Abstract
Objective
To quantify the health and economic outcomes associated with changes in folic acid consumption following fortification of enriched grain products in the United States.
Design
Cost-effectiveness analysis.
Setting
Annual burden of disease, quality-adjusted-life-years (QALYs), and costs were projected for four steady-state strategies: no fortification or fortifying with 140, 350, or 700 micrograms (mcg) folic acid per 100 grams (g) enriched grain. The analysis considered four health outcomes: neural tube defects (NTDs); myocardial infarctions (MIs); colon cancers; and B-12 deficiency maskings.
Subjects
U.S. adult population subgroups defined by age, gender, and race/ethnicity, with folate intake distributions from the National Health and Nutrition Examination Surveys (1988-1992 and 1999-2000), and reference sources for disease incidence, utility, and economic estimates.
Results
The greatest benefits from fortification were predicted in MI prevention, with 16,862 and 88,172 cases averted per year in steady state for the 140-mcg and 700-mcg fortification levels, respectively. These projections were 6,261 and 38,805 for colon cancer and 182 and 1,423 for NTDs, while 15 to 820 additional B-12 cases were predicted. Compared with no fortification, all post-fortification strategies provided QALY gains and cost savings for all subgroups, with predicted population benefits of 266,649 QALYs gained and $3.6 billion saved in the long run by changing the fortification level from 140-mcg/100-g enriched grain to 700-mcg/100-g.
Conclusions
This study indicates that the health and economic gains of folic acid fortification far outweigh the losses for the U.S. population, and that increasing the level of fortification deserves further consideration to maximize net gains.
Keywords: Cost-effectiveness analysis, folic acid fortification, prenatal and maternal nutrition, health policy
Introduction
Increasing intake of folate or folic acid during preconception and early in pregnancy can significantly reduce the risk of neural tube defects (NTDs) in newborns.1, 2 Increased intake may also reduce the risk of myocardial infarction (MI) and colon cancer3-16 and increase the risk that symptoms of vitamin B-12 deficiency are masked,4, 5, 7, 12 thereby allowing the neurological manifestations of the disease to progress.4
In 1998, the FDA mandated that manufacturers add 140 mcg of folic acid per 100 grams (g) of enriched cereal-grain product,11, 13, 14, and several studies have shown that such fortification provides substantial health and economic benefits.6, 8, 9, 13, 14, 17-21 However, the potential economic and health effects of this and alternative fortification policies have not been evaluated using national post-policy data adjusted for measurement error, while considering all four relevant health outcomes among population subgroups.
Accordingly, this analysis quantifies the projected health and economic outcomes for NTDs, MIs, colon cancers, and B-12 maskings associated with the changes in folic acid consumption following fortification in the United States, as well as for alternative fortification levels.
Methods
Overview
Population-wide disease burden and the associated costs and quality-adjusted life years (QALYs) were projected under four scenarios: no fortification, or fortification with 140, 350, or 700 mcg of folic acid per 100 g enriched grain. The no-fortification strategy reflects pre-fortification levels of folate intake, the 140-mcg strategy reflects current post-fortification intake in the U.S., and fortifying with 350 and 700 mcg are hypothetical scenarios.13, 19, 20 The four scenarios differ only in terms of the distribution of folate intake in the population, which we model in four categories: ≤200, 201-300, 301-400, and >400 mcg/day.
For each scenario, we projected the steady-state number of NTDs, myocardial infarctions (MIs), colon cancers, and B-12 maskings among a U.S. population of non-institutionalized, non-Hispanic white (heretofore referred to as “white”), non-Hispanic black (“black”), and Mexican-American persons aged 15 or older. Other racial/ethnic subgroups were not included because of insufficient sample size on which to base folate intake estimates. For each folate-intake category, we estimated age-, gender-, and race/ethnicity-specific risks of developing each of the four health outcomes. We then assigned lifetime QALY losses and disease-related net costs for each health outcome, using either published estimates or a Markov modeling approach, to calculate the population-wide impact of each strategy.
Folate Intake Distributions
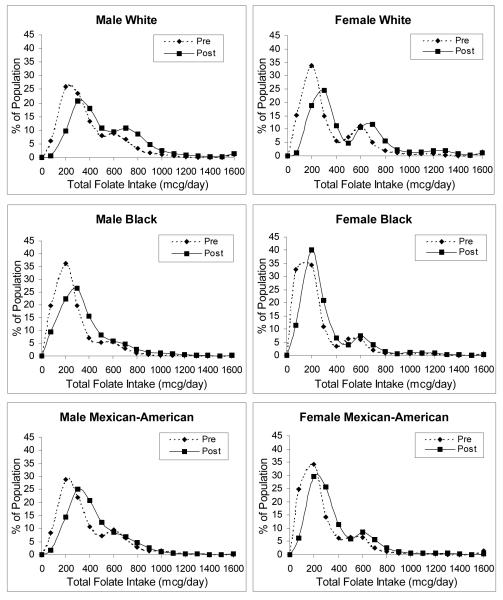
Estimates of population-based folate intake distributions were previously derived using food and dietary supplement data from two periods of the National Health and Nutrition Examination Surveys (NHANES).22 Briefly, data from NHANES III (1988-1994) were used to estimate pre-fortification food folate intake, and those from NHANES 1999-2000 were used to estimate both dietary supplement intake and post-fortification food folate intake. Because nutrient intake data are based primarily on one 24-hour dietary recall measure, which does not represent an individual’s average long-term daily intake, population distribution estimates of dietary folate intake were corrected for measurement error by using a sub-sample of NHANES III subjects who had provided two 24-hour recalls. Total folate intake distributions before and after the fortification policy, corrected for measurement error, are shown in Figure 1 and the Appendix. Folate intake for the two hypothetical scenarios was estimated as the product of the pre-post differences in corrected food folate intake and the ratio of the higher to the current levels (e.g., 350/140 for the 350-mcg strategy), plus the post-fortification supplement intake.
Figure 1.
Daily total folate intake distributions pre-versus-post fortification by gender and race/ethnicity, corrected for measurement error. Reprinted with permission from the American Public Health Association from Bentley TGK, Willett WC, Weinstein WC, and Kuntz KM. Population-Level Changes in Folate Intake by Age, Gender, and Race/Ethnicity after Folic Acid Fortification. Am J Public Health.96:2040-2047.
Disease Incidence
Annual incidence of the four disease outcomes prior to fortification was estimated as a function of age range, gender, and race/ethnicity (Table 1). When data were not available for the Mexican-American population, the rates for the Hispanic population were used.
Table 1.
Estimates of annual disease risk per 100,000 personsa
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Whiteb | Blackb | Mexican- American |
Whiteb | Blackb | Mexican- American |
|
| Neural tube defects | ||||||
| 15-44 y | ----- | ----- | ----- | 6.10 | 4.58 | 13.31 |
| Myocardial infarctions | ||||||
| 15-44 y | 60.6 | 89.8 | 23.6 | 19.1 | 50.7 | 6.3 |
| 45-64y | 698.3 | 1,100.0 | 449.7 | 349.0 | 918.1 | 253.3 |
| 65+ y | 1,627.7 | 2,047.7 | 1,306.8 | 886.3 | 1,363.8 | 759.4 |
| Colon cancer | ||||||
| 15-44 y | 2.98 | 3.13 | 1.96 | 2.21 | 3.70 | 1.47 |
| 45-64 y | 48.51 | 59.86 | 24.05 | 34.53 | 49.69 | 20.88 |
| 65+ y | 247.59 | 222.24 | 147.80 | 199.03 | 230.91 | 96.50 |
| Vitamin B-12 masking c | ||||||
| 15-44 y | 0.012 | 0.029 | ||||
| 45-64 y | 0.078 | 0.185 | ||||
| 65+ y | 0.181 | 0.427 | ||||
Some age categories have been combined for ease of presentation.
