4-PE-Cy7, CD3-PE, and CD3-APC. Samples were acquired until at least 800,000 events in a live lymphocyte gate. The flow cytometry analyses allow identifying through specific markers the presence of these cell populations.18–22 The following monoclonal antibodies were used in the FACS assays and some antibodies used in this study for mesenchymal cells are recognized in literature by CD90 and CD105, for epithelial cells are CD 166 and CD29 and for stem cells, CD34, from BD Biosciences. The cell characterizations were performed before the seeding to certify the presence of the hDSC population.
Diamond film preparation
NCD films were deposited on single-crystal silicon substrates using microwave plasma-assisted chemical vapor deposition (CVD) with a methane (CH4)/H2 gaseous source. Immediately after removal from the CVD chamber, the diamond film surface bonds become terminated with hydrogen. This is due to the fact that the hydrogen concentration in the plasma synthesis process is very high (94%), with only 3% nitrogen as a possible surface contamination, and the remaining gas is CH4, composed of carbon (diamond precursor) and hydrogen.
The diamond surfaces were prepared to contain alternate and adjacent regions of oxygen and hydrogen terminations.23 Initially, samples of diamond film with hydrogen termination were coated with polymethylmethacrylate polymer (PMMA) used as a resist for electron beam lithography. Next, a selected area of the PMMA was electron beam scanned in the SEM (JSM-6460 LV), creating a pattern by using an e-beam nanolithography system (Nanometer Pattern Generation System). The samples were then immersed in a developing solution and rinsed in isopropyl alcohol. Using these methods, the PMMA surface areas scanned by the electron beam were removed, and the remaining area was preserved. To replace the hydrogen terminations with oxygen, the lithographed diamond surface was subsequently exposed for several minutes to the oxygen plasma stream produced by a small hollow cathode plasma gun.24 The remaining PMMA were then removed in an acetone ultrasonic bath. In this way, the diamond samples with adjacent regions presenting hydrogen and oxygen terminations were obtained.
The wettability of the different surfaces was quantified using contact angle measurements,23 using a KSV Modular CAM 200 System. Samples with a diamond surface containing just one kind of termination were used for contact angle measurements. The sessile drop method was employed, using deionized water.
hDSC seeding onto diamond film with H- and O-terminated surfaces
Cells were resuspended in the same media and plated into six-well culture plates (Costar), each well containing a diamond disc previously prepared with selected H-terminated and O-terminated areas. Approximately 2×105 cells were plated into each well, and grown in 5% CO2 at 37°C until the cells reached confluence, with culture medium changes two times per week. At confluence, after 28 days, hDSCs were removed washing with distilled water and mechanic action by tooth brushing application and the diamond surfaces were analyzed using SEM and EDS. Each sample was analyzed in duplicate, using cells harvested from two individual patients.
Optical microscopy analysis
Optical microscopic analyses were performed using an Olympus microscope Model BX51 TRF.
SEM and EDS analysis
SEM and EDS analyses of the samples were performed using a JSM-6460 LV (Jeol) and EDS Thermo Noran software Noran six (Waltham).
Results and Discussion
The hDSCs were characterized first by a flow cytometer (Fig. 1), where we evidenced the presence of dental stem cells (Fig. 1a, b) and mixed cell populations of dental mesenchymal cells (Fig. 1c) and dental epithelial cells (Fig. 1d).
FIG. 1.
(a) Tooth germ when removed and the pulp region (the arrow shows the area where the soft tissue was removed for extracting the stem cells); (b) stem cells; (c) dental mesenchymal cells; (d) dental epithelial cells. Color images available online at www.liebertpub.com/tea
A typical SEM image of the H-terminated diamond films used in this work is presented (Fig. 2).
FIG. 2.

Scanning electron microscopy (SEM) image of the diamond films.
The diamond surfaces containing alternate and adjacent regions of oxygen and hydrogen terminations were characterized by KFM (Fig. 3) presenting a potential difference of about 80 mV.23 Secondary electron imaging (Fig. 4) allowed for identification of the regions with different terminations.23 Note that in Figure 4, the contrast is not related to the morphology, it is due to different electrical properties of the O- and H-terminated surfaces. The contact angle for diamond surfaces with oxygen terminations was 75°, and for H-terminated surfaces was 83°.23
FIG. 3.