Non- Hispanic white and non-Hispanic black.
Annual rate of B-12 masking and pernicious anemia and total folate intake >1,000 micrograms (mcg) per day.
All women between the ages of 15-4423-26 were considered at risk for an NTD-affected pregnancy. NTD incidence as a function of race/ethnicity was based on estimates from the Centers for Disease Control and Prevention (CDC) of 10.6 cases of spina bifida and anencephaly per 10,000 live births.9, 27 Live birth rates from the National Center for Health Statistics28 were used to calculate NTD incidence per 100,000 women aged 15-44.
MI incidence was based on calculations by gender and age from the Framingham Risk Equations.29 The relative racial/ethnic distribution of MI incidence was assumed to be the same as that of CHD-specific death among each age and gender-specific subgroup.30 Subgroup-specific annual colon cancer incidence rates were derived from the Surveillance, Epidemiology, and End Results Program (SEER) of the National Cancer Institute.31-33
Vitamin B-12 masking was defined as the delayed diagnosis of B-12 deficiency followed by the development of neurological complications. Estimates of masking risk incorporated the probability of consuming greater than 1,000 mcg/day of folate22 – the “tolerable upper intake level” for folic acid10, 11 – and the risk of pernicious anemia (PA) – a common cause of B-12 deficiency.19, 34, 35
Folate-specific incidence of each disease was calculated by using data on the percent of the population in each folate-intake category (Figure 1) and the relative risks of disease by folate intake to split out the subgroup-specific disease rates. The risk of NTD was reduced by 50% for women with folate intake levels of greater than 400 mcg/day,13, 14, 19, 20,1, 2 and the relative reduction of MI risk for individuals with folate intake greater than 400 mcg/day was 24%.36 The risk ratios for colon cancer diagnosis by folate intake were 0.92, 0.79, and 0.69 for 201-300, 301-400, and >400 mcg/day, respectively, compared with ≤200 mcg/day.37 Relative risks for MI and colon cancer were assumed to be the same for men and women.
Valuing Outcomes
The number of disease events associated with each strategy were estimated as the product of incidence and national population estimates for each subgroup.38 The numbers of events were multiplied by the associated QALYs lost and net costs per event (Table 2) to estimate the net health and economic impact of each fortification strategy. All QALY and cost estimates were discounted by 3% per year.
Table 2.
Net costs incurred and QALYs lost associated with NTD, MI, colon cancer, and B-12 masking eventsa
|
Disease
outcome |
Ranges for sensitivity
analyses |
Source | |||
|---|---|---|---|---|---|
| Men | Women | Men | Women | ||
| QALYS | |||||
| NTD | – | 18.91 | – | 13 - 28 | 9, 20, 39 |
| MI | 2.12 | 2.03 | 1.17 - 3.07 | 1.12 - 2.94 | 40-45 |
| CC | 2.54 | 2.96 | 1.40 - 3.68 | 1.63 - 4.29 | 41, 46 |
| B-12 | 0.31 | 0.17 - 0.45 | 20, 39 | ||
| COSTS (thousands of dollars) | |||||
| NTD | – | $185.5 | – | $5.0 - $185.5 | 9, 21 |
| MI | $32.6 | $32.7 | $5.0 - $32.7 | 43, 45, 47, 48, 89 | |
| CC | $31.8 | $5.0 - $31.8 | 49-56 | ||
| B-12 | $5.3 | $5.0 - $50.0 | 20 | ||
Assuming a 3% discount rate.
QALYs, quality-adjusted-life-years; NTDs, neural tube defects; MIs, myocardial infarctions; CC, colon cancer; B-12, Vitamin B-12 masking; mcg, micrograms; g, grams
Health Related Quality-of-Life
Estimates of QALYs lost for NTDs and B-12 masking outcomes were based on a CDC cost-effectiveness analysis20 that used the Quality of Well-Being Index.39 To estimate the QALYs lost associated with MI and colon cancer we used a Markov modeling approach in which we incorporated disease-specific mortality, health-related quality-of-life weights, and mortality from other causes.40-42 For MI, we assumed the same utility for patients with coronary artery disease (CAD) of 0.84, calculated from previous analyses as the mean of mild and severe angina, weighted by the proportion of angina patients with CAD.43-45 For colon cancer, life expectancy after diagnosis was weighted by stage-specific mortality and we assumed a stage-weighted utility of 0.76.46
Costs
All costs were adjusted to 2005 dollars using the Consumer Price Index. For costs incurred with NTDs, estimates from a published analysis21 were used, weighted for relative proportions of spina bifida and anencephaly.9 Costs incurred with MI events incorporated short-term care43 as well as annual outpatient, medications, and diagnostic costs for a typical CAD patient,45, 47, 48 which were assumed to be applicable to MI patients. Costs incurred with colon cancer incorporated stage-weighted estimates from the Institute of Medicine,49-56 and those associated with cases of masked B-12 deficiency were based on calculations by the CDC.20 Estimates of annual fortification costs for the 140-mcg fortification strategy ($3.3 million) were based on those used by Grosse and colleagues,21 and those for the two hypothetical scenarios ($6.0 and $10.6 million for 350-mcg and 700-mcg fortification strategies, respectively) incorporated the fixed cost estimates from Grosse et al. and the CDC’s estimates of bulk folic acid costs, adjusted for cost declines since 1996.20,21 The fortification costs were doubled in sensitivity analysis.
Results
Incidence
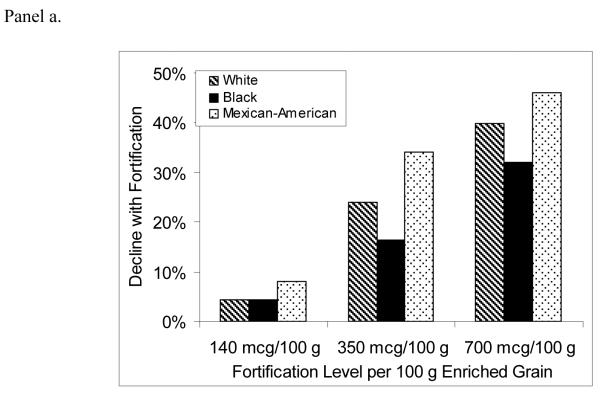
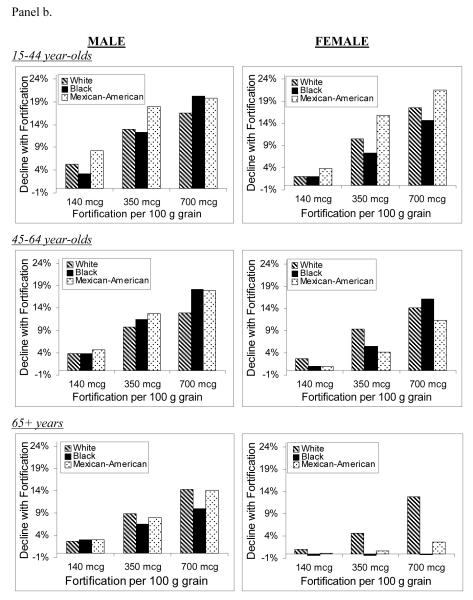
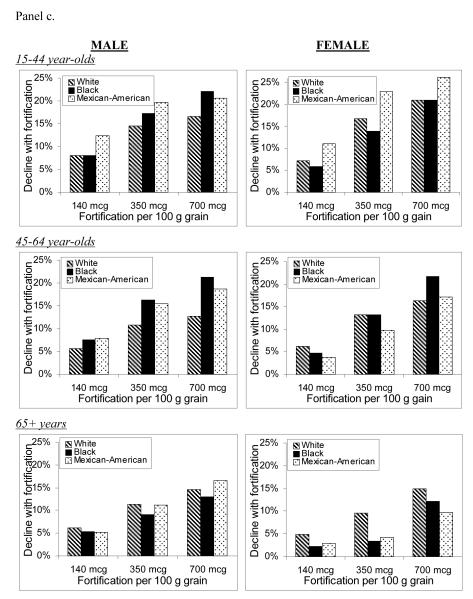
Figure 2 shows the projected percent decline in annual disease incidence for NTDs, MIs, and colon cancer, comparing the post-fortification scenarios with pre-fortification.
Figure 2.
Percent decline in annual incidence of neural tube defects (Panel a), myocardial infarctions (Panel b), and colon cancers (Panel c) after folic acid fortification, by age, gender, race/ethnicity, and fortification strategy.
For NTD-affected pregnancies, average annual incidence for all racial/ethnic groups was predicted to decrease by 5%, 24%, and 39% for the 140, 350, and 700 mcg/100 g fortification scenarios, respectively. Mexican-Americans were consistently projected to have the largest percent declines and blacks the lowest. Average annual MI incidence was predicted to decrease by 2%, 8%, and 14% for the lowest-to-highest post-fortification levels, while projected declines of colon cancer were 2%, 11%, and 15%. The racial/ethnic subgroups with the greatest predicted benefit were those with the largest post-fortification increases in percent reaching the risk-reduction folate intake thresholds for each disease outcome. Among older females, for example, whites were estimated to experience the largest increases in percent consuming greater than 400 mcg/day of total folate, and were predicted to experience the greatest declines in disease incidence. On the other hand, older black females were predicted to experience a 0%-1% increase in MI incidence, due to a decrease in the proportion consuming more than 400 mcg/day.
Table 3 shows the projected total annual number of events averted, QALYs gained, and costs incurred for the U.S. population. The model predicted that the greatest benefits would be in MI prevention, with 16,862 cases averted per year at the 140-mcg fortification level and 88,172 at the highest fortification level. Between 6,261 and 38,805 annual cases of colon cancer and 182 to 1,423 annual NTDs would be prevented, while 15 to 820 new annual cases of B-12 masking would be caused.
Table 3.
Annual QALYs and costs associated with U.S. folic acid fortification, by fortification strategy and outcome
| Strategya | NTDs | MIs | CC | B-12 | Net | |
|---|---|---|---|---|---|---|
| Events averted | ||||||
| 140 mcg/100 g | 182 | 16,862 | 6,261 | −15 | 23,289 | |
| 350 mcg/100 g | 883 | 53,011 | 20,110 | −184 | 73,821 | |
| 700 mcg/100 g | 1,423 | 88,172 | 38,805 | −820 | 127,579 | |
| QALYs gained | ||||||
| 140 mcg/100 g | 3,436 | 35,458 | 17,402 | −5 | 56,291 | |
| 350 mcg/100 g | 16,697 | 111,121 | 57,403 | −57 | 185,165 | |
| 700 mcg/100 g | 26,899 | 184,149 | 112,146 | −254 | 322,940 | |
| Costs incurred (millions of dollars) b |
Fortification
costs |
Net costs | ||||
| 140 mcg/100 g | −$33.7 | −$550.8 | −$199.4 | $0.1 | $3.3 | −$780.5 |
| 350 mcg/100 g | −$163.8 | −$1,731.5 | −$640.4 | $1.0 | $6.0 | −$2,528.8 |
| 700 mcg/100 g | −$263.9 | −$2,880.0 | −$1,235.7 | $4.3 | $10.5 | −$4,364.8 |
Strategies labeled by amount of fortification in mcg of folic acid added per 100 g of enriched grain product.
Disease-specific costs do not include fortification costs.
QALYs, quality-adjusted-life-years; NTDs, neural tube defects; MIs, myocardial infarctions; CC, colon cancer; B-12, Vitamin B-12 masking; mcg, micrograms; g, grams
Quality-of-Life and Cost Measures
Fortification was predicted to be cost-saving and to provide positive net QALY gains at all fortification levels, and the 700 mcg/100 g strategy was projected to have the largest health gain and cost savings, with over 320,000 QALYs gained and over $4 billion saved per year (Table 3). The predicted annual gains of over 26,000 QALYs and savings of over $263 million from NTD prevention alone far outweighed the QALYs lost and costs incurred from B-12 masking and fortification itself, which combined were predicted to result in annual losses of fewer than 260 QALYs and $15 million even at the highest fortification level. QALY gains and cost savings due to MIs and colon cancers averted each year would be even greater, with MI prevention alone predicted to save 184,000 QALYs and $3 billion annually at the 700-mcg level.
The model predicted the 700-mcg fortification level to yield the greatest net QALY gains and cost savings for all age, gender, and racial/ethnic subgroups (Table 4). Benefits were projected to increase with age, with males predicted to benefit more than females in most populations. At all fortification levels, the highest gains were expected in white males aged 65 and older, with predicted annual gains of over 13,000 QALYs and $190 million at the currently enacted level and over 59,000 QALYs and $888 million at the 700-mcg level. Among racial/ethnic categories, whites were projected to experience the greatest gains and Mexican-Americans the fewest.
Table 4.
Annual QALYs and costs (millions of dollars) associated with U.S. folic acid fortification, by gender, age, and race/ethnicity.
| Strategya | Non-Hispanic White | Non-Hispanic Black | Mexican-American | |||
|---|---|---|---|---|---|---|
| QALYs | Costs | QALYs | Costs | QALYs | Costs | |
| Men aged 15-44 y | ||||||
| 140 mcg/100 g | 3,041 | −$45.23 | 506 | −$7.50 | 265 | −$3.86 |
| 350 mcg/100 g | 7,241 | −$108.51 | 1,849 | −$27.84 | 551 | −$8.11 |
| 700 mcg/100 g | 9,078 | − $135.52 | 2,996 | − $45.19 | 600 | − $8.72 |
| Men aged 45-64 y | ||||||
| 140 mcg/100 g | 12,939 | −$194.42 | 2,336 | −$35.05 | 642 | −$9.65 |
| 350 mcg/100 g | 31,967 | −$482.75 | 6,846 | −$103.34 | 1,700 | −$25.68 |
| 700 mcg/100 g | 41,703 | − $630.77 | 10,745 | − $162.67 | 2,358 | − $35.68 |
| Men aged 65+ y | ||||||
| 140 mcg/100 g | 13,204 | −$190.82 | 1,663 | −$24.61 | 334 | −$4.94 |
| 350 mcg/100 g | 38,477 | −$569.02 | 3,394 | −$50.56 | 860 | −$12.79 |
| 700 mcg/100 g | 59,677 | − $888.08 | 5,129 | − $76.52 | 1,483 | − $22.15 |
| Women aged 15-44 y | ||||||
| 140 mcg/100 g | 2,627 | −$27.21 | 532 | −$6.18 | 1,068 | −$10.17 |
| 350 mcg/100 g | 13,404 | −$141.13 | 1,923 | −$22.68 | 4,426 | −$43.23 |
| 700 mcg/100 g | 22,210 | − $233.64 | 3,722 | −$43.99 | 5,964 | − $57.89 |
| Women aged 45-64 y | ||||||
| 140 mcg/100 g | 5,335 | −$78.24 | 802 | −$11.60 | 113 | −$1.61 |
| 350 mcg/100 g | 16,605 | −$251.04 | 3,998 | −$60.75 | 412 | −$6.10 |
| 700 mcg/100 g | 24,415 | − $372.64 | 10,916 | − $169.90 | 1,017 | − $15.45 |
| Women aged 65+ y | ||||||
| 140 mcg/100 g | 6,295 | −$79.78 | 87 | −$0.38 | 48 | −$0.58 |
| 350 mcg/100 g | 18,833 | −$262.02 | 192 | −$1.52 | 95 | −$1.26 |
| 700 mcg/100 g | 42,384 | − $618.09 | 924 | − $9.45 | 279 | − $3.86 |
Strategies labeled by amount of fortification in mcg of folic acid added per 100 g of enriched grain product.