Atomic force microscopy (a) and Kelvin force microscopy (b) of the diamond surface.
FIG. 4.
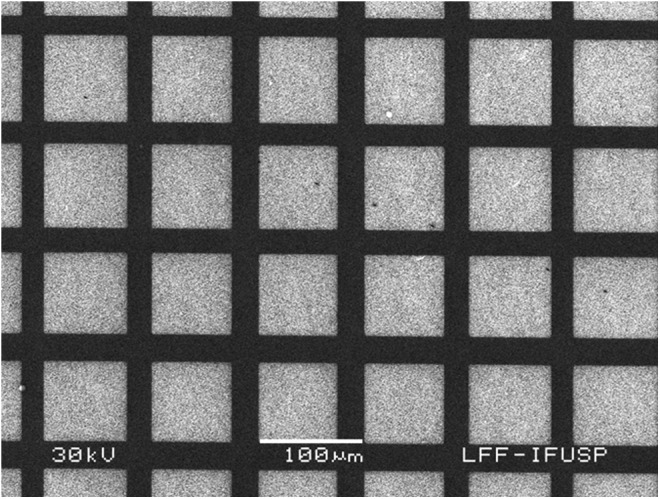
Scanning electron microscopy (SEM) of the diamond surfaces.
Tooth bud cell yields from impacted third molar teeth varied depending on the age of the individual and developmental stage of the tooth.9 Teeth less developed extracted usually are present in younger individuals. These were also less mineralized structures and provided more cells due to the presence of more soft tissue; this kept the stemness ability detected on the analysis of colony-forming units in early tooth development stage while other more developed teeth had more mineralized structures and less soft tissue quantity. We obtained on average cell yields of ∼1.0×106 cell/impacted third molar tooth.9
SEM images of ECM deposited by hDSCs grown on H- and O-terminated diamond surfaces are shown (Fig. 5). The contrasting H- and O-terminated diamond surface areas are clearly visible, due to the different electrical properties of these different regions, and as shown in Figure 4.
FIG. 5.

SEM micrograph of human dental stem cells (hDSCs) extracellular matrix elaborated on H- and O-terminated diamond surfaces.
A typical EDS analysis result is presented (Fig. 6a, b). The Figure 6a shows SEM micrograph of the H- and O-terminated diamond surfaces upon which hDSCs were grown and then removed by washing with distilled water and mechanic action. Note that it is possible to observe that the organic material was completely removed from this region. EDS analyses, performed in the arrow shown in Figure 6a, demonstrate that O-terminated surfaces contained much higher concentrations of calcium, oxygen, and phosphorus (Fig. 6b). Note that the monolayer oxygen surface terminations are not detectable by EDS analyses, indicating that the detected oxygen was deposited from the cultured hDSCs. Similar experiments were performed, subjecting the same substrate with H- and O-terminated surfaces to the culture medium, but without cells for 28 days. The result, in this case, is shown in Figure 6c and d, where clearly no difference is detected through regions with H- and O-terminated surfaces. In Figure 6b, it is possible to observe a periodicity spaced by about 100 μm, presenting higher concentrations of calcium, oxygen, and phosphorus, exactly in the regions with oxygen termination. On the other hand, in Figure 6d, no periodicity is observed, and the elements concentrations are randomized, showing no preference in the calcium, oxygen, and phosphorus deposition. From these results, we can conclude that the ECM was considerably more developed in the O-terminated regions, as compared with the H-terminated diamond surface regions.
FIG. 6.
(a) SEM micrograph of the diamond; (b) energy dispersive spectroscopy (EDS) analysis for the line drawn in (a); (c) SEM micrograph of the diamond with H- and O-terminated surfaces subjected to the culture medium, but without cells, during 28 days; (d) EDS analysis for the arrow drawn in (c).