QALYs, quality-adjusted-life-years; mcg, micrograms; g, grams
Sensitivity Analyses
This analysis projected substantial fortification benefits despite predicting at the currently enacted fortification level a 5% reduction in NTD rates, which is far less than the 20-30% declines estimated from observed data.6, 8, 9, 17 Due to lack of adequate dose-response data, the model allows only for reduced NTD risk at folate consumption of >400 mcg/day, yet there may be benefits in NTD risk-reduction at lower levels.
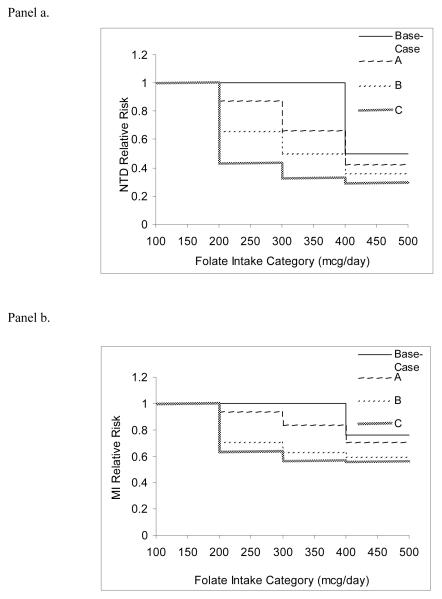
To evaluate the effect of this possibility, three additional NTD dose-response assumptions were tested (Figure 3a), allowing women to benefit from folate intake over 200 mcg/day.57-60 Curve A assumes a dose-response gradient similar to that of colon cancer – albeit through different mechanisms – while B and C were calculated by decreasing the risk ratios of function A by 25% and 50%, respectively. All three functions maintained the base-case assumption of a 50% reduced risk for intake >400 mcg/day compared with ≤400 mcg/day. Given the use of a similar 400-mcg/day threshold for reducing the risk of MI by folate intake and evidence of potential benefit at lower levels without a threshold effect,61 an analogous sensitivity analysis on this dose-response function was performed (Figure 3b).
Figure 3.
Dose-response assumptions used in sensitivity analyses for neural tube defects (NTDs, Panel a) and myocardial infarctions (MIs, Panel b). Risk is relative to an average folate intake of <200 micrograms (mcg) per day.
When risk reduction benefits for NTDs and MIs were allowed at lower folate intakes, the model predicted that more events would be prevented and there would be greater reductions in disease incidence. The use of NTD function B predicted at the 140-mcg level a 23% risk reduction, the closest approximation to observed data. When applying these functions for both NTD and MI dose-response, the 700-mcg strategy was predicted to save 370,000 QALYs and $5 billion, compared with 320,000 QALYs and $4 billion in the base case (Table 5).
Table 5.
QALYs and costs (millions of dollars) associated with folic acid fortification, using alternative NTD and MI dose-response functionsa
| Annual QALY Gains | ||||||
|---|---|---|---|---|---|---|
| Strategyb | NTDs | MIs | CC | B-12 | Net QALY gain |
Annual net costs |
| No fortification | 0 | 0 | 0 | 0 | 0 | $0 |
| 140 mcg/100 g | 15,842 | 114,532 | 17,402 | −5 | 147,770 | −$2,154 |
| 350 mcg/100 g | 28,445 | 193,475 | 57,403 | −57 | 279,267 | −$3,958 |
| 700 mcg/100 g | 33,268 | 224,325 | 112,146 | −254 | 369,485 | −$5,078 |
NTD dose-response function assumes the following relative risks of NTD-affected pregnancy: 0.36; 0.50; and 0.65 for maternal folate intake of >400, 301-400, and 201-300 micrograms (mcg) per day, respectively, compared to ≤200 mcg/day. MI dose-response function assumes the following relative risks of MI: 0.59; 0.63; and 0.70 for folate intake of >400, 301-400, and 201-300 micrograms (mcg) per day, respectively, compared to ≤200 mcg/day.
Strategies labeled by amount of fortification in mcg of folic acid added per 100 g of enriched grain product.
QALYs, quality-adjusted-life-years; NTDs, neural tube defects; MIs, myocardial infarctions; CC, colon cancer; B-12, Vitamin B-12 masking; mcg, micrograms; g, grams
To evaluate the effect of the model’s other assumptions, a range of estimates were applied for QALYs and costs (Table 2), relative risk of NTD-affected pregnancy (10% and 90%), masking risk (30%-200% of base case), female PA risk (150% of base case),34, 35, 62 and discount rate (0% and 5%). Even with extreme estimates that would bias results away from fortification, none of these variations – applied individually or concurrently – changed the rankings between strategies or the conclusion that QALY gains and cost savings would result from fortification up to the highest level considered. When biasing against fortification overall, the conclusions remained the same, even though the predicted QALYs gained and costs saved were smaller: for all subgroups, fortification would remain cost saving, and the 700-mcg strategy would provide the greatest total QALY gains, with $486 million saved and over 196,000 QALYs gained.
Discussion
It was predicted that for three post-fortification strategies, the projected health and economic benefits gained from preventing NTDs, MIs, and colon cancers in the U.S. population far exceeded those lost due to fortification itself and increased B-12 masking risk, with significant variations by age, gender, and race/ethnicity. For all health outcomes, the QALY and cost benefits to whites were projected to be significantly greater than those to blacks and Mexican-Americans. With predicted population benefits of 322,940 QALYs gained and $4.4 billion saved, fortifying at 700 mcg/100 g enriched grain product – the highest level considered in this analysis – strongly dominated all other scenarios. The benefits of higher fortification levels were predicted to far outweigh the associated risks for all populations, and in all sensitivity analyses.
The substantial racial/ethnic differences predicted in disease outcomes were caused primarily by differences in total folate intake. Although disease incidence was not projected to decrease among all populations, this effect was caused by the unrealistic discontinuous risk functions used to avoid interpolating the epidemiologic data analyzed in risk strata. Targeted supplement-use interventions may be necessary to further mitigate disparities and reduce disease prevalence, and future research should aim to identify racial/ethnic differences in intake of fortified and non-fortified foods.
The results of this analysis provide evidence for recommending that fortification be increased to at least 700 mcg of folic acid per 100 g of enriched grain product, corroborating prior research that predicted greater economic gains at higher fortification levels.20 This analysis demonstrates that the benefits of higher fortification would exceed the risks even in the most unfavorable subgroups. It also addresses other important considerations by using estimates of folate intake that are national, subgroup-specific, and corrected for the bias caused by the use of one-day dietary intake data.
There are several limitations to consider when interpreting these results. The use of limited data on the dose-response relationships between folate intake and disease risk may have underestimated the post-fortification health benefits. However, even when using more realistically continuous – albeit uncertain – dose-response assumptions for both NTDs and MIs, the conclusions of positive benefit-risk tradeoffs for all subgroups – and of greater benefit at higher fortification levels – did not change, and the model predicted that more NTDs and MIs would be prevented and that the NTD reduction would be consistent with that observed post-policy.6, 8, 9, 17
A related source of uncertainty is that synthetic folic acid is more bioavailable to absorption by the human body than is naturally occurring folate. While this factor can be incorporated using Dietary Folate Equivalents (DFEs) – a measure that adjusts intake estimates for these absorption differences – we were unable to include DFEs in our analysis due to data limitations. With fortification resulting in greater proportions of intake from synthetic folic acid, the model’s use of total folate may thus have caused the benefits of fortification to be underestimated, and the risks to be overestimated. Conversely, because this factor was not considered in estimating folate-specific risks of MI and colon cancer, the benefits for dietary sources of folate may be overstated. The model’s projected cost savings associated with fortification may have been underestimated, as lifetime caregiving costs of NTD-affected individuals were not included, reduced costs associated with the proportion of NTDs ending in a terminated pregnancy were excluded, and MI costs were used that may not consider the increased costs of today’s standards of care. By the same token, we may have overestimated QALY losses due to MI because survival has been improved by today’s standards of care. Taken as a whole, it is unlikely that any such positive or negative effects would substantially impact the results of the analysis, or alter the conclusions of overall benefit.
Given the suggestion of possible insignificant or even adverse effects of increased folate intake on MI risk and on colorectal cancer progression among individuals with preexisting disease,3, 63-67 the benefits to MI as well as to colon cancer could be less than predicted by our model. However, recent evidence also indicates a positive folate-stroke association 68-70 and an overall cardiovascular benefit,16 and our results may thus underestimate benefits. The potential risk to colorectal cancer progression may appear to be supported by recent published research indicating a possible temporary delay in the ongoing decline in colorectal cancer incidence,71 but this could be in part an artifact of increased use of colonoscopy. In addition, a recent report from the National Cancer Institute indicates that not only is incidence still decreasing at 2% per year, but also mortality – which one would expect to increase if fortification were accelerating growth of existing tumors – is also declining at an annual rate of close to 4%.72 This analysis is thus important for motivating further trials among people without existing disease, while simultaneously suggesting caution among policymakers who may be considering potential fortification increases.
By not formally allowing competing risks between disease outcomes while allocating benefit for each disease event averted, the model may have double-counted some of the benefits gained because multiple events may be occurring per individual. This analysis did not incorporate potential associations of folic acid intake with increased twinning,73-76 with other cancers,77-79 or with cognitive decline.80 Given the lack of consistent evidence for such outcomes, it is unclear in which direction their inclusion may impact results, yet the strength of the findings from this analysis suggests that the conclusion of overall benefit associated with increased folate intake would not be changed.
While these benefit-risk estimates assume a steady state, in reality fortification’s effect on NTDs and B-12 maskings would be relatively immediate, while that for MIs and colon cancer could take up to five36 and 15 years,37 respectively. However, not only were fortification’s benefits for NTDs alone predicted to outweigh the potential B-12 masking risk, this risk may in fact have been overestimated given our use of a conservative risk threshold of 1,000 mcg/day despite no evidence of harm below 5,000 mcg/day.10 It is also important to note that while there is conflicting evidence regarding whether masking has increased since fortification,81-83 current medical knowledge regarding appropriate screening measures for B-12 deficiency suggests that the fear of delayed diagnosis by physicians may not in fact be realized. The risk, however, may be that symptom improvement due to masking could reduce patients’ likelihood of seeking medical advice until after neurological complications have occurred.84 Nevertheless, with the low prevalence of potential masking – estimated at 0.09% in older women before fortification and 0.61% after83 – even with conservative estimates our model predicted that this risk would be outweighed by the benefits.
Given the uncertainty involved, future research should clarify the dose-response relationships and benefit-risk associations between folate intake and disease risk. This is especially important for outcomes such as MIs, colon cancer, stroke, cognitive decline and B-12 masking, for which causality has not yet been established; there has been conflicting evidence on potential risks, in particular among individuals with pre-existing disease; or there remains debate over the validity of the evidence.3, 16, 67-70, 81-88 In addition, future policy decisions may consider B-12 co-fortification or a more stringent screen for B-12 deficiency to offset the potentially elevated B-12 masking risks due to higher fortification, and may evaluate a broader range of fortification levels to better determine the optimal fortification strategy.
In summary, folic acid fortification was implemented in the United States in 1998 to reduce the chance of NTDs in newborns. While there are potential risks of increased folate intake to populations with vitamin B-12 deficiency, there may also be benefits in preventing MIs and colon cancer. Overall, in considering the benefit-risk tradeoffs of folic acid fortification, this study suggests that the health and economic gains may outweigh the losses for the U.S. population as a whole, and that additional studies on the potential benefits and hazards associated with folate intake – as well as an in-depth evaluation of the level of fortification – deserve further consideration in order to maximize net gains among all racial/ethnic, age, and gender-specific subgroups.
Appendix.
Appendix.
Population total folate consumption by age, gender, race/ethnicity, and folic acid fortification level
| Males | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No fortification | 140 mcg/100 ga | 350 mcg/100 g | 700 mcg/100 g | |||||||||||||
| Folate Intakeb | ||||||||||||||||
| ≤200 |
201 300 |
301-400 | >400 | ≤200 |
201- 300 |
301- 400 |
>400 | ≤200 |
201- 300 |
301- 400 |
>400 | ≤200 |
201- 300 |
301- 400 |
>400 | |
| Non-Hispanic White | ||||||||||||||||
| 15-44 y | 19% | 28% | 17% | 36% | 0% | 17% | 23% | 57% | 0% | 3% | 11% | 86% | 0% | 0% | 1% | 99% |
| 45-64 y | 13% | 21% | 14% | 52% | 3% | 13% | 18% | 66% | 0% | 3% | 9% | 88% | 0% | 0% | 1% | 99% |
| 65+ y | 17% | 23% | 15% | 45% | 4% | 19% | 21% | 55% | 0% | 5% | 17% | 78% | 0% | 0% | 1% | 99% |
| Non-Hispanic Black | ||||||||||||||||
| 15-44 y | 26% | 46% | 14% | 14% | 5% | 35% | 33% | 27% | 0% | 8% | 28% | 64% | 0% | 0% | 4% | 96% |
| 45-64 y | 41% | 24% | 12% | 23% | 15% | 27% | 21% | 38% | 2% | 11% | 19% | 68% | 0% | 1% | 5% | 94% |
| 65+ y | 49% | 23% | 10% | 19% | 35% | 20% | 12% | 32% | 26% | 16% | 12% | 46% | 19% | 12% | 10% | 59% |
| Mexican-American | ||||||||||||||||
| 15-44 y | 27% | 35% | 17% | 21% | 2% | 17% | 27% | 54% | 0% | 1% | 6% | 93% | 0% | 0% | 0% | 100% |
| 45-64 y | 25% | 29% | 16% | 29% | 6% | 23% | 24% | 47% | 0% | 5% | 16% | 78% | 0% | 0% | 2% | 98% |
| 65+ y | 26% | 28% | 16% | 30% | 13% | 25% | 21% | 41% | 3% | 16% | 20% | 60% | 0% | 4% | 11% | 84% |
Strategies labeled by amount of fortification in mcg of folic acid added per 100 g of enriched grain product.
Folate intake categories defined by total average folate intake in mcg/day.