Optical micrograph of hDSCs cultured on the diamond substrate with H- and O-terminated surfaces after 28 days shows that it is possible to observe preferential cell alignment and organization. Although the cells cover most of the surface, in the regions of lower cellular concentration, we can see orthogonal lines, suggesting that the cells appear to be following the O-terminated patterned regions (Fig. 7), where the ECM was developed.
FIG. 7.

Optical micrograph of hDSCs cultured for 28 days (10×), the arrows show the cells' orientation.
Summary and Conclusions
Mixed cell populations obtained from human tooth pulp were characterized by the flow cytometer and grown on diamond films with H- and O-terminated surfaces for 28 days, and then removed by lysis and washing with distilled water. EDS analyses showed that the regions with O-terminated surfaces contained much higher levels of deposited calcium, oxygen, and phosphorus. These results suggest that the ECM was considerably more developed in the O-terminated regions, as compared with the H-terminated regions. In addition, optical microscopy of hDSCs cultured on the diamond substrate with H- and O-terminated surfaces, before washing with distilled water, showed preferential directions of the cells' arrangement, where orthogonal lines suggest that the cells appeared to be following the O-terminated regions. In our control group, where we observed by EDS analyses, the diamond films only with the culture medium without cells, we can see no deposits of calcium and phosphorus. In fact, we can conclude that the cells deposited higher levels of calcium, oxygen, and phosphorus on O-terminated surfaces after 28 days in culture. These findings suggest that O-terminated diamond surfaces treated on biodegradable scaffolds can be useful for mineralized dental tissue formation.
Acknowledgments
We would like to thank Dr. Esper Georges Kallas for providing technical support of the LIM 60, Medical Investigation Laboratories (Kallas Lab), University of São Paulo. We thank the Brazilian sponsors UNIFESP, Plastic Surgery Department, FAPESP, CNPq, Instituto Nacional de Ciência e Tecnologia (INCT)–Biofabrication (CNPq 5736661/2008-1 and FAPESP 08/57860-3), the Rede Biofab, Ibero-American Network of Biofabrication-BIOFAB-CYTED (208RT0340), and NIH/NIDCR/NIBIB for their continued support (DE016132, TW007665 [P.C.Y.]). We also thank CNPq for scholarship productivity of S.E.D. and M.T.D. (310048/2011-7 e 310049/2011-3).
Disclosure Statement
The authors declare that no conflicts of interest, actual or potential, or competing financial interests exist.
References
- 1.Kaigler D. Mooney D. Tissue engineering's impact on dentistry. J Dent Educ. 2001;65:456. [PubMed] [Google Scholar]
- 2.Young C.S. Honda M. Terada S. Vacanti J.P. Bartlett J.D. Yelick P.C. Tissue engineering of complex tooth structures on biodegradable polymer scaffolds. J Dent Res. 2002;81:695. doi: 10.1177/154405910208101008. [DOI] [PubMed] [Google Scholar]
- 3.Duailibi M.T. Duailibi S.E. Young C.S. Vacanti J.P. Bartlett J.D. Yelick P.C. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 4.Young C.S. Abukawa H. Ariscan R. Ravens M. Troulis M.J. Kaban L.B. Vacanti J.P. Yelick P.C. Tissue-engineered hybrid tooth and bone. Tissue Eng. 2005;11:1599. doi: 10.1089/ten.2005.11.1599. [DOI] [PubMed] [Google Scholar]
- 5.Young C.S. Kim S.-W. Qin C. Baba O. Butler W.T. Taylor R.R. Bartlett J.D. Vacanti J.P. Yelick P.C. Developmental analysis and computer modeling of bioengineered teeth. Arch Oral Biol. 2005;50:259. doi: 10.1016/j.archoralbio.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 6.Duailibi S.E. Duailibi M.T. Vacanti J.P. Yelick P.C. Prospects for tooth regeneration. Periodontology. 2000;2006;41:177. doi: 10.1111/j.1600-0757.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 7.Snead M.L. Whole–tooth regeneration: it takes a village of scientists, clinicians, and patients. J Dent Educ. 2008;72:903. [PMC free article] [PubMed] [Google Scholar]