Mcg, micrograms; g, grams
| Females | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No fortification | 140 mcg/100 gc | 350 mcg/100 g | 700 mcg/100 g | |||||||||||||
| Folate Intaked | ||||||||||||||||
| ≤200 |
201- 300 |
301- 400 |
>400 | ≤200 |
201- 300 |
301- 400 |
>400 | ≤200 |
201- 300 |
301- 400 |
>400 | ≤200 |
201- 300 |
301- 400 |
>400 | |
| Non-Hispanic White | ||||||||||||||||
| 15-44 y | 41% | 23% | 5% | 31% | 9% | 32% | 20% | 39% | 0% | 7% | 22% | 71% | 0% | 0% | 1% | 99% |
| 45-64 y | 28% | 19% | 8% | 46% | 7% | 21% | 16% | 56% | 0% | 5% | 14% | 80% | 0% | 0% | 1% | 98% |
| 65+ y | 23% | 21% | 8% | 48% | 8% | 25% | 15% | 52% | 0% | 11% | 24% | 65% | 0% | 0% | 5% | 95% |
| Non-Hispanic Black | ||||||||||||||||
| 15-44 y | 60% | 18% | 4% | 18% | 31% | 29% | 14% | 26% | 10% | 22% | 20% | 48% | 2% | 8% | 13% | 76% |
| 45-64 y | 52% | 19% | 5% | 24% | 24% | 34% | 14% | 28% | 3% | 23% | 29% | 46% | 0% | 1% | 11% | 88% |
| 65+ y | 53% | 23% | 7% | 17% | 41% | 35% | 7% | 16% | 11% | 65% | 7% | 16% | 0% | 23% | 61% | 16% |
| Mexican-American | ||||||||||||||||
| 15-44 y | 63% | 21% | 4% | 12% | 12% | 35% | 25% | 28% | 0% | 6% | 18% | 77% | 0% | 0% | 1% | 99% |
| 45-64 y | 44% | 21% | 6% | 29% | 25% | 29% | 13% | 33% | 8% | 25% | 22% | 45% | 1% | 8% | 19% | 73% |
| 65+ y | 30% | 25% | 12% | 33% | 22% | 29% | 15% | 34% | 11% | 31% | 22% | 36% | 2% | 19% | 36% | 43% |
Strategies labeled by amount of fortification in mcg of folic acid added per 100 g of enriched grain product.
Folate intake categories defined by total average folate intake in mcg/day.
Mcg, micrograms; g, grams
References
- 1.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–5. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 2.MRC Vitamin Study Research Group Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet. 1991;338:131–7. [PubMed] [Google Scholar]
- 3.Bazzano LA, Reynolds K, Holder KN, He J. Effect of folic acid supplementation on risk of cardiovascular diseases: a meta-analysis of randomized controlled trials. JAMA. 2006;296:2720–6. doi: 10.1001/jama.296.22.2720. [DOI] [PubMed] [Google Scholar]
- 4.Bentley JR, Ferrini RL, Hill LL. American College of Preventive Medicine public policy statement. Folic acid fortification of grain products in the U.S. to prevent neural tube defects. Am J Prev Med. 1999;16:264–7. doi: 10.1016/s0749-3797(98)00156-1. [DOI] [PubMed] [Google Scholar]
- 5.Dickinson CJ. Does folic acid harm people with vitamin B12 deficiency? QJM. 1995;88:357–64. [PubMed] [Google Scholar]
- 6.Erickson JD. Folic acid and prevention of spina bifida and anencephaphly. MMWR Recomm Rep. 2002;51:1–3. [PubMed] [Google Scholar]
- 7.Koehler KM, Pareo-Tubbeh SL, Romero LJ, Baumgartner RN, Garry PJ. Folate nutrition and older adults: challenges and opportunities. J Am Diet Assoc. 1997;97:167–73. doi: 10.1016/S0002-8223(97)00044-8. [DOI] [PubMed] [Google Scholar]
- 8.Mathews TJ, Honein MA, Erickson JD. Spina bifida and anencephaly prevalence--United States, 1991-2001. MMWR Recomm Rep. 2002;51:9–11. [PubMed] [Google Scholar]
- 9.Mersereau P, Kilker K, Carter H, et al. Spina bifida and anencephaly before and after folic acid mandate -- United States, 1995-1996 and 1999-2000. MMWR Morb Mortal Wkly Rep. 2004;53:362–5. [PubMed] [Google Scholar]
- 10.National Academy of Sciences IoM, Food and Nutrition Board . Dietary Reference Intakes: Thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. National Academy Press; Washington, DC: 1998. [PubMed] [Google Scholar]
- 11.National Institutes of Health Clinical Nutrition Service . Facts about dietary supplements: folate. National Institutes of Health; Bethesda, MD: [Accessed November 2004]. 2001. [Internet] at: http://ods.od.nih.gov/factsheets/cc/folate.html. [Google Scholar]
- 12.Tucker KL, Mahnken B, Wilson PW, Jacques P, Selhub J. Folic acid fortification of the food supply. Potential benefits and risks for the elderly population.[comment] JAMA. 1996;276:1879–85. doi: 10.1001/jama.1996.03540230029031. [DOI] [PubMed] [Google Scholar]
- 13.U.S. Food and Drug Administration Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1993;58:53305–12. [Google Scholar]
- 14.U.S. Food and Drug Administration Food standards: amendment of standards of identity for enriched grain products to require addition of folic acid. Fed Regist. 1996;61:8781–97. [Google Scholar]
- 15.U.S. Food and Drug Administration [Accessed April 2003];Folic acid fortification fact sheet. [Internet] Office of Public Affairs. 1996 at: http://vm.dfsan.fda.gov/~dms/wh folic.html.
- 16.Wald DS, Wald NJ, Morris JK, Law M. Folic acid, homocysteine, and cardiovascular disease: judging causality in the face of inconclusive trial evidence. BMJ. 2006;333:1114–7. doi: 10.1136/bmj.39000.486701.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams LJ, Mai CT, Edmonds LD, et al. Prevalence of spina bifida and anencephaly during the transition to mandatory folic acid fortification in the United States. Teratology. 2002;66:33–9. doi: 10.1002/tera.10060. [DOI] [PubMed] [Google Scholar]
- 18.Tice JA, Ross E, Coxson PG, Rosenberg I, Weinstein MC, Hunink MG, Goldman PA, Williams L, Goldman L. Cost-effectiveness of vitamin therapy to lower plasma homocysteine levels for the prevention of coronary heart disease: effect of grain fortification and beyond. JAMA. 2001;286:936–43. doi: 10.1001/jama.286.8.936. [DOI] [PubMed] [Google Scholar]
- 19.Romano PS, Waitzman NJ, Scheffler RM, Pi RD. Folic acid fortification of grain: an economic analysis. Am J Public Health. 1995;85:667–76. doi: 10.2105/ajph.85.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly A, Haddix A, Scanlon K, Helmick C, Mulinare J. Worked example: cost-effectiveness of strategies to prevent neural tube defects. In: Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-effectiveness in Health and Medicine. Oxford University Press; New York: 1996. pp. 313–48. [Google Scholar]
- 21.Grosse SD, Waitzman NJ, Romano PS, Mulinare J. Reevaluating the benefits of folic acid fortification in the United States: economic analysis, regulation, and public health. Am J Public Health. 2005;95:1917–22. doi: 10.2105/AJPH.2004.058859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bentley TGK, Willett WC, Weinstein MC, Kuntz KM. Population-level changes in folate intake by age, gender, and race/ethnicity after folic acid fortification. Am J Public Health. 2006;96:2040–7. doi: 10.2105/AJPH.2005.067371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Folate status in women of childbearing age --- United States, 1999. MMWR Morb Mortal Wkly Rep. 2000;49:962–5. [PubMed] [Google Scholar]
- 24.Johnston R, Staples D. Knowledge and use of folic acid by women of childbearing age -- United States, 1997. MMWR Recomm Rep. 1997;46:721–3. [PubMed] [Google Scholar]
- 25.Erickson JD, Mulinare J, Yang Q, Johnson C, Pfeiffer CM, Giles W, Bowman B. Folate status in women of childbearing age, by race/ethnicity -- United States, 1999-2000. MMWR Morb Mortal Wkly Rep. 2002;51:808–10. [Google Scholar]
- 26.Petrini J, Damus K, Johnston R, Mattison D. Knowledge and use of folic acid by women of childbearing age -- United States, 1995 and 1998. MMWR Recomm Rep. 1999;48:325–7. [PubMed] [Google Scholar]
- 27.Williams L, Rasmussen SA, Flores A, Kirby R, Edmonds LD. Decline in the prevalence of spina bifida and anencephaly by race/ethnicity: 1995-2002. Pediatrics. 2005;116:580–6. doi: 10.1542/peds.2005-0592. [DOI] [PubMed] [Google Scholar]
- 28.Hamilton B, Sutton P, Ventura S. Revised birth and fertility rates for the 1990s and new rates for Hispanic populations, 2000 and 2001: United States. Natl Vital Stat Rep National Center for Health Statistics. 2003;51 [PubMed] [Google Scholar]
- 29.Anderson KM, Odell PM, Wilson PW, Kannel WB. Cardiovascular disease risk profiles. Am Heart J. 1991;121:293–8. doi: 10.1016/0002-8703(91)90861-b. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention . Health data for all ages -- adult mortality by cause: U.S./State, 1999-2002. National Center for Health Statistics; Hyattsville, MD: [Accessed October 2006]. 2006. [Internet] at: http://www.cdc.gov/nchs/health_data_for_all_ages.htm. [Google Scholar]
- 31.Surveillance, Epidemiology, and End Results (SEER) Program SEER*Stat Database: Incidence - SEER 11 Regs Public-Use, Aug 1999 Sub for Hispanics (1992-1997) National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch; [Accessed February 2005]. 1999. at: www.seer.cancer.gov. [Google Scholar]
- 32.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Edwards Be. SEER cancer statistics review, 1973-1999. National Cancer Institute; [Accessed May 2002]. 2002. at: http://seer.cancer.gov/csr/1973_1999. [Google Scholar]
- 33.Ries L, Eisner M, Kosary C, Hankey B, Miller B, Clegg L, Mariotto A, Feuer E, editors. EB. SEER cancer statistics review, 1975-2001. National Cancer Institute; [Accessed October 2004]. 2004. at: http://seer.cancer.gov/csr/1975_2001. [Google Scholar]
- 34.Borch K, Liedberg G. Prevalence and incidence of pernicious anemia. An evaluation for gastric screening. Scand J Gastroenterol. 1984;19:154–60. [PubMed] [Google Scholar]
- 35.Pedersen AB, Mosbech J. Morbidity of pernicious anaemia. Incidence, prevalence, and treatment in a Danish county. Acta Med Scand. 1969;185:449–52. [PubMed] [Google Scholar]
- 36.Rimm EB, Willett WC, Hu FB, Sampson L, Colditz GA, Manson JE, Hennekens C, Stampfer MJ. Folate and vitamin B6 from diet and supplements in relation to risk of coronary heart disease among women. JAMA. 1998;279:359–64. doi: 10.1001/jama.279.5.359. [DOI] [PubMed] [Google Scholar]
- 37.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Census Bureau, Population Division . Annual Population Estimates. Washington DC: [Accessed July 2005]. 2005. [Internet] at: http://www.census.gov/popest/national/ [Google Scholar]
- 39.Kaplan RM, Anderson JP. A general health policy model: update and applications. Health Serv Res. 1988;23:203–35. [PMC free article] [PubMed] [Google Scholar]
- 40.Abby SL, Harris IM, Harris KM. Homocysteine and cardiovascular disease. J Am Board Fam Pract. 1998;11:391–8. doi: 10.3122/15572625-11-5-391. [DOI] [PubMed] [Google Scholar]
- 41.Arias E. United States life tables, 2001. Natl Vital Stat Rep National Center for Health Statistics. 2004;52 [PubMed] [Google Scholar]
- 42.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 43.Cohen DJ, Taira DA, Berezin R, Cox DA, Morice M-C, Stone GW, Grines CL. Cost-effectiveness of coronary stenting in acute myocardial infarction: results from the Stent Primary Angioplasty in Myocardial Infarction (Stent-PAMI) Trial. Circulation. 2001;104:3039–45. doi: 10.1161/hc5001.100794. [DOI] [PubMed] [Google Scholar]
- 44.Nease RF, Jr., Kneeland T, O’Connor GT, Sumner W, Lumpkins C, Shaw L, Pryor D, Sox HC. Variation in patient utilities for outcomes of the management of chronic stable angina. Implications for clinical practice guidelines. Ischemic Heart Disease Patient Outcomes Research Team. JAMA. 1995;273:1185–90. [PubMed] [Google Scholar]
- 45.Kuntz KM, Fleischmann KE, Hunink MGM, Douglas PS. Cost-effectiveness of diagnostic strategies for patients with chest pain. Ann Intern Med. 1999;130:709–18. doi: 10.7326/0003-4819-130-9-199905040-00002. [DOI] [PubMed] [Google Scholar]
- 46.Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. Am J Gastroenterol. 1999;94:1650–7. doi: 10.1111/j.1572-0241.1999.01157.x. [DOI] [PubMed] [Google Scholar]
- 47.Wong JB, Sonnenberg FA, Salem DN, Pauker SG. Myocardial revascularization for chronic stable angina. Ann Intern Med. 1990;113:852. doi: 10.7326/0003-4819-113-11-852. [DOI] [PubMed] [Google Scholar]
- 48.Stinnett A, Mittleman M, Weinstein M, et al. Appendix C: The cost-effectiveness of dietary and pharmacologic therapy for cholesterol reduction in adults. In: Gold M, Siegel J, Russell L, Weinstein M, editors. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. pp. 349–91. [Google Scholar]
- 49.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [DOI] [PubMed] [Google Scholar]
- 50.Khandker RK, Dulski JD, Kilpatrick JB, Ellis RP, Mitchell JB, Baine WB. A decision model and cost-effectiveness analysis of colorectal cancer screening and surveillance guidelines for average-risk adults. Int J Technol Assess Health Care. 2000;16:799–810. doi: 10.1017/s0266462300102077. [DOI] [PubMed] [Google Scholar]
- 51.Loeve F, Brown ML, Boer R, van Ballegooijen M, van Oortmarssen GJ, Habbema JD. Endoscopic colorectal cancer screening: a cost-saving analysis. J Natl Cancer Inst. 2000;92:557–63. doi: 10.1093/jnci/92.7.557. [DOI] [PubMed] [Google Scholar]
- 52.Ness RM, Holmes AM, Klein R, Dittus R. Cost-utility of one-time colonoscopic screening for colorectal cancer at various ages. Am J Gastroenterol. 2000;95:1800–11. doi: 10.1111/j.1572-0241.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 53.Pignone M, Russell L, Wagner J, editors. Economic models of colorectal cancer screening in average-risk adults: workshop summary. Institute of Medicine, National Academies Press; 2005. [PubMed] [Google Scholar]
- 54.Pignone M, Saha S, Hoerger T, Mandelblatt J. Cost-effectiveness analyses of colorectal cancer screening: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:96–104. doi: 10.7326/0003-4819-137-2-200207160-00007. [DOI] [PubMed] [Google Scholar]
- 55.Vijan S, Hwang EW, Hofer TP, Hayward RA. Which colon cancer screening test? A comparison of costs, effectiveness, and compliance. Am J Med. 2001;111:593–601. doi: 10.1016/s0002-9343(01)00977-9. [DOI] [PubMed] [Google Scholar]
- 56.Wagner J, Tunis S, Brown M, Ching A, Almeida R. Cost-effectiveness of colorectal cancer screening in average-risk adults. In: Young G, Rozen P, Levin B, editors. Prevention and Early Detection of Colorectal Cancer. Saunders; London: 1996. pp. 321–56. [Google Scholar]