- 8.Duailibi S.E. Duailibi M.T. Zhang W. Asrican R. Vacanti J.P. Yelick P.C. Bioengineered dental tissue grown in the rat jaw. J Dent Res. 2008;87:745. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duailibi M.T. Duailibi S.E. Duailibi Neto E.F. Negreiros R.M. Jorge W.A. Ferreira L.M. Vacanti J.P. Yelick P.C. Tooth tissue engineering: optimal dental stem cell harvest based on tooth development. Artif Organs. 2011;35:129. doi: 10.1111/j.1525-1594.2010.01200.x. [DOI] [PubMed] [Google Scholar]
- 10.Langer R. Vacanti J.P. Tissue engineering. Science. 1993;260:920. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 11.Terada S. Sato M. Sevy A. Vacanti J.P. Tissue engineering in the twenty-first century. Yonsei Med J. 2000;41:685. doi: 10.3349/ymj.2000.41.6.685. [DOI] [PubMed] [Google Scholar]
- 12.Jernvall J. Thesleff I. Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev. 2000;92:19. doi: 10.1016/s0925-4773(99)00322-6. [DOI] [PubMed] [Google Scholar]
- 13.Thesleff I. Epithelial-mesenchymal signaling regulating tooth morphogenesis. J Cell Sci. 2003;116:1647. doi: 10.1242/jcs.00410. [DOI] [PubMed] [Google Scholar]
- 14.Bluteau G. Luder H.U. De Bari C. Mitsiadis T.A. Stem cells for tooth engineering. Eur Cell Mater. 2008;16:1. doi: 10.22203/ecm.v016a01. [DOI] [PubMed] [Google Scholar]
- 15.Show Y. Swope V.M. Swain G.M. The effect of the CH4 level on the morphology, microstructure, phase purity and electrochemical properties of carbon films deposited by microwave-assisted CVD from Ar-rich source gas mixtures. Diamond Relat Mater. 2009;2009;18:1426. [Google Scholar]
- 16.Amaral M. Gomes P.S. Lopes M.A. Santos J.D. Silva R.F. Fernandes M.H. Cytotoxicity evaluation of nanocrystalline diamond coatings by fibroblast cell cultures. Acta Biomater. 2009;5:755. doi: 10.1016/j.actbio.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 17.Tachiki M. Kaibara Y. Sumikawa Y. Shigeno M. Banno T. Song K.S. Umezawa H. Kawarada H. Diamond nanofabrication and characterization for biosensing application. Phys Stat Sol. 2003;199:39. [Google Scholar]
- 18.Gronthos S. Mankani M. Brahim J. Robey P.G. Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. PNAS. 2000;2000;97:13625. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Gronthos S. Brahim J. Li W. Fisher L.W. Cherman N. Boyde A. DenBesten P. Robey P. Shi S. Stem cell properties of human dental pulp stem cells. J Dent Res. 2002;81:531. doi: 10.1177/154405910208100806. [DOI] [PubMed] [Google Scholar]
- 21.Shi S. Bartold P.M. Miura M. Seo B.M. Robey P.G. Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofacial Res. 2005;8:191. doi: 10.1111/j.1601-6343.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 22.Gronthos S. Mrozik K. Shi S. Bartold P.M. Ovine periodontal ligament stem cells: isolation, characterization, and differentiation potential. Calcif Tissue Int. 2006;79:310. doi: 10.1007/s00223-006-0040-4. [DOI] [PubMed] [Google Scholar]
- 23.Salvadori M.C. Araújo W.W.R. Teixeira F.S. Cattani M. Pasquarelli A. Oks E.M. Brown I.G. Termination of diamond surfaces with hydrogen, oxygen and fluorine using a small, simple plasma gun. Diamond Relat Mater. 2010;19:324. [Google Scholar]
- 24.Vizir A. Oks E.M. Salvadori M.C. Teixeira F.S. Brown I.G. Small plasma source for materials application. Rev Sci Instrum. 2007;78:86103. doi: 10.1063/1.2766837. [DOI] [PubMed] [Google Scholar]