- 57.Werler M, Shapiro S, Mitchell A. Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA. 1993;269:1257–61. [PubMed] [Google Scholar]
- 58.Daly LE, Kirke PN, Molloy A, Weir DG, Scott JM. Folate levels and neural tube defects. Implications for prevention.[comment] JAMA. 1995;274:1698–702. doi: 10.1001/jama.1995.03530210052030. [DOI] [PubMed] [Google Scholar]
- 59.Daly S, Mills JL, Molloy AM, Conley M, Lee YJ, Kirke PN, Weir DG, Scott JM. Minimum effective dose of folic acid for food fortification to prevent neural-tube defects. Lancet. 1997;350:1666–9. doi: 10.1016/S0140-6736(97)07247-4. [DOI] [PubMed] [Google Scholar]
- 60.Wald NJ, Law MR, Morris JK, Wald DS. Quantifying the effect of folic acid. Lancet. 2001;358:2069–73. doi: 10.1016/s0140-6736(01)07104-5. [DOI] [PubMed] [Google Scholar]
- 61.Drogan D, Klipstein-Grobusch K, Dierkes J, Weikert C, Boeing H. Dietary intake of folate equivalents and risk of myocardial infarction in the European Prospective Investigation into Cancer and Nutrition (EPIC)--Potsdam study. Public Health Nutr. 2006;9:465–71. doi: 10.1079/phn2005863. [DOI] [PubMed] [Google Scholar]
- 62.Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med. 1996;156:1097–100. [PubMed] [Google Scholar]
- 63.Novakovic P, Stempak JM, Sohn KJ, Kim YI. Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis. 2006;27:916–24. doi: 10.1093/carcin/bgi312. [DOI] [PubMed] [Google Scholar]
- 64.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. Jama. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 65.Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut. 2006;55:1461–6. doi: 10.1136/gut.2005.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 67.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 68.Carlsson CM. Lowering homocysteine for stroke prevention. Lancet. 2007;369:1841–2. doi: 10.1016/S0140-6736(07)60830-7. [DOI] [PubMed] [Google Scholar]
- 69.Wang X, Qin X, Demirtas H, Li J, Mao G, Huo Y, Sun N, Liu L, Xu X. Efficacy of folic acid supplementation in stroke prevention: a meta-analysis. Lancet. 2007;369:1876–82. doi: 10.1016/S0140-6736(07)60854-X. [DOI] [PubMed] [Google Scholar]
- 70.Toole J, Malinow M, Chambless L, Spence JD, Pettigrew LC, Howard VJ, Sides EG, Wang C-H, Stampfer M. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: The Vitamin Intervention for Stroke Prevention (VISP) Randomized Controlled Trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 71.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–9. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 72.Ries L, Melbert D, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2004. National Cancer Institute; Bethesda, MD: [Accessed December 2007]. 2007. at: http://seer.cancer.gov/csr/1975_2004/ [Google Scholar]
- 73.Czeizel AE, Metneki J, Dudas I. The higher rate of multiple births after periconceptional multivitamin supplementation: an analysis of causes. Acta Genet Med Gemellol (Roma) 1994;43:175–84. doi: 10.1017/s0001566000001938. [DOI] [PubMed] [Google Scholar]
- 74.Li Z, Gindler J, Wang H, Berry RJ, Li S, Correa A, Zheng JC, Erickson JD, Wang Y. Folic acid supplements during early pregnancy and likelihood of multiple births: a population-based cohort study. Lancet. 2003;361:380–4. doi: 10.1016/s0140-6736(03)12390-2. [DOI] [PubMed] [Google Scholar]
- 75.Lawrence JM, Watkins ML, Chiu V, Erickson JD, Petitti DB, Kim YI. Food fortification with folic acid and rate of multiple births, 1994-2000. Birth Defects Res A Clin Mol Teratol. 2004;70:948–52. doi: 10.1002/bdra.20088. [DOI] [PubMed] [Google Scholar]
- 76.Kucik J, Correa A. Trends in twinning rates in metropolitan Atlanta before and after folic acid fortification. J Reprod Med. 2004;49:707–12. [PubMed] [Google Scholar]
- 77.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–8. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 78.Henao O, Piyathilake C, Waterbor J, Funkhouser E, Johanning G, Heimburger D, Partridge E. Women with polymorphisms of methylenetetrahydrofolate reductase (MTHFR) and methionine synthase (MS) are less likely to have cervical intraepithelial neoplasia (CIN) 2 or 3. Int J Cancer. 2005;113:991–7. doi: 10.1002/ijc.20695. [DOI] [PubMed] [Google Scholar]
- 79.Larsson S, Giovannucci E, Wolk A. Folate and risk of breast cancer: a meta-analysis. J Natl Cancer Inst. 2007;99:64–76. doi: 10.1093/jnci/djk006. [DOI] [PubMed] [Google Scholar]
- 80.Durga J, van Boxtel MP, Schouten EG, Kok FJ, Jolles J, Katan MB, Verhoef P. Effect of 3-year folic acid supplementation on cognitive function in older adults in the FACIT trial: a randomised, double blind, controlled trial. Lancet. 2007;369:208–16. doi: 10.1016/S0140-6736(07)60109-3. [DOI] [PubMed] [Google Scholar]
- 81.Mills JL, Von Kohorn I, Conley MR, Zeller JA, Cox C, Williamson RE, Dufour DR. Low vitamin B-12 concentrations in patients without anemia: the effect of folic acid fortification of grain. Am J Clin Nutr. 2003;77:1474–7. doi: 10.1093/ajcn/77.6.1474. [DOI] [PubMed] [Google Scholar]
- 82.Morris MS, Jacques PF, Rosenberg IH, Selhub J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am J Clin Nutr. 2007;85:193–200. doi: 10.1093/ajcn/85.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ray JG, Vermeulen MJ, Langman LJ, Boss SC, Cole DE. Persistence of vitamin B12 insufficiency among elderly women after folic acid food fortification. Clin Biochem. 2003;36:387–91. doi: 10.1016/s0009-9120(03)00061-4. [DOI] [PubMed] [Google Scholar]
- 84.Brouwer I, Verhoef P. Folic acid fortification: is masking of vitamin B-12 deficiency what we should really worry about? Am J Clin Nutr. 2007;86:897–8. doi: 10.1093/ajcn/86.4.897. [DOI] [PubMed] [Google Scholar]
- 85.Liem A, Reynierse-Buitenwerf GH, Zwinderman AH, Jukema JW, van Veldhuisen DJ. Secondary prevention with folic acid: effects on clinical outcomes. J Am Coll Cardiol. 2003;41:2105–13. doi: 10.1016/s0735-1097(03)00485-6. [DOI] [PubMed] [Google Scholar]
- 86.The Heart Outcomes Prevention Evaluation Investigators Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 87.Wyckoff KF, Ganji V. Proportion of individuals with low serum vitamin B-12 concentrations without macrocytosis is higher in the post folic acid fortification period than in the pre folic acid fortification period. Am J Clin Nutr. 2007;86:1187–92. doi: 10.1093/ajcn/86.4.1187. [DOI] [PubMed] [Google Scholar]
- 88.Powers HJ. Folic acid under scrutiny. Br J Nutr. 2007;98:665–6. doi: 10.1017/S0007114507795326. [DOI] [PubMed] [Google Scholar]
- 89.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics--2006 Update. A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006 doi: 10.1161/CIRCULATIONAHA.105.171600. CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]